Abstract
Pathogen recognition is a critical function of immune sentinel cells. Naïve macrophages or dendritic cells (DCs) undergo pathogen-directed activation and maturation, and as mature antigen-presenting cells (APCs), they contribute essential functions to both innate and adaptive immunity. Using recombinant adenovirus (rAdV) as a model for murine APC activation by DNA viruses, we demonstrate a critical role for stress kinase activation in cell intrinsic and extrinsic antiviral signaling cascades. We propose two viral triggers, viral capsid and viral DNA, are required for APC activation. Endosomal escape and presentation of cytosolic rAdV DNA induces phosphorylation of TANK-binding kinase 1 (TBK1) at serine 172 but does not induce IκB kinase ɛ activity as determined by in vitro kinase assays. However, induction of TBK1 alone is not sufficient for interferon regulatory factor 3 (IRF3) phosphorylation. We show that capsid-dependent activation of Jun N-terminal kinase (JNK) stress kinase is a necessary step, licensing TBK1 phosphorylation of IRF3 at Ser 396. A second later phase of JNK activity is required to coordinate phosphorylation of JNK-dependent transcription factors (c-Jun/ATF2) with activated IRF3 in the induction of primary IRF3-responsive transcripts. Finally, we demonstrate that maximal JNK/TBK1/IRF3 stimulation by rAdV depends on an intact type I interferon (IFN) signaling cascade. By requiring multiple viral triggers and type I IFN autocrine regulation, APCs have an inherent fail-safe mechanism against inappropriate activation and maturation.
Activation of antigen-presenting cells (APCs) by recombinant adenovirus (rAdV) occurs by means of cell intrinsic and autocrine/paracrine mechanisms. Cell intrinsic signals result from the direct interaction of rAdV with pattern recognition receptors (PRRs). Although viral capsid components may offer extracellular targets of host cell recognition, adenoviral nucleic acid has been proposed as a viral element targeted by PRRs in APCs (11, 29, 52). Two types of PRRs are capable of sensing adenoviral nucleic acids: endosomal PRRs and cytosolic PRRs. Toll-like receptor 9 (TLR9) recognizes DNA motifs in endosomally localized viral genomes and is the main mode of rAdV DNA recognition in plasmacytoid DCs (3, 52). In contrast to plasmacytoid DCs, primary macrophages and conventional DCs react to rAdV DNA through TLR-independent cytosolic receptors (11, 29, 52). Double-stranded DNA, irrespective of CpG motifs and other sequence constraints, has been shown to induce a type I interferon (IFN) antiviral response when introduced into the cytoplasm of cells (20, 25, 41).
The natures of the putative cytosolic DNA sensor(s) and downstream adaptor molecules are under investigation (21, 44), but several studies have shown that the interferon-stimulating DNA cascade targets activation of interferon regulatory factor 3 (IRF3) (20, 29, 41, 44). IRF3 is present in the cytosol as a dormant transcription factor in an autoinhibited configuration (24, 35, 43). In response to viral infection or exposure to nucleic acid, IRF3 becomes hyperphosphorylated, dimerizes, and translocates to the nucleus as an active transcription factor (reviewed in references 16 and 17). The identity of the protein kinases involved and the conformational consequences resulting from IRF3 phosphorylation are areas of intense investigation (6, 32, 38, 40). Two IκB kinase (IKK)-related kinases Tank-binding kinase 1 (TBK1) and IKKɛ have been identified as responsible for phosphorylating critical sites at the C-terminal IRF3 domain (12, 21, 26). Among these sites is serine 396 that is required for IRF3 dimerization and translocation to the nucleus (39). In addition to C-terminal phosphorylation, N-terminal phosphorylation of IRF3 occurs and has been linked to the activation of stress-activated kinases c-Jun N-terminal kinases (JNKs) (38, 40). JNKs are serine/threonine protein kinases originally identified as stress kinases activated by UV irradiation leading to phosphorylation of the c-Jun and ATF transcription factors (15). Activated c-Jun complexes with activating transcription factor 2 (ATF2) to participate with IRF3/7 and NF-κB in formation of the β-interferon enhanceosome (1, 10, 31). The biological significance of N-terminal IRF3 phosphorylation or the involvement of stress-activated kinases in IRF3 activation is currently unclear.
The intrinsic APC response to rAdV results in a cascade of events including secretion of inflammatory chemokines and cytokines (reviewed in reference 28). Maturation of APCs and subsequent induction of the adaptive immune response by rAdV requires the action of extrinsic autocrine/paracrine signals (9, 46, 52). However, administration of exogenous cytokines to wild-type (wt) APCs does not recapitulate the innate immune activation of APCs observed during the viral infection (18, 27). In the case of rAdV, the signaling mechanisms that coordinate coupling of intrinsic and extrinsic cascades leading to APC maturation remain to be elucidated.
In the present study, we have investigated the molecular details by which AdV activates cell intrinsic innate immune cascades and the signaling mechanisms that couple them with intercellular autocrine/paracrine cascades. We demonstrate that APC activation by rAdV occurs through two viral triggers, a capsid-mediated stress signal and introduction of the viral genome to a cytosolic sensor, and that both steps are shown to engage distinct kinase cascades, leading to activation of IRF3. The direct response to virus infection is augmented by the type I IFN signaling cascade. Type I IFN signaling through STAT1/2 (signal transducer and activator of transcription factor 1 or 2) activation provides a JNK platform that is highly responsive to rAdV infection. The antiadenoviral APC response presents important insights into the molecular mechanisms that control the immunogenicity to AdV, which may be shared by other double-stranded DNA viruses or naked DNA immunogens.
MATERIALS AND METHODS
Viruses and rAdV DNA.
Ad5CiG, Ad2 defective in endosomal escape (ts1), wt Ad2, and Ad5CiG-ΔRGD were previously described (37). Viruses were grown in HEK-293 cells according to standard protocols, purified through two rounds of CsCl gradient ultracentrifugation, and stored at −70°C in storage buffer (10 mM Tris, 2 mM MgCl2, 40% sucrose [pH 7.5]). All viral preparations contained less than 0.1 endotoxin unit per ml, as determined by the Limulus amebocyte lysate assay. AdV empty capsids (eAdV) were purified as previously described (29). AdV DNA was purified from double CsCl-purified adenoviral stocks incubated in 10 mM Tris (pH 8.0), 1 mM EDTA, 0.25 M 2-mercaptoethanol, and 1% sodium dodecyl sulfate for 30 min at 37°C with occasional vortexing. Self- digested pronase (Sigma) was added at a final concentration of 500 μg/ml, and the samples were incubated at 37°C for 1 h followed by three phenol-chloroform extractions. Viral DNA (vDNA) was precipitated with NaCl-ethanol and suspended in nuclease-free water. The DNA used was tested and was free of endotoxin (less than 0.001 U/mg of DNA). Psoralen UV inactivation was performed by mixing 0.01 volume of 33-mg/ml 8-methoxy-psoralen (Sigma) with the purified viral vector and exposing it to a 365-nm UV light source 4 cm from the light filter for 60 min on ice. Residual psoralen was removed by dialysis.
Animals.
Eight- to 12-week-old male and female C57BL/6 mice were obtained from Jackson Laboratories. IRF3 KO (knockout), interferon alpha/beta receptor chain 1 (IFNAR1) KO, and STAT1 KO mice on C57BL/6 background were generously provided by Tadatsugu Taniguchi, David Levy, and Andrew Larner, respectively.
Bone marrow-derived macrophages and BMDC.
Bone marrow cells were extracted from the femurs and tibiae of mice, and red cells were removed with lysis solution (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM EDTA). For bone marrow-derived macrophages, bone marrow cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 20% fetal bovine serum (FBS) and 25% supernatant derived from L929 confluent cells. On day 7, immature macrophages were collected. For bone marrow-derived dendritic cells (BMDC), bone marrow cells were cultured in DMEM containing 10% FBS and 30% supernatant derived from J558L cells. On day 2, two-thirds of the supernatant was replaced to remove granulocytes. On day 7, nonadherent cells were collected. The typical purity of each population was above 80% as determined by fluorescence-activated cell sorting analysis of CD11b and CD11c surface markers, respectively.
Cell treatments.
Bone marrow-derived cells that had been allowed to differentiate for 7 days were replated onto 60-mm plates at a density of 5 × 106 cells/plate in 3 ml of DMEM supplemented with 10% FBS. On day 9, the volume of the medium was reduced to 1.5 ml, and cells were treated with Ad5CiG at a ratio of 25,000 particles per cell unless otherwise indicated. For transfection experiments, the medium was replaced on day 8 with OPTI-MEM I (Invitrogen). On day 9, 10 μg of adenovirus DNA or poly(I·C) (Amersham Biosciences) and 10 μl of Lipofectamine 2000 (Invitrogen) were incubated in 100 μl of OPTI-MEM I for 15 min before using for stimulation. For inhibition studies, primary macrophages were pretreated for 60 min with drugs prior to exposure to various stimuli. Cell viability was above 85% for all treatments as confirmed by trypan blue exclusion assay. The phosphatidylinositol 3-kinase (PI3K)/Akt pathway was restrained with the panselective PI3K inhibitors LY294002 (100 μM) (catalog no. 440202) and wortmannin (2 μM) (catalog no. 681675). The mitogen-activated protein kinase (MAPK) inhibitors SB202190 (20 μM), JNK inhibitor II SP600125 (50 μM) and U0126 (50 μM) were used to inhibit the p38, JNK, and extracellular signal-regulated kinase (ERK) pathways, respectively. All the above inhibitors were obtained from Calbiochem. For de novo protein synthesis inhibition, cycloheximide (CHX) (50 μg/ml) and puromycin at the indicated concentrations were from Sigma-Aldrich.
Fluorescence-activated cell sorting analysis.
Monoclonal antibodies used to identify mouse DC populations included fluorescein isothiocyanate-conjugated anti-CD11c and phycoerythrin-conjugated anti-CD40, anti-CD80, and anti-CD86 antibodies. Primary, isotype controls and anti-CD16/CD32 antibodies were from BD Pharmingen. After blocking Fc receptors with anti-CD16/CD32 for 5 min, cells were stained with fluorescence-labeled primary or isotype control antibodies in phosphate-buffered saline (PBS) buffer with 2% FBS. Cells were then fixed in 1% paraformaldehyde. Data acquisition and analysis were performed using a FACScan flow cytometer with CellQuest software (BD Biosciences, San Jose, CA). Depending on the number of cells available, 10,000 to 50,000 events per sample were analyzed. For viability assessment, 2 × 105 cells suspended in 200 μl PBS were mixed 30 min before analysis with 200 μl of 1 μg/ml propidium iodide (catalog no. P-4170; Sigma Chemical) in PBS.
SYBR green I quantitative RT-PCR.
For quantitative reverse transcription-PCR (RT-PCR), total cellular RNA was isolated from 5 × 106 cells grown in 60-mm tissue culture plates using Tri-Reagent (Molecular Research Center) as instructed by the manufacturer. Amplifications were carried out in a total volume of 15 μl by using a one-step QuantiTect SYBR green kit (Qiagen, Valencia, CA) in an Applied Biosystems Prism 7900H sequence detection system with SDS 2.1 software. Cycles consisted of an initial incubation at 95°C for 15 min, followed by 35 cycles (1 cycle consisting of 15 s at 94°C, 30 s at 60°C, and 20 s at 72°C), with a final incubation at 72°C for 7 min. All determinations were performed in triplicate. Nontemplate controls run with every assay consistently had no cycle threshold values before 35 cycles of PCR. The abundance of each mRNA was normalized to β-actin and compared to untreated cells to calculate the relative induction. Sequences of primers are available on request.
SYBR green I quantitative PCR for AdV genomes per cell.
Amplifications were carried out in a total volume of 20 μl by using a QuantiTect SYBR green kit (Qiagen, Valencia, CA). Briefly, forward (5′-TTCCGCTTCACTGGACTCTT-3′) and reverse (5′-GTGGACAGCGAGGAAGAAAG −3′) adenovirus type 5 (Ad5) primers amplified a 180-bp fragment of the hexon gene. To detect the levels of the housekeeping gene, forward (5′-AGGTCGGTGTGAACGGATTTG-3′) and reverse (5′-TGTAGACCATGTAGTTGAGGTCA-3′) primers amplified a 123-bp fragment of the glyceraldehyde-3-phosphate dehydrogenase gene.
Western blots.
Whole-cell extracts were prepared by washing cells twice with ice-cold PBS and harvesting them in lysis buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1% NP-40) with the addition of the phosphatase inhibitors (30 mM sodium fluoride, 100 μM sodium orthovanadate, 100× cocktails 1 and 2 [Sigma-Aldrich]) and protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/ml microcystin-LR, 1 mM benzamidine). The lysates were cleared by centrifugation at 13,000 × g for 15 min at 4°C, and protein quantification was performed with the DC protein assay kit (Bio-Rad Laboratories). For Western blot analysis, gel boxes, 10% NuPage-bis-tris gels, and buffers were purchased from Invitrogen and Immobilon-P membranes were purchased from Millipore. All blots were blocked in Tris-buffered saline containing Tween (0.1%) and 5% skim milk at room temperature for 1 h. Antibodies specific for phosphorylated IRF3 (pIRF3) (Ser396; catalog no. 4961), phosphorylated JNK (pJNK) (Thr183/Tyr185; catalog no. 9251), phosphorylated c-Jun (p-cJun) (Ser63; catalog no. 9261), phosphorylated STAT1 (pSTAT1) (Tyr701; catalog no. 9171), total TBK1 (catalog no. 3013), total IKKɛ (catalog no. 2690), β-actin (catalog no. 4967), and horseradish peroxidase-linked anti-rabbit immunoglobulin G (catalog no. 7074) were from Cell Signaling Technology. Anti-phospho-specific pTBK1 (pSer172; catalog no. 558397) was from BD Biosciences, Total IRF3 antibodies (catalog no. SC-9082 and SC-15991) were from Santa Cruz Laboratories, and pSTAT2 (Tyr689; catalog no. 07-224) was from Upstate Biotechnology. All primary antibodies were used at a dilution of 1:1,000 in Tris-buffered saline containing 5% bovine serum albumin, except for β-actin antibody, which was used at a dilution of 1:2,000. Signals were visualized by ECLplus kit (Amersham Biosciences).
In vitro kinase assay.
JNK, TBK1, and IKKɛ immunokinase assays were performed using endogenous molecules immunoprecipitated from primary APCs. After the indicated treatments, cells were lysed with TNE buffer (20 mM Tris-HCl [pH 7.5], 350 mM NaCl, 1.0% Triton X-100, 2 mM EDTA, 2 mM EGTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 50 mM NaF, 1.0 mM sodium vanadate, 10 mM β-glycerolphosphate, and 1× cocktail protease inhibitors), immunopurified with anti-JNK (sc-1648 antibody), anti-TBK1 (sc-9910), or anti-IKKɛ (sc-5694) Santa Cruz antibodies and subjected to an in vitro kinase assays using glutathione S-transferase (GST)-Jun1-89 (Jun protein with amino acids 1 to 89), or GST-IRF3 as respective substrates. Kinase reactions were carried out in 1× kinase buffer (2 mM HEPES [pH 7.4], 100 mM NaCl, 10 mM MgCl2, 0,25% NP-40, 0.5 mM EGTA, 1 mM dithiothreitol, 10% glycerol) in the presence of 2 μCi [γ-32P]ATP and 10 to 30 μM cold ATP for 30 min at 30°C. Reaction mixtures were then separated on sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene difluoride membranes, and the phosphorylation states of the corresponding GST-fused peptides were detected and quantified using a phosphorimager. Quantification of JNK activity is based on the degree of c-Jun phosphorylation relative to the level of JNK in the immunoprecipitates. The same membranes were then used for immunoblotting with total JNK (sc-571; Santa Cruz) or total TBK1 or total IKKɛ Cell Signaling Technology antibodies described above.
Statistical analysis.
Data were expressed as means ± standard errors of the means.
RESULTS
rAdV induces TBK1, but not IKKɛ, activity following delivery of vDNA to the cytosolic compartment.
APC maturation is the result of pathogen stimulation characterized by presentation of costimulatory molecules on the cell membrane and expression of an array of chemokines and cytokines. We have previously shown that APCs respond to rAdV by expression of surface costimulatory molecules and secretion of modest levels of proinflammatory chemokines and high levels of type I interferons compared to cells exposed to the TLR ligand lipopolysaccharide (29). We also demonstrated that IRF3 phosphorylation was strongly induced by rAdV infection, where IRF3 activation was a primary response to AdV infection and occurred in a DNA-dependent, TLR-independent manner (29). The adaptor/kinase cascade that mediates IRF3 phosphorylation by rAdV has not been identified. Signaling cascades that respond to RNA virus infection (TLR3 ligation [via TRIF adaptor] or intracellular RNA [via RIG-I adaptor]) similarly stimulate IRF3 phosphorylation and do so through the TANK-1-binding kinase complex TBK1/IKKɛ (reviewed in reference 34). Therefore, we investigated activation of TBK1 and IKKɛ in response to rAdV infection.
TBK1 activation is associated with phosphorylation of serine 172 (22), which can be detected using an antibody directed against TBK1 with serine 172 phosphorylated (PSer172TBK1). Primary bone marrow-derived murine macrophages were exposed to rAdV, and protein lysates were harvested at various time points through a 36-hour time course. Western blot analysis with anti-PSer172TBK1 antibody revealed induction of PSer172TBK1 by 2 hours and that it peaked around 5 h postinfection, an activation time course that parallels C-terminal phosphorylation of IRF3 (Fig. 1A). IRF3 activation is a primary response to rAdV exposure and occurs in the presence of the translation inhibitor cycloheximide (29). Since TBK1 activation is predicted to be a necessary upstream event in IRF3 activation, then it should also be insensitive to CHX inhibition. Primary macrophages were pretreated with CHX followed by exposure to rAdV for 5 h. Cell lysates were harvested and characterized for induction of PSer172TBK1 (Fig. 1B). CHX treatment results in a strong enhancement of rAdV-induced PSer172TBK1 and PSer396IRF3 but a complete inhibition of rAdV-induced PTyrSTAT1 and PTyrSTAT2. STAT1/2 phosphorylation indicates expression/secretion of type I IFNs by rAdV-infected cells through autocrine stimulation and provides a control for the effectiveness of the CHX translational blockade. To determine whether both TBK1 and IKKɛ kinases are involved in the primary response to AdV, macrophages were treated with rAdV in the presence or absence of CHX, and lysates were harvested at 5 h postinfection. Total TBK1 or IKKɛ enzymes were immune precipitated, and kinase activity was quantified using a C-terminal IRF3 segment substrate (36). TBK1 immunoprecipitates from rAdV-treated cells generated high levels of phosphorylated, C-terminal IRF3 compared to mock-treated samples (Fig. 1C). In contrast, there was no indication that the IKKɛ beads were able to phosphorylate the C-terminal IRF3 substrate. In a parallel assay, lysates generated from poly(I·C)-stimulated macrophages reveal strong induction of IKKɛ activity when the C-terminal IRF3 substrate was used (data not shown). These data support the notion that TBK1 functions as a primary antiviral response kinase leading to IRF3 activation.
FIG. 1.
TBK1, but not IKKɛ, mediates C-terminal phosphorylation of IRF3 in response to AdV DNA. (A) rAdV induces TBK1 and C-terminal IRF3 phosphorylation with complementary kinetics. Immunoblot of lysates from macrophages treated with vehicle (mock) or rAdV for the indicated times (in hours). Activation of TBK1 and IRF3 was detected with anti-phospho-specific Ser172-pTBK1, and Ser396-pIRF3 antibodies, respectively. Endogenous levels of TBK1 and IRF3 were characterized with polyclonal antibodies against the total proteins. (B) Primary and secondary signaling events elicited by AdV infection. Immunoblot of macrophage lysates harvested 4 h after mock and AdV treatments in the absence or presence of cycloheximide. (C) TBK1 and IKKɛ activities induced by AdV. Protein extracts (600 μg) from macrophages treated for 5 h were subjected to immunoprecipitation (IP) by using antibodies to TBK1 or IKKɛ followed by in vitro kinase assays with GST-IRF3188-427as the substrate. C-IRF3, C-terminal IRF3; WB, Western blotting. (D) AdV internalized genomes, but not capsids, activate TBK1 and IRF3. Western blots showing levels of phospho-TBK1, phospho-IRF3, and corresponding β-actin in macrophage lysates harvested at 5 h posttreatment with vehicle (mock), AdV (psoralen-UV-inactivated Ad2), AdV defective in endosomal escape (ts1) (psoralen UV inactivated), AdV empty capsid (eAdV), RGDmtAdV (RGD), and transfected viral DNA. Representative data from one of two experiments are shown for each panel.
If TBK1 were a primary target of the cytosolic sensor, then TBK1 phosphorylation should occur only following release of virion from the endosomal compartment. To examine this question, macrophages were infected with one of three rAdV variants: UV-psoralen-inactivated ts1Ad2, which due to a mutation in the viral p23 protease is compromised in endosomal escape (13); adenoviral empty capsids, which are greatly reduced in viral DNA content but undergo endosome internalization (42) but are not known to escape the endosome (7); or a rAdV mutated in the RGD integrin-binding domain of the capsid protein penton, which contributes to stimulation of AdV endocytosis (49). Because the ts1 virus is an Ad2 E1-containing virus, the control AdV in this experiment is Ad2 that has been psoralen UV inactivated to block any possible E1 influence in the experiment. For comparison, a separate assay of macrophages transfected with vDNA was also carried out.
Induction of PSer172TBK1 and PSer396IRF3 was observed with UV-psoralen-inactivated rAdV and with transfected vDNA (Fig. 1D). However, neither PSer172TBK1 nor PSer396IRF3 was induced in macrophages exposed to either UV-psoralen-inactivated ts1Ad or AdV empty capsid. Therefore, we have shown that phosphorylation of TBK1 is dependent on viral escape from the endosomal compartment, that viral infection induces levels of TBK1 activity, and that TBK1 activation is a primary response to rAdV exposure.
Capsid-mediated stress kinase activation is required for C-terminal IRF3 phosphorylation.
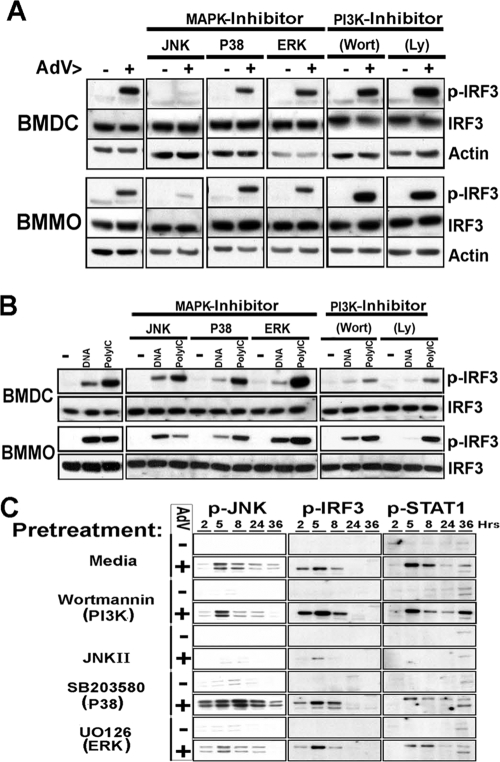
Some studies have suggested that multiple kinase activities may be involved in activation of IRF family members (32, 38). AdV infection has been shown to stimulate several kinase pathways, including PI3K (23), the stress kinase p38 (45), and the ERK cascade (45) in various cell types, as well as in primary murine macrophages (29). A recent study (11) found JNK MAPK inhibitor treatment of Ad3 induced BMDC to result in suppression of type I interferon secretion. To determine whether activation of an accessory kinase could influence activation of IRF3, primary APCs were pretreated with MAPK or PI3K inhibitors and harvested 5 h after rAdV infection to determine the levels of PSer396IRF3. For comparison, cells were transfected with vDNA or poly(I·C) and harvested 2 h posttransfection as previously described (29). Pretreatment of APCs with PI3K inhibitor wortmannin or LY2928 did not inhibit rAdV-induced PSer396IRF3 (Fig. 2A). However, PI3K inhibitor pretreatment of APCs transfected with vDNA resulted in a strong reduction of PSer396IRF3 levels (Fig. 2B). PSer396IRF3 induction by poly(I·C) was less sensitive to PI3K inhibitor treatment in macrophages than in BMDC.
FIG. 2.
Roles of the MAPK and PI3K pathways in C-terminal phosphorylation of IRF3 by rAdV. (A) C-terminal phosphorylation of IRF3 induced by AdV infection requires JNK, but not PI3K, signals. Ser396-pIRF3, IRF3, and actin Western blots of lysates from dendritic cells and bone marrow-derived macrophages (BMMO) treated for 5 h with vehicle (−) or AdV (+) in the presence of the indicated inhibitor. The inhibitors were 20 μM SB202190 for p38, 50 μM SP600125 for JNK, ERK for JNK inhibitor II, 50 μM UO126 for 2 μM wortmannin (Wort), and 100 μM LY294002 (Ly). (B) C-terminal phosphorylation of IRF3 induced by transfecting nucleic acids requires PI3K signaling. Ser396-pIRF3, IRF3, and actin immunoblots of lysates from macrophages or DCs treated for 2 h with Lipofectamine (− lanes), Lipofectamine plus adenoviral DNA (DNA lanes), or Lipofectamine plus poly(I·C) (PolyIC lanes) in the presence of the indicated inhibitors. (C) Time course experiment of steady-state levels of Thr183/Tyr185-pJNK, Ser396-pIRF3, and Tyr701-pSTAT1 during AdV infection and effect of signaling inhibitors. Lysates from macrophages treated with vehicle or AdV in the presence of indicated inhibitors were collected at successive time points (in hours) and immunoblotted with anti-Thr183/Tyr185-pJNK, pIRF3, or pSTAT1 phospho-specific antibodies. Representative data from one of two experiments are shown for each panel.
In contrast, pretreatment with the JNK II inhibitor effectively blocked rAdV induction of PSer396IRF3. JNK II inhibition of PSer396IRF3 occurred in both primary macrophages and dendritic cells (Fig. 2A); however, the RAW 286.1 murine macrophage cell line IRF3 activation by rAdV was largely unaffected by JNK II inhibitor pretreatment (data not shown). In comparison to JNK inhibition, the effects of p38 or ERK inhibition were modest following rAdV infection and slightly more pronounced following vDNA or poly(I·C) transfection (Fig. 2B). To confirm the influence of PI3K or MAPK inhibitors on IRF3 phosphorylation, an extended rAdV infection time course experiment was carried out in the presence of the indicated inhibitors (Fig. 2C). Consistent with the 5-h treatment condition, JNK II inhibitor blocks activation of primary (IRF3) and secondary (STAT1) antiviral signaling through the entire time course of APC maturation.
To rule out the possibility that the kinase inhibitors may be indirectly influencing the level of IRF3 phosphorylation by compromising cell viability or virus uptake, macrophages were pretreated with MAPK inhibitors or wortmannin and then infected for either 5 or 24 h. Cells harvested at 5 h were stained with propidium iodide and immediately assessed by flow cytometry for cell viability or harvested for total DNA (Fig. 3A). Based on propidium iodide staining, pretreatment with JNK II inhibitor had a minimal negative effect on cell viability 5 h postinfection. Measurement of vDNA/total cellular DNA by quantitative PCR revealed that MAPK inhibitors had minimal influence on vDNA uptake, although there was a significant increase in vDNA/cell when the macrophages were treated with wortmannin. Macrophages harvested 24 h postinfection were fixed, and flow cytometry was used to measure enhanced green fluorescent protein transgene expression and surface expression of the costimulatory molecule CD86. rAdV transgene expression was not diminished by treatment with JNK II inhibitor. However, macrophages exposed to JNK II inhibitor were compromised in expression of the maturation marker CD86 (Fig. 3A). The data collectively argue against an indirect JNK-dependent effect on virus transduction and lead us to conclude that JNK is directly involved in the cascade leading to IRF3 phosphorylation and activation of the antiviral response in APCs.
FIG. 3.
JNK signaling controls accumulation of C-terminal pIRF3 in a manner that is independent of Ser272 TBK1 phosphorylation. (A) Specificity of JNK and PI3K inhibitors at modulating IRF3 activation. The PI panel shows viability, by propidium iodide (PI) staining, of macrophages after 5-h treatment with vehicle (−) or AdV (+) in the presence of the indicated inhibitors (inhib) (Wort, wortmannin). The Relative DNA panel illustrates viral DNA in 5 h AdV-infected macrophages with the indicated inhibitors. The eGFP panel depicts 36 h transfection efficiency in macrophages treated with indicated inhibitors. eGFP, enhanced green fluorescent protein; MFI, mean fluorescence intensity. The CD86 panel demonstrates the effectiveness of indicated inhibitors at blocking the activation of macrophages by AdV. Macrophage activation was assessed by flow cytometric analysis of CD86 upregulation 36 h after infection. (B) Effect of JNK and PI3K inhibitor on TBK1 activation at 5 h postinfection. Ser396-pIRF3, Ser272-pTBK1 Western blots of lysates from macrophages treated with vehicle (Mock), AdV, Lipofectamine plus viral DNA (DNA), or Lipofectamine plus poly(I·C) (PolyI:C) in the presence of the indicated inhibitors. Representative data from one of two experiments are shown for each panel.
JNK II inhibitor blocks PSer396IRF3, but not PSer172TBK1, following rAdV infection.
To determine where JNK and wortmannin intersect the IRF3 activation pathway, cells were pretreated with JNK inhibitor or wortmannin and exposed to AdV, vDNA, or poly(I·C). Western analysis using anti-PSer172TBK1 antibody revealed minimal compromise in PSer172TBK1 when cells were pretreated with JNK II inhibitor, but accumulation of PSer396IRF3 was abolished in rAdV-treated samples (Fig. 3B). In contrast, pretreatment with wortmannin resulted in elevated levels of PSer172TBK1, including mock-treated samples. Consistent with previous assays, wortmannin had no effect on rAdV-induced phospho-IRF3 but results in diminished PSer396IRF3 following vDNA transfection. Of note, although levels of PSer172TBK1 were elevated in wortmannin mock-treated cells, PSer396IRF3 was at background levels. Simple activation of TBK1 alone does not result in increased PSer396IRF3 levels. These data suggest that rAdV-induced JNK acts in parallel with TBK1 to allow access to the C-terminal IRF3 domain. Additionally, the mode of vDNA introduction to the cell may impact on the accessory kinase that collaborates with TBK1 in the sequence leading to IRF3 activation (vDNA transfection involves PI3K, and rAdV infection requires JNK).
Distinct transcription factor cascades are coordinated by JNK.
To better understand the relationship between the JNK and IRF3 signaling pathways, an early rAdV infection time course experiment (20 min to 5 h) was performed (Fig. 4A). Consistent with earlier studies (29), low levels of pJNK phosphorylation were detected within 20 min of virus exposure, and these levels persist for 2 h. At 5 h postinfection, peak levels of pJNK occur, and as expected, all AdV-induced JNK activation is inhibited by the addition of JNK II inhibitor (Fig. 4A, −2h JNKII). The peak JNK activation profile is coincident with peak levels of activated c-Jun, IRF3, and STAT1. We next asked whether early JNK induction contributed to the later activation profile (5 h). To address this question, cells were infected with rAdV, and at 1 h postinfection, JNK II inhibitor was added. After an additional 4-h incubation, cell lysates were harvested and analyzed by Western blotting (Fig. 4A, +1h JNKII). Treatment with JNKII inhibitor 1 h postinfection allowed the initial weak JNK activation but resulted in a loss of the pJNK peak at 5 h as expected. Lack of the pJNK peak after 5 h coincides with diminished p-cJun and pSTAT1 induction at 5 h, consistent with established pJNK/p-cJun/IFN induction cascades. However, PSer396IRF3 was still strongly induced in this experiment. Thus, immediate-early pJNK is important in establishing a framework for TBK1 IRF3 phosphorylation at later time points.
FIG. 4.
Immediate-early and early AdV-mediated JNK induction is essential for C-terminal IRF3 phosphorylation and promoter activity, respectively. (A) Effect of immediate-early and early JNK induction on downstream signaling events. JNK activation was selectively blocked in macrophages by adding the JNK inhibitor 2 h before (−2h) or 1 h after AdV infection (+1h). Where indicated, macrophages were pretreated with CHX to enhance JNK activation. 20′, 40′, and 90′, 20, 40, and 90 minutes, respectively; 2 and 5, 2 and 5 hours. (B) Puromycin stress activation of JNK superinduces AdV-mediated C-terminal phosphorylation of IRF3. Macrophages were mock treated or infected with AdV in the presence of media alone or puromycin (Puro) (50 mM). At the indicated times (in hours), whole-cell lysates were prepared, and Western blots were analyzed for JNK signaling (p-JNK and p-cJun) and C-terminal IRF3 activation (pIRF3). Preservation of de novo protein synthesis was monitored using pSTAT1 antibody. (C) Effects of immediate-early and early JNK inhibition on transcriptional induction of IRF3-driven genes (Fold Induction). RT-PCR analysis of macrophages treated for 5 h with vehicle or AdV in the presence of indicated inhibitory conditions (IFN-α4, IFN-β, ISG15, ISG54, ISG56, Vig1, and IP10). Representative data from one of two experiments are shown for each panel.
To confirm that the two-step activation sequence can occur in the absence of translation, the same experiment was carried out with the addition of CHX 1 hour before virus exposure (Fig. 4A, −1h CHX+1h JNKII). Pretreatment with CHX resulted in a strong increase in pJNK. Coincident with the increase in pJNK, we observed a strong increase in p-cJun. The enhanced level of pJNK and p-cJun were lost upon the addition of the JNKII inhibitor, demonstrating the rapid turnover of pJNK and p-cJun in the rAdV APC model. The high level of CHX-induced early pJNK is followed by hyperinduction of PSer396IRF3 at 5 h postinfection (Fig. 4A). Importantly, there is an obvious kinetic separation between direct pJNK phosphorylation of p-cJun and the indirect enhancement of pIRF3 at 5 h postinfection. As previously indicated, pSTAT1 is dependent on translation and is inhibited by CHX. To confirm the biphasic influence of JNK induction on IRF3 and c-Jun, cells were treated with a concentration of puromycin that specifically induces JNK activation but does not block translation. Consistent with pJNK contributing to both c-Jun and IRF3 activation, treatment with puromycin induced high levels of pJNK, p-cJun, and pIRF3 under conditions that allowed STAT1 activation (Fig. 4B).
The importance of coordinating PSer396IRF3 with JNK-induced transcription factors (pc-Jun) was revealed by measuring the transcriptional activation levels of IRF3-dependent genes (type I IFNs, ISG15, ISG54, ISG56, Vig1, and IP10 [IFN-inducible protein of 10 kDa]). Total RNA was harvested from cells treated as described in the legend to Fig. 4A, and IRF3-responsive transcripts were measured by quantitative RT-PCR (Fig. 4C). Under all JNK II inhibitor treatment conditions, rAdV induction of type I IFN transcripts as well as the IRF3-dependent transcripts Vig1, IP10, ISG15, ISG54, and ISG56 were greatly reduced compared to control RNA samples. The data demonstrate a biphasic model of JNK activation in response to rAdV, where low levels of early pJNK are sufficient to facilitate IRF3 activation; however, sustained or higher levels of pJNK are required at later time points to induce p-cJun which collaborates with pIRF3 to bring about full transcriptional activation of the antiviral response.
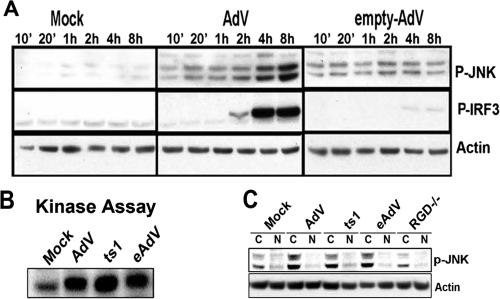
AdV capsid induces early JNK, but not IRF3.
We next asked whether early JNK activation by rAdV involved simple capsid membrane interactions. Macrophages were treated with viral empty capsid or rAdV and characterized for levels of pJNK at the indicated time points (Fig. 5A). pJNK was induced by both rAdV and empty capsid in lysates harvested through 2 h postinfection. However, macrophages treated with empty capsids did not approach the peak levels achieved with rAdV (4 to 8 h) (Fig. 5A). To confirm that early pJNK induction is a capsid-dependent event, an in vitro JNK kinase activity assay was carried out using cell lysates generated from macrophages exposed to rAdV, empty capsid, or the ts1 mutant virus for 1 h. Streptavidin bead-purified total JNK was used in a kinase assay to phosphorylate the JNK substrate GST-Jun. JNK activity levels at 1 h postinfection were above background and essentially identical for wt rAdV, ts1, or empty capsid-treated macrophages (Fig. 5B). As a follow-up, macrophages were treated for 1 h with rAdV, ts1AdV, empty capsid, or RGDmtAdV (mutant AdV that lacks the penton integrin-binding domain) and harvested into cytosolic and nuclear fractions. Consistent with the previous experiment, lysates screened by Western blotting for p-JNK revealed rAdV, ts1, and empty capsid each induced low levels of p-JNK (Fig. 5C). Cellular lysates harvested from RGDmtAdV were compromised in levels of pJNK, indicating a role for penton/integrin ligation in pJNK induction. Based on the observations that early JNK activation is necessary for rAdV-induced PSer396IRF3 and that during the early activation period, all capsid-containing particles equally stimulate JNK with the notable exception of the RGDmtAdV, the data indicate that APC engagement of the viral capsid mediates early JNK activation and that integrin ligation may be involved in stimulation of the stress kinase pathway. The data also indicate that in the absence of TBK1 activation (empty capsid [Fig. 1D]), IRF3 phosphorylation and second stage peak induction of pJNK do not occur (Fig. 5A).
FIG. 5.
Immediate-early JNK activation is triggered by extracellular adenoviral capsid and requires RGD penton motifs. (A) Kinetics of JNK and IRF3 induction following infection with rAdV and empty AdV. Immunoblot analysis of lysates from macrophages treated with vehicle (Mock), rAdV, or empty AdV for the indicated periods of time (from 10 minutes [10′] to 8 hours). (B) rAdV, AdV defective in endosomal escape (ts1), and empty capsid (eAdV) activate JNK. Protein extracts (600 μg) from macrophages treated for 1 h were subjected to immunoprecipitation by using a monoclonal antibody to JNK1 followed by in vitro kinase assays with GST-c-Jun1-89 as the substrate. (C) Western blot analysis of cytosolic (C) and nuclear (N) extracts from macrophages treated for 1 h with vehicle (Mock), rAdV (AdV), AdV defective in endosomal escape (ts1), empty AdV capsid (eAdV), and AdV devoid of penton base RGD motifs (RGD−/−). Representative data from one of two experiments are shown for each panel.
Type I IFN amplification of the JNK/IRF3 cascade.
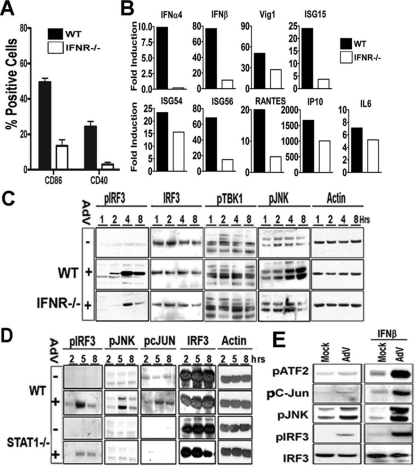
Type I IFN signaling contributes to antiadenoviral immunity in vivo (51) and to APC activation in vitro (14). Macrophages from IFNR−/− mice were used to determine how the absence of type I IFN signaling would influence induction of the TBK/JNK/IRF3 activation cascade. IFNAR−/− or control macrophages were infected with rAdV, and surface expression of CD86 and CD40 was determined by flow cytometry 36 h postinfection (Fig. 6A). Compromised levels of CD86 and CD40 in IFNR−/− macrophages confirm the importance of autocrine/paracrine type I IFN signaling in the APC maturation response to rAdV. Quantitative RNA measurement of IRF3-responsive transcripts harvested at 5 h postinfection reveals reduced transcript induction in IFNR−/− macrophages below that in wt macrophages (Fig. 6B). These data suggest that a large fraction of IRF3-dependent transcript induction depends on intact type I IFN signaling in the target APCs. A time course experiment of wt and IFNR−/− macrophages was carried out, and samples were harvested for Western blot analysis. When we probed for PSer396IRF3 (Fig. 6C), we found that PSer396IRF3 levels in IFNR−/− lysates were compromised compared to the wt. Furthermore, the levels of PSer172TBK1 were similar in wt and IFNR−/− rAdV-infected macrophages throughout the experimental time course, indicating that autocrine signaling was not influencing TBK1 activation. However, when filters were probed for pJNK, there was a clear pJNK deficiency in IFNR−/− macrophages at the later infection time points (Fig. 6C). The data indicate that maximal JNK activation in response to rAdV is compromised in cells that lack type I IFN signaling capability.
FIG. 6.
AdV-mediated IRF3 activation is controlled by type I IFN signaling. (A) AdV-induced surface expression of costimulatory molecules is compromised in IFNR−/− APCs. Macrophages from wild-type (WT) and IFNR−/− mice were treated as previously described and analyzed by flow cytometry for presence of costimulatory molecules CD40 and CD86. (B) IRF3-mediated gene expression is enhanced by type I IFN signaling. Real-time RT-PCR of IRF3-dependent mRNAs isolated from 5-h rAdV infection of WT or IFNR−/− macrophages. IL6, interleukin 6. (C) Type I IFN signaling is required for full JNK activation by AdV. Immunoblot of lysates from macrophages treated with vehicle (−) or AdV (+) for the indicated time points (in hours) were analyzed with anti-phospho-specific (Ser396-pIRF3, Thr183/Tyr185-pJNK, Ser172-pTBK1), anti-IRF3, or anti-β-actin antibodies. (D) Compromised pIRF3, pJNK, and p-cJun peak activation in STAT1−/− macrophages. The results of a time course experiment and Western blot analysis of AdV infection of lysates from WT and STAT1−/− macrophages are shown. (E) Pretreatment with IFN-β augments the JNK/IRF3 response to rAdV. Macrophages were treated for 18 h with 1,000 U/ml IFN-β or vehicle (Mock) followed by rAdV. Lysates harvested 5 h postinfection were characterized by immunoblotting as indicated. Representative data from one of two experiments are shown for each panel.
IFNR signaling leads to activation of Jak/STAT pathways, and we have shown that STAT1 and STAT2 transcription factors undergo tyrosine phosphorylation at 5 h postinfection. To determine whether STAT1 activation is required for the optimal induction of the JNK/TBK1/IRF3 cascade, macrophages isolated from STAT1−/− mice were used in a rAdV activation experiment. Consistent with the IFNAR−/− results, Western blot analysis of lysates from the STAT1−/− macrophage time course indicate a compromise in the peak levels of JNK and IRF3 activation (Fig. 6D).
Since type I IFN signaling is an established element contributing to induction of an antiviral state in uninfected cells, we next asked how macrophage pretreatment with type I IFNs influenced APC responsiveness to rAdV. Macrophages were pretreated for 18 h with 103 U/ml beta interferon (IFN-β) and exposed to mock or rAdV treatment. After 5 h, cells were harvested, and the lysates were analyzed by Western blotting. There was no indication of JNK or IRF3 activation in mock-infected cells. However, cells preincubated with IFN-β and treated with rAdV demonstrate a heightened activation response at the levels of pJNK, pIRF3, p-cJun, and pATF2 with no impact on the steady-state level of IRF3 (Fig. 6E). These results demonstrate that APCs undergo an autocrine amplification phase mediated by type I IFNs that impact on JNK networks. Enhancing JNK responsiveness following rAdV infection leads to an increased pool of IRF3 that is accessible to TBK1 phosphorylation.
DISCUSSION
Using rAdV as a model for interferon-stimulating DNA activation of APCs, we have shown how stress kinase cascades make a critical contribution to the recognition response to infecting viral DNA (see the model shown in Fig. 7). The IRF3 activation response to rAdV is proposed to involve two triggers as follows. (i) Capsid-cell membrane interactions contribute to a very modest activation of stress kinases, including JNK. (ii) Subsequent endosomal escape and presentation of vDNA to the cytoplasm engages a putative cytosolic DNA sensor that activates TBK1 and causes C-terminal IRF3 phosphorylation. Additionally, pJNK contributes directly to phosphorylation of c-Jun and ATF2 that collaborate with pIRF3 to activate IFN-β expression and secretion. To complete APC activation by rAdV, we have shown that the pJNK/TBK1/IRF3 priming cascade is an amplification target of the extrinsic type I IFN signaling cascade. Using the rAdV model as a paradigm, we predict that any cytosolic IFN-stimulating DNA response in APCs will require a similar combination of events to accomplish an effective inflammatory and or adaptive immune response.
FIG. 7.
Activation of innate immunostimulatory cascades during infection with adenovirus. Interaction of adenovirus capsid with the plasma membrane induces a JNK-mediated stress response (step 1) that via N-terminal phosphorylation or recruitment of scaffold proteins (step 2) would change the IRF3 configuration to unmask its C-terminal end. Simultaneously, the presence of double-stranded DNA in the cytosol (step 3) activates an as-yet-unidentified receptor and adaptor system that results in the induction of the TBK1 enzyme. Activated TBK1 induces the phosphorylation of IRF3 (step 4) on specific serine residues clustered on the now-exposed C-terminal part of the molecule, resulting in its homo- or heterodimerization. These dimers then translocate to the nucleus and in coordination with JNK-activated AP-1 induce the transcription of type I IFN genes (IFN-α4 and IFN-β) (step 5). Secreted IFNs activate the type I IFN receptor (step 6). This interaction leads to Tyk2/JAK activation of STAT1 and STAT2 and formation of the transcription factor complex IFN-stimulated gene factor 3 (ISGF3; a heterotrimer of p-STAT1, p-STAT2, and IRF9). Type I IFN-STAT1 activation leads directly or indirectly to) a positive-feedback amplification loop of the JNK/TBK1/IRF3 cytosolic DNA-sensing response cascade (step 7. The integration of the primary activation cascade (intrinsic) with the autocrine (extrinsic) cascade(s) leads to endpoint maturation of the APCs. AP-1, activator protein-1; ISRE, IFN-stimulated response element.
Although IRF3 activation has been extensively characterized in a variety of viral and nonviral systems, aspects of the IRF3 activation process remain unresolved. Early studies demonstrated the importance of C-terminal phosphorylation in dimerization and nuclear localization which lead to the proposal that the C-terminal phosphorylation site clusters were masked by protein folding in the inactive cytosolic state (24). The formation of a monomeric autoinhibitory complex in the C-terminal protein-interacting domain was confirmed by structure analysis (35, 43). A major unresolved component of IRF3 activation is identifying the mechanism that facilitates disruption of the autoinhibitory complex. In vitro studies have proposed a dual phosphorylation switch where TBK1 phosphorylation at cluster II (Ser396) leads to opening of the autoinhibitory structure, revealing access to cluster I and possibly cluster III (32). Our studies indicate an additional level of complexity to in vivo IRF3 phosphorylation. We have shown that rAdV induces TBK1, but not IKKɛ, and that upregulation of TBK1 leads to phosphorylation of IRF3, consistent with the in vitro model. However, inhibition of JNK disrupts rAdV-induced PSer396IRF3, but not activation of TBK1. Therefore, we have identified a form of IRF3 autoinhibition that involves TBK1 gaining access to IRF3 through the action of the JNK stress kinase.
The mechanism through which active JNK is able to facilitate exposure of Ser396IRF3 to TBK1 is unknown. Studies by Servant et al. (40) demonstrated involvement of stress kinases, DNA damage response, and calcium-dependent kinases in the generation of a phosphorylated form of IRF3 where the target site was located in the N-terminal region (amino acids 185 to 195); however, this response was not directly tied to the activation event occurring in the C-terminal domain. Although we have not detected JNK-dependent phosphorylation of IRF3 in rAdV-infected cell kinase assays (data not shown), the formal possibility of a direct interaction between JNK and IRF3 still exists and will require further investigation. Alternatively, JNK may function indirectly by either influencing an IRF3 N-terminus-specific kinase or by facilitating the formation of a restructured IRF3 complex. The indirect JNK/IRF3 activation model offers a more flexible strategy for tissue- and stimulus-specific IRF3 responses. Indeed, our studies with macrophages and DCs show that Lipofectamine transfection of AdV DNA induces a strong IRF3 phosphorylation that is largely independent of JNK inhibitors but highly susceptible to PI3K inhibitors. Interestingly, DNA-PK, a wortmannin- and LY294002-sensitive member of the PI3K family, has previously been implicated in the activation and N-terminal phosphorylation of IRF3 (5). In an earlier study characterizing murine DC maturation with constant growth factor stimulation, we had identified a P13K-dependent influence on CD86 surface expression (33). Stress signaling leading to IRF3 phosphorylation has been also reported for lipopolysaccharide where TLR4-mediated nuclear translocation of IRF3 was shown to be sensitive to inhibition of stress-activated protein kinase p38 (30). Together, these data suggest that induction of stimulus-specific and/or cell-specific stress pathways is a vital component when generating an innate antiviral response through IRF3. Determining how these stress cascades are influencing IRF3 presentation to TBK1 or vice versa will provide insight into how the antiviral response to IFN-inducing DNA is fine-tuned. The cascades that are described for IRF3 may also impact on other IFN response factors, including IRF7. Little is known about IRF7 activation in response to rAdV, but it clearly makes an important contribution to the antiviral state. A recent study by Fejer et al. (11) demonstrated a strong loss of type I IFN induction following a systemic administration of Ad3 to IRF7−/− mice, whereas IFN secretion following Ad3 administration in IRF3−/− mice was no different from secretion in wt mice.
How does the type 1 IFN cascade impact on rAdV activation of APCs? The data indicate that primary viral detection by cytosolic PRRs triggers the phosphorylation of IRF3 to generate IFN-β and IFN-α4. These type I IFNs act through their cognate receptor, IFNAR1/IFNAR2, and activate STAT1 and STAT2 to induce IRF7 transcription. Upregulation of IRF7 results in a further induction of IFN-β and IFN-α4 and the remaining IFN-α subtypes (19, 31). In this study, we found that the type I IFN positive-feedback loop also controls the level of C-terminal phosphorylated IRF3 achieved during viral infection. Type I IFN signaling does not affect changes in total levels of JNK, IRF3, or TBK1 but stimulates the JNK-mediated stress response to the virus. We have shown for the first time that JNK induction is a rate-limiting step during this stage of the antiviral response (Fig. 4) and that the levels of pJNK induced in wt cells and IFNR−/− rAdV-infected cells reflect type I IFN enhancement (Fig. 6). Similar observations were made when STAT1−/− macrophages were exposed to rAdV. In agreement with these observations, we (Fig. 6) and others (8, 48) have observed that extrinsic type I IFN enhances the activation of the JNK stress pathway. The current studies demonstrate a requirement for type I IFN signaling for optimal JNK/TBK1/IRF3 response; they do not distinguish between establishment of an optimal JNK response network through basal IFN signaling or through a rAdV-induced response network.
On the basis of the potency of the innate and adaptive immune response to rAdV, we propose that the IFN-inducing adenoviral DNA pathway should serve as a blueprint for the optimization of DNA vaccine strategies. So far, optimization of naked DNA vaccines has involved the use of methods, such as electroporation (47) or methods using cationic liposomes (4) to significantly enhance DNA uptake. In addition to delivering DNA to cytosolic sensors, these methods of DNA transfection induce cellular stress responses (2). Cationic liposomes induce a variety of kinase cascades, including MAPK, ERK, and PI3K (50). Electroporation induces a strong stress response (2), and DNA vaccines administered by these methods utilize the TBK1/IRF3 cascade as an essential element of DNA-dependent innate immunity (21). Differences in stress response timing and/or duration as well as coordination with presentation of vaccine antigenic epitopes may contribute to the different immunogenicities observed for DNA delivered by rAdV and nonviral systems. As previously described, the stress cascades involved in IRF3 activation by DNA transfection are not identical to those activated by rAdV, and these differences may have a profound influence in the adjuvency of the cytosolic DNA signal. Enhancing our understanding of how to coordinate the intersection between cytosolic DNA sensing and stress cascades may provide an opportunity to direct and fine-tune the adjuvency and consequently the adaptive immune response to antigens delivered by naked DNA or rAdV vectors.
Acknowledgments
We are grateful to Andrew Larner, Carolina Lopez, Thomas Mosman, and Lionel Ivashkiv for generously providing reagents used in this study and to A.L. for manuscript critique.
This work was supported by the National Institutes of Health grants R01 AI63142 to E.F.-P. and KO1 HL70438 to M.N.
Footnotes
Published ahead of print on 11 February 2009.
REFERENCES
- 1.Agalioti, T., S. Lomvardas, B. Parekh, J. Yie, T. Maniatis, and D. Thanos. 2000. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-beta promoter. Cell 103667-678. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, J. M., G. C. Newbound, and M. D. Lairmore. 1997. Transcriptional modulation of viral reporter gene constructs following induction of the cellular stress response. Nucleic Acids Res. 251082-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basner-Tschakarjan, E., E. Gaffal, M. O'Keeffe, D. Tormo, A. Limmer, H. Wagner, H. Hochrein, and T. Tuting. 2006. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production. J. Gene Med. 81300-1306. [DOI] [PubMed] [Google Scholar]
- 4.Chen, W. C., and L. Huang. 2005. Non-viral vector as vaccine carrier. Adv. Genet. 54315-337. [DOI] [PubMed] [Google Scholar]
- 5.Chu, W., X. Gong, Z. Li, K. Takabayashi, H. Ouyang, Y. Chen, A. Lois, D. J. Chen, G. C. Li, M. Karin, and E. Raz. 2000. DNA-PKcs is required for activation of innate immunity by immunostimulatory DNA. Cell 103909-918. [DOI] [PubMed] [Google Scholar]
- 6.Clément, J. F., A. Bibeau-Poirier, S. P. Gravel, N. Grandvaux, E. Bonneil, P. Thibault, S. Meloche, and M. J. Servant. 2008. Phosphorylation of IRF-3 on Ser 339 generates a hyperactive form of IRF-3 through regulation of dimerization and CBP association. J. Virol. 823984-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotten, M., and J. M. Weber. 1995. The adenovirus protease is required for virus entry into host cells. Virology 213494-502. [DOI] [PubMed] [Google Scholar]
- 8.Cull, V. S., P. A. Tilbrook, E. J. Bartlett, N. L. Brekalo, and C. M. James. 2003. Type I interferon differential therapy for erythroleukemia: specificity of STAT activation. Blood 1012727-2735. [DOI] [PubMed] [Google Scholar]
- 9.Elkon, K. B., C. C. Liu, J. G. Gall, J. Trevejo, M. W. Marino, K. A. Abrahamsen, X. Song, J. L. Zhou, L. J. Old, R. G. Crystal, and E. Falck-Pedersen. 1997. Tumor necrosis factor alpha plays a central role in immune-mediated clearance of adenoviral vectors. Proc. Natl. Acad. Sci. USA 949814-9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falvo, J. V., B. S. Parekh, C. H. Lin, E. Fraenkel, and T. Maniatis. 2000. Assembly of a functional beta interferon enhanceosome is dependent on ATF-2-c-jun heterodimer orientation. Mol. Cell. Biol. 204814-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fejer, G., L. Drechsel, J. Liese, U. Schleicher, Z. Ruzsics, N. Imelli, U. F. Greber, S. Keck, B. Hildenbrand, A. Krug, C. Bogdan, and M. A. Freudenberg. 2008. Key role of splenic myeloid DCs in the IFN-alphabeta response to adenoviruses in vivo. PLoS Pathog. 4e1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald, K. A., S. M. McWhirter, K. L. Faia, D. C. Rowe, E. Latz, D. T. Golenbock, A. J. Coyle, S. M. Liao, and T. Maniatis. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4491-496. [DOI] [PubMed] [Google Scholar]
- 13.Greber, U. F., P. Webster, J. Weber, and A. Helenius. 1996. The role of the adenovirus protease on virus entry into cells. EMBO J. 151766-1777. [PMC free article] [PubMed] [Google Scholar]
- 14.Hensley, S. E., W. Giles-Davis, K. C. McCoy, W. Weninger, and H. C. Ertl. 2005. Dendritic cell maturation, but not CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J. Immunol. 1756032-6041. [DOI] [PubMed] [Google Scholar]
- 15.Hibi, M., A. Lin, T. Smeal, A. Minden, and M. Karin. 1993. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 72135-2148. [DOI] [PubMed] [Google Scholar]
- 16.Hiscott, J. 2007. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 28215325-15329. [DOI] [PubMed] [Google Scholar]
- 17.Hiscott, J., P. Pitha, P. Genin, H. Nguyen, C. Heylbroeck, Y. Mamane, M. Algarte, and R. Lin. 1999. Triggering the interferon response: the role of IRF-3 transcription factor. J. Interferon Cytokine Res. 191-13. [DOI] [PubMed] [Google Scholar]
- 18.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 10010872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honda, K., and T. Taniguchi. 2006. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6644-658. [DOI] [PubMed] [Google Scholar]
- 20.Ishii, K. J., C. Coban, H. Kato, K. Takahashi, Y. Torii, F. Takeshita, H. Ludwig, G. Sutter, K. Suzuki, H. Hemmi, S. Sato, M. Yamamoto, S. Uematsu, T. Kawai, O. Takeuchi, and S. Akira. 2006. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 740-48. [DOI] [PubMed] [Google Scholar]
- 21.Ishii, K. J., T. Kawagoe, S. Koyama, K. Matsui, H. Kumar, T. Kawai, S. Uematsu, O. Takeuchi, F. Takeshita, C. Coban, and S. Akira. 2008. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451725-729. [DOI] [PubMed] [Google Scholar]
- 22.Kishore, N., Q. K. Huynh, S. Mathialagan, T. Hall, S. Rouw, D. Creely, G. Lange, J. Caroll, B. Reitz, A. Donnelly, H. Boddupalli, R. G. Combs, K. Kretzmer, and C. S. Tripp. 2002. IKK-i and TBK-1 are enzymatically distinct from the homologous enzyme IKK-2: comparative analysis of recombinant human IKK-i, TBK-1, and IKK-2. J. Biol. Chem. 27713840-13847. [DOI] [PubMed] [Google Scholar]
- 23.Li, E., D. Stupack, R. Klemke, D. A. Cheresh, and G. R. Nemerow. 1998. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH kinase. J. Virol. 722055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin, R., Y. Mamane, and J. Hiscott. 1999. Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol. Cell. Biol. 192465-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin, D. A., and K. B. Elkon. 2006. Intracellular mammalian DNA stimulates myeloid dendritic cells to produce type I interferons predominantly through a Toll-like receptor 9-independent pathway. Arthritis Rheum. 54951-962. [DOI] [PubMed] [Google Scholar]
- 26.McWhirter, S. M., K. A. Fitzgerald, J. Rosains, D. C. Rowe, D. T. Golenbock, and T. Maniatis. 2004. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl. Acad. Sci. USA 101233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, G., S. Lahrs, A. B. Shah, and R. P. DeMatteo. 2003. Optimization of dendritic cell maturation and gene transfer by recombinant adenovirus. Cancer Immunol. Immunother. 52347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muruve, D. A. 2004. The innate immune response to adenovirus vectors. Hum. Gene Ther. 151157-1166. [DOI] [PubMed] [Google Scholar]
- 29.Nociari, M., O. Ocheretina, J. W. Schoggins, and E. Falck-Pedersen. 2007. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 814145-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noyce, R. S., S. E. Collins, and K. L. Mossman. 2006. Identification of a novel pathway essential for the immediate-early, interferon-independent antiviral response to enveloped virions. J. Virol. 80226-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panne, D., T. Maniatis, and S. C. Harrison. 2007. An atomic model of the interferon-beta enhanceosome. Cell 1291111-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panne, D., S. M. McWhirter, T. Maniatis, and S. C. Harrison. 2007. Interferon regulatory factor 3 is regulated by a dual phosphorylation-dependent switch. J. Biol. Chem. 28222816-22822. [DOI] [PubMed] [Google Scholar]
- 33.Philpott, N. J., M. Nociari, K. B. Elkon, and E. Falck-Pedersen. 2004. Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-alpha induction pathway. Proc. Natl. Acad. Sci. USA 1016200-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pichlmair, A., and C. Reis e Sousa. 2007. Innate recognition of viruses. Immunity 27370-383. [DOI] [PubMed] [Google Scholar]
- 35.Qin, B. Y., C. Liu, S. S. Lam, H. Srinath, R. Delston, J. J. Correia, R. Derynck, and K. Lin. 2003. Crystal structure of IRF-3 reveals mechanism of autoinhibition and virus-induced phosphoactivation. Nat. Struct. Biol. 10913-921. [DOI] [PubMed] [Google Scholar]
- 36.Reily, M. M., C. Pantoja, X. Hu, Y. Chinenov, and I. Rogatsky. 2006. The GRIP1:IRF3 interaction as a target for glucocorticoid receptor-mediated immunosuppression. EMBO J. 25108-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoggins, J. W., and E. Falck-Pedersen. 2006. Fiber and penton base capsid modifications yield diminished adenovirus type 5 transduction and proinflammatory gene expression with retention of antigen-specific humoral immunity. J. Virol. 8010634-10644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Servant, M. J., N. Grandvaux, and J. Hiscott. 2002. Multiple signaling pathways leading to the activation of interferon regulatory factor 3. Biochem. Pharmacol. 64985-992. [DOI] [PubMed] [Google Scholar]
- 39.Servant, M. J., N. Grandvaux, B. R. tenOever, D. Duguay, R. Lin, and J. Hiscott. 2003. Identification of the minimal phosphoacceptor site required for in vivo activation of interferon regulatory factor 3 in response to virus and double-stranded RNA. J. Biol. Chem. 2789441-9447. [DOI] [PubMed] [Google Scholar]
- 40.Servant, M. J., B. ten Oever, C. LePage, L. Conti, S. Gessani, I. Julkunen, R. Lin, and J. Hiscott. 2001. Identification of distinct signaling pathways leading to the phosphorylation of interferon regulatory factor 3. J. Biol. Chem. 276355-363. [DOI] [PubMed] [Google Scholar]
- 41.Stetson, D. B., and R. Medzhitov. 2006. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 2493-103. [DOI] [PubMed] [Google Scholar]
- 42.Stilwell, J. L., D. M. McCarty, A. Negishi, R. Superfine, and R. J. Samulski. 2003. Development and characterization of novel empty adenovirus capsids and their impact on cellular gene expression. J. Virol. 7712881-12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahasi, K., N. N. Suzuki, M. Horiuchi, M. Mori, W. Suhara, Y. Okabe, Y. Fukuhara, H. Terasawa, S. Akira, T. Fujita, and F. Inagaki. 2003. X-ray crystal structure of IRF-3 and its functional implications. Nat. Struct. Biol. 10922-927. [DOI] [PubMed] [Google Scholar]
- 44.Takaoka, A., Z. Wang, M. K. Choi, H. Yanai, H. Negishi, T. Ban, Y. Lu, M. Miyagishi, T. Kodama, K. Honda, Y. Ohba, and T. Taniguchi. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448501-505. [DOI] [PubMed] [Google Scholar]
- 45.Tibbles, L. A., J. C. Spurrell, G. P. Bowen, Q. Liu, M. Lam, A. K. Zaiss, S. M. Robbins, M. D. Hollenberg, T. J. Wickham, and D. A. Muruve. 2002. Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J. Virol. 761559-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trevejo, J. M., M. W. Marino, N. Philpott, R. Josien, E. C. Richards, K. B. Elkon, and E. Falck-Pedersen. 2001. TNF-alpha-dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proc. Natl. Acad. Sci. USA 9812162-12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsang, C., S. Babiuk, S. van Drunen Littel-van den Hurk, L. A. Babiuk, and P. Griebel. 2007. A single DNA immunization in combination with electroporation prolongs the primary immune response and maintains immune memory for six months. Vaccine 255485-5494. [DOI] [PubMed] [Google Scholar]
- 48.van Boxel-Dezaire, A. H., M. R. Rani, and G. R. Stark. 2006. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25361-372. [DOI] [PubMed] [Google Scholar]
- 49.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73309-319. [DOI] [PubMed] [Google Scholar]
- 50.Yan, W., W. Chen, and L. Huang. 2007. Mechanism of adjuvant activity of cationic liposome: phosphorylation of a MAP kinase, ERK and induction of chemokines. Mol. Immunol. 443672-3681. [DOI] [PubMed] [Google Scholar]
- 51.Zhu, J., X. Huang, and Y. Yang. 2008. A critical role for type I IFN-dependent NK cell activation in innate immune elimination of adenoviral vectors in vivo. Mol. Ther. 161300-1307. [DOI] [PubMed] [Google Scholar]
- 52.Zhu, J., X. Huang, and Y. Yang. 2007. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J. Virol. 813170-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]