Abstract
Noroviruses are the major cause of nonbacterial gastroenteritis in humans. However, little is known regarding the norovirus life cycle, including cell binding and entry. In contrast to human noroviruses, the recently discovered murine norovirus 1 (MNV-1) readily infects murine macrophages and dendritic cells in culture. Many viruses, including the related feline calicivirus, use terminal sialic acids (SA) as receptors for infection. Therefore, we tested whether SA moieties play a role during MNV-1 infection of murine macrophages. Competition with SA-binding lectins and neuraminidase treatment led to a reduction in MNV-1 binding and infection in cultured and primary murine macrophages, suggesting a role for SA during the initial steps of the MNV-1 life cycle. Because SA moieties can be attached to glycolipids (i.e., gangliosides), we next determined whether MNV-1 uses gangliosides during infection. The gangliosides GD1a, GM1, and asialo-GM1 (GA1) are natural components of murine macrophages. MNV-1 bound to ganglioside GD1a, which is characterized by an SA on the terminal galactose, but not to GM1 or asialo-GM1 in an enzyme-linked immunosorbent assay. The depletion of gangliosides using an inhibitor of glycosylceramide synthase (d-threo-P4) led to a reduction of MNV-1 binding and infection in cultured and primary murine macrophages. This defect was specifically rescued by the addition of GD1a. A similar phenotype was observed for MNV field strains WU11 (GV/WU11/2005/USA) and S99 (GV/Berlin/2006/DE). In conclusion, our data indicate that MNV can use terminal SA on gangliosides as attachment receptors during binding to murine macrophages.
Noroviruses are nonenveloped, positive-sense RNA viruses in the family Caliciviridae (14). Human noroviruses (HuNoV) cause most of the sporadic cases and outbreaks of infectious gastroenteritis worldwide in people of all ages (3, 5, 12, 24, 32, 33, 60). However, little is known about early events in HuNoV infection due to the lack of an efficient cell culture system or small animal model (10, 52). Murine norovirus (MNV) is the only norovirus that grows well in tissue culture and has a tropism for murine macrophages and dendritic cells (62). It is an important pathogen and the most prevalent virus in research mice (17, 18, 35). MNVs comprise at least 15 distinct strains that differ less than 15% at the nucleotide level and belong to one genogroup and serotype (57). MNV, like its human counterparts, is an enteric virus that is highly infectious after oral inoculation, replicates in the intestine, and is shed in the stool, resulting in fecal-oral transmission (63). MNV-1 was initially isolated from immunocompromised mice (21), but we have since shown that MNV-1 can also infect inbred wild-type 129 and C57/BL6 mice (36, 57). This ability of MNV to infect a small animal host (21) and grow in cell culture (62), together with the availability of a reverse genetic system (6, 59), lays the foundation for detailed studies of various aspects of norovirus biology, including host factors required for binding as in this study.
Within the calicivirus family, binding and entry have best been studied for feline calicivirus (FCV). The virus infects the upper respiratory tract by attaching to α2,6-linked sialic acids (SA) and using the junctional adhesion molecule-1 for internalization (31, 53). Less is known about norovirus entry. Histo-blood group antigens (reviewed in references 11, 28, and 55), α2,3-sialylated carbohydrates of the type 2 chain (e.g., sialyl-Lewis x [44]), and glycosaminoglycan heparan sulfate (54) are carbohydrates that function as attachment molecules for HuNoV strains, but cellular cofactors that determine permissiveness have yet to be identified (15).
Virus entry often is a multistep process that is usually initiated by binding to an attachment receptor, but an interaction with a specific entry receptor(s) is necessary for internalization (4, 50). Carbohydrate moieties of host cell glycoproteins and glycolipids, e.g., SA, as well as proteoglycans, constitute a widely used strategy of viruses to attach to epithelial cells (4). In certain cases, SA can account for virus host range, tissue tropism, and pathogenesis (4, 27, 41). Most SA receptors utilized by viruses contain terminal SA attached to the penultimate galactose by an α2,6 or α2,3 linkage, including reovirus, rotavirus, and enterovirus, which infect their host through the intestinal tract (reviewed in reference 41).
Gangliosides are acidic glycosphingolipids (GSL) that are composed of ceramide and oligosaccharide side chains that contain one or more SA, primarily in the α2,3 and α2,8 orientation. They are differentially expressed on the cell surface and are involved in diverse biological functions (30). Multiple viruses, bacteria, and bacterial toxins have been shown to use gangliosides as receptors (reviewed in references 2 and 41). Interestingly, enteric rotaviruses, the leading cause of childhood diarrhea, can use gangliosides for attachment (reviewed in reference 20).
In this report, we analyze the role of SA particularly on gangliosides as attachment receptors for MNV. We show that MNV-1 binds to SA moieties on cultured and primary murine macrophages. In particular, binding to terminal SA on the ganglioside GD1a is important during the attachment phase in the viral life cycle in what we propose is a multistep binding process.
MATERIALS AND METHODS
Cell culture and mice.
RAW 264.7 cells were purchased from ATCC (Manassas, VA) and maintained as described previously (62). Swiss Webster mice were purchased from Charles River and primary bone marrow-derived macrophages (BM-MΦ) were isolated and cultured as described previously (62).
Virus stocks.
The MNV strains WU11 (GV/WU11/2005/USA) and S99 (Berlin/2006/DE) were used at passage 3 and the plaque-purified MNV-1 clone (GV/MNV1/2002/USA) MNV-1.CW3 at passage 6 (35, 57).
Virus quantification by quantitative reverse transcriptase PCR (qRT-PCR).
For the quantification of different MNV strains, a real-time RT-PCR assay was established using TaqMan technology, amplifying a conserved region in open reading frame 1 with the following primers and probe: sense primer 5′-GTGCGCAACACAGAGAAACG-3′, antisense primer 5′-CGGGCTGAGCTTCCTGC-3′, and probe 5′-FAM-CTAGTGTCTCCTTTGGAGCACCTA-3′-TAMRA-FAM. The extracted total RNA was resuspended in 15 μl DNase/RNase-free water. Viral cDNA was amplified (42°C, 50 min; 70°C, 15 min) using Moloney murine leukemia virus RT (Invitrogen, CA) with the antisense oligonucleotide, according to the manufacturer's recommendations, using 4 μl RNA suspension in a reaction volume of 35 μl. The TaqMan reaction was carried out using Taq DNA polymerase (NEB, CA) according to the manufacturer's recommendations, using 5 μl cDNA, 500 nM sense and antisense oligonucleotides and probes, 3 mM MgCl2, 0.5 μl ROX reference dye (Invitrogen, CA) and 1 U Taq polymerase (NEB) in a reaction volume of 20 μl. The TaqMan PCR was performed on an MX-Pro qPCR system (Stratagene, TX) with the following conditions: one cycle at 95°C for 2 min, followed by 40 cycles of 94°C for 15 s, 55°C for 30 s, and 72°C for 15 s. To quantify the genome equivalents, an external standard curve was established using a 10-fold serial dilution of a plasmid containing the MNV-1 genome ranging from 4 × 108 to 4 × 102 copies.
Treatment and infection of cells.
Macrophages were plated in 12-well dishes at 2 × 105 cells per well. The cells were pretreated with lectins (Sambucus nigra lectin [SNL], Maackia amurensis lectin [MAL], and concanavalin A [ConA]; Sigma-Aldrich, MO), neuraminidase (Vibrio cholera neuraminidase, N7885; Sigma-Aldrich, MO), d-threo-1-ethylenedioxyphenyl-2-palmitoyl-3-pyrrolidino-propanol (D-threo-P4) (26), gangliosides (GD1a, GM1 [Matreya, PA], and GA1), purified B pentamer of wild-type (wt) or mutant (W92A) LT-IIb enterotoxin (38), or antibodies (anti-GD1a [Sigma-Aldrich, MO], MAB5606 [Millipore, IL], anti-GM1 [Sigma-Aldrich, MO], 1954 [Matreya, PA], and mouse and rabbit immunoglobulin G [IgG] as isotype controls [ZyMed, CA]) as described for each individual experiment. The cells were washed two times with 2 ml phosphate-buffered saline (PBS) per well and infected with MNV at the indicated multiplicities of infection (MOI) for 60 min on ice in a volume of 0.5 ml per well. For the lectins and enterotoxins, the cells were directly infected in the presence of competitor. The unbound virus was removed by washing the cells three times with 2 ml ice-cold PBS per well. For the binding assays, the cells were lysed by immediately adding 0.5 ml Trizol (Invitrogen, CA) to each well. The total RNA was extracted according to the manufacturer's recommendations, and genome equivalents were measured by TaqMan. For the infectivity assays, 0.5 ml (1 ml for lectins and enterotoxin) of medium was added to each well and the cells were incubated at 37°C and 5% CO2 for 8 h (RAW 264.7) or 10 h (primary BM-MΦ). The viral titers were measured by plaque assay as previously described (62).
Quantification of infected cells by immunofluorescence analysis (IFA).
To quantify the number of primary BM-MΦ infected with MNV-1, the cells expressing the nonstructural protein VPg (viral protein, genome linked) were counted. One day prior to infection, BM-MΦ were seeded in a six-well plate on sterile glass coverslips at 106 cells per well. The cells were pretreated and infected as described above. At 12 h postinfection (hpi), the cells were fixed in 4% paraformaldehyde for 15 min. After being washed in PBS, the cells were blocked in blocking buffer (PBS, 10% bovine serum albumin, 0.1% Triton X-100) for 1 h and subsequently probed for 1 h with a monoclonal antibody against MNV-1 VPg (59) diluted 1:5,000 in wash buffer (PBS, 1% bovine serum, 1% goat serum, 0.1% Triton X-100). After three PBS washes, the cells were incubated for 45 min with a fluorescein isothiocyanate-conjugated goat anti-mouse antibody (Invitrogen, CA; 1:5,000 dilution in wash buffer) and embedded with Prolong Gold antifade with DAPI (4′,6-diamidino-2-phenylindole) (Invitrogen, CA). Fluorescently labeled cells were examined using the Olympus IX70 inverted microscope at the Center for Live Cell Imaging at the University of Michigan. A total of 700 cells as indicated by DAPI staining were counted per condition and scored for MNV-1 VPg gene expression. After image analysis with Metamorph Premier v6.3 software (Molecular Devices, Downingtown, PA), the infected cells had a fluorescent intensity at least three times the fluorescent intensity of the uninfected controls. Their percentage was normalized to that of the untreated control.
Ganglioside depletion and rescue.
Macrophages were plated in 12-well dishes at 2 × 105 cells per well. The cells were pretreated in serum-free medium for 72 h with 200 nM D-threo-P4, dissolved in dimethyl sulfoxide (26), or mock-treated with equal amounts of dimethyl sulfoxide. At 48 h prior to infection, 3 μM free gangliosides (GD1a, GM1 [Matreya, PA], and GA1 [Sigma-Aldrich, MO] dissolved in double-distilled water) were added to the medium. Medium containing inhibitor and gangliosides was replaced every 24 h prior to infection. The cells were infected and harvested as described above. Cell viability was monitored in parallel by WST-1 reagent (Roche, NJ), following the manufacturer's recommendations and measuring the absorbance after 120 min.
Ganglioside extraction and quantification.
Total cellular lipids were extracted from cells as previously described (49) with the following modifications. Gangliosides were extracted from RAW 264.7 cells and primary BM-MΦ by chloroform-methanol-water extraction (2:1:0.8, vol/vol/vol), sonicated for 15 min in a bath sonicator, and centrifuged at 4,000 × g for 30 min. The lower chloroform phase was washed two times, separately from the upper liquid phase, with methanol and 0.9% NaCl (1:0.8, vol/vol). All liquid phases containing the gangliosides were recombined and desalted on a Sep-Pak C18 column as previously described (61), eluted with chloroform-methanol (1:1, vol/vol), and evaporated under a stream of nitrogen, and the residues were resuspended in chloroform:methanol (2:1, vol/vol). The total cellular phospholipids were estimated using a phosphate assay (42). Gangliosides were separated by thin-layer chromatography (TLC) using a solvent system containing chloroform-methanol-0.2% CaCl2 · 2H2O (55:45:10, vol/vol/vol) and detected by charring with 8% copper sulfate in 8% phosphoric acid. The quantification was performed by densitometric scanning and comparison with authentic standards run in parallel on the same plates.
Ganglioside ELISA.
The binding of MNV-1 to the purified gangliosides GD1a, GM1 (Matreya, PA) and GA1 was measured by enzyme-linked immunosorbent assay (ELISA) as previously described (21) with the following modifications. The ELISA plates were coated with 100 ng ganglioside per well in carbonate buffer (pH 9.6) overnight at 4°C. After washing and blocking, the plates were incubated with increasing concentrations of cesium chloride (CsCl)-purified MNV-1 (62) for 1 h at 37°C. The amount of purified virus was estimated by comparing the intensity of the viral capsid protein to a bovine serum albumin standard on a Coomassie-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel. The bound virus was detected with a rabbit anti-MNV-1 polyclonal antiserum (1:10,000 dilution [62]), followed by peroxidase-conjugated secondary goat anti-rabbit IgG (Jackson Immunoresearch; 1:5,000 dilution). For the enterotoxin competition experiments, ganglioside-coated polyvinyl microtiter plates were incubated overnight at 4°C with 10 or 100 μg/ml LT-IIb (wt), mutant LT-IIb (W92A), or PBS. After washing and blocking, the plates were then incubated with 5 μg/well CsCl-purified MNV-1 overnight at 4°C.
Statistics.
Differences in the binding and infection assays are shown as percentages relative to the control treatment and are presented as means ± standard errors (SE) of duplicate samples from at least three independent experiments. Statistical analysis was performed using a two-tailed paired t test with two degrees of freedom. All statistical analyses were carried out using the Prism software package (GraphPad Software, CA).
RESULTS
MNV-1 infection is partially blocked by SA-binding lectins.
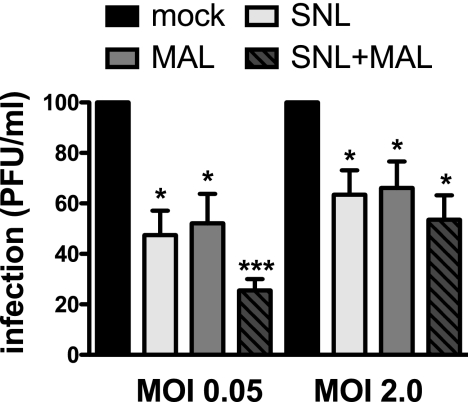
To investigate whether SA plays a role in MNV infection, we pretreated cultured murine macrophages (RAW 264.7 cells) with 100 μg/ml of two SA-binding lectins. Infections of cells with MNV-1 in the presence of MAL, which preferentially binds α2,3-linked SA (58), and SNL, which preferentially binds α2,6-linked SA (47), resulted in a significant drop in viral titers 8 hpi (Fig. 1). No statistically significant difference in the degree of inhibition was observed comparing low and high MOI. However, a combined lectin treatment with 50 μg/ml SNL and MAL each had a statistically significant synergistic effect, particularly at a low MOI (Fig. 1). Competition with 100 μg/ml ConA, which binds to α-linked mannose and terminal glucose residues (43), did not inhibit MNV-1 infection (data not shown). Unlike FCV, no preference between α2,3- and α2,6-linked SA was observed (53), as both SNL and MAL inhibited MNV-1 infection to an extent similar to that of the mock treatment at a given MOI. The partial inhibition by the two lectins may be due to the incomplete inhibition of all surface-exposed SA residues, as we were unable to increase the amount of lectin further due to deleterious effects on cell viability. Alternatively, additional binding factors may be important during MNV-1 infection as partial inhibition is also observed with other viruses that use SA as attachment receptors (40, 51, 53).
FIG. 1.
MNV-1 infection is partially blocked by lectins from Maackia amurensis and Sambucus nigra. RAW 264.7 cells were pretreated with 100 μg/ml SNL or MAL or a combined 50 μg/ml SNL and MAL each or mock treated for 1 h at 37°C prior to infection with MNV-1. Infection was carried out for 1 h on ice to prevent the virus from internalizing. Unbound virus was washed off, and cells were incubated for 8 h at 37°C to allow for a single round of infection. Viral titers were determined by plaque assay and are shown as percentages of infection relative to the no-lectin treatment (mock). Cell viability throughout the experiment was monitored using WST-1 reagent and remained above 80% (data not shown). The results are presented as means ± SE of the results of duplicate samples from four independent experiments. Statistical analysis was performed using the paired t test. *, P < 0.05; ***, P < 0.001.
MNV-1 binding and infection are neuraminidase sensitive.
To further verify the role for SA in MNV-1 binding and infection, RAW 264.7 cells and primary murine BM-MΦ were pretreated with Vibrio cholera neuraminidase, which cleaves α2,3- and α2,6-linked terminal SA as well as α2,8-linked internal SA. The cells were infected with MNV-1 for 60 min on ice. The amount of viral genome bound to cells was determined by qRT-PCR at 0 hpi, the infectious virus was measured 8 hpi (RAW 264.7) or 10 hpi (BM-MΦ) by plaque assay, or the number of infected cells expressing the nonstructural protein VPg was determined by IFA at 12 hpi. The removal of SA moieties from the cell surface of cultured or primary macrophages led to a significant dose-dependent loss in viral binding at high and low MOI (Fig. 2a and b). This decrease in binding resulted in a significant reduction in MNV-1 titers of approximately 75% in primary and cultured murine macrophages (Fig. 2c and d). Treatment of RAW 264.7 cells with up to 400 U/ml Salmonella enterica serovar Typhimurium neuraminidase, which preferentially cleaves α2,3-linked SA, resulted in a statistically significant inhibition of infection of approximately 30% (data not shown). To determine whether the amount of virus bound to cells correlated with the number of infected cells instead of the number of infectious virions, IFA was performed for VPg in primary macrophages at high MOI (Fig. 2e). VPg attached to the incoming viral genome could not be detected at 0 hpi (data not shown). A modest decrease in the percentage of infected cells was observed at 12 hpi after neuraminidase treatment. In general, neuraminidase treatment resulted in a stronger inhibition of MNV-1 infection than binding, but a good correlation was seen between the percentage of VPg expressing cells and binding. However, neither lectin competition nor sialidase treatment abolished the infection completely.
FIG. 2.
MNV-1 binding and infection are neuraminidase sensitive. RAW 264.7 cells (a and c) and primary murine BM-MΦ (b, d, and e) were pretreated with 0, 2.5, and 25 mU/ml Vibrio cholera neuraminidase prior to infection with MNV-1. Infection was carried out for 1 h on ice, and unbound virus was washed off. (a, b) To determine viral binding, cells were lysed immediately after infection and genome equivalents (genome eq) were determined by qRT-PCR (TaqMan). (c, d) To determine the effect on infection, cells were further incubated for 8 h (RAW264.7) and 10 h (BM-MΦ) at 37°C. Viral titers were determined by plaque assay and are shown as percentages of infection relative to that of the mock treatment. Cell viability throughout the experiment was monitored using WST-1 reagent and remained above 90% (data not shown). (e) To determine the number of cells infected with MNV-1 (MOI = 2.0), the number of BM-MΦ expressing VPg was determined by IFA at 12 hpi. Results are shown as percentages of infected cells relative to that of the mock treatment. Results of all assays are presented as means ± SE from three independent experiments. Statistical analysis was performed using the paired t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
MNV-1 binds terminal SA on gangliosides.
SA moieties are found on glycoconjugated proteins and lipids. Gangliosides are glycosphingolipids that can contain SA moieties and serve as attachment factors for many viruses, including rotaviruses (20, 41). Therefore, we determined whether MNV-1 could bind to gangliosides present in the cell membranes of murine macrophages. First, we used TLC to determine which membrane gangliosides are present on murine macrophages. A comparison of the bands to a standard of known gangliosides (Matreya, PA) showed that GD1a, GM1, and GA1 (asialo-GM1) are natural components of RAW 264.7 cells and primary BM-MΦ (Fig. 3a). The other bands likely represent structural variants in the ceramide moieties. A similar pattern of gangliosides was found in mouse peritoneal macrophages which express primarily GD1a and GM1 gangliosides (65). Figure 3b schematically depicts the structures of GD1a, GM1, and the nonsialylated ganglioside GA1. GD1a contains two α2,3-linked SA, one terminal SA that is accessible to neuraminidase and one internal SA resistant to neuraminidase treatment (20). GM1 contains only one internal α2,3-linked SA and GA1 contains none. Second, to determine whether MNV-1 can bind to these three gangliosides, microtiter plates were coated with GD1a, GM1, or GA1, and the binding of increasing concentrations of purified MNV-1 was determined by ELISA (Fig. 3c). The B pentamers of cholera toxin, which bind GM1 (13, 25), and LT-IIb, an enterotoxin from Escherichia coli which binds GD1a (38), were used as positive controls (data not shown). In the ELISA, MNV-1 only bound efficiently to GD1a but not GM1 or GA1, suggesting that MNV-1 can bind to the terminal SA on GD1a but not to the internal SA on GM1.
FIG. 3.
MNV-1 binds to the terminal SA on the penultimate galactose on GD1a. (a) Gangliosides were extracted from total lipids of RAW 264.7 cells or primary BM-MΦ, separated by TLC, and compared to known ganglioside standards. GD1a, GM1, and GA1 were identified as components of RAW 264.7 cells and primary BM-MΦ. (b) Structures of the gangliosides GD1a, GM1, and GA1. Glc, glucose; Gal, galactose; GalNAc, N-acetylgalactosamine. (c) MNV-1 binding to specific gangliosides was analyzed in an ELISA. GD1a, GM1, and GA1 were coated onto microtiter plates and incubated with increasing concentrations of purified MNV-1 or PBS (0 μg/well). (d) LT-IIb competition of MNV-1 binding to GD1a by ELISA. Microtiter plates were coated with GD1a and incubated with B pentamer of LT-IIb wt or the W92A mutant or PBS prior to the addition of purified MNV-1. (c and d) Virus was detected with anti-MNV-1 rabbit polyclonal serum, followed by a peroxidase-labeled secondary antibody and developed using ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid]. Absorbance was measured at an optical density at 405 nm. The results are presented as means ± SE of the results of duplicate samples from three independent experiments. The dashed line is drawn at twice the background level, and values below it were considered negative. Statistical analysis was performed using the paired t test. *, P < 0.05.
To verify the binding specificity of MNV-1 for GD1a, we tested the ability of the LT-IIb B pentamer to compete for MNV-1 binding to GD1a by ELISA (Fig. 3d). The B pentamer of a GD1a-nonbinding mutant of LT-IIb (W92A) with a single tryptophan-to-alanine substitution was used as a control (H. F. Nawar and T. D. Connell, unpublished data). Microtiter plates were coated with GD1a and incubated with 10 or 100 μg/ml of enterotoxins or PBS prior to the addition of MNV-1. The addition of wt LT-IIb but not its binding-deficient W92A mutant specifically reduced MNV-1 binding to GD1a. This indicated that MNV-1 binding to GD1a is specific and raised the possibility that MNV-1 can bind to GD1a during the infection of macrophages.
The GD1a-binding enterotoxin LT-IIb and an anti-GD1a antibody block MNV-1 binding.
To determine whether MNV-1 binds to GD1a on murine macrophages, we performed competition experiments with the B pentamer of LT-IIb and its GD1a-nonbinding W92A mutant, or an anti-GD1a and anti-GM1 antibody (Fig. 4). RAW 264.7 cells were preincubated with increasing concentrations of the respective LT-IIb B pentamer or antibody and infected with MNV-1 in the presence of a competitor. The amount of viral genome bound to cells was measured by our qRT-PCR binding assay as described above. A dose-dependent decrease in MNV-1 binding was seen in the presence of the GD1a-binding LT-IIb B pentamer (wt) but not the isogenic binding-deficient W92A mutant (Fig. 4a) as well as an anti-GD1a but not an anti-GM1 antibody (Fig. 4b). These data demonstrate that the wt LT-IIb B pentamer and an anti-GD1a antibody specifically compete for MNV-1 binding. This suggests that MNV-1 binding to GD1a plays a biologically important role during MNV-1 infection of macrophages.
FIG. 4.
MNV-1 binding to murine macrophages is inhibited by the GD1a-binding LT-IIb B pentamer from E. coli and an anti-GD1a antibody. (a) RAW 264.7 cells were pretreated with 10 or 100 μg/ml B pentamer of wild-type (wt) LT-IIb or the non-GD1a binding LT-IIb W92A mutant or mock treated for 1 h at 37°C. (b) RAW 264.7 cells were pretreated for 1 h at 37°C with 2 or 20 μg/ml anti-GD1a (mouse), anti-GM1 (rabbit), or the corresponding isotype controls (2 or 20 μg/ml mouse/rabbit IgG) prior to infection with MNV-1. All cells were infected at an MOI of 2 as described in the legend to Fig. 2, and viral binding was determined by qRT-PCR measuring genome copies at 0 hpi. Cell viability throughout the experiment was monitored using WST-1 reagent and remained above 90% (data not shown). Results of the enterotoxin competition are shown as percentages relative to that of the mock treatment and are presented as means ± SE of the results of duplicate samples from five independent experiments. Results of the antibody competition are shown as percentages relative to those of their corresponding isotype controls, which were set to 100%, and are presented as means ± SE of the results of duplicate samples from three independent experiments. Statistical analysis was performed using the paired t test. *, P < 0.05; **, P < 0.01.
MNV-1 binding and infection of murine macrophages are reduced by the depletion of gangliosides and are rescued by the addition of GD1a.
To verify the role of GD1a during MNV-1 binding and infection, we used a specific inhibitor of glycosylceramide synthase, D-threo-P4, which depletes cells of GSL including gangliosides (26). A 48-h pretreatment of RAW 264.7 cells with 200 nM D-threo-P4 depleted 93% of GD1a and 92% of GM1 compared to that of the solvent control as determined by TLC (data not shown). Since D-threo-P4 only inhibits GSL synthesis, missing gangliosides can be selectively added back by incubating cells with free gangliosides that are incorporated into the plasma membrane (34). Murine macrophages were treated for 72 h with 200 nM D-threo-P4 to deplete GSL. The depletion of GSL reduced MNV-1 binding to RAW 264.7 cells and primary BM-MΦ by 90% and 50%, respectively, compared to that of the mock treatment (Fig. 5a and b). GSL depletion resulted in reduced MNV-1 titers (Fig. 5c and d) and VPg-expressing primary BM-MΦ (Fig. 5e).
FIG. 5.
Treatment of murine macrophages with D-threo-P4 inhibits MNV-1 binding and infection but can be rescued by the addition of GD1a. RAW 264.7 cells (a and c) and primary murine BM-MΦ (b, d, and e) were pretreated with 200 nM D-threo-P4 or mock treated for 72 h prior to infection. Free ganglioside GD1a or GA1 was added into the culture medium 48 h prior to infection. Cell viability throughout the experiment was monitored using WST-1 reagent and remained above 80% (data not shown). Cells were infected as described in the legend to Fig. 2. Viral binding was determined by qRT-PCR measuring genome copies at 0 hpi (a and b). Infection was determined by measuring MNV-1 titers by plaque assay at 8 hpi (RAW 264.7) and 10 hpi (BM-MΦ) (c and d) or counting the number of MNV-1-infected cells (MOI = 2.0) expressing VPg by IFA at 12 hpi (e). Results are shown as percentages relative to that of the mock treatment and are presented as means ± SE of the results from at least three independent experiments. Statistical analysis was performed using the paired t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To assess whether GD1a alone was sufficient to rescue the binding and infection defects observed after GSL depletion, depleted cells were reconstituted with specific gangliosides by adding 3 μM GD1a or GA1 as a control to the culture medium 48 h prior to infection. Under these conditions, TLC analysis showed a 2.4-fold increase in the amount of GD1a compared to that of the nonreconstituted RAW 264.7 cell membranes (data not shown). Reconstituting macrophage membranes with GD1a, but not GA1, specifically restored the binding of MNV-1 to RAW 264.7 cells and primary BM-MΦ (Fig. 5a and b). A similar trend was also observed for MNV-1 titers (Fig. 5c and d) and the number of infected cells (Fig. 5e). The ability of GD1a but not GA1 to rescue MNV-1 binding in cultured and primary murine macrophages provides a biological confirmation of the ELISA data (Fig. 3), demonstrating that MNV-1 binding to GD1a is biologically relevant during the infection of murine macrophages.
GD1a acts as an attachment receptor for MNV field strains WU11 and S99.
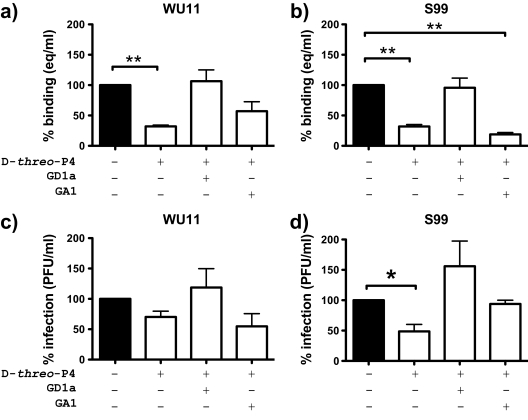
MNV-1 was isolated after repeated passage through the brains of immunocompromised mice (21) and may have acquired different characteristics from fecally isolated strains. Furthermore, MNV strains, while being members of a single genogroup, differ in their genetic and biological properties, including persistence in wild-type mice (57). Since binding to SA can alter the pathogenesis of other viruses (1, 19, 29, 37, 48), we analyzed whether GSL and, in particular, GD1a also play a role during the binding and infection of primary BM-MΦ with field strains WU11 and S99. The strains were chosen for their amino acid sequence diversity in the capsid protein. WU11, a nonpersistent MNV-1 strain (57), and S99, with an unknown persistence phenotype, share 93.9% and 94.8% amino acid identity with the MNV-1 capsid protein, respectively. Cells were depleted of GSL and reconstituted with GD1a or GA1, and the binding and infection of WU11 and S99 were assessed as described for MNV-1. Binding and infection in primary BM-MΦ depleted of GSL were reduced for both strains (Fig. 6). However, as observed previously with MNV-1 in RAW 264.7 cells (Fig. 5a and c), binding was reduced more significantly than infection. The addition of GD1a, but not GA1, to GSL-depleted BM-MΦ increased the viral binding and infection of WU11 and S99 (Fig. 6). These data demonstrated that similarly to MNV-1, the MNV strains WU11 and S99 depend in part on the ganglioside GD1a during the binding and infection of permissive macrophages. In the case of S99, however, GA1 in addition to GD1a may also play a role during infection, but not binding, as the infection defect observed after GSL depletion was rescued by GA1 (Fig. 6d).
FIG. 6.
MNV field strains WU11 and S99 use GD1a during attachment to primary murine macrophages. Primary BM-MΦ were depleted of GSL and reconstituted with GD1a and GA1 as described in the legend to Fig. 5. Cells were infected with WU11 and S99. Binding and infection were measured as described in the legend to Fig. 2. Cell viability throughout the experiment was monitored using WST-1 reagent and remained above 80% (data not shown). Results are shown as percentages relative to that of the mock treatment and are presented as means ± SE of the results of duplicate samples from three independent experiments. Statistical analysis was performed using the paired t test. *, P < 0.05; **, P < 0.01.
DISCUSSION
The study of norovirus entry has been particularly hampered by the lack of an efficient cell culture system. MNV is the only norovirus that replicates well in cell culture and therefore lends itself to a detailed molecular analysis of norovirus entry. In this study, we focused on the identification of molecules involved in MNV-1 attachment to cells. Very recently, sialylated neoglycoproteins like sialyl-Lewis x were identified as an additional HuNoV attachment factor (44). FCV, another member of the Caliciviridae family, attaches to α2,6-linked SA and uses JAM-1 as a receptor (31, 53). Many viruses, including rotaviruses, also use terminal SA as attachment receptors for infection. Therefore, we investigated the role of SA and gangliosides, SA-containing lipids, during the MNV-1 binding and infection of permissive murine macrophages.
Two main conclusions can be drawn from the data presented in this study. First, SA plays an important role during the MNV-1 infection of murine macrophages. Lectin competition and the treatment of cells with sialidase (Vibrio cholera neuraminidase) resulted in reduced viral titers or VPg-expressing cells (Fig. 1 and 2). This suggests that the binding of MNV-1 to both α2,3- and α2,6-linked SA is important during the viral life cycle. The utilization of SA independent of its linkage is also seen with adeno-associated virus types 1 and 6 (64) and the polyomaviruses BK virus and JC virus (9). Furthermore, using a newly developed binding assay, we demonstrated that the reduced viral titers after sialidase treatment in cultured and primary macrophages can in large part be attributed to the first step in the viral life cycle, i.e., virion binding. Second, our data demonstrate that the terminal SA on GD1a can serve as an attachment receptor during the infection of murine macrophages. Of the gangliosides identified in cultured and primary murine macrophages, MNV-1 can bind to GD1a in ELISAs and in tissue culture, but not GM1 or GA1, which lack terminal SA. Furthermore, we observed the specific competition of MNV-1 binding to GD1a with LT-IIb B pentamer, a GD1a-binding enterotoxin, in the ELISA (Fig. 3d) or in cultured macrophages and with an anti-GD1a antibody in culture (Fig. 4). This confirms a role for GD1a in MNV-1 attachment to cells. In addition, the biological relevance of gangliosides, specifically GD1a, during MNV-1 binding can be extended to some MNV field strains. Similarly to that with MNV-1, the depletion of gangliosides resulted in the reduced binding and infection of primary murine macrophages with field strains WU11 and S99, but this defect could be rescued by the addition of GD1a. In summary, MNV-1 binding to murine macrophages depends on terminal SA moieties and the SA-containing ganglioside GD1a is utilized by MNV-1 during the initial binding phase of the viral life cycle in murine macrophages.
Besides terminal SA on the ganglioside GD1a as a receptor for MNV, our data also suggest the involvement of additional receptor molecules. Our lectin data showed a role for both α2,3- and α2,6-linked SA during MNV-1 infection. However, the gangliosides identified in murine macrophages to date do not have α2,6-linked SA. In addition, SA and gangliosides are broadly present on many cell types, including MNV-1 nonpermissive intestinal cells and permissive murine macrophages (45, 65). Therefore, it is unlikely that binding to these moieties is the cause of the restricted tropism of MNV. Hence, additional nonlipid glycoconjugates with α2,6-linked SA may play a role during MNV entry. A similar phenomenon is observed for JC virus, which also uses different oligosaccharides, including glycoproteins and glycolipids, as receptors (23). Furthermore, the inhibition of binding and infection after the neuraminidase treatment of murine macrophages was incomplete, potentially indicating a role for additional receptor molecules. These molecules may or may not be sialylated, since not all SA are accessible to neuraminidase (20).
SA residues are important during virus attachment and entry (27, 41). Many viruses use SA as attachment molecules but rely on additional receptors for internalization (8, 50). The inhibition of GSL synthesis resulted in a significant inhibition of MNV binding to murine macrophages, but the effect on viral titers was less pronounced compared to neuraminidase treatment. In contrast, there was a good correlation between the percentage of infected cells as determined by IFA and of bound viral genome in the binding assay in primary macrophages. These discrepancies in the levels of inhibition may be explained by one or more of the following possibilities. (i) They reflect inherent differences between cultured and primary cells in their cell surface composition. (ii) A significant degree of experimental variation was observed for some conditions. (iii) The employed assays measure different populations of virions. The binding assay measures genome equivalents that may or may not lead to a productive infection. The plaque assay measures infectious particles produced within a replicative cycle (i.e., 8 h), irrespective of the number of infected cells, while IFA measures the number of infected cells. (iv) TLC data showed that ganglioside depletion was incomplete. This would leave potential binding sites exposed on the cell surface that in turn could be bound by an infectious particle and result in the production of many virions. (v) The contribution of gangliosides to the phases of binding and infection is different. If the entry of MNV into cells is similar to that of FCV and other viruses in that the virus binds to an attachment receptor but infection only occurs after binding to an entry receptor(s), gangliosides play an important role during binding because they allow MNV to attach to the cell. However, other nonganglioside molecules would be predicted to function as entry receptors to allow MNV to internalize and lead to an infection. This is consistent with observations for rotaviruses, which use gangliosides, including GD1a, as attachment receptors, and additional molecules, including integrins, as entry receptors (7, 20). However, further analysis is needed to fully understand the role of GD1a in MNV infection.
Multiple reports now indicate that binding to SA is a key factor for tropism and the pathogenesis of many viruses. An analysis of sialyltransferase expression shows that α2,6-linked SA are predominantly found in the lungs and liver, whereas α2,3-linked SA have a broader distribution (22). This is particularly well studied in the case of influenza virus, where the differential binding patterns toward α2,3- and α2,6-linked SA play a major role during infection and the transmission of human and emerging avian strains (19, 39, 48). Similarly, the respiratory FCV only uses α2,6-linked SA as attachment receptors, potentially explaining the tropism for the respiratory tract (53). Other examples are found among viruses that infect via the gastrointestinal tract. For example, type 1 reovirus binds to α2,3-linked SA present on apical surfaces of M cells in the Peyer's patch mucosa, and SA binding influences the systemic spread of type 3 reoviruses (1, 16). In the case of enteric transmissible gastroenteritis coronavirus, the ability to bind to SA is linked to its enteropathogenicity, potentially by facilitating the retention of virions in the SA-rich mucin of the intestine (46). MNV is an enteric virus that infects cells in the lamina propria of the small intestine (36). Whether SA plays a role in the enteric tropism of MNV and whether variability in the carbohydrate usage leads to the different biological characteristics (e.g., persistence) seen with individual strains remain currently unknown and are interesting questions for future investigations.
Little is yet known about early events during MNV infection. It has been proposed that the key factors for tropism and host specificity of noroviruses are linked to attachment, entry, and uncoating (15). For members of the calicivirus family, it has been shown that carbohydrates constitute attachment receptors for FCV, i.e., α2,6-linked SA (53), and HuNoV, i.e., histo-blood group antigens, heparan sulfate, and sialyl-Lewis x (44, 54, 56). Similarly, we report here that MNV uses carbohydrates, i.e., terminal SA moieties on the GD1a ganglioside and potentially other unidentified molecules, during attachment. This is the first report of an MNV receptor molecule in what is most likely a multistep binding and internalization process. Identifying additional receptor molecules will greatly enhance our knowledge of norovirus entry mechanisms. Utilizing the power of the MNV model system by beginning a detailed analysis of the molecular determinants of receptor binding in vitro and in vivo may lead to a better understanding of norovirus pathogenesis and the development of efficient antiviral strategies.
Acknowledgments
This work was sponsored in part by the NIH/NIAID Regional Center of Excellence for Bio-Defense and Emerging Infectious Diseases Research (RCE) Program, Region V “Great Lakes” RCE (NIH award 1-U54-AI-057153), to C.E.W. Work in the laboratory of T.D.C. is supported by NIH R01 DE013833 and DE017138.
We thank Eckart Schreier (Robert Koch-Institute, Berlin, Germany) for the MNV strain S99, Skip Virgin (University of Washington, St. Louis, MO) for the MNV strain WU11, and members of the Center for Statistical Consultation and Research at the University of Michigan for advice on statistical analysis. We are indebted to Bill Tsai and Michael Imperiale (University of Michigan) for critical reading of the manuscript and helpful comments.
Footnotes
Published ahead of print on 25 February 2009.
REFERENCES
- 1.Barton, E. S., B. E. Youree, D. H. Ebert, J. C. Forrest, J. L. Connolly, T. Valyi-Nagy, K. Washington, J. D. Wetzel, and T. S. Dermody. 2003. Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease. J. Clin. Investig. 1111823-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco, L. P. 2008. Gangliosides’ role in cellular interactions with microbes and immune cells activation, p. 29-52. In D. Sasaki (ed.), Glycolipids: new research, vol. 1. Nova Science Publishers, Inc., New York, NY. [Google Scholar]
- 3.Blanton, L. H., S. M. Adams, R. S. Beard, G. Wei, S. N. Bulens, M. A. Widdowson, R. I. Glass, and S. S. Monroe. 2006. Molecular and epidemiologic trends of caliciviruses associated with outbreaks of acute gastroenteritis in the United States, 2000-2004. J. Infect. Dis. 193413-421. [DOI] [PubMed] [Google Scholar]
- 4.Bomsel, M., and A. Alfsen. 2003. Entry of viruses through the epithelial barrier: pathogenic trickery. Nat. Rev. Mol. Cell Biol. 457-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. 2007. Norovirus activity—United States, 2006-2007. MMWR Morb. Mortal. Wkly. Rep. 56842-846. [PubMed] [Google Scholar]
- 6.Chaudhry, Y., M. A. Skinner, and I. G. Goodfellow. 2007. Recovery of genetically defined murine norovirus in tissue culture by using a fowlpox virus expressing T7 RNA polymerase. J. Gen. Virol. 882091-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciarlet, M., S. E. Crawford, E. Cheng, S. E. Blutt, D. A. Rice, J. M. Bergelson, and M. K. Estes. 2002. VLA-2 (α2β1) integrin promotes rotavirus entry into cells but is not necessary for rotavirus attachment. J. Virol. 761109-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimitrov, D. S. 2004. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2109-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dugan, A. S., M. L. Gasparovic, and W. J. Atwood. 2008. Direct correlation between sialic acid binding and infection of cells by two human polyomaviruses (JC virus and BK virus). J. Virol. 822560-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duizer, E., K. J. Schwab, F. H. Neill, R. L. Atmar, M. P. Koopmans, and M. K. Estes. 2004. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 8579-87. [DOI] [PubMed] [Google Scholar]
- 11.Estes, M. K., B. V. Prasad, and R. L. Atmar. 2006. Noroviruses everywhere: has something changed? Curr. Opin. Infect. Dis. 19467-474. [DOI] [PubMed] [Google Scholar]
- 12.Fankhauser, R. L., S. S. Monroe, J. S. Noel, C. D. Humphrey, J. S. Bresee, U. D. Parashar, T. Ando, and R. I. Glass. 2002. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J. Infect. Dis. 1861-7. [DOI] [PubMed] [Google Scholar]
- 13.Fukuta, S., J. L. Magnani, E. M. Twiddy, R. K. Holmes, and V. Ginsburg. 1988. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect. Immun. 561748-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green, K. Y. 2006. Caliciviridae, p. 949-980. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 5th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 15.Guix, S., M. Asanaka, K. Katayama, S. E. Crawford, F. H. Neill, R. L. Atmar, and M. K. Estes. 2007. Norwalk virus RNA is infectious in mammalian cells. J. Virol. 8112238-12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helander, A., K. J. Silvey, N. J. Mantis, A. B. Hutchings, K. Chandran, W. T. Lucas, M. L. Nibert, and M. R. Neutra. 2003. The viral σ1 protein and glycoconjugates containing α2-3-linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. J. Virol. 777964-7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson, K. S. 2008. Murine norovirus, a recently discovered and highly prevalent viral agent of mice. Lab. Anim. (New York) 37314-320. [DOI] [PubMed] [Google Scholar]
- 18.Hsu, C. C., C. E. Wobus, E. K. Steffen, L. K. Riley, and R. S. Livingston. 2005. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin. Diagn. Lab. Immunol. 121145-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibricevic, A., A. Pekosz, M. J. Walter, C. Newby, J. T. Battaile, E. G. Brown, M. J. Holtzman, and S. L. Brody. 2006. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J. Virol. 807469-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isa, P., C. F. Arias, and S. Lopez. 2006. Role of sialic acids in rotavirus infection. Glycoconj. J. 2327-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karst, S. M., C. E. Wobus, M. Lay, J. Davidson, and H. W. Virgin IV. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 2991575-1578. [DOI] [PubMed] [Google Scholar]
- 22.Kitagawa, H., and J. C. Paulson. 1994. Differential expression of five sialyltransferase genes in human tissues. J. Biol. Chem. 26917872-17878. [PubMed] [Google Scholar]
- 23.Komagome, R., H. Sawa, T. Suzuki, Y. Suzuki, S. Tanaka, W. J. Atwood, and K. Nagashima. 2002. Oligosaccharides as receptors for JC virus. J. Virol. 7612992-13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koopmans, M., J. Vinje, M. de Wit, I. Leenen, W. van der Poel, and Y. van Duynhoven. 2000. Molecular epidemiology of human enteric caliciviruses in The Netherlands. J. Infect. Dis. 181(Suppl. 2)S262-S269. [DOI] [PubMed] [Google Scholar]
- 25.Kuziemko, G. M., M. Stroh, and R. C. Stevens. 1996. Cholera toxin binding affinity and specificity for gangliosides determined by surface plasmon resonance. Biochemistry 356375-6384. [DOI] [PubMed] [Google Scholar]
- 26.Lee, L., A. Abe, and J. A. Shayman. 1999. Improved inhibitors of glucosylceramide synthase. J. Biol. Chem. 27414662-14669. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann, F., E. Tiralongo, and J. Tiralongo. 2006. Sialic acid-specific lectins: occurrence, specificity and function. Cell. Mol. Life Sci. 631331-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Pendu, J., N. Ruvoen-Clouet, E. Kindberg, and L. Svensson. 2006. Mendelian resistance to human norovirus infections. Semin. Immunol. 18375-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipton, H. L., A. S. Kumar, S. Hertzler, and H. V. Reddi. 2006. Differential usage of carbohydrate co-receptors influences cellular tropism of Theiler's murine encephalomyelitis virus infection of the central nervous system. Glycoconj. J. 2339-49. [DOI] [PubMed] [Google Scholar]
- 30.Lloyd, K. O., and K. Furukawa. 1998. Biosynthesis and functions of gangliosides: recent advances. Glycoconj. J. 15627-636. [DOI] [PubMed] [Google Scholar]
- 31.Makino, A., M. Shimojima, T. Miyazawa, K. Kato, Y. Tohya, and H. Akashi. 2006. Junctional adhesion molecule 1 is a functional receptor for feline calicivirus. J. Virol. 804482-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno-Espinosa, S., T. Farkas, and X. Jiang. 2004. Human caliciviruses and pediatric gastroenteritis. Semin. Pediatr. Infect. Dis. 15237-245. [DOI] [PubMed] [Google Scholar]
- 34.Moss, J., P. H. Fishman, V. C. Manganiello, M. Vaughan, and R. O. Brady. 1976. Functional incorporation of ganglioside into intact cells: induction of choleragen responsiveness. Proc. Natl. Acad. Sci. USA 731034-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller, B., U. Klemm, A. Mas Marques, and E. Schreier. 2007. Genetic diversity and recombination of murine noroviruses in immunocompromised mice. Arch. Virol. 1521709-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mumphrey, S. M., H. Changotra, T. N. Moore, E. R. Heimann-Nichols, C. E. Wobus, M. J. Reilly, M. Moghadamfalahi, D. Shukla, and S. M. Karst. 2007. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J. Virol. 813251-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nam, H. J., B. Gurda-Whitaker, W. Y. Gan, S. Ilaria, R. McKenna, P. Mehta, R. A. Alvarez, and M. Agbandje-McKenna. 2006. Identification of the sialic acid structures recognized by minute virus of mice and the role of binding affinity in virulence adaptation. J. Biol. Chem. 28125670-25677. [DOI] [PubMed] [Google Scholar]
- 38.Nawar, H. F., S. Arce, M. W. Russell, and T. D. Connell. 2005. Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities. Infect. Immun. 731330-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholls, J. M., R. W. Chan, R. J. Russell, G. M. Air, and J. S. Peiris. 2008. Evolving complexities of influenza virus and its receptors. Trends Microbiol. 16149-157. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson, E. C., F. Jamshidi, S. M. Johansson, M. S. Oberste, and N. Arnberg. 2008. Sialic acid is a cellular receptor for coxsackievirus A24 variant, an emerging virus with pandemic potential. J. Virol. 823061-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olofsson, S., and T. Bergstrom. 2005. Glycoconjugate glycans as viral receptors. Ann. Med. 37154-172. [DOI] [PubMed] [Google Scholar]
- 42.Rani, C. S., A. Abe, Y. Chang, N. Rosenzweig, A. R. Saltiel, N. S. Radin, and J. A. Shayman. 1995. Cell cycle arrest induced by an inhibitor of glucosylceramide synthase. Correlation with cyclin-dependent kinases. J. Biol. Chem. 2702859-2867. [DOI] [PubMed] [Google Scholar]
- 43.Reeke, G. N., Jr., J. W. Becker, B. A. Cunningham, G. R. Gunther, J. L. Wang, and G. M. Edelman. 1974. Relationships between the structure and activities of concanavalin A. Ann. N. Y. Acad. Sci. 234369-382. [DOI] [PubMed] [Google Scholar]
- 44.Rydell, G. E., J. Nilsson, J. Rodriguez-Diaz, N. Ruvoen-Clouet, L. Svensson, J. Le Pendu, and G. Larson. 2009. Human noroviruses recognize sialyl Lewis x neoglycoprotein. Glycobiology 19309-320. [DOI] [PubMed] [Google Scholar]
- 45.Sato, E., T. Uezato, M. Fujita, and K. Nishimura. 1982. Developmental profiles of glycolipids in mouse small intestine. J. Biochem. 912013-2019. [DOI] [PubMed] [Google Scholar]
- 46.Schwegmann-Wessels, C., and G. Herrler. 2006. Sialic acids as receptor determinants for coronaviruses. Glycoconj. J. 2351-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shibuya, N., K. Tazaki, Z. W. Song, G. E. Tarr, I. J. Goldstein, and W. J. Peumans. 1989. A comparative study of bark lectins from three elderberry (Sambucus) species. J. Biochem. 1061098-1103. [DOI] [PubMed] [Google Scholar]
- 48.Shinya, K., M. Ebina, S. Yamada, M. Ono, N. Kasai, and Y. Kawaoka. 2006. Avian flu: influenza virus receptors in the human airway. Nature 440435-436. [DOI] [PubMed] [Google Scholar]
- 49.Shu, L., L. Lee, Y. Chang, L. B. Holzman, C. A. Edwards, E. Shelden, and J. A. Shayman. 2000. Caveolar structure and protein sorting are maintained in NIH 3T3 cells independent of glycosphingolipid depletion. Arch. Biochem. Biophys. 37383-90. [DOI] [PubMed] [Google Scholar]
- 50.Smith, A. E., and A. Helenius. 2004. How viruses enter animal cells. Science 304237-242. [DOI] [PubMed] [Google Scholar]
- 51.Stevenson, R. A., J. A. Huang, M. J. Studdert, and C. A. Hartley. 2004. Sialic acid acts as a receptor for equine rhinitis A virus binding and infection. J. Gen. Virol. 852535-2543. [DOI] [PubMed] [Google Scholar]
- 52.Straub, T. M., K. Honer zu Bentrup, P. Orosz-Coghlan, A. Dohnalkova, B. K. Mayer, R. A. Bartholomew, C. O. Valdez, C. J. Bruckner-Lea, C. P. Gerba, M. Abbaszadegan, and C. A. Nickerson. 2007. In vitro cell culture infectivity assay for human noroviruses. Emerg. Infect. Dis. 13396-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stuart, A. D., and T. D. Brown. 2007. Alpha2,6-linked sialic acid acts as a receptor for feline calicivirus. J. Gen. Virol. 88177-186. [DOI] [PubMed] [Google Scholar]
- 54.Tamura, M., K. Natori, M. Kobayashi, T. Miyamura, and N. Takeda. 2004. Genogroup II noroviruses efficiently bind to heparan sulfate proteoglycan associated with the cellular membrane. J. Virol. 783817-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan, M., and X. Jiang. 2007. Norovirus-host interaction: implications for disease control and prevention. Expert Rev. Mol. Med. 91-22. [DOI] [PubMed] [Google Scholar]
- 56.Tan, M., and X. Jiang. 2005. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 13285-293. [DOI] [PubMed] [Google Scholar]
- 57.Thackray, L. B., C. E. Wobus, K. A. Chachu, B. Liu, E. R. Alegre, K. S. Henderson, S. T. Kelley, and H. W. Virgin IV. 2007. Murine noroviruses comprising a single genogroup exhibit biological diversity despite limited sequence divergence. J. Virol. 8110460-10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, W. C., and R. D. Cummings. 1988. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked alpha-2,3 to penultimate galactose residues. J. Biol. Chem. 2634576-4585. [PubMed] [Google Scholar]
- 59.Ward, V. K., C. J. McCormick, I. N. Clarke, O. Salim, C. E. Wobus, L. B. Thackray, H. W. Virgin IV, and P. R. Lambden. 2007. Recovery of infectious murine norovirus using pol II-driven expression of full-length cDNA. Proc. Natl. Acad. Sci. USA 10411050-11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Widdowson, M. A., S. S. Monroe, and R. I. Glass. 2005. Are noroviruses emerging? Emerg. Infect. Dis. 11735-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams, M. A., and R. H. McCluer. 1980. The use of Sep-Pak C18 cartridges during the isolation of gangliosides. J. Neurochem. 35266-269. [DOI] [PubMed] [Google Scholar]
- 62.Wobus, C. E., S. M. Karst, L. B. Thackray, K. O. Chang, S. V. Sosnovtsev, G. Belliot, A. Krug, J. M. Mackenzie, K. Y. Green, and H. W. Virgin. 2004. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wobus, C. E., L. B. Thackray, and H. W. Virgin IV. 2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. J. Virol. 805104-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu, Z., E. Miller, M. Agbandje-McKenna, and R. J. Samulski. 2006. α2,3 and α2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J. Virol. 809093-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yohe, H. C., S. Ye, B. B. Reinhold, and V. N. Reinhold. 1997. Structural characterization of the disialogangliosides of murine peritoneal macrophages. Glycobiology 71215-1227. [DOI] [PubMed] [Google Scholar]