Abstract
During some persistent viral infections, virus-specific T-cell responses wane due to the antigen-specific deletion or functional inactivation (i.e., exhaustion) of responding CD8 T cells. T-cell exhaustion often correlates with high viral load and is associated with the expression of the inhibitory receptor PD-1. In other infections, functional T cells are observed despite high levels of pathogen persistence. The reasons for these different T-cell fates during chronic viral infections are not clear. Here, we tracked the fate of virus-specific CD8 T cells in lymphocytic choriomeningitis virus (LCMV)-infected mice during viral clearance, the persistence of wild-type virus, or the selection and persistence of a viral variant that abrogates the presentation of a single epitope. Viral clearance results in PD-1lo functional virus-specific CD8 T cells, while the persistence of wild-type LCMV results in high PD-1 levels and T-cell exhaustion. However, following the emergence of a GP35V→A variant virus that abrogates the presentation of the GP33 epitope, GP33-specific CD8 T cells remained functional, continued to show low levels of PD-1, and reexpressed CD127, a marker of memory T-cell differentiation. In the same animals and under identical environmental conditions, CD8 T cells recognizing nonmutated viral epitopes became physically deleted or were PD-1hi and nonfunctional. Thus, the upregulation of PD-1 and the functional inactivation of virus-specific T cells during chronic viral infection is dependent upon continued epitope recognition. These data suggest that optimal strategies for vaccination should induce high-magnitude broadly specific T-cell responses that prevent cytotoxic T-lymphocyte escape and highlight the need to evaluate the function of vaccine-induced T cells in the context of antigens presented during virus persistence.
Mounting evidence suggests that CD8 T cells are crucial for the control or elimination of a wide variety of human viral infections and malignancies (1). However, many viruses are able to establish a chronic infection, and this persistence has been associated with a loss of CD8 T-cell responses due to either the physical elimination (deletion) (26) or functional inactivation (exhaustion) of distinct epitope-specific CD8 T-cell populations (44). Antigen-specific CD8 T-cell unresponsiveness was first defined during chronic lymphocytic choriomeningitis virus (LCMV) infection (49). T-cell deletion and exhaustion subsequently have been observed for many chronic viral infections, including during the infection of mice with mouse hepatitis virus or gammaherpesvirus (6, 24), during simian immunodeficiency virus (SIV) and hepatitis C virus (HCV) infection of monkeys (12, 43, 47), and also during HCV and human immunodeficiency virus (HIV) infection of humans (18, 22, 38). CD8 T-cell unresponsiveness also has been reported for tumor-specific CD8 T cells responding to melanoma antigens or to Epstein-Barr virus in non-Hodgkin's lymphoma patients (23, 42).
The fact that CD8 T-cell dysregulation has been observed in mice, nonhuman primates, and humans, as well as the number of different infections and antigens at which these responses are directed, makes it likely that the functional inactivation of antigen-specific CD8 T cells is a conserved mechanism for silencing prolonged T-cell responses and limiting cytotoxic T-lymphocyte (CTL)-mediated pathology. In seeming direct contrast to these observations, functional virus-specific T cells also have been detected in some patients and certain animal models despite high levels of viral replication (17, 27). The reasons why functional T-cell responses persist in some settings of chronic viral infections but not others remain poorly understood. The functional exhaustion of CD8 T cells has been shown to correlate with PD-1 expression, and the blockade of PD-1 can reverse exhaustion, but the factors that govern PD-1 expression and T-cell exhaustion in such settings are also not well defined (4, 33). While some evidence supports the idea that T-cell exhaustion during chronic viral infections is a progressive process (40, 46), PD-1 expression also could result from aberrant signals received early during T-cell priming. During persisting infections, ongoing antigen stimulation can be abrogated by the clearance/control of infection (either immunological or pharmacological) or by the mutation of relevant T-cell epitopes, and this has been suggested to result in the maintenance of T-cell function during chronic infection (40). However, it is clear that viruses as well as tumors use the epitope escape strategy to facilitate persistence (15, 30), and it is not entirely clear how such escape impacts T-cell exhaustion and memory T-cell differentiation.
We have studied the role of epitope escape on PD-1 expression, CD8 T-cell exhaustion, and memory T-cell differentiation during persistent LCMV infection by examining CD8 T-cell responses following epitope mutation and responses when epitopes are preserved. We show here that the continued expression of PD-1 and the loss of function by LCMV DbGP33-specific CD8 T cells are dependent on continued epitope presentation during chronic LCMV infection. Virus clearance results in the development of PD-1lo CD127hi functional memory T cells, while the persistence of wild-type LCMV results in the formation of PD-1hi CD127lo exhausted T cells. When the presentation of the GP33 epitope is abrogated by mutation early during chronic viral infection, DbGP33-specific CD8 T cells in viremic mice become PD-1lo and maintain the ability to respond functionally and upregulate CD127, a marker associated with memory T-cell development, similarly to mice that have cleared acute LCMV infection. In these same mice, however, under identical cytokine conditions and a lack of CD4 T-cell help, CD8 T cells recognizing other nonmutated viral epitopes become either functionally unresponsive (DbGP276, KbNP205) and are PD-1hi CD127lo or are clonally deleted (DbNP396) irrespective of the fate of DbGP33-specific cells. Thus, these studies demonstrate the importance of persisting antigenic stimulation in driving T-cell exhaustion. These results also suggest that early epitope mutation during chronic infection allows the development of functional T-cell responses, suggesting that early priming differences between acute and chronic infection are not solely responsible for the development of functional memory T cells and exhausted CD8 T cells, respectively.
MATERIALS AND METHODS
Animals and virus.
Six- to 8-week-old female C57Bl/6J (B6) or P14 B6.D2-TgN (T-cell receptor transgenic [TCR-Tg]) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). TCR-Tg mice were backcrossed to the B6 background (>10 generations) at the Emory University animal facility prior to use. All experiments were conducted under Emory University IACUC approval and followed all relevant federal guidelines and institutional policies. The clone 13 strain of LCMV was grown as previously described (25). For adoptive T-cell transfers, single-cell suspensions of splenocytes from naïve P14 TCR-Tg mice were delivered intravenously (i.v.) to naïve CD4-depleted B6 mice as previously described (49). Recipients were infected with 2 × 106 PFU of LCMV (clone 13) i.v. 2 days posttransfer. Virus titers were determined by plaque assay on Vero cell monolayers as previously described (49).
Antibodies and MHC tetramers.
All antibodies were purchased from BD Biosciences (San Diego, CA). Major histocompatibility complex class I (MHC-I) tetramers were refolded with synthetic peptides and prepared as previously described (3).
Cells and flow cytometry.
For fluorescence-activated cell sorter analysis, cells were resuspended in phosphate-buffered saline (2% bovine serum albumin, 0.2% sodium azide) and stained with the indicated reagents at a final concentration of 1 mg/ml for 30 min at 4°C. Cells then were washed twice, fixed in 2% paraformaldehyde solution, and immediately acquired on a FACSCalibur flow cytometer (BD Biosciences). Intracellular staining for gamma interferon (IFN-γ) and tumor necrosis factor (TNF) was done as previously described (28).
Viral sequencing.
Viral isolates were plaque purified on Vero cell monolayers and propagated to high titers in BHK cells. RNA from viral supernatants was extracted by guanidine isothiocyanate-phenol disruption, and cDNA synthesis was performed (using 5′-GCTCGAAACTATACTCATGA-3′). Viral templates were amplified by PCR (40 cycles, 55°C annealing temperature) using primers 5′-TTCCTCTAGATCAACTGGGTGTCA-3′ and 5′-GCAGAGGTCAGATTGCAAAAGTTG-3′. Sequences were obtained using an internal primer (5′-AATGTTTGAGGCTCTGCCTC-3′) on an ABI-377 (Applied Biosystems, Foster City, CA) automated sequencer.
RESULTS
Titration of donor TCR-Tg cells and patterns of viral infection.
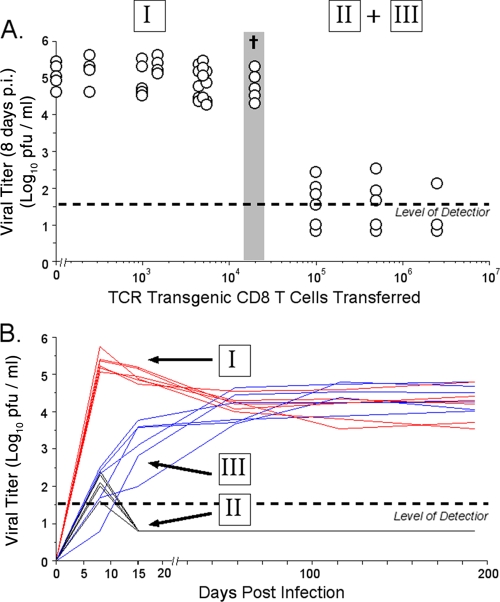
To begin to investigate how the precursor frequency of virus-specific naïve CD8 T cells influenced the outcome of chronic viral infection, we utilized the adoptive transfer of small numbers of LCMV-specific TCR-Tg CD8 T cells from the P14 mouse bearing a TCR specific for the DbGP33-41 epitope of LCMV. We generated TCR-Tg chimeric mice by the adoptive transfer of graded numbers of CD8 T cells from P14 TCR-Tg mice into intact, nonirradiated, syngeneic B6 recipients. Since many chronic viral infections, including LCMV, are more severe in the absence of efficient CD4 T-cell help, recipient mice were depleted of CD4 T cells using the GK1.5 antibody and then infected with the virulent clone 13 strain of LCMV. The transfer of up to 2 × 104 donor CD8 T cells, followed by LCMV infection, did not alter the initial virus titer 8 days postinfection (p.i.) from that seen during the infection of B6 mice (Fig. 1A). Mice given this dose of cells were unable to control LCMV infection and thereafter maintained high levels of viremia (Fig. 1B). Thus, mice given <2 × 104 TCR-Tg cells had viral kinetics indistinguishable from those of LCMV clone 13-infected B6 mice that did not receive TCR-Tg cells. However, when ≥105 donor P14 TCR-Tg cells were transferred, initial viremia (8 days p.i.) was dramatically lower (Fig. 1A). Approximately 50% of mice in this group efficiently controlled the LCMV infection, and we were unable to detect virus in sera or tissues from these mice >15 days p.i. (Fig. 1B). Surprisingly, the remainder of mice receiving ≥105 donor TCR-Tg cells did not control the LCMV infection but became persistently infected (Fig. 1B). Thus, mice given higher doses of TCR-Tg cells either cleared the infection or, after early initial transient control, became persistently infected with virus at levels reaching those of mice receiving fewer cells. Interestingly, the transfer of an intermediate dose of TCR-Tg donor cells (2 × 104) followed by LCMV infection was lethal at 9 to 10 days p.i. (Fig. 1A). Thus, we observed three distinct phenotypes of viremia during the infection of TCR-Tg chimeric mice with LCMV clone 13: (i) high initial viremia followed by persistence and high viral load, (ii) initial virus control and clearance, and (iii) initial partial control of the infection but the establishment of a persistent infection and high viral load.
FIG. 1.
Transfer of different numbers of virus-specific TCR-Tg cells, followed by LCMV infection, results in different patterns of viral infection. (A) Mice received the indicated number of CD8 TCR-Tg cells and were depleted of CD4 T cells 2 days prior to infection with LCMV clone 13. Serum virus titers were determined 8 days p.i. by plaque assay. A dagger indicates that all of the mice receiving this dose of cells died at 9 to 10 days p.i. in five separate experiments. (B) LCMV kinetics in individual mice that received <20,000 donor cells and had high initial viremia followed by persistence (group I), mice that received >20,000 donor cells and had low initial viremia and control (group II), or mice that had low initial viremia but the establishment of a persistent infection with high virus levels (group III). Virus levels in serum were determined by plaque assay at the indicated times p.i. Five representative mice from each group are shown.
Fate of virus-specific CD8 T cells.
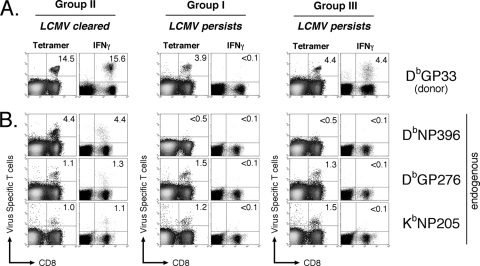
To understand the reasons for the different patterns of viral infection established in these different groups of mice, we first examined the fate of donor DbGP33-specific CD8 T cells in recipient mice. Mice that controlled the viral infection had similar numbers of donor DbGP33-specific CD8 T cells as measured by MHC-I tetramers or in functional assays measuring the intracellular production of IFN-γ (Fig. 2A, group II). Thus, similarly to endogenous polyclonal CD8 T-cell responses in acutely infected mice, the efficient control of LCMV infection results in a functionally competent TCR-Tg memory virus-specific CD8 T-cell population.
FIG. 2.
Fate and function of virus-specific CD8 T cells in LCMV-infected mice. Groups are the same as those described in the legend to Fig. 1B. Splenocytes from LCMV-infected mice were isolated at >30 days p.i. and analyzed by flow cytometry following staining with the indicated tetramers ex vivo or after 5 h of restimulation in vitro with the indicated peptide, followed by intracellular staining for IFN-γ. The physical presence (tetramer) and function (IFN-γ) of donor TCR-Tg CD8 T cells (A) and endogenous polyclonal virus-specific CD8 T cells (B) are shown. Percentages indicate the fraction of CD8 T cells. Data are representative of five separate experiments (group I, n = 48; group II, n = 11; group III, n = 37).
We also were able to detect DbGP33-specific CD8 T cells by MHC-I tetramers in mice that had high initial levels of viremia and subsequently were unable to control the infection (Fig. 2A, group I). However, very few DbGP33-specific CD8 T cells in these mice were capable of producing either IFN-γ or TNF-α in response to peptide stimulation in vitro, and these cells were only weakly cytolytic (Fig. 2A and data not shown). Thus, donor DbGP33-specific CD8 T cells in mice persistently infected with LCMV that had high initial viremia become functionally unresponsive, which is similar to the fate of polyclonal DbGP33-specific CD8 T cells in chronically infected B6 mice.
Mice that initially had low levels of viremia but whose virus levels eventually reached ∼104 to 105 PFU/ml also had numbers of DbGP33-specific CD8 T cells that were detectable by MHC-I tetramer staining (Fig. 2A, group III). Surprisingly, the vast majority of DbGP33-specific CD8 T cells in these mice also could be accounted for in functional assays, such as intracellular staining for IFN-γ, and were capable of cytolysis despite the high levels of viremia (Fig. 2A, group III, and data not shown). Approximately half of these cells also were able to produce TNF-α following peptide restimulation ex vivo (data not shown). Thus, we have generated mice that are persistently infected with LCMV and in which donor DbGP33-specific CD8 T cells either maintain function (group III) or become functionally unresponsive (group I).
In mice that controlled the LCMV infection, endogenous DbNP396-, DbGP276-, and KbNP205-specific CD8 T cells were detectable both by MHC-I tetramers and cytokine production following peptide restimulation ex vivo; i.e., all epitope-specific responses were functional (Fig. 2B, group II). However, in both group I and group III of mice persistently infected with LCMV, regardless of the functionality of the DbGP33-specific CD8 T cells, DbNP396-specific CD8 T cells became clonally deleted, as we were not able to detect these cells by MHC tetramer staining, while DbGP276- and KbNP205-specific CD8 T cells were detectable by MHC-I tetramer but failed to elicit effector functions upon antigen restimulation (Fig. 2B, groups I and III). Thus, the deletion or functional exhaustion of endogenous CD8 T cells specific for other viral epitopes remained unchanged in mice persistently infected with LCMV independently of the functional status of DbGP33-specific CD8 T cells.
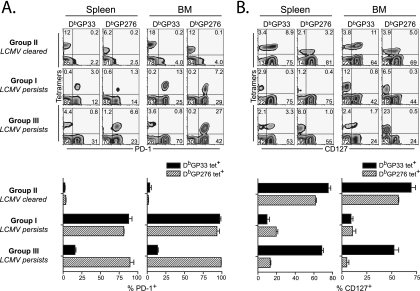
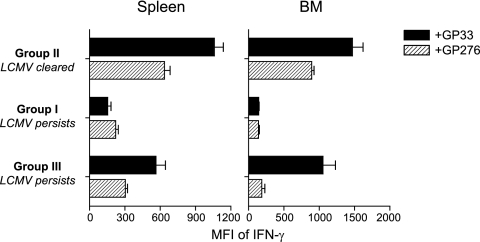
We next examined whether virus-specific CD8 T cells with different functional qualities in groups I, II, and III differed in the expression of molecules associated with T-cell exhaustion (PD-1) and functional memory T-cell development (CD127). In mice that controlled the infection, both donor DbGP33-specific as well as endogenous DbGP276-specific CD8 T cells from either the spleen or bone marrow expressed low levels of PD-1, and a majority of these cells also expressed CD127 (Fig. 3, group II). CD127 is the alpha chain of the interleukin-7 receptor, is necessary for memory T-cell homeostatic proliferation, and is a surrogate marker of memory T-cell development (20). Conversely, in mice that had high initial viremia and became persistently infected with LCMV, both DbGP33-specific and DbGP276-specific CD8 T cells in either the spleen or bone marrow expressed high levels of PD-1, while most of these cells were CD127 negative, which is consistent with functional exhaustion (Fig. 3, group I). In contrast, in mice that had low initial viremia but which became persistently infected with LCMV, DbGP33-specific CD8 T cells present >30 days p.i. expressed low levels of PD-1, and many of these cells had upregulated CD127 (Fig. 3, group III). The percentage and mean fluorescence intensity (MFI) of CD127 expression on these DbGP33-specific CD8 T cells in group III were substantially higher than that found in the viremic mice from group I, but they never reached the levels observed in the mice that rapidly cleared the infection in group II. In addition, the MFI of IFN-γ produced by splenic or bone marrow DbGP33-specific CD8 T cells from group III also was intermediate between those from viremic mice in group I (i.e., no IFN-γ) and those in group II, suggesting that while the DbGP33-specific CD8 T cells in these mice persistently infected with LCMV were not exhausted and expressed markers of memory CD8 T-cell differentiation, these cells were not as optimal as DbGP33-specific CD8 T cells generated in mice following acute infection (Fig. 4). In these same mice from group III, however, DbGP276-specific T cells from both the spleen and bone marrow were PD-1hi and CD127lo, which is consistent with a functionally exhausted phenotype despite the fact that DbGP33-specific CD8 T cells in these mice were PD-1lo and CD127hi. Thus, low initial viremia but eventual viral persistence not only favored the persistence of functional DbGP33-specific CD8 T cells, but these cells also showed evidence of differentiation toward a memory T-cell fate, including the downregulation of PD-1 and the upregulation of CD127, while CD8 T cells responding to other epitopes in the same animals remained exhausted (PD-1hi CD127lo).
FIG. 3.
PD-1 and CD127 expression on virus-specific CD8 T cells is dependent on antigen persistence. Groups are the same as those described in the legend to Fig. 1B. Lymphocytes of spleen and bone marrow were isolated from mice at day 55 p.i. and stained with the indicated reagents. PD-1 (A) and CD127 (B) expression on the indicated tetramer (DbGP33 or DbGP276) were shown by representative plots gated on CD8 T cells and by percent expression (average ± standard deviation). Data are representative of mice from each group (n = 5).
FIG. 4.
Altered function of CD8 T cells during different patterns of viral infection. Lymphocytes isolated from spleen and bone marrow (BM) of mice generated as described in the legend to Fig. 3 were stimulated with GP33 or GP276 peptide for 5 h in vitro, followed by intracellular staining for IFN-γ. The MFI of IFN-γ expression in cells capable of making IFN-γ responsive to corresponding peptide was shown as the average ± standard deviation (n = 5).
Viral isolation and sequencing.
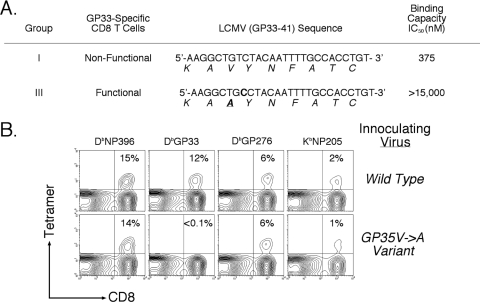
Given that we have increased the precursor frequency for only the GP33 epitope and the function of GP33-specific T cells is different between mice in group I and group III, we reasoned that the differences in donor CD8 T-cell function in TCR-Tg chimeric mice could be due to the selection of a GP33-mutated LCMV variant virus. Therefore, we sequenced virus isolates from chronically infected TCR-Tg chimeric mice in which donor DbGP33-specific CD8 T-cell function was either maintained (group III) or lost (group I). We found only the original GP33-41 viral sequence in persistently infected mice (n = 6/6) with nonfunctional DbGP33-specific CD8 T cells (Fig. 5A). However, viral sequences from mice in which DbGP33-specific T cells maintained functional responsiveness, despite high viral burden, contained a point mutation in the GP33-41 epitope that resulted in a V→A mutation at position 35 (n = 6/6). This mutation results in a >500-fold decrease in the binding affinity of this peptide to H-2Db (36). Thus, the persistence of the wild-type sequence of virus correlated with functional unresponsiveness by GP33-specific CD8 T cells, while the selection of the GP35V→A variant virus correlated with the maintenance of function by GP33-specific CD8 T cells.
FIG. 5.
Selection of GP35V→A CTL escape variant virus explains differences in GP33-specific CD8 T cells in persistently infected mice. Groups are the same as those described in the legend to Fig. 1B. (A) Serum was obtained from six mice each from group I and group III, and virus levels were determined by plaque assay. Six plaques were selected and grown to high titer in BHK cells and then were subjected to isolation and sequencing. Shown are the nucleotide sequences obtained as well as the resulting changes in amino acid coding sequences. Fifty percent inhibitory concentrations are derived from Puglielli et al. (36). (B) GP35V→A variant LCMV does not elicit a DbGP33 CD8 T-cell response. Mice were infected 8 days prior with the wild-type sequence clone 13 or the GP35V→A variant LCMV. Virus-specific CD8 T cells were quantitated by staining splenocytes with MHC-I tetramers as indicated. Percentages indicate the fraction of CD8 T cells. Data are from representative mice (n = 6 for each group).
To test whether the GP35V→A mutation abrogates GP33-41 presentation by H-2Db, and also to examine whether other epitopes remained nonmutated and unaffected, we infected nontransgenic B6 mice with a wild-type LCMV sequence derived from mice with nonfunctional DbGP33-specific CD8 T cells or with the variant GP35V→A LCMV derived from mice with functional DbGP33-specific CD8 T cells and examined the resulting LCMV-specific CD8 T-cell responses. In mice that received the wild-type sequence virus, all immunodominant responses, including those specific for the DbGP33 epitope, were detectable at 8 days p.i. (Fig. 5B). However, in mice that received the GP35V→A variant virus, no DbGP33-specific CD8 T cells were detected despite the fact that all other epitope-specific responses were normal; i.e., there were no mutations in other epitopes that resulted in their inability to stimulate CD8 T cells. This strongly suggests that the GP35V→A mutation results in the loss of the presentation of GP33-41 by H-2Db and also that other epitopes continue to be presented in mice that select for the GP35V→A variant of LCMV.
Taken together, these observations suggest that epitope escape during chronic viral infection can result in the persistence of functional, nonexhausted CD8 T cells specific for the original epitope sequence. It is interesting that the escape in this LCMV TCR-Tg system likely occurs quite early, as the viral load is similar between groups I and III by ∼3 to 4 weeks post infection. It is likely that this early escape is part of the reason for the persistence of functional T cells. The development of escape mutations at later times during chronic viral infections after T cells have been chronically stimulated for prolonged periods may result in different outcomes in terms of T-cell function and differentiation. Another point worth considering is what factors facilitate epitope escape. In the TCR-Tg adoptive transfer experiments presented here, an increase in T-cell precursor frequency likely means stronger selective pressure on the GP33 epitope to escape. However, the transfer of TCR-Tg T cells could suppress endogenous T-cell responses, and indeed the numbers of NP396- and GP276-specific CD8 T cells are reduced at early time points when P14 cells are transferred (data not shown). Thus, one factor that may favor epitope escape is a highly focused CD8 T-cell response where the mutation of a single determinant provides a replication advantage to the pathogen.
DISCUSSION
We have shown that, during chronic LCMV infection, continued epitope recognition is a major factor in determining the functional status of CD8 T cells. Viral clearance results in competent memory CD8 cells that are positive for effector functions, while viral persistence correlates with the deletion or loss of function in virus-specific CD8 T cells. However, if an individual viral epitope is no longer presented efficiently in the present study due to the selection of the GP35V→A variant virus early during chronic infection, only CD8 T cells specific for that epitope maintain or regain function. CD8 T cells specific for other viral epitopes that continue to be presented are deleted or rendered nonfunctional (Table 1). These results suggest that the continued recognition of antigen is a major determinant of T-cell function and differentiation during chronic viral infection. Moreover, increased PD-1 levels and a lack of CD127 expression in virus-specific T cells correlated with antigen recognition but not overall viral load. These results show that at least three distinct mechanisms may operate simultaneously to attenuate the antiviral T-cell response during chronic viral infection in vivo: clonal deletion (death) (DbNP396), functional unresponsiveness (exhaustion) (DbGP276 and DbGP33), or viral persistence due to the selection of a variant virus that evades CTL recognition (ignorance) (DbGP33). These observations may be useful for understanding chronic viral infections in humans, such as those by HIV and HCV. For example, some studies have shown that CD8 T cells specific for these pathogens have reduced cytolytic activity and perforin and/or cytokine expression, while others have reported that functional exhaustion during these infections may not play a major role (16, 17, 18, 21, 22). This discrepancy may be due to differences in the selection of CTL escape variant viruses; if the selection of a CTL escape variant has occurred, virus-specific CD8 T cells may appear functional during in vitro assays. In contrast, if the original-sequence virus persists, CD8 T cells will be functionally impaired and display poor functional responses to antigen following restimulation in ex vivo assays. Indeed, during SIV infection, PD-1 expression is reduced on CD8 T-cell populations responding to an epitope that mutates early during infection (32). In this study, the authors examined an epitope highly prone to mutate in chronically infected primates. However, the impact of epitope mutation on T-cell functionality, avoiding T-cell exhaustion, and memory CD8 T-cell differentiation was not examined.
TABLE 1.
Summary of T-cell functional status during virus clearance, the persistence of the wild-type sequence virus, and the persistence of the GP35V→A variant virusa
| Result of infection | DbGP33-41 | DbNP396-404 | DbGP276-285 | KbNP205-215 |
|---|---|---|---|---|
| Clearance | Functional | Functional | Functional | Functional |
| Persistence | ||||
| Wild-type GP33-41 | Exhausted | Deleted | Exhausted | Exhausted |
| GP35V→A | Functional | Deleted | Exhausted | Exhausted |
Functional indicates the positive detection of epitope-specific CD8 T cells by MHC tetramer and by IFN-γ production following peptide stimulation ex vivo. Exhausted indicates detection by tetramer only, and deleted indicates detection by neither tetramer nor IFN-γ production.
It has been shown in some experimental and clinical viral infections that T-cell responses are lost following the selection of a CTL escape virus (11). This has been proposed to be due to the lack of TCR stimulation required to maintain effector CTL populations. Here, we show that the selection of an LCMV escape virus results in CD8 T cells that retain effector function despite high levels of viremia, and that these CD8 T cells persist and begin to acquire markers of memory CD8 T-cell differentiation. A major difference between our current observations and those in other systems is that in the current study, escape occurs very early during infection (during the first 1 to 2 weeks of infection) when CD8 T cells are still functional, while in other infections virus escape often occurs after several months or years. Prolonged exposure to chronic infection and antigen stimulation can lead to virus-specific CD8 T cells that fail to develop normal memory T-cell properties and become dependent on antigen for their long-term maintenance (29, 45). Thus, epitope mutation after prolonged infection may leave T cells committed to an antigen-dependent and functionally suboptimal differentiation state, while early epitope escape, when the T-cell response still is malleable, may favor the development of a more functional population of virus-specific CD8 T cells. This model suggests that there is a finite window early during infection in which PD-1 downregulation, as a result of viral clearance due to immunological responses or pharmacological intervention or the selection of an epitope escape virus, results in functional memory T cells. Other cell signals delivered during prolonged stimulation prior to virus escape also may play a role in modulating the ability of T cells to survive and maintain function. Thus, the level, duration, and timing of antigen stimulation may be critical parameters in determining the survival and functional status of virus-specific CD8 T cells during chronic infections.
In the experiments outlined above, we have increased the selective pressure for the GP35V→A variant virus by increasing the number of DbGP33-specific precursors. While LCMV CTL escape variant viruses can arise during the infection of wild-type mice, these rare events are prevented from becoming the dominant virus population by cells responding to other LCMV epitopes (34). In contrast, LCMV CTL escape normally occurs and becomes the dominant virus sequence during the direct infection of TCR-Tg mice in which virtually all of the CD8 T cells are specific for the GP33 epitope (36). Here, we show the novel finding that selective pressure is capable of being titrated. Increasing the precursor frequency of naïve virus-specific CD8 T cells clearly can impact the strength of the early epitope-specific CD8 T-cell response, and the resulting increase in T-cell competition can limit endogenous responses of the same specificity (7, 10, 35). It is somewhat less clear how increasing the vigor of CD8 T-cell responses to one determinant will impact the early CD8 T-cell response to other viral determinants, though immunodomination or the suppression of weaker T-cell responses by more vigorous antiviral populations can occur (48). One possibility is that a more narrowly focused CTL response against a virus early during infection provides sufficient selective advantage for the virus to mutate the major target of the T-cell response, while a more broadly focused early response may decrease the favorability of mutating any single epitope. CTL escape has been documented in HIV- and HCV-infected individuals with polyclonal CD8 T-cell responses, and recent studies of SHIV-immunized and HCV-immunized primates have shown that vaccine failure correlates with the selection of CTL escape variant viruses as well as T-cell dysfunction (5, 37, 40). These results have implications for vaccine development and suggest that multivalent vaccines that induce a broad antiviral T-cell response are preferable to narrowly focused vaccines that would favor the selection of variant viruses.
At the interface of the persistence of wild-type LCMV and the selection of the CTL escape virus, we observed a very narrow range of donor TCR-Tg cells that (following LCMV infection) was lethal to the host (Fig. 1A). The initial CD8 T-cell response at this dose is extremely large (∼107 DbGP33-specific CD8 T cells/spleen) but sufficiently diverse so that CD8 T cells specific for other viral epitopes prevent the outgrowth of the CTL escape virus. Since LCMV is noncytopathic, death observed at this dose of cells is due to an extremely large immune response meeting an immovable viral infection, resulting in gross immunopathology, organ failure, and death. As either of these forces becomes dominant, the other is compelled to adapt; if the initial viremia is high, virus-specific CD8 T cells are forced to become unresponsive, while if the initial T-cell response is highly focused on a single epitope, the selection of the CTL escape variant virus occurs. In either case, host pathology is curtailed.
In addition to persisting antigen, other aspects of chronic viral infection could modulate the responsiveness and/or differentiation of virus-specific CD8 T cells. While DbGP33-specific CD8 T cells in mice that select the GP35V→A mutant virus are functional and PD-1lo, these cells produce lower levels of IFN-γ after restimulation than true memory DbGP33-specific CD8 T cells from mice that control the infection (Fig. 4). This altered functional profile could be due to differences in the initial strength or duration of the TCR activation signals delivered to these cells, environmental factors present such as damage to the lymphoid compartment or immune accessory cells, or the cytokine milieu present in chronically infected mice (8). Indeed, recent findings indicate that interleukin-10 produced early during chronic viral infection can influence some functions of CD8 T cells and the outcome of chronic LCMV infection (9, 14). Alternatively, although the GP35V→A mutation results in a dramatic reduction in the ability of the peptide to bind to MHC, some residual persistence of the wild-type virus, periodic reversion to wild-type sequence, or low-level presentation of the GP35V→A peptide in vivo may occur. However, we have previously shown that the transfer of T cells from LCMV immune mice into mice infected with the GP35V→A variant virus results in the proliferation of DbGP276-specific, but not DbGP33-specific, CD8 T cells (39). Although these results do not necessarily rule out the residual persistence of the wild-type LCMV or the presentation of low levels of the GP35V→A variant epitope in these mice, they do indicate that there is not sufficient CD8 T-cell recognition of this epitope in mice infected with the GP35V→A variant virus to stimulate these T cells.
We have shown that the continued presentation of viral antigen is a major determining factor in the functional status of virus-specific CD8 T cells. The reversal of this functional impairment in virus-specific T cells remains an ideal strategy for therapeutic intervention in many chronic infections and tumors. We already have shown that the blockade of PD-1 later during infection can significantly improve the functional capacity of virus-specific T cells in mice chronically infected with LCMV (4, 13, 19), while others have shown similar findings during HIV infection of humans (2, 33, 41). Given our results, one likely strategy for maintaining or restoring function in virus-specific CD8 T cells would be the suppression of viral antigen early during infection. It has been previously reported that highly active antiretroviral therapy early during HIV infection is correlated with the improved maintenance of CD8 T-cell responses (31, 33). Indeed, recent evidence has shown that T-cell exhaustion was reduced upon the removal of antigen during persistent HIV infection, either by antiviral therapy or during CTL escape, and suggests that vaccination strategies inducing broad CD8 T-cell responses combined with antiviral therapy provide a novel approach to preventing disease progression (40).
Acknowledgments
We thank B. T. Konieczny for excellent technical assistance and members of the Ahmed laboratory for helpful discussions.
This work was supported by funding from the Foundation for the National Institutes of Health (NIH) through the Grand Challenges in Global Health Initiative and NIH grants AI30048 and AI56299 (to R.A.) and by the Cancer Research Institute (to E.J.W. and J.N.B.) and NIH grant AI071309 (to E.J.W.).
We have no conflicting financial interests.
Footnotes
Published ahead of print on 11 February 2009.
REFERENCES
- 1.Ahmed, R., L. A. Morrison, and D. M. Knipe. 1996. Viral persistence, p. 181-206. In N. Nathanson (ed.), Viral pathogenesis. Lippincott-Raven, Philadelphia, PA.
- 2.Alter, G., N. Teigen, R. Ahern, H. Streeck, A. Meier, E. S. Rosenberg, and M. Altfeld. 2007. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J. Infect. Dis. 1951452-1460. [DOI] [PubMed] [Google Scholar]
- 3.Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 27494-96. [DOI] [PubMed] [Google Scholar]
- 4.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439682-687. [DOI] [PubMed] [Google Scholar]
- 5.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415335-339. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann, C. C., C. Ramakrishna, M. Kornacki, and S. A. Stohlman. 2001. Impaired T cell immunity in B cell-deficient mice following viral central nervous system infection. J. Immunol. 1671575-1583. [DOI] [PubMed] [Google Scholar]
- 7.Blattman, J. N., R. Antia, D. J. Sourdive, X. Wang, S. M. Kaech, K. Murali-Krishna, J. D. Altman, and R. Ahmed. 2002. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 195657-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrow, P., C. F. Evans, and M. B. Oldstone. 1995. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J. Virol. 691059-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks, D. G., M. J. Trifilo, K. H. Edelmann, L. Teyton, D. B. McGavern, and M. B. Oldstone. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 121301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butz, E. A., and M. J. Bevan. 1998. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao, J., J. McNevin, U. Malhotra, and M. J. McElrath. 2003. Evolution of CD8+ T cell immunity and viral escape following acute HIV-1 infection. J. Immunol. 1713837-3846. [DOI] [PubMed] [Google Scholar]
- 12.Cooper, S., A. L. Erickson, E. J. Adams, J. Kansopon, A. J. Weiner, D. Y. Chien, M. Houghton, P. Parham, and C. M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10439-449. [DOI] [PubMed] [Google Scholar]
- 13.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443350-354. [DOI] [PubMed] [Google Scholar]
- 14.Ejrnaes, M., C. M. Filippi, M. M. Martinic, E. M. Ling, L. M. Togher, S. Crotty, and M. G. von Herrath. 2006. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 2032461-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilboa, E. 1999. How tumors escape immune destruction and what we can do about it. Cancer Immunol. Immunother. 48382-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, Jr., I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 7410249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goulder, P. J., Y. Tang, C. Brander, M. R. Betts, M. Altfeld, K. Annamalai, A. Trocha, S. He, E. S. Rosenberg, G. Ogg, C. A. O'Callaghan, S. A. Kalams, R. E. McKinney, Jr., K. Mayer, R. A. Koup, S. I. Pelton, S. K. Burchett, K. McIntosh, and B. D. Walker. 2000. Functionally inert HIV-specific cytotoxic T lymphocytes do not play a major role in chronically infected adults and children. J. Exp. Med. 1921819-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 755550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ha, S. J., S. N. Mueller, E. J. Wherry, D. L. Barber, R. D. Aubert, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2008. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J. Exp. Med. 205543-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 41191-1198. [DOI] [PubMed] [Google Scholar]
- 21.Kostense, S., G. S. Ogg, E. H. Manting, G. Gillespie, J. Joling, K. Vandenberghe, E. Z. Veenhof, D. van Baarle, S. Jurriaans, M. R. Klein, and F. Miedema. 2001. High viral burden in the presence of major HIV-specific CD8+ T cell expansions: evidence for impaired CTL effector function. Eur. J. Immunol. 31677-686. [DOI] [PubMed] [Google Scholar]
- 22.Kostense, S., K. Vandenberghe, J. Joling, D. Van Baarle, N. Nanlohy, E. Manting, and F. Miedema. 2002. Persistent numbers of tetramer+ CD8+ T cells, but loss of interferon-gamma+ HIV-specific T cells during progression to AIDS. Blood 992505-2511. [DOI] [PubMed] [Google Scholar]
- 23.Lee, P. P., C. Yee, P. A. Savage, L. Fong, D. Brockstedt, J. S. Weber, D. Johnson, S. Swetter, J. Thompson, P. D. Greenberg, M. Roederer, and M. M. Davis. 1999. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 5677-685. [DOI] [PubMed] [Google Scholar]
- 24.Liu, H., S. Andreansky, G. Diaz, T. Hogg, and P. C. Doherty. 2002. Reduced functional capacity of CD8+ T cells expanded by post-exposure vaccination of gamma-herpesvirus-infected CD4-deficient mice. J. Immunol. 1683477-3483. [DOI] [PubMed] [Google Scholar]
- 25.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 688056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskophidis, D., F. Lechner, H. Pircher, and R. M. Zinkernagel. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362758-761. [DOI] [PubMed] [Google Scholar]
- 27.Mueller, Y. M., S. C. De Rosa, J. A. Hutton, J. Witek, M. Roederer, J. D. Altman, and P. D. Katsikis. 2001. Increased CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells. Immunity 15871-882. [DOI] [PubMed] [Google Scholar]
- 28.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8177-187. [DOI] [PubMed] [Google Scholar]
- 29.Murali-Krishna, K., L. L. Lau, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 2861377-1381. [DOI] [PubMed] [Google Scholar]
- 30.Oldstone, M. B. 1997. How viruses escape from cytotoxic T lymphocytes: molecular parameters and players. Virology 234179-185. [DOI] [PubMed] [Google Scholar]
- 31.Oxenius, A., D. A. Price, H. F. Gunthard, S. J. Dawson, C. Fagard, L. Perrin, M. Fischer, R. Weber, M. Plana, F. Garcia, B. Hirschel, A. McLean, and R. E. Phillips. 2002. Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc. Natl. Acad. Sci. USA 9913747-13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrovas, C., D. A. Price, J. Mattapallil, D. R. Ambrozak, C. Geldmacher, V. Cecchinato, M. Vaccari, E. Tryniszewska, E. Gostick, M. Roederer, Douek, S. H. Morgan, S. J. Davis, G. Franchini, and R. A. Koup. 2007. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood 110928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2032281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pircher, H., D. Moskophidis, U. Rohrer, K. Burki, H. Hengartner, and R. M. Zinkernagel. 1990. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature 346629-633. [DOI] [PubMed] [Google Scholar]
- 35.Probst, H. C., T. Dumrese, and M. F. van den Broek. 2002. Cutting edge: competition for APC by CTLs of different specificities is not functionally important during induction of antiviral responses. J. Immunol. 1685387-5391. [DOI] [PubMed] [Google Scholar]
- 36.Puglielli, M. T., A. J. Zajac, R. G. van der Most, J. L. Dzuris, A. Sette, J. D. Altman, and R. Ahmed. 2001. In vivo selection of a lymphocytic choriomeningitis virus variant that affects recognition of the GP33-43 epitope by H-2Db but not H-2Kb. J. Virol. 755099-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadagopal, S., R. R. Amara, S. Kannanganat, S. Sharma, L. Chennareddi, and H. L. Robinson. 2008. Expansion and exhaustion of T-cell responses during mutational escape from long-term viral control in two DNA/modified vaccinia virus Ankara-vaccinated and simian-human immunodeficiency virus SHIV-89.6P-challenged macaques. J. Virol. 824149-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8+ T cells in chronic human immunodeficiency virus infection. Blood 963094-3101. [PubMed] [Google Scholar]
- 39.Shin, H., S. D. Blackburn, J. N. Blattman, and E. J. Wherry. 2007. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204941-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Streeck, H., Z. L. Brumme, M. Anastario, K. W. Cohen, J. S. Jolin, A. Meier, C. J. Brumme, E. S. Rosenberg, G. Alter, T. M. Allen, B. D. Walker, and M. Altfeld. 2008. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 5e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 121198-1202. [DOI] [PubMed] [Google Scholar]
- 42.van Baarle, D., E. Hovenkamp, M. F. Callan, K. C. Wolthers, S. Kostense, L. C. Tan, H. G. Niesters, A. D. Osterhaus, A. J. McMichael, M. H. van Oers, and F. Miedema. 2001. Dysfunctional Epstein-Barr virus (EBV)-specific CD8+ T lymphocytes and increased EBV load in HIV-1 infected individuals progressing to AIDS-related non-Hodgkin lymphoma. Blood 98146-155. [DOI] [PubMed] [Google Scholar]
- 43.Vogel, T. U., T. M. Allen, J. D. Altman, and D. I. Watkins. 2001. Functional impairment of simian immunodeficiency virus-specific CD8+ T cells during the chronic phase of infection. J. Virol. 752458-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welsh, R. M. 2001. Assessing CD8 T cell number and dysfunction in the presence of antigen. J. Exp. Med. 193F19-F22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wherry, E. J., D. L. Barber, S. M. Kaech, J. N. Blattman, and R. Ahmed. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA 10116004-16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wherry, E. J., S. J. Ha, S. M. Kaech, W. N. Haining, S. Sarkar, V. Kalia, S. Subramaniam, J. N. Blattman, D. L. Barber, and R. Ahmed. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27670-684. [DOI] [PubMed] [Google Scholar]
- 47.Xiong, Y., M. A. Luscher, J. D. Altman, M. Hulsey, H. L. Robinson, M. Ostrowski, B. H. Barber, and K. S. MacDonald. 2001. Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8+ T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J. Virol. 753028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yewdell, J. W., and J. R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 1751-88. [DOI] [PubMed] [Google Scholar]
- 49.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1882205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]