Abstract
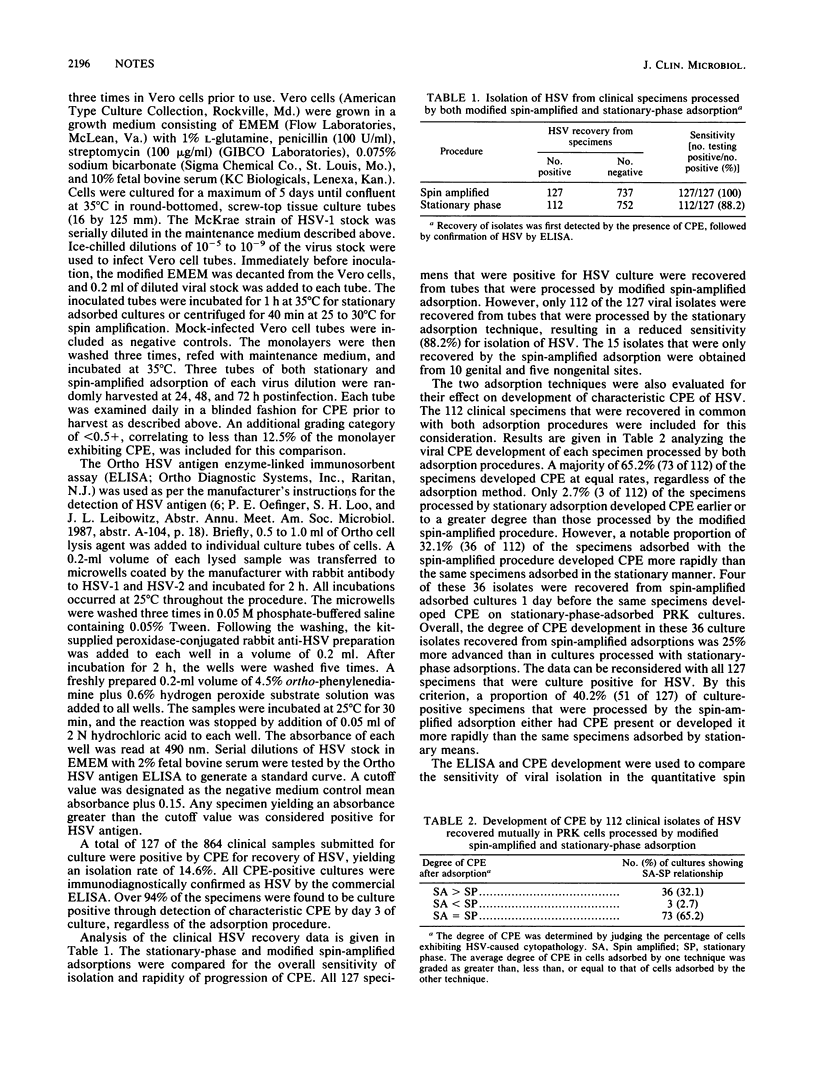
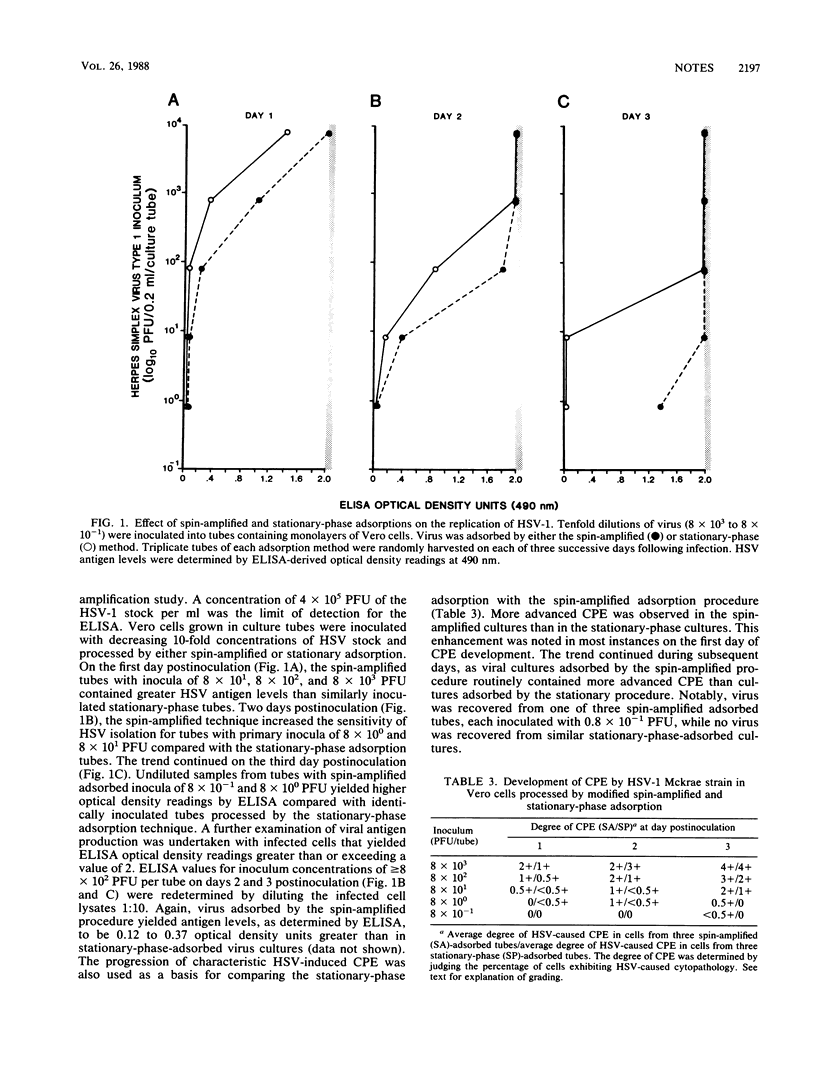
Conventional culture tubes were used in a modification of the spin-amplified adsorption procedure for recovery of herpes simplex virus (HSV) from clinical specimens. The sensitivity of isolation of HSV from 864 specimens adsorbed by the spin-amplified method was 100% (127 of 127), compared with 88.2% (112 of 127) for stationary-phase-adsorbed specimens. Cytopathic effect developed more rapidly in 32.1% (36 of 112) of isolates adsorbed by spin amplification than in those adsorbed by stationary means. In a separate quantitative study, cultures of HSV type 1 adsorbed by spin amplification yielded higher antigen levels and greater cytopathic effect than stationary-phase-adsorbed cultures. Cells grown in conventional tissue culture tubes may be used in a spin-amplified adsorption for rapid detection and increased sensitivity of HSV isolation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GORDON F. B., QUAN A. L., TRIMMER R. W. Morphologic observations on trachoma virus in cell cultures. Science. 1960 Mar 11;131(3402):733–734. doi: 10.1126/science.131.3402.733. [DOI] [PubMed] [Google Scholar]
- Gleaves C. A., Smith T. F., Shuster E. A., Pearson G. R. Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J Clin Microbiol. 1984 Jun;19(6):917–919. doi: 10.1128/jcm.19.6.917-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleaves C. A., Wilson D. J., Wold A. D., Smith T. F. Detection and serotyping of herpes simplex virus in MRC-5 cells by use of centrifugation and monoclonal antibodies 16 h postinoculation. J Clin Microbiol. 1985 Jan;21(1):29–32. doi: 10.1128/jcm.21.1.29-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. B., Misra V., Mosmann T. R. Cytomegalovirus infectivity: analysis of the phenomenon of centrifugal enhancement of infectivity. Virology. 1976 Jul 1;72(1):235–243. doi: 10.1016/0042-6822(76)90326-3. [DOI] [PubMed] [Google Scholar]
- Michalski F. J., Shaikh M., Sahraie F., Desai S., Verano L., Vallabhaneni J. Enzyme-linked immunosorbent assay spin amplification technique for herpes simplex virus antigen detection. J Clin Microbiol. 1986 Aug;24(2):310–311. doi: 10.1128/jcm.24.2.310-311.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. A., Smith T. F. Evaluation of an enzyme-linked immunosorbent assay for the detection of herpes simplex virus antigen. J Clin Microbiol. 1984 Jun;19(6):730–732. doi: 10.1128/jcm.19.6.730-732.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn J. E., Walker D. L. Enhancement of infectivity of murine cytomegalovirus in vitro by centrifugal inoculation. J Virol. 1968 Sep;2(9):853–858. doi: 10.1128/jvi.2.9.853-858.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruneda R. C., Almanza I. Centrifugation-shell vial technique for rapid detection of herpes simplex virus cytopathic effect in Vero cells. J Clin Microbiol. 1987 Feb;25(2):423–424. doi: 10.1128/jcm.25.2.423-424.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon V. C., Turner R. B., Speranza M. J., Overall J. C., Jr Rapid detection of herpes simplex virus in clinical specimens by centrifugation and immunoperoxidase staining. J Clin Microbiol. 1986 Apr;23(4):683–686. doi: 10.1128/jcm.23.4.683-686.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster E. A., Beneke J. S., Tegtmeier G. E., Pearson G. R., Gleaves C. A., Wold A. D., Smith T. F. Monoclonal antibody for rapid laboratory detection of cytomegalovirus infections: characterization and diagnostic application. Mayo Clin Proc. 1985 Sep;60(9):577–585. doi: 10.1016/s0025-6196(12)60979-3. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Gerba C. P. Development of a method for detection of human rotavirus in water and sewage. Appl Environ Microbiol. 1982 Jun;43(6):1440–1450. doi: 10.1128/aem.43.6.1440-1450.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson P. D., Kaplan M. H. Comparison of two rapid culture methods for detection of cytomegalovirus in clinical specimens. J Clin Microbiol. 1987 Dec;25(12):2445–2446. doi: 10.1128/jcm.25.12.2445-2446.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS E., DRESSLER H. R. Centrifugation and Rickettsiae and viruses onto cells and its effect on infection. Proc Soc Exp Biol Med. 1960 Apr;103:691–695. doi: 10.3181/00379727-103-25637. [DOI] [PubMed] [Google Scholar]


