Abstract
A major challenge for human immunodeficiency virus (HIV)/AIDS vaccines is the elicitation of anti-Env antibodies (Ab) capable of neutralizing the diversity of isolates in the pandemic. Here, we show that high-avidity, but nonneutralizing, Abs can have an inverse correlation with peak postchallenge viremia for a heterologous challenge. Vaccine studies were conducted in rhesus macaques using DNA priming followed by modified vaccinia Ankara boosting with HIV type 1 (HIV-1) immunogens that express virus-like particles displaying CCR5-tropic clade B (strain ADA) or clade C (IN98012) Envs. Rhesus granulocyte-macrophage colony-stimulating factor was used as an adjuvant for enhancing the avidity of anti-Env Ab responses. Challenge was with simian/human immunodeficiency virus (SHIV)-162P3, a CCR5-tropic clade B chimera of SIV and HIV-1. Within the groups receiving the clade B vaccine, a strong inverse correlation was found between the avidity of anti-Env Abs and peak postchallenge viremia. This correlation required the use of native but not gp120 or gp140 forms of Env for avidity assays. The high-avidity Ab elicited by the ADA Env had excellent breadth for the Envs of incident clade B but not clade C isolates, whereas the high-avidity Ab elicited by the IN98012 Env had excellent breadth for incident clade C but not clade B isolates. High-avidity Ab elicited by a SHIV vaccine with a dual-tropic clade B Env (89.6) had limited breadth for incident isolates. Our results suggest that certain Envs can elicit nonneutralizing but high-avidity Ab with broad potential for blunting incident infections of the same clade.
A central challenge for the development of a human immunodeficiency virus (HIV)/AIDS vaccine is the elicitation of protective antibodies (Ab) capable of recognizing the diversity of isolates that drive the worldwide pandemic (6). HIV infections elicit a neutralizing Ab that is patient rather than clade or epidemic specific (3, 27, 39). This reflects sequence variability and complex masking and thermodynamic characteristics of the HIV type 1 (HIV-1) envelope glycoprotein (Env) limiting Ab responses against conserved targets for neutralization.
Ab-mediated protection against viral infections is most frequently measured as titers of neutralizing Ab or Ab that can block the entry of virus into cultured cells. However, in whole animals and in cultures to which effector cells or complement have been added, Ab can also protect by initiating mechanisms of virus and cell killing that are dependent on the display of the Fc region of bound Ab (19, 26). Coating of virus with Ab, or opsonization, facilitates virus uptake and destruction by phagocytes. Binding of Ab to infected cells is the first step for lysis by activation of killer cells in a process called antibody-dependent cellular cytotoxicity. Cytolysis as well as other nonlytic mechanisms of virus control, such as the stimulation of inhibitory chemokines, are active in antibody-dependent cell-mediated virus inhibition assays. Binding of complement to Ab on virions or infected cells can initiate complement-mediated lysis (4).
The potential of a polyclonal serum to initiate nonneutralizing mechanisms of protection requires that the antibodies in the serum have sufficient titer and avidity (tightness of binding) for Fc-dependent mechanisms of killing to be activated. Antibodies to the Envs of immunodeficiency viruses undergo slow-affinity maturation because of high levels of glycosylation that interfere with the ability of immunoglobulin (Ig) to recognize Env and because HIV infections preferentially kill the antiviral CD4 T cells (9) whose help is required for B cells to enter into germinal center reactions for hypermutation and avidity maturation.
The vaccine trials reported here were undertaken to test candidate HIV-1 vaccines for their ability to elicit neutralizing Ab for incident isolates. All of the immunization regimens consisted of priming with recombinant DNA vaccines and boosting with recombinant modified vaccinia Ankara (MVA) vaccines. Both of the recombinant vaccines expressed noninfectious virus-like particles (VLPs) displaying native forms of HIV-1 Env. One group of macaques was used to test the immunogens present in the clinical product for a vaccine being tested in HIV Vaccine Trials Network protocol 065 (29). These immunogens express the CCR5-tropic strain ADA Env and are termed JS7 DNA and MVA62B. A second group was used to test an MVA boost expressing higher levels of the ADA Env (MVA62Sma) (42). The third and fourth groups were used to test the ability of granulocyte-macrophage colony-stimulating factor (GM-CSF) to enhance the immune responses elicited by the clade B vaccine expressing the higher level of the ADA Env and a clade C vaccine (IN3DNA/MVA71) expressing the CCR5-tropic 98IN02 Env. GM-CSF was used as an adjuvant because in earlier trials codelivery of GM-CSF had enhanced the breadth of the neutralizing activity and the avidity of the anti-Env Ab elicited by simian/human immunodeficiency virus 89.6 (SHIV-89.6) immunogens (28) (21).
At the end of the immunization phase of the trial, a high-dose intrarectal SHIV-162P3 challenge (15, 24) was given to boost Ab responses and explore the potential of the HIV-1 vaccines to protect against a heterologous CCR5-tropic SHIV challenge. The SHIV Gag protein was 22.9% identical to the HIV Gag in the clade B vaccine and 54.9% identical to the Gag in the clade C vaccine. The HIV-1 Envs in the vaccines and challenge were more closely related: 87.6% identity for clade B and 74.8% identity for clade C.
Disappointingly, none of the candidate HIV vaccines elicited neutralizing activity for strains representing incident clade B or clade C isolates (22, 23). However, a nonneutralizing Ab-mediated effect on protection was suggested by a strong inverse correlation between the avidity of the elicited anti-Env Ab and peak postchallenge viremia. This correlation required the conduct of avidity assays using the native but not gp140 or gp120 forms of Env. Encouragingly, the avidity of the Ab that had correlated with reductions in peak viremia had broad intraclade activity for Envs of incident isolates.
MATERIALS AND METHODS
DNA vaccines.
The clade B (JS7) and clade C (IN3) DNA vaccines expressed Gag, protease (PR), reverse transcriptase (RT), Env, Vpu, Tat, and Rev proteins and produced noninfectious VLPs (33). Point mutations inactivated both zinc fingers in gag, the active site of PR, and the reverse transcription, RNase H, and strand transfer activities of RT. The clade B vaccine was constructed from HIV-1-IIIB (HXB-2) gag sequences, HIV-1-IIIB (BH10) PR and RT sequences, and a recombinant of HXB-2 and ADA tat, rev, vpu, and env sequences. Most of the JS7 Env sequence was from the CCR5-tropic ADA Env. The clade C vaccine, pGA1/IN3, was constructed from 98IN012 sequences and also has a CCR5-tropic Env. Both DNA vaccines expressed full-length Envs. Vaccine inserts coexpressing GM-CSF were constructed by inserting rhesus GM-CSF sequences in the position of nef in the JS7 and IN3 vaccine inserts. This position achieved the expression of 40 to 100 ng of GM-CSF in transient transfections of ∼1 × 106 293T cells. This level of expression is within 30- to 300-ng per 1 × 106 cells, a range that has effectively served as an adjuvant for tumor vaccines but is well below 1,500 ng per 1 × 106 cells, an amount that has proven suppressive (31). The vaccine inserts were expressed in pGA2, which has the cytomegalovirus immediate-early promoter in the absence of intron A, or in pGA1, which has the cytomegalovirus immediate-early promoter in the presence of intron A. Use of pGA1 or pGA2 for insert expression was determined empirically, and the version of pGA that gave the highest level of expression for each insert was used.
MVA vaccines.
The clade B (MVA62 or MVA62Sma) and C (MVA71) vaccines were constructed from the same sequences as their clade-matched DNA vaccines. Gag, PR, and RT were expressed in deletion III and Env sequences in deletion II of MVA (42). Gag-PR and -RT sequences contained inactivating point mutations in the RT, RNase H, and strand transfer activities of RT but did not contain the zinc finger and PR mutations present in the DNA. In contrast to the DNA vaccines, Envs were truncated for 115 amino acids of the cytoplasmic tail to increase the stability of the recombinant MVAs and the expression of Env on the plasma membrane of MVA-infected cells (40). Both the Gag-RT and Env inserts used the mH5 early/late promoter (41). Two clade B MVA vaccines were constructed that differed in their levels of expression of Env. Attenuated expression was achieved by placing a premature start codon (ATG) upstream of the Env start codon (42). This attenuating start codon encoded a presumed 33-amino-acid out-of-frame protein. The MVA with attenuated expression of Env ([EnvAtt] MVA62B) expressed about one-fifth the level of Env expressed by the unattenuated MVA (MVA62Sma) (42). The clade C-expressing MVA, MVA71, was not attenuated for Env expression.
Trial design.
Young adult male rhesus macaques from the Yerkes National Primate Research Center breeding colony were used for immunizations and cared for under guidelines established under the Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals using protocols approved by the Emory University Institutional Animal Care and Use Committee. Animals were typed for the A*01 allele by PCR analysis and randomized into groups based on weight and A*01 status (20). Two groups received immunizations in the presence of GM-CSF, and two received immunizations in the absence of GM-CSF. Groups receiving immunizations in the absence of GM-CSF received two DNA immunizations at weeks 0 and 8 and two MVA boosts at weeks 16 and 24. Groups receiving the GM-CSF adjuvant received one DNA immunization at week 0 and two MVA boosts at weeks 8 and 24. All DNA immunizations delivered 1 ml of saline containing 3 mg of DNA intramuscularly (i.m.) in a single thigh, and all MVA immunizations delivered 1 ml of saline containing 1 × 108 PFU of MVA in a single thigh. For the GM-CSF adjuvanted groups, the DNA priming immunization was with DNA-coexpressing GM-CSF, and the boost codelivered MVA with rhesus GM-CSF protein (5 μg per kg of body weight). A second shot of GM-CSF in the absence of MVA was delivered i.m. 4 days later. Rhesus GM-CSF protein was provided by the Resource for Nonhuman Primate Immune Reagents (Emory University). Two animals in the GM-CSF-adjuvanted groups were removed from the trial; one (animal RDf-9) had cardiomyopathy and was euthanized. The second (RWy-8) developed a skin rash immediately after the second GM-CSF-adjuvanted MVA boost.
The high-dose SHIV-162P3 challenge (100 times the 50% tissue culture infective dose in 0.5 ml) was delivered atraumatically 16 weeks after the last MVA inoculation 15 to 20 cm into the colorectal area of the intestines using a pediatric feeding tube. At the time of challenge, the groups from the trials both with and without GM-CSF were randomized into challenge groups with six additional naïve macaques, which were added to serve as unvaccinated controls.
Phlebotomy was by venipuncture with blood collected into sodium citrate cell preparation tubes for preparation of peripheral blood mononuclear cells (PBMC), into serum sample tubes for preparation of serum, and into EDTA tubes for samples for viral loads for routine hematological assays. Colorectal biopsies were obtained from the rectum using biopsy forceps. For colonic biopsies, a scope was placed a short distance into the colon, and biopsies were obtained using biopsy forceps. Up to 20 biopsy samples were taken at a time, with the decision as to the number of biopsies left to the discretion of the veterinarian. Biopsies were placed in ice-cold RPMI medium without fetal bovine serum and transported on ice to the lab.
Cell isolation and staining.
PBMC were isolated from blood using standard methods for blood collected in cell preparation tubes. Lymphocytes were harvested from colorectal biopsies by washing biopsy pellets twice with Hanks' buffered saline solution and then resuspending the biopsies in RPMI 1640 medium containing antibiotics, 10 U/ml DNase I (Roche), and 300 U/ml collagenase IV (Worthington). Tissues were digested for 2 h at room temperature with slow shaking. Digested tissues were then further disrupted by passing them three to five times through an 18-gauge needle, followed by three to five passages through a 23-gauge needle, and then filtering the cells through a 70-μm-pore-size cell strainer. For analyses for lymphocyte subsets, 1 × 106 cells were resuspended in 100 μl of phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS) and stained according to standard procedures for flow cytometry. The following antibodies were used for staining of lymphocytes: CD3 (SP34-2), CD4 (L200), CD8 (SK1), CD28 (CD28.2), CD95 (DX2), gamma interferon ([IFN-γ] B27), interleukin-2 (MQ1-17H1), and tumor necrosis factor alpha (MAb11). Stained cells were acquired using a LSR II Flow Cytometer (BD Biosciences). All flow cytometry data were analyzed using FlowJo software (Tree Star, Inc.).
Intracellular cytokine staining.
Approximately 2 × 106 PBMC were stimulated with pools of HIV-1 clade B consensus Gag and Env peptides, SIVmac239 Gag peptides, and SHIV162P3 Env peptides. Clade C peptides were also used, but the data are not presented because the peptides were recalled by the NIH AIDS Repository. Stimulations were conducted in the presence of costimulation by 1 μg per ml of antibody to human CD28 and CD49d in a final volume of 200 μl at 37°. After 2 h of stimulation, brefeldin A was added, and cells were cultured for an additional 4 h at 37°C. Cells were washed with PBS containing 2% FBS, fixed with Cytofix/Cytoperm, permeabilized with 1× Perm/Wash, and incubated with a cocktail of fluorochrome-conjugated antibodies to CD3 (SP34-2)-Pacific Blue, CD4 (L200)-fluorescein isothiocyanate, CD8 (SK1)-peridinin chlorophyll protein, IFN-γ (B27)-Alexa Fluor 700, interleukin-2 (MQ1-17H1)-allophycocyanin, and tumor necrosis factor alpha (MAb11)-phycoerythrin-Cy7 for 30 min at 4°C in the dark. Cells were washed once with 1× Perm/Wash and once with PBS containing 2% FBS and resuspended in 1% paraformaldehyde in PBS. Approximately 500,000 lymphocytes were acquired for analysis using an LSR II Flow Cytometer.
Anti-Env Ab.
Full-length Env antigens as well as the ADA gp120 Env were produced for binding and avidity assays using transient transfections of 293T cells with DNAs expressing VLPs or pseudovirions. Expressed Envs were captured onto enzyme-linked immunosorbent assay (ELISA) plates using concanavilin A (Vector Laboratories) (7, 21). gp140 was expressed using MVA/ADA.gp140 (P. L. Earl and B. Moss, unpublished data) and purified as previously described (10). The expressed gp140 (0.6 μg per ml) was captured with sheep anti-gp120 Ab (Cliniqa, San Marcos, CA) at 0.5 μg per well in coat buffer (Immunochemistry Technologies, Minneapolis, MN). Avidity assays used parallel enzyme-linked immunosorbent assays, one treated with PBS and the other with 1.5 M sodium thiocyanate for 10 min at room temperature (21, 38). Avidity indices, or the ratio of the dilution of serum giving an optical density of 0.5 for the sodium thiocyanate wash to the dilution giving an optical density of 0.5 for the PBS wash times 100, showed excellent reproducibility for an in-house reference vaccine serum included in all assays (mean ± standard deviation [SD], 27.3 ± 1.6). Binding Ab, reported in micrograms of anti-Env Ab per ml of serum relative to a standard curve of rhesus IgG captured by goat anti-rhesus Ab, also showed good reproducibility for a reference standard (mean ± SD, 4.1± 1.0 μg per ml) (21, 33).
Neutralizing Ab.
Vaccines were tested for the elicitation of neutralizing Ab using a luciferase reporter gene assay in TZM-bl cells (25).
Viral RNA.
Assays for viral RNA were conducted using a quantitative real-time PCR as previously described (17). All specimens were extracted and assayed in duplicate, and the mean results are reported.
Statistical analyses.
A Pearson correlation test was used to assess the relationship between peak levels of viral RNA and the avidity of elicited anti-Env Ab. A Spearman rank-correlation test was used to assess correlations between T-cell responses and reductions in peak viremia. A Wilcoxon rank sum test was used for correlations between Ab responses and A*01 status. Statistical analyses were performed using the software programs S-PLUS, version 7.0, and SAS, version 9.1.
RESULTS
Some protection for the SHIV challenge.
Four trial groups were used to test the ability of the HIV vaccines to elicit neutralizing Ab and provide protection against a CCR5-tropic SHIV-162P3 challenge (Table 1). Two non-GM-CSF-adjuvanted groups, termed B and B(EnvAtt), were used to test HIV-1 clade B vaccines with different levels of Env expression in the MVA boost. Two GM-CSF-adjuvanted groups, termed B+GM and C+GM, were used to test HIV-1 clades B and C vaccines in the presence of a GM-CSF adjuvant, respectively. The GM-CSF adjuvant was present as a coexpressed DNA at the time of the prime inoculation and as protein (5 μg per kg) at the time of each of the MVA boosts and again 4 days later (without additional MVA).
TABLE 1.
Summary of trials and immunogens
| Trial and/or group | No. of animals in group (no. of A*01-positive animals) | Immunization regimena
|
|
|---|---|---|---|
| DNA prime | MVA boost | ||
| Without adjuvant | |||
| B(EnvAtt) | 6 (3) | pGA2/JS7 | MVA62B |
| B | 6 (3) | pGA2/JS7 | MVA62Sma |
| With adjuvant | |||
| B+GM | 3 (1) | pGA2/JS7.GM-CSF | MVA62Sma plus GM-CSF protein |
| C+GM | 3 (1) | pGA1/IN3.GM-CSF | MVA71 plus GM-CSF protein |
| Naive | 6 (3) | None | None |
DNA immunizations delivered 3 mg of DNA i.m.; MVA immunizations delivered 1 × 108 PFU of MVA i.m.
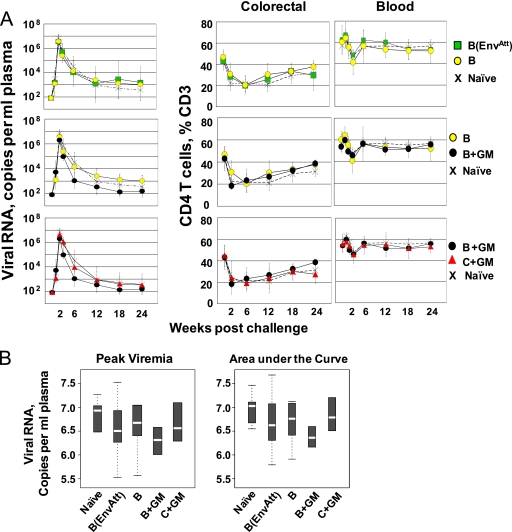
Protection against the SHIV challenge was assessed by measuring over time the number of copies of viral RNA per ml of blood and the CD4 T-cell counts (Fig. 1A). All vaccinated groups had lower median peaks of viral RNA and lower overall levels of infection, measured by areas under the curve, than the unvaccinated controls (Fig. 1B). Among the groups, the B+GM group showed the lowest peak, the most rapid pull-down, the lowest set point level of viral RNA, and the smallest area under the viral curve. Analyses for protection of colorectal CD4 T cells revealed the anticipated depletion of these cells, which display the CCR5 coreceptor for SHIV-162P3 (16). By 24 weeks postchallenge, the frequencies of intestinal CD4 T cells had recovered to normal levels in the B and B+GM groups but had undergone less complete recoveries in the other groups. Total CD4 T-cell counts in blood (where many cells do not bear the CCR5 coreceptor) underwent a transient and modest dip at 3 weeks postchallenge. These frequencies had recovered in all groups by 6 weeks postchallenge.
FIG. 1.
Postchallenge control of SHIV-162P3 viral RNA and protection of CD4 T cells. (A) Titers of viral RNA and frequencies of CD4 T cells at the indicated time points. Titers of viral RNA in blood are geometric means ± SDs. Frequencies of CD4 T cells are means ± SDs. Data for colorectal CD4 T cells are available for only some time points due to restrictions on the frequency of biopsies. (B) Levels of viremia in different groups. Box plots provide graphic summaries for peak viral RNA and areas under the curve (0 to 24 weeks). The white lines in the boxes are medians; the upper and lower limits of the boxes indicate the 75th and 25th percentiles, respectively. Minimum and maximum values are indicated by the upper and lower brackets. For numbers of animals per group, see Table 1.
Similar levels of postvaccine T-cell responses to Gag and Env, but higher levels of postchallenge responses to Env than Gag.
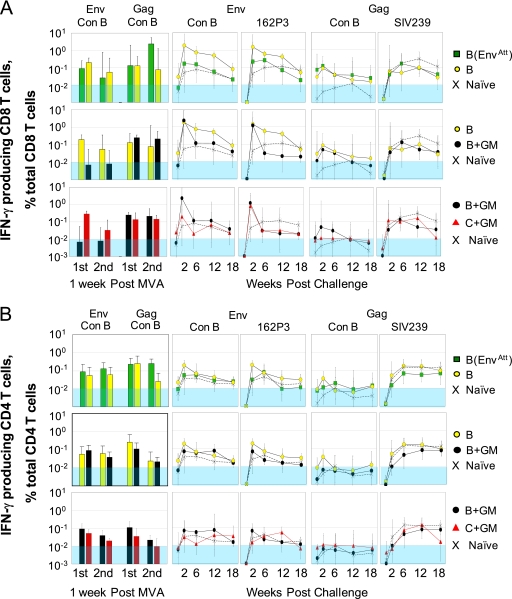
The different groups had overall similar frequencies of responding T cells during the vaccination phase of the trial (Fig. 2). These responses represented about 0.1% of total CD4 or CD8 T cells. The highest response was for the CD8 Gag response after the second MVA boost in the B(EnvAtt) group. This response represented 2.3% of total CD8 T cells. The GM-CSF adjuvant did not measurably affect CD4 or CD8 responses. The stimulation of higher anti-Env CD8 responses in the clade C than the clade B vaccine by the consensus B pool was not reflected in the CD8 Gag response or the CD4 responses and presumably reflected a chance effect of histocompatibility type in the small group sizes. Consistent with our prior trials using i.m. inoculations of DNA, T-cell responses were not detected until after the first MVA boost (data not shown) (21, 34).
FIG. 2.
Responding T cells measured by intracellular cytokine staining for IFN-γ. CD8 (A) and CD4 (B) responses over time measured as IFN-γ producing cells as a percentage of total CD4 or CD8 T cells are presented as geometric means ± SDs. The bar graphs on the left of figures show peak responses after each MVA boost following stimulation with consensus clade B peptide pools for Gag or Env. The line graphs show postchallenge responses to pools of consensus clade B (Con B) Env peptides, 162P3 Env peptides, consensus clade B Gag peptides, and SIV239 Gag peptides (indicated at the tops of panels). Symbols for groups are to the right of panels. The shaded blue area indicates responses below the limit for accurate quantification.
Postchallenge, the frequencies of anti-Env T cells were much higher than the frequencies of anti-Gag T cells. This is consistent with the HIV vaccine having greater homology with HIV Env than with the SIV Gag of the SHIV challenge. The CD8 T-cell responses to Env were highest in the groups vaccinated with the B vaccine or with the B vaccine plus GM-CSF (B and B+GM groups, respectively), reaching frequencies of over 1% of total CD8 T cells at 2 weeks postchallenge. The CD4 T-cell responses were also highest in these groups but, in contrast to the prechallenge data, had frequencies that were 5- to 10-fold lower than those for the CD8 T cells. Responses to stimulations with the 162P3 Env pool were similar to those for the consensus B pool. In contrast to Env, anti-HIV-1 Gag CD8 responses had very low postchallenge peaks (∼0.1% of total CD8 T cells). Both CD8 and CD4 responses to the SIV Gag pool rose over the first 6 weeks postchallenge, reflecting a primary response to the infection.
Titers, avidity, and neutralizing activity of Ab responses.
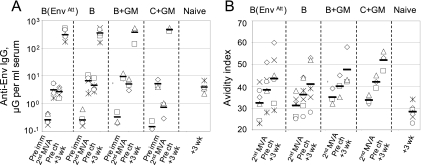
Anti-Env binding Ab responses achieved measurable levels after the second MVA boost, at which time they ranged from 3 to 10 μg of anti-Env binding Ab per ml of serum (Fig. 3A). Consistent with its attenuated expression of Env, the lowest titers of binding Ab were found in the B(EnvAtt) group. By the time of the challenge at 16 weeks after the second MVA boost, Ab responses had declined. Following challenge, the titers of anti-Env Ab expanded rapidly, reaching median titers of several hundred micrograms per milliliter of serum in all groups. At this same time, the titers of the primary binding Ab response in the unvaccinated controls were 100 times lower.
FIG. 3.
Anti-Env binding Ab and avidity for the indicated time points. (A) Titers of binding Ab relative to a standard curve for macaque IgG. (B) Avidities of binding Ab. Horizontal lines in panel A indicate geometric means; in panel B, horizontal lines indicate medians. All assays were conducted using Env captured from VLPs produced in transient transfections by the clade-matched DNA vaccine. Vaccine groups are indicated at the tops of panels. Prechallenge sera were not taken for the naïve group. These responses were likely similar to those in preimmune macaques in other groups. Pre imm, preimmunizations; 2nd MVA, 2 weeks after the second MVA boost; pre ch, prechallenge; and +3 wk, 3 weeks postchallenge.
In contrast to the titers of binding Ab that rose and fell with immunizations, the avidity of the anti-Env Ab slowly increased over the duration of the trial (Fig. 3B). Among the clade B vaccine groups, the B+GM group achieved the highest avidity (median of 48), the B(EnvAtt) had the next highest avidity (median of 43), and the B group had the lowest avidity (median of 41). The rank orders of avidities within groups tended to hold with time. Animals with lower-avidity Ab at 2 weeks after the second MVA boost also had lower-avidity responses at 3 weeks postchallenge. At 3 weeks postchallenge, the avidity of Ab in the unvaccinated control group for the clade B Env was low (a median of 28), 13 to 20 points below the indices in the vaccinated groups. Avidities for the clade C+GM vaccine measured against a clade C Env were overall similar to those for the clade B+GM vaccine measured against the clade B Env. These avidities were higher than those elicited by the nonadjuvanted clade B vaccines and likely reflected the GM-CSF adjuvant effect on avidity maturation.
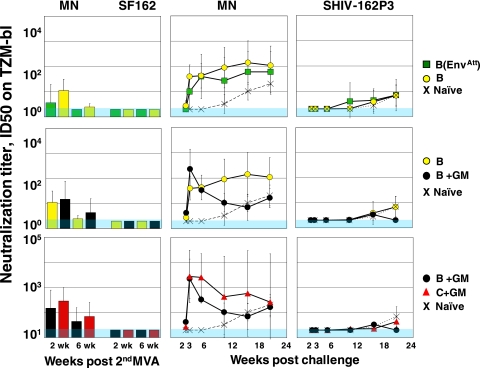
Both the clade B and C vaccines elicited low titers of neutralizing Ab for the easily neutralized HIV-1 MN isolate but failed to elicit neutralizing Ab for the SHIV-162P3 challenge or incident isolates that were more difficult to neutralize (Fig. 4 and data not shown). The titers of MN-neutralizing activity were highest for the C+GM group (titer of 300), second highest for the B+GM groups (150) and B (100) groups, and lowest for the B(EnvAtt) group. Postchallenge, neutralizing titers for MN rapidly expanded. This expansion had an acute peak at 3 weeks postchallenge in the B+GM group, the group with the best viral control. In the unvaccinated controls, neutralizing Ab for MN did not appear until 12 weeks postchallenge.
FIG. 4.
Neutralizing Ab production over time. Neutralization titers are the reciprocal for serum dilutions at which relative luminescence units were reduced 50% compared to virus control wells. The threshold value of 20 was used for negative values. This threshold is shown in shaded blue. The isolates used in neutralization assays are indicated at the tops of panels. Symbols for groups are identified at right. MN, HIV-1 MN; SF162, HIV-1 SF162; ID50, 50% infectious dose.
Neutralizing Ab for the SHIV-162P3 challenge was slow to appear. This activity had appeared in all groups except the C+GM group by 18 weeks postchallenge. By 24 weeks postchallenge, it was present at geometric mean titers ranging from 40 to just under 100 except in the B+GM group, where it was undetectable, a phenomenon that could reflect this group's having the best-controlled infection.
Correlation of avidity with reduced peak viremia.
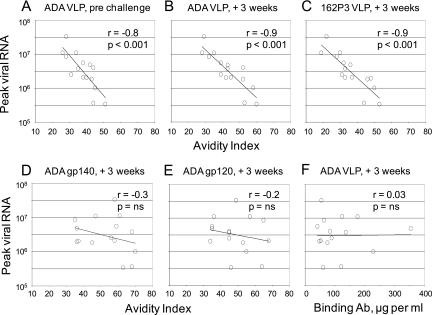
Correlations between immune responses and protection were conducted for data pooled from the three groups receiving the clade B vaccines. These correlations tested avidity indices against the titers of viral RNA at 2 weeks postchallenge. At this time, individual animals in the vaccine groups had up to 100-fold differences in their levels of postchallenge viremia, which ranged from 3 × 105 to 3 × 107 copies of viral RNA per ml. These analyses revealed strong inverse correlations between peak viremia and the avidity indices of the clade B anti-Env Ab for the immunizing ADA Env. Strong correlations were found for serum harvested prechallenge as well as at 3 weeks postchallenge (Fig. 5A and B). A strong inverse correlation also was found between peak postchallenge viremia and avidity indices for the 162P3 Env in the SHIV challenge (Fig. 5C). The inverse correlation between avidity and reductions in peak postchallenge viremia did not hold if secreted gp140 or gp120 forms of the immunizing ADA Env were used as targets for avidity analyses (Fig. 5D and E). There was also no correlation between the titers of binding Ab and peak postchallenge viremia (Fig. 5F).
FIG. 5.
Inverse correlation between the avidity of anti-Env binding Ab and peak viremia. Inverse correlations between peak viremia and the avidity index for ADA Env captured from VLPs for Ab in serum harvested prechallenge (A) or at 3 weeks postchallenge (B). (C) Inverse correlation between peak viremia and the 162P3 challenge Env captured from pseudovirions for Ab harvested at 3 weeks postchallenge. There was no correlation between peak viremia and the avidity index of Ab harvested at 3 weeks postchallenge for ADA gp140 (D) or ADA gp120 (E) forms of Env. (F) Lack of a correlation between peak viremia and the relative titers of binding Ab at 3 weeks postchallenge. Correlations were conducted for the 15 animals in clade B-vaccinated groups using the Pearson method. The form of ADA Env used in assays and the sera being tested in the assay (+3 weeks, 3 weeks postchallenge) are given at the tops of the panels. See Materials and Methods for assays. ns, not significant.
Analyses for correlations between peak CD8 or CD4 T-cell responses and peak levels of viral RNA revealed no correlations. These analyses were conducted for responses stimulated by the consensus B peptide pools after each of the MVA boosts as well as postchallenge (see Fig. S1A in the supplemental material). The titers of neutralizing Ab for HIV-1 MN or HIV-1 SF2, either prior to or at 3 weeks postchallenge, also had no correlation with the peak titer of the challenge infection (see Fig. S1B in the supplemental material).
Avidity for Envs in incident isolates.
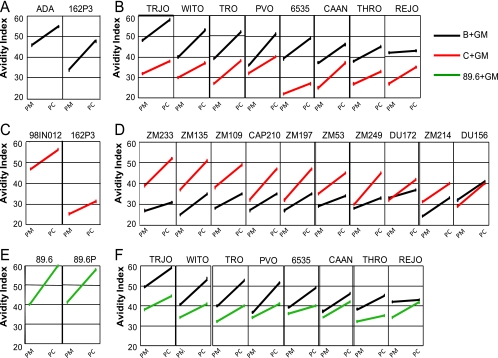
Serum elicited by the trials presented here as well as serum from an earlier trial using SHIV-89.6 immunogens and a SHIV-89.6P challenge (21) were next tested for the avidity of elicited anti-Env Ab for their immunizing Envs, the Envs in their challenge infection, and Envs in panels of incident clade B and clade C isolates (Fig. 6) (22, 23). These tests were conducted on sera elicited in GM-CSF-adjuvanted immunizations because these sera had the highest avidity indices (Fig. 3) (21). Each of the immunizing Envs elicited high-avidity anti-Env Ab for itself (Fig. 6A, C, and E). The highest avidity for the challenge Env was seen for the 89.6 serum for its closely related 89.6P challenge Env (Fig. 6E). In this trial, avidity correlated with up to a 1,000-fold reduction in peak viremia (21). Intermediate avidity was found between the ADA Env and its 162P3 challenge (Fig. 6A). This level of avidity, however, correlated with up to a 100-fold reduction in peak viremia (Fig. 5C). Only low avidity was observed between the sera elicited by the clade C IN98012 Env and the clade B 162P3 challenge Env (Fig. 6C).
FIG. 6.
Intraclade but not interclade activity of high-avidity Ab elicited by clades B and C HIV-1 vaccines. Avidity tests were conducted with pooled sera from GM-CSF-adjuvanted immunizations. Panels A, C, and E show avidities of vaccine-elicited sera for the Env in the vaccine and its challenge. Panels B and D show avidities of the clade B and C vaccine-elicited sera for clade B incident Envs (B) and for clade C incident Envs (D). Panel F shows avidities of clade B and SHIV-89.6 vaccine-elicited sera for clade B incident Envs. Color codes for the vaccines are identified at right; the Envs tested in avidity assays are identified at the tops of panels. PM, serum harvested after the second MVA boost; PC, serum harvested at 3 weeks postchallenge.
Remarkably, the high-avidity ADA-elicited Ab had broad activity for a reference panel of incident clade B Envs (Fig. 6B), whereas the IN98012 serum had broad activity for the reference clade C Envs (Fig. 6D). Neither the B nor C serum had good avidity (index of >40) for the incident Envs of the other clade (Fig. 6B and D). For the ADA-elicited serum, one Env, from isolate TRJO, exhibited even higher avidity binding for the ADA serum than the ADA Env (Fig. 6B). This Env, as well as Envs of four more incident isolates (WITO, TRO, PVO, and 6535), had as good, or better, avidity binding for the ADA-elicited Ab than the 162P3 Env, for which a strong correlation between avidity and decreases in peak viremia had been shown (Fig. 5C). Only one of the eight tested Envs (REJO) bound the clade B-elicited serum with only moderate avidity.
None of the clade C incident Envs proved to be a better target for the binding of the elicited clade C Ab than the 98IN012 Env in the vaccine (Fig. 6D). However, 5 of the 10 tested Envs (ZM2233, ZM135, ZM109, CAP210, and ZM197) bound the clade C serum with avidities as high, or higher, than had been associated with reductions in peak viremia in the clade B-vaccinated and SHIV-162P3-challenged macaques (Fig. 5C). Three of the clade C incident Envs (DU172, ZM214, and DU156) had more moderate avidity binding. The Env (DU156) with the lowest avidity binding showed comparable avidities for the clades C and B sera.
In contrast to the serum elicited by the CCR5-tropic ADA Env, serum elicited by the dual tropic clade B 89.6 Env did not show high avidity binding for the incident clade B viruses (Fig. 6F). Thus, the ability to elicit serum with high-avidity intraclade binding for incident Envs is not a characteristic of all Envs within a clade.
DISCUSSION
Traditional evaluations of experimental HIV vaccines for protective Ab responses have focused on neutralizing Ab. Here, we have tested for both the neutralizing activity and the avidity of elicited anti-Env Ab and found a strong inverse correlation between avidity and peak viremia for a heterologous challenge. Avidity indices for the immunizing Env ranging from 20 to 60 correlated with postchallenge peak viremias ranging from 3 × 107 to 3 × 105 copies of viral RNA per ml of plasma. This inhibition of acute viral replication was for a challenge with only 87% homology between its envelope glycoprotein and the immunizing Env. The protection did not appear to require neutralizing activity because it took place in the absence of detectable levels of neutralizing Ab for the SHIV-162P3 challenge. The correlation held for the avidity of the Ab at the time of challenge as well as the avidity of the Ab at 3 weeks postchallenge. We suggest that avidity correlated with protection because it facilitated the initiation by bound Ab of Fc-mediated mechanisms of viral control such as complement-mediated lysis (1, 14, 35), antibody-dependent cellular cytotoxicity (2, 8, 12, 30, 37), antibody-dependent cell-mediated virus inhibition (11), and phagocytosis. Presumably the more tightly Ab bound to Env on virions and infected cells, the more effective it was at initiating Fc-mediated mechanisms of virus control.
Importantly, for the correlation to be seen, avidity needed to be measured against the full-length form of Env captured from VLPs or pseudovirions and not against secreted gp140 or gp120 forms of the vaccine Env. This implies that the native form of Env was more effective at displaying the epitopes representative of those found on virions and infected cells than gp120 and gp140 subunits of Env. In our assays, both the gp120 and gp140 forms of Env were excellent targets for Ab binding, with anti-Env Ab exhibiting even higher avidities for these secreted forms than the native form of Env. This binding, however, did not represent Ab that was contributing to protection as measured by our correlations.
Encouragingly, the avidity of the Ab elicited by the ADA Env in our immunogens had indices for the Envs of incident clade B isolates that in many cases were even higher than those that correlated with reductions in peak viremia for the heterologous SHIV-162P3 challenge. Thus, unlike neutralizing Ab, which is largely isolate specific, high-avidity Ab may be capable of blunting acute viremia for a diversity of isolates. This is consistent with studies on Fc- and complement-mediated virolysis and cytolysis that have demonstrated that patient serum mediating these activities recognizes a range of Envs (1, 2, 5, 12, 18, 32, 35). Our studies also show that not all clade B Envs elicit high-avidity Ab for incident clade B isolates. The anti-Env Ab elicited by the SHIV-89.6 Env had high avidity for itself but only low avidity for incident Envs. Importantly, the high-avidity Ab elicited by our clade B ADA Env vaccine failed to show high-avidity binding for incident clade C Envs, whereas the anti-Env Ab elicited by our clade C vaccine showed high-avidity binding for incident clade C but not clade B Envs. Thus, the high-avidity Ab that we have raised appears to have broad intraclade but not interclade activity. This suggests that vaccines designed to elicit Ab that protects by virtue of high-avidity binding to Env may need to use clade-specific Envs.
Although consistent with classical findings that high levels of antigen can reduce the avidity of Ab responses, we had not anticipated that the level of Env expression in our MVA boost might affect the avidity of anti-Env Ab. Although we do not have sufficient data to achieve statistical significance, our results suggest that the attenuated Env expression in our B(EnvAtt) vaccine may have the ability to elicit higher-avidity (albeit lower titer) Ab than our B vaccine with unattenuated Env expression. The B(EnvAtt) vaccine (JS7/MVA62) is our vaccine that is currently in clinical trials through the HIV Vaccine Trials Network.
Our studies show vaccination playing a strong role in molding the Ab response in the immediate postchallenge phase of infection. The vaccine-primed anamnestic Ab response rapidly placed high titers (an estimated 500 μg per ml) of good avidity (indices of >40) of anti-Env Ab in play. At 3 weeks postchallenge these responses had ∼100 times higher titers and ∼ 20 points higher avidities than the Ab responses in unvaccinated controls. We do not know how the epitopes recognized by the vaccine-primed serum compare to those recognized in recent infections (36). What we do know is that the epitopes primed by our full-length Envs included ones capable of producing a correlate between avidity and peak postchallenge viremia.
The inverse correlation between the avidity of anti-Env binding Ab and peak postchallenge viremia has been a consistent finding across our macaque challenge studies using SHIV or HIV immunogens and SHIV-89.6P or SHIV-162P3 challenges (21; also this study). This correlation has been present for prechallenge as well as postchallenge immune responses (21; also this study). In contrast, correlations with elicited CD8 T cells have not always been observed (this study) and, when observed, have been limited to peak postchallenge CD8 responses (21).
Summary.
In summary, we have shown that the avidity of an anti-Env Ab response for the native form of Env can blunt peak viremia for a heterologous challenge in the absence of neutralizing Ab. We have also shown that a clade B and a clade C CCR5-tropic Env can elicit high-avidity Ab with broad intraclade but not interclade specificity for incident Envs. We suggest that the induction of such Ab by HIV vaccines represents the induction of a nonneutralizing activity capable of contributing to virus control during the critical early period of HIV infection (13).
Supplementary Material
Acknowledgments
This research was supported by Integrated Preclinical/Clinical AIDS Vaccine Development program project, P01 AI 49364, to H. Robinson; Emory Center for AIDS Research, P30 DA 12121; Yerkes National Primate Research Center base grant P51 RR00165; NIH grant AI 30034 to D. Montefiori; and by the Division of Intramural Research, NIAID, NIH.
We thank Dev Chandran for initial cloning and recombinant virus construction that were used to make the clade C recombinant, MVA71. We are indebted to Jeffrey Americo for preparation of the purified ADA gp140 protein. We are grateful to the Yerkes Division of Research Resources for the consistent excellence of veterinary care and pathology support, GeoVax Inc. for the provision of good manufacturing practice-produced clinical product for JS7 and MVA62 vaccines, and the NIH AIDS Research and Reference Reagent Program for the provision of peptides. We are indebted to H. Drake-Perrow for outstanding administrative assistance.
Footnotes
Published ahead of print on 18 February 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Aasa-Chapman, M. M., S. Holuigue, K. Aubin, M. Wong, N. A. Jones, D. Cornforth, P. Pellegrino, P. Newton, I. Williams, P. Borrow, and A. McKnight. 2005. Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection. J. Virol. 792823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad, A., and J. Menezes. 1996. Antibody-dependent cellular cytotoxicity in HIV infections. FASEB J. 10258-266. [DOI] [PubMed] [Google Scholar]
- 3.Albert, J., B. Abrahamsson, K. Nagy, E. Aurelius, H. Gaines, G. Nystrom, and E. M. Fenyo. 1990. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS 4107-112. [DOI] [PubMed] [Google Scholar]
- 4.Blue, C. E., O. B. Spiller, and D. J. Blackbourn. 2004. The relevance of complement to virus biology. Virology 319176-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumberg, R. S., T. Paradis, K. L. Hartshorn, M. Vogt, D. D. Ho, M. S. Hirsch, J. Leban, V. L. Sato, and R. T. Schooley. 1987. Antibody-dependent cell-mediated cytotoxicity against cells infected with the human immunodeficiency virus. J. Infect. Dis. 156878-884. [DOI] [PubMed] [Google Scholar]
- 6.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5233-236. [DOI] [PubMed] [Google Scholar]
- 7.Cole, K. S., J. L. Rowles, B. A. Jagerski, M. Murphey-Corb, T. Unangst, J. E. Clements, J. Robinson, M. S. Wyand, R. C. Desrosiers, and R. C. Montelaro. 1997. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J. Virol. 715069-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connick, E., D. G. Marr, X. Q. Zhang, S. J. Clark, M. S. Saag, R. T. Schooley, and T. J. Curiel. 1996. HIV-specific cellular and humoral immune responses in primary HIV infection. AIDS Res. Hum Retrovir. 121129-1140. [DOI] [PubMed] [Google Scholar]
- 9.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 41795-98. [DOI] [PubMed] [Google Scholar]
- 10.Earl, P. L., W. Sugiura, D. C. Montefiori, C. C. Broder, S. A. Lee, C. Wild, J. Lifson, and B. Moss. 2001. Immunogenicity and protective efficacy of oligomeric human immunodeficiency virus type 1 gp140. J. Virol. 75645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forthal, D. N., G. Landucci, K. S. Cole, M. Marthas, J. C. Becerra, and K. Van Rompay. 2006. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J. Virol. 809217-9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forthal, D. N., G. Landucci, and B. Keenan. 2001. Relationship between antibody-dependent cellular cytotoxicity, plasma HIV type 1 RNA, and CD4+ lymphocyte count. AIDS Res. Hum. Retrovir. 17553-561. [DOI] [PubMed] [Google Scholar]
- 13.Gasper-Smith, N., D. M. Crossman, J. F. Whitesides, N. Mensali, J. S. Ottinger, S. G. Plonk, M. A. Moody, G. Ferrari, K. J. Weinhold, S. E. Miller, C. F. Reich, 3rd, L. Qin, S. G. Self, G. M. Shaw, T. N. Denny, L. E. Jones, D. S. Pisetsky, and B. F. Haynes. 2008. Induction of plasma (TRAIL), TNFR-2, Fas ligand, and plasma microparticles after human immunodeficiency virus type 1 (HIV-1) transmission: implications for HIV-1 vaccine design. J. Virol. 827700-7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauduin, M. C., R. Weir, M. S. Fung, and R. A. Koup. 1998. Involvement of the complement system in antibody-mediated post-exposure protection against human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 14205-211. [DOI] [PubMed] [Google Scholar]
- 15.Harouse, J. M., A. Gettie, T. Eshetu, R. C. Tan, R. Bohm, J. Blanchard, G. Baskin, and C. Cheng-Mayer. 2001. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIVSF162P3. J. Virol. 751990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harouse, J. M., A. Gettie, R. C. Tan, J. Blanchard, and C. Cheng-Mayer. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284816-819. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann-Lehmann, R., R. K. Swenerton, V. Liska, C. M. Leutenegger, H. Lutz, H. M. McClure, and R. M. Ruprecht. 2000. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res. Hum. Retrovir. 161247-1257. [DOI] [PubMed] [Google Scholar]
- 18.Huber, M., M. Fischer, B. Misselwitz, A. Manrique, H. Kuster, B. Niederost, R. Weber, V. von Wyl, H. F. Gunthard, and A. Trkola. 2006. Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection. PLoS Med. 3e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber, M., and A. Trkola. 2007. Humoral immunity to HIV-1: neutralization and beyond. J. Intern. Med. 2625-25. [DOI] [PubMed] [Google Scholar]
- 20.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50657-661. [DOI] [PubMed] [Google Scholar]
- 21.Lai, L., D. Vodros, P. A. Kozlowski, D. C. Montefiori, R. L. Wilson, V. L. Akerstrom, L. Chennareddi, T. Yu, S. Kannanganat, L. Ofielu, F. Villinger, L. S. Wyatt, B. Moss, R. R. Amara, and H. L. Robinson. 2007. GM-CSF DNA: An adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology 369153-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 7910108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, M., J. F. Salazar-Gonzalez, C. A. Derdeyn, L. Morris, C. Williamson, J. E. Robinson, J. M. Decker, Y. Li, M. G. Salazar, V. R. Polonis, K. Mlisana, S. A. Karim, K. Hong, K. M. Greene, M. Bilska, J. Zhou, S. Allen, E. Chomba, J. Mulenga, C. Vwalika, F. Gao, M. Zhang, B. T. Korber, E. Hunter, B. H. Hahn, and D. C. Montefiori. 2006. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol. 8011776-11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luciw, P. A., E. Pratt-Lowe, K. E. Shaw, J. A. Levy, and C. Cheng-Mayer. 1995. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV). Proc. Natl. Acad. Sci. USA 927490-7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montefiori, D. C. 2004. Evaluating neutralizing antibodies against HIV, SIV and SHIV in a luciferase reporter gene assay. John Wiley and Sons, New York, NY. [DOI] [PubMed]
- 26.Montefiori, D. C. 1997. Role of complement and Fc receptors in the pathogenesis of HIV-1 infection. Springer Semin. Immunopathol. 18371-390. [DOI] [PubMed] [Google Scholar]
- 27.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 1004144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson, H. L., D. C. Montefiori, F. Villinger, J. E. Robinson, S. Sharma, L. S. Wyatt, P. L. Earl, H. M. McClure, B. Moss, and R. R. Amara. 2006. Studies on GM-CSF DNA as an adjuvant for neutralizing Ab elicited by a DNA/MVA immunodeficiency virus vaccine. Virology 352285-294. [DOI] [PubMed] [Google Scholar]
- 29.Robinson, H. L., S. Sharma, J. Zhao, S. Kannanganat, L. Lai, L. Chennareddi, T. Yu, D. C. Montefiori, R. R. Amara, L. S. Wyatt, and B. Moss. 2007. Immunogenicity in macaques of the clinical product for a clade B DNA/MVA HIV vaccine: elicitation of IFN-gamma, IL-2, and TNF-alpha coproducing CD4 and CD8 T Cells. AIDS Res. Hum. Retrovir. 231555-1562. [DOI] [PubMed] [Google Scholar]
- 30.Sawyer, L. A., D. A. Katzenstein, R. M. Hendry, E. J. Boone, L. K. Vujcic, C. C. Williams, S. L. Zeger, A. J. Saah, C. R. Rinaldo, Jr., J. P. Phair, et al. 1990. Possible beneficial effects of neutralizing antibodies and antibody-dependent, cell-mediated cytotoxicity in human immunodeficiency virus infection. AIDS Res. Hum. Retrovir. 6341-356. [DOI] [PubMed] [Google Scholar]
- 31.Serafini, P., R. Carbley, K. A. Noonan, G. Tan, V. Bronte, and I. Borrello. 2004. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 646337-6343. [DOI] [PubMed] [Google Scholar]
- 32.Shepp, D. H., S. Chakrabarti, B. Moss, and G. V. Quinnan, Jr. 1988. Antibody-dependent cellular cytotoxicity specific for the envelope antigens of human immunodeficiency virus. J. Infect. Dis. 1571260-1264. [DOI] [PubMed] [Google Scholar]
- 33.Smith, J. M., R. R. Amara, D. Campbell, Y. Xu, M. Patel, S. Sharma, S. T. Butera, D. L. Ellenberger, H. Yi, L. Chennareddi, J. G. Herndon, L. S. Wyatt, D. Montefiori, B. Moss, H. M. McClure, and H. L. Robinson. 2004. DNA/MVA vaccine for HIV type 1: effects of codon-optimization and the expression of aggregates or virus-like particles on the immunogenicity of the DNA prime. AIDS Res. Hum. Retrovir. 201335-1347. [DOI] [PubMed] [Google Scholar]
- 34.Smith, J. M., R. R. Amara, H. M. McClure, M. Patel, S. Sharma, H. Yi, L. Chennareddi, J. G. Herndon, S. T. Butera, W. Heneine, D. L. Ellenberger, B. Parekh, P. L. Earl, L. S. Wyatt, B. Moss, and H. L. Robinson. 2004. Multiprotein HIV-1 clade B DNA/MVA vaccine: construction, safety and immunogenicity. AIDS Res. Hum. Retrovir. 20654-665. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan, B. L., E. J. Knopoff, M. Saifuddin, D. M. Takefman, M. N. Saarloos, B. E. Sha, and G. T. Spear. 1996. Susceptibility of HIV-1 plasma virus to complement-mediated lysis. Evidence for a role in clearance of virus in vivo. J. Immunol. 1571791-1798. [PubMed] [Google Scholar]
- 36.Tomaras, G. D., N. L. Yates, P. Liu, L. Qin, G. G. Fouda, L. L. Chavez, A. C. Decamp, R. J. Parks, V. C. Ashley, J. T. Lucas, M. Cohen, J. Eron, C. B. Hicks, H. X. Liao, S. G. Self, G. Landucci, D. N. Forthal, K. J. Weinhold, B. F. Keele, B. H. Hahn, M. L. Greenberg, L. Morris, S. S. Karim, W. A. Blattner, D. C. Montefiori, G. M. Shaw, A. S. Perelson, and B. F. Haynes. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 8212449-12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tyler, D. S., H. K. Lyerly, and K. J. Weinhold. 1989. Anti-HIV-1 ADCC. AIDS Res. Hum. Retrovir. 5557-563. [DOI] [PubMed] [Google Scholar]
- 38.Vermont, C. L., H. H. van Dijken, C. J. van Limpt, R. de Groot, L. van Alphen, and G. P. van Den Dobbelsteen. 2002. Antibody avidity and immunoglobulin G isotype distribution following immunization with a monovalent meningococcal B outer membrane vesicle vaccine. Infect. Immun. 70584-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 40.Wyatt, L. S., I. M. Belyakov, P. L. Earl, J. A. Berzofsky, and B. Moss. 2008. Enhanced cell surface expression, immunogenicity and genetic stability resulting from a spontaneous truncation of HIV Env expressed by a recombinant MVA. Virology 372260-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wyatt, L. S., S. T. Shors, B. R. Murphy, and B. Moss. 1996. Development of a replication-deficient recombinant vaccinia virus vaccine effective against parainfluenza virus 3 infection in an animal model. Vaccine 141451-1458. [DOI] [PubMed] [Google Scholar]
- 42.Wyatt, R. S., P. L. Earl, J. Vogt, L. A. Eller, D. Chandran, J. Liu, H. L. Robinson, and B. Moss. 2008. Correlation of immunogenicities and in vitro expression levels of recombinant modified vaccinia Ankara HIV vaccines. Vaccine 26486-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.