Abstract
Converging TGF-β and insulin-like neuroendocrine signaling pathways regulate whether Caenorhabditis elegans develops reproductively or arrests at the dauer larval stage. We examined whether neurotransmitters act in the dauer entry or recovery pathways. Muscarinic agonists promote recovery from dauer arrest induced by pheromone as well as by mutations in the TGF-β pathway. Dauer recovery in these animals is inhibited by the muscarinic antagonist atropine. Muscarinic agonists do not induce dauer recovery of either daf-2 or age-1 mutant animals, which have defects in the insulin-like signaling pathway. These data suggest that a metabotropic acetylcholine signaling pathway activates an insulin-like signal during C. elegans dauer recovery. Analogous and perhaps homologous cholinergic regulation of mammalian insulin release by the autonomic nervous system has been noted. In the parasitic nematode Ancylostoma caninum, the dauer larval stage is the infective stage, and recovery to the reproductive stage normally is induced by host factors. Muscarinic agonists also induce and atropine potently inhibits in vitro recovery of A. caninum dauer arrest. We suggest that host or parasite insulin-like signals may regulate recovery of A. caninum and could be potential targets for antihelminthic drugs.
Animals have developed many adaptations that allow them to survive sporadic or seasonal declines in growth conditions. Diapause, an alternative developmental state of low metabolic activity, is an example of such an adaptation that is found in a wide range of species (1). In the nematode, Caenorhabditis elegans, the dauer diapause stage is induced by unfavorable environmental conditions (2). Under normal growth conditions, C. elegans develops through four larval stages (L1–L4) to the reproductive adult stage. However, in response to a secreted pheromone, low food levels, and high temperature (i.e., unfavorable growth conditions) during the L1 larval stage, animals enter an alternative developmental pathway and arrest at the dauer larval L3 stage. The C. elegans dauer larva has morphological, physiological, and anatomical changes that differ from a reproductive L3 stage animal including: altered neuroanatomy, radial shrinkage of a thick cuticle, sealing of mouth and anal openings, suspension of feeding, and changes in metabolism (2). Animals can remain arrested as dauer larvae for many months. When pheromone levels and temperature decline, and food levels increase, the animals molt and reenter the life cycle as an L4 larva that is indistinguishable from animals that have not arrested at the dauer stage (2). In parasitic nematodes, such as the hookworm, Ancylostoma caninum, diapause arrest is an obligatory part of the life history. The dauer stage in parasitic nematodes is the infective stage that is morphologically, behaviorally, and functionally analogous to the C. elegans dauer (3–5). Recovery to the reproductive stages in the parasitic nematodes is induced upon host infection by unknown host factors.
Mutants that affect C. elegans dauer formation (daf) fall into two categories: dauer constitutive and dauer defective. Dauer constitutive mutations cause animals to enter the dauer stage even under favorable growth conditions (low pheromone, high food, and low temperature). Conversely, mutants that are dauer defective do not arrest at the dauer stage even under conditions of high pheromone (2). The mutations affecting dauer arrest have been ordered into a genetic epistasis pathway (2). The pathway represents the steps in a neuroendocrine signaling system from sensory detection of food, pheromone, and temperature to overall remodeling of the animal to form a full dauer.
The initiation and recovery of dauer arrest is regulated by exposed sensory neurons. First, several mutants that do not arrest as dauer larvae in response to the dauer pheromone have structural abnormalities in the sensory amphid sensillum (2). Second, killing particular sensory neurons with a laser microbeam induces dauer arrest, suggesting these neurons normally signal to induce reproductive development (2).
Molecular genetic analysis has identified a TGF-β pathway (2, 6) and an insulin-like signaling pathway (7) thought to couple the sensory neural inputs to other secretory cell or to the target tissues that are remodeled and metabolically shifted in the dauer larvae (2, 7). DAF-7, the TGF-β ligand, is expressed in a pair of exposed sensory neurons in reproductively growing animals but not expressed in animals under conditions of high pheromone (2). DAF-4, one of the TGF-β receptors, and DAF-3, a Smad protein implicated in coupling TGF-β signals to the nucleus, are expressed broadly in secretory as well as target tissues (6). Predicted insulin-like ligands of the DAF-2 insulin receptor have been identified in the genome sequence but not yet shown to engage DAF-2 or regulate dauer arrest (8).
Temperature also modulates dauer arrest because even null mutations in the TGF-β pathway genes are temperature-sensitive and most daf-2 alleles are temperature-sensitive (2, 9, 10). The temperature input to this neuroendocrine pathway is mediated at least partially by the thermoregulatory AIY and AIZ interneurons (11, 12), but how the activity of these neurons is coupled to neuroendocrine outputs is unknown.
We reasoned that the neural pathways from the dauer regulatory sensory neurons and interneurons to neurosecretory cells that signal target tissues are likely to utilize known neurotransmitters. Glutamate, acetylcholine, γ-aminobutyric acid, serotonin, FMRFamide, and dopamine have been implicated in particular C. elegans behaviors (13, 14), and drugs that interact with the mammalian receptors of these neurotransmitters affect particular C. elegans behaviors (13, 14). Moreover, the C. elegans genome sequence has identified members of the receptor superfamilies for most of these neurotransmitters (15).
To identify neurotransmitter inputs to the dauer neuroendocrine pathways, we tested a variety of neurotransmitter agonists and antagonists for induction of dauer arrest or recovery. We find that muscarinic agonists specifically promote dauer recovery in pheromone-induced dauer larvae as well as particular classes of dauer constitutive mutants. The muscarinic agonists do not induce recovery of either daf-2- or age-1-induced dauer larvae, which have defective insulin-like signaling. We show that this muscarinic pathway also regulates A. caninum recovery from dauer arrest. In mammals, muscarinic agonists promote insulin release both in vivo and in vitro (17, 18). We suggest that insulin-like secretory cells in the nematodes are regulated by cholinergic inputs in a metabolic control pathway homologous to mammalian autonomic input to pancreatic beta cell activity.
Materials and Methods
Strains and Growth Conditions.
Animals were grown on standard NG agar plates. In this study, the mutations in C. elegans used were: LGI, daf-8(e1393); LGII, daf-22(m130), unc-4(e120), sqt-1(sc13), age-1(m333 and mg44); LGIII, daf-7(e1372), daf-2(e1370, e1391), daf-4(m63); LGIV, daf-1(m40), daf-10(e1387); LGX, daf-12(m20). age-1 animals were maintained as marked heterozygous strains [sqt-1(sc13) age-1(mg44)/mnC1 or unc-4(e120) age-1(m333)/mnC1]. A. caninum were maintained as described previously (18).
Dauer Recovery Assay.
Minimal media plates were used for the drug assays: 3.0 g NaCl, 20 g agarose (Sigma Type II A6877), 0.025 M KPO4 (pH 6.0), 1 mM CaCl2, 1 mM MgSO4, and 5 μg/ml cholesterol were added. In some assays, Escherichia coli (DH5∝) bacteria arrested with streptomycin was added to each plate. All of the drugs used were from Sigma. Dauer-stage animals were transferred to drug plates at 25° without the addition of food. For experiments presented in Figs. 1 and 2 and Table 1, about 10,000 L1s were placed in 10 ml of S medium (19) containing 1–2 ml of a 0.4% (wt/vol) solution of E. coli DH5∝ bacteria in M9 solution (19) arrested with streptomycin in a 25-ml flask on a rotating, heated water bath at 25°C. For wild-type dauer larvae, 600 μl of the 0.4% (wt/vol) bacterial solution and 20–50 μl of pheromone also were added to the flask as described in ref. 20. After 72 hr, the cultures were centrifuged and the supernatant was removed. Animals were resuspended in a preheated 25° solution of 1% SDS and placed on a rocker at 25° for 30 min, and the SDS was removed. Animals were washed with either M9 or S medium (19) four to six times. After a final spin, 100–200 dauer larvae were placed onto the drug plates without food and scored 24 and 48 hr later for dauer larvae and non-dauer adults. Because mutations in age-1 are maternally rescued, age-1 dauer larvae were isolated either by plating synchronized L1s or picking maternally rescued unc-4 age-1 or sqt-1 age-1 non-dauer animals to plates at 25°C and, after 3 days, picking dauer larvae to the drug and control plates. Consequently, the plates with age-1 had some bacteria on them.
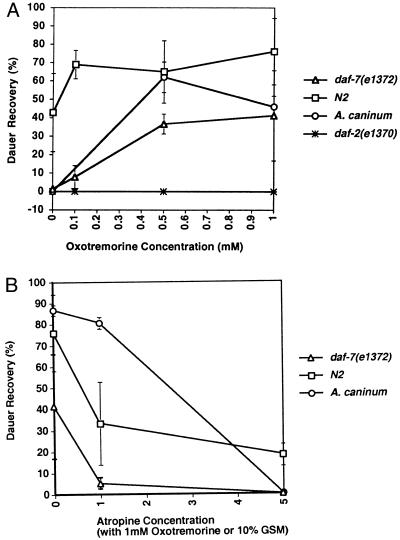
Figure 1.
The effect of muscarinic agonists and an antagonist on dauer recovery in C. elegans and A. caninum. (A) Oxotremorine, a synthetic muscarinic agonist, promotes dauer recovery in both C. elegans and A. caninum. daf-2(e1370) fails to recover at all drug concentrations. The scale for the drug concentration for A. caninum is 10×. All points are the average of two experiments, where at least two plates were scored with the exception of 100 mM daf-7, N2, daf-2, where only one experiment was done. (B) Atropine can specifically inhibit the muscarinic agonist-induced response. In C. elegans, at 1 mM oxotremorine, as the concentration of atropine, a muscarinic antagonist, is increased, dauer recovery is completely inhibited. In A. caninum L3 incubated with serum and GSM plus atropine (5 mM), dauer recovery was inhibited by 99.5% (from 86.7% to 0.5%). Similarly, in A. caninum larvae, 0.5 mM arecoline and increasing amounts of atropine cause dauer recovery to be completely inhibited. Concentrations of 1–5 mM of a drug are used routinely in drug assays in C. elegans (13, 14, 24). The unusually high doses may be due to a cuticle permeability barrier. Drugs in the following classes were tested on daf-7(e1372) and daf-2(e1370) mutant strains for dauer recovery and on wild type and the daf-22(m130) mutant for dauer induction and had no reproducible effect on dauer recovery or formation. (a) Noradrenaline/adrenaline: cocaine*, imipramine*, reserpine*, tetrabenazine*, clonidine, isoproteronol, epinephrine, propanolol, and metopolol; (b) serotonin: 5-hydroxytryptamine, p-chlorophenylalanine, and spiperone; (c) opioids: hydromorphone, meperidine, nalorphine, PCP*, and methaqualone; (d) dopamine: dopamine and metoclopramide; (e) γ-aminobutyric acid: muscimol, diazepam, pentobarbitol, bicuculline, and picrotoxin; (f) glutamate: NMDA, kainate, α-amino-adipate, glutamate diethyl ester, and quisqualate. *, Affects multiple signaling pathways.
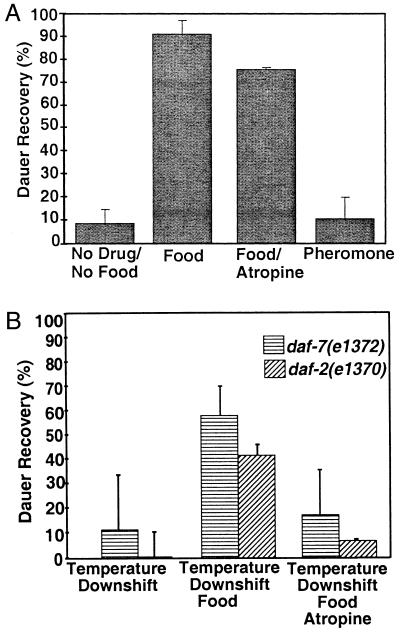
Figure 2.
Atropine specifically inhibits dauer recovery in C. elegans and A. caninum. (A) Wild-type pheromone-induced dauer larvae placed on plates containing bacterial food, no bacteria and no pheromone, bacteria and 1 mM atropine, and pheromone at 25°C and scored 24–42 hr later for dauer larvae and reproductive L4/adults. Experiments were performed at least twice. With no food or pheromone, 91% of the animals remain arrested (n = 1,141). Dauer larvae placed on plates with food recovered efficiently, with less than 10% remaining arrested at the dauer stage (n = 2,596). The addition of 1 mM atropine in the presence of food partially inhibited dauer recovery: 25% remained arrested at the dauer stage (n = 1,311). Eighty-nine percent of the animals maintained on plates with pheromone but no food (n = 1,027) remained arrested at the dauer stage. The pheromone preparation contained bacterial contaminants that may have been used as a food source. In A. caninum incubated with 10% serum and 25 mM GSM, 9% of the infective larvae remained as dauer larvae and did not resume feeding. The addition of atropine (0.5 mM) to the serum and GSM completely inhibited recovery of A. caninum L3, and no worms resumed feeding (data not shown). (B) daf-7(e1372) (horizontally striped bars) and daf-2(e1370) (diagonally striped bars) dauer larvae from 25°C liquid cultures were placed onto plates at 15°C. Animals were scored for the presence of dauer larvae and reproductive adults after 2 days. Temperature downshift induced dauer recovery only very slightly in daf-2(1370) animals (4%, n = 320) and not in either daf-7(1372) (0.2%, n = 330) or daf-2(1391) animals, where 100% of the animals remained as dauers (n = 164). At 15°C with food, 65% of the daf-2(e1370) (n = 384) and 43% of the daf-7(e1372) dauer larvae (n = 587) recovered and 76% of daf-2(e1391) animals recovered (n = 458). Atropine at 1 mM potently inhibits dauer recovery of daf-2(e1370) to 18% (n = 228), daf-7(e1372) to 6% (n = 405), and daf-2(e1391) to 23% (n = 363) dauer larvae on plates at 15°C with food. For daf-2(1370) and daf-7(e1372), each experiment was performed two or three times, whereas for daf-2(e1391), the numbers are from only one trial. The difference between the effects of atropine on pheromone-induced dauer larvae and either daf-2- or daf-7-induced dauer larvae may be because the pheromone-induced dauer larvae had been arrested longer than the daf-2 and daf-7 dauer larvae. Older dauer larvae will recover when exogenous pheromone is removed, even without the addition of food, but younger dauer larvae do not (2). Alternatively, pheromone may inhibit both the TGF-β- and insulin-like-signaling pathways (and perhaps other signals) whereas daf-2 or daf-7 mutants may only decrease one endocrine signal.
Table 1.
Induction of dauer recovery by muscarinic agonists
| Drug | daf-7(e1372) | daf-8(e1377) | daf-14(m77) | daf-2(e1370) | age-1(m333) | age-1(mg44) |
|---|---|---|---|---|---|---|
| No drug/no food | 1.3 ± 1.3 | 4.5 ± 0.1 | 0 | 0 | 0 | 0 |
| Pilocarpine | 8.8 ± 6.0 | 17.7 ± 0.4 | 2.5 ± 0.1 | 0 | ND | ND |
| Arecoline | 34.3 ± 24.4 | 34.0 ± 0.7 | 0.2 ± <0.1 | 0 | 0 | 0 |
| Oxotremorine | 41.2 ± 24.5 | 36.2 ± 0.3 | 14.3 ± <0.1 | 0 | 0 | 0 |
| Oxotremorine atropine | 5.2 ± 2.7 | 4.8 ± 0.01 | <0.1 ± <0.1 | 0 | ND | ND |
Experiments were performed as in Materials and Methods. Animals were scored 24–48 h after being placed as dauer larvae on plates containing drugs. Values are mean ± SE. Numbers shown indicate the percent dauer recovery from two trials, where each plate was at least in duplicate for daf-7(e1372), daf-2(e1370), and age-1(m333). The remaining strains show values from one experiment in which each drug was tested on at least two plates, although similar trends have been observed previously. For each drug tested the number of animals are in the following order: no drug no food; 1 mM pilocarpine; 1 mM arecoline; 1 mM oxotremorine; and 1 mM oxotremorine with 1 mM atropine. Number of animals tested are: daf-7(e1372), 2345, 692, 756, 1363, 1227; daf-8(e1377), 1582, 354, 652, 753, 925; daf-14(m77), 1300, 676, 698, 902, 1101; daf-2(e1370), 872, 222, 258, 207, 460; age-1(m333), 113, not done (ND), 74, 74, ND; age-1(mg44), 71, ND, 57, 52, ND.
Drug Assay in A. caninum.
Hookworm-infective L3 animals were collected from 1- to 4-week-old coproculture by the Baermann technique and decontaminated with 1% HCl in BU buffer (50 mM Na2PO4/22 mM KH2PO4/70 mM NaCl, pH 6.8) (5, 21) for 30 min at 22°C. Approximately 250 L3 animals were incubated in each individual wells of a 96-well plate containing 0.1 ml of RPMI 1640 tissue culture medium, supplemented with 0.25 mM Hepes, pH 7.2/100 units/ml penicillin/100 μg/ml streptomycin/100 μg/ml gentamycin/2.5 μg/ml amphotericin B. The L3 animals were activated to resume development and feeding by including 10% (vol/vol) canine serum and 25 mM S-methyl-glutathione (GSM; ref. 22) in the medium. Nonactivated L3 animals were incubated in RPMI 1640 alone. The agonists were tested for activation by incubation with the L3 animals in the absence of the normal stimulus (i.e., serum + GSM), whereas atropine was tested in the presence of either the normal stimulus or the agonists. Animals were incubated at 37°C in 5% CO2 for 24 hr. The percentage of feeding was determined by incubating the animals with 2.5 mg/ml FITC-BSA for 2–3 hr and counting the number of L3 animals that ingested the labeled BSA by microscopic examination under epifluorescent illumination (23). Each treatment was done in triplicate, and each experiment was repeated at least once.
Dauer Arrest Assay.
Animals were grown at 15° and placed in a bleach solution to isolate eggs. One hundred to 200 eggs or synchronized L1 larvae then were added to plates containing a drug and food at 20°C. When the non-dauer larvae had reached the gravid adult hermaphrodite stage and were beginning to lay eggs, each plate was examined visually for the presence of dauer larvae and non-dauer larvae. After this, animals were rinsed off the plate into a plastic dish containing 1% SDS (dauer larvae are the only larval stage resistant to this treatment). After 30 min, dishes were examined for the presence of dauer larvae and non-dauer larvae.
Results
Cholinergic Input to Dauer Recovery.
We tested drugs that affect the following mammalian neuronal pathways for effects on C. elegans dauer induction and dauer recovery: adrenergic/noradrenergic, serotonergic, cholinergic, glutaminergic, dopaminergic, GABAergic, and opioid. Most drugs tested did not affect induction or recovery from dauer arrest (Fig. 1). However, multiple, unrelated muscarinic agonists promote dauer recovery. All four muscarinic agonists tested, carbachol, oxotremorine, pilocarpine, and arecoline, promote recovery of dauer larvae induced by mutation as well as by pheromone although oxotremorine was the most potent (Fig. 1 and Table 1). Muscarinic agonists induce recovery of dauer larvae induced by defective TGF-β signaling in the daf-7(e1372) mutant, with a defect in the TGF-β ligand, as well as daf-8 and daf-14 mutants, which encode members of the Smad family of signal transduction transcription factors that act downstream of the daf-7 ligand (A. Estevez, M. Sundermeyer, M. Estevez, K. V. King, and D. L. Riddle, personal communication; T. Inoue, and J. H. Thomas, personal communication) (Table 1). The muscarinic agonists do not induce recovery of daf-2 mutants, which have defects in the C. elegans homologue of the mammalian insulin receptor gene, or in age-1 mutants, which have a defect in a PI-3-kinase that acts downstream of the daf-2 receptor (refs. 2 and 9; Fig. 1 and Table 1). Thus, the muscarinic recovery pathway depends on insulin-like signaling.
The infective “dauer” L3 of the hookworm A. caninum can be stimulated to resume feeding and development in vitro by incubation with canine serum and GSM, but not by tissue culture medium alone (18). When A. caninum L3 were incubated with either oxotremorine or arecoline without canine serum or GSM in the tissue culture medium, 60–80% of the animals recovered, as indicated by the resumption of feeding (Fig. 1A). Therefore, muscarinic agonists mimicked the recovery induced by serum and GSM.
Fig. 1A shows the oxotremorine dose-response curve for wild-type pheromone-induced dauer larvae, daf-7(e1372), daf-2(e1370), and A. caninum dauer larvae. Oxotremorine and pilocarpine induce maximum recovery of daf-7(e1372) dauer larvae at 5 mM concentration, whereas pheromone-induced dauer larvae reach maximum recovery at 1 mM (Fig. 1A and data not shown). A. caninum L3 dauer larvae also reach maximum recovery at 5 mM oxotremorine (Fig. 1A), but fail to recover when incubated with pilocarpine (data not shown). Pilocarpine had the least effect on all the strains tested (Table 1). Additionally, it had no effect on A. caninum (data not shown). The maximal response for arecoline is 10-fold lower than for the other agonists in both C. elegans and A. caninum (Table 1 and data not shown).
Atropine Specifically Inhibits Dauer Recovery.
To determine the specificity of the muscarinic response, we added both oxotremorine and atropine, a muscarinic antagonist, varying the concentration of antagonist (Fig. 1B). In 1 mM oxotremorine, 40% of the daf-7(e1372) dauer larvae recover at 25°C, the nonpermissive temperature for daf-7(e1372ts). However, in combination with 1 mM atropine, 1 mM oxotremorine induced only 5% recovery; at 5 mM atropine, the 1 mM oxotremorine response is completely abolished. For wild-type pheromone-induced dauer larvae, the results are almost identical (Fig. 1B). This suggests that the drug-induced recovery is a specific muscarinic response, because in mammals, atropine affects muscarinic and not nicotinic receptors (25, 26).
Atropine can partially inhibit C. elegans dauer recovery induced by food signals. When bacteria are added to pheromone-induced dauer larvae at 25°C, 91% of the animals recover, compared with 9% recovery without food (Fig. 2A). Atropine partially inhibits the food-induced recovery of dauer larvae, from 91% without drug to 75% with drug. However, pheromone reduced this response much more severely (from 91% to 20%). Thus, food induces more than the muscarinic pathway to trigger dauer recovery.
In A. caninum, recovery induced by serum and GSM was inhibited nearly completely by atropine (5 mM) (Fig. 2A; from 86.7% to 0.5%). Moreover, A. caninum L3 incubated with 0.5 mM arecoline and 1.0 mM atropine failed to recover. These data indicate that the muscarinic pathway is a major recovery signal from arrest in hookworm.
Temperature downshifts in the presence of food induce dauer recovery in animals bearing mutations in the TGF-β- or insulin-like-signaling pathways (2, 6, 9). Temperature downshift in the absence of food partially induces dauer recovery in daf-2 mutants and does not induce dauer recovery in daf-7 mutants (Fig. 2B). Similarly, bacterial food at 25°C does not allow reproductive development of either daf-2 or daf-7 mutants (ref. 2; data not shown). However, temperature downshift in the presence of food induces more than 40% recovery of both mutants (Fig. 2B). This temperature shift plus food recovery in both daf-7 and daf-2 mutants is potently inhibited by atropine (Fig. 2B). Similar inhibition of dauer recovery was observed with another daf-2 allele, daf-2(e1391) (Fig. 2B).
Neurotransmitter Regulation of Dauer Arrest.
We examined whether exogenous application of neurotransmitters could mimic the dauer pheromone to induce dauer arrest. Drugs that affect mammalian neuronal pathways including adrenergic/noradrenergic, serotonergic, cholinergic, glutaminergic, dopaminergic, GABAergic, and opioid were examined for effects on C. elegans dauer induction (Table 1). We tested these drugs for induction of dauer arrest in wild-type and daf-22 mutants, a mutant that does not secrete pheromone, but arrests at the dauer stage when exposed to exogenous pheromone (2). None of the drugs tested induced dauer arrest under favorable growth conditions. The drugs were active because several of the drugs caused either paralysis, death, or egg-laying defects.
Discussion
Arrest at the dauer stage is a nematode survival strategy that is a specific example of the phyletically general diapause arrest (1–3). In C. elegans, dauer arrest occurs under harsh environmental conditions whereas in the hookworm, A. caninum, a parasitic nematode, diapause is a nonconditional stage in the life cycle (3, 4). Dauer recovery is regulated by levels of pheromone, food, and temperature in C. elegans, whereas in A caninum, unknown host factors induce dauer recovery upon infection (3–5).
We have shown that muscarinic agonists cause dauer recovery in both C. elegans and A. caninum and that this recovery is inhibited specifically by the muscarinic antagonist atropine (Fig. 1). The endogenous neurotransmitter at muscarinic receptors is acetylcholine, which, in vertebrates, functions at cholinergic synapses in both the peripheral and central nervous system (26). Acetylcholine has a wide variety of functions in vertebrate signaling including sympathetic and parasympathetic ganglion cells as well as the adrenal medulla, synapses within the central nervous system, and motor end plates on skeletal muscle innervated by somatic motoneurons (26). Muscarinic receptors are found in muscle, the autonomic ganglia, the central nervous system, and secretory glands. These receptors couple to G proteins and signal on longer time scales than nicotinic receptors. Both muscarinic and nicotinic receptors also have been found in invertebrates such as Drosophila and C. elegans (13, 14, 26–28).
The nicotinic receptor has been the primary focus of the studies on cholinergic signaling in the worm. The drug levamisole, a nicotinic agonist, is toxic to C. elegans, causing muscle hypercontraction (13). Mutants that are resistant to this drug have revealed components of a nicotinic-signaling cascade (13). Levamisole has no effect on dauer recovery (data not shown), suggesting that the nicotinic receptor pathway does not regulate dauer arrest.
Fewer studies have been done on muscarinic signaling in C. elegans. Binding studies on crude homogenates of C. elegans have shown that they contain muscarinic receptors that have the potential to bind to the muscarinic ligands [3H]QNB (29) and [3H]N-methylscopalamine (30) with high affinity (13). These receptors were found in both C. elegans adults and L1 and L2 larvae (29). Several muscarinic receptor homologues have been identified in the C. elegans genome sequence database (ref. 28; H.A.T., G. Sandoval, and G.R., unpublished observation).
Both arecoline and pilocarpine are naturally occurring drugs from the betel nut seed and the Pilocarpus leaf, respectively, whereas oxotremorine and carbachol are synthetic drugs (26). Arecoline, pilocarpine, and oxotremorine have the same sites of action on mammalian metabotropic cholinergic receptors, but arecoline also acts on nicotinic receptors (26). Atropine, a natural product, specifically inhibits mammalian muscarinic responses (26). Because all of the drug-induced dauer recovery was inhibited by atropine, and this drug does not inhibit nicotinic signaling in C. elegans, we conclude that dauer recovery is mediated by muscarinic signaling.
Muscarinic agonists potently induced recovery of dauer larvae induced by pheromone or mutations in the daf-7 TGF-β group of genes, but did not induce recovery of the daf-2 and age-1 insulin-signaling mutants. Thus, the cholinergic input to dauer recovery depends on insulin-like signaling. We suggest that muscarinic agonists induce recovery of the TGF-β pathway mutant dauer larvae or pheromone-induced dauer larvae by stimulating signaling in the daf-2 insulin-like pathway. In this way, cholinergic stimulation can induce recovery in animals with defective TGF-β pathway genes but not in animals with defective insulin-like pathway genes.
In vertebrates, studies link the muscarinic and insulin signaling pathways. Both adrenergic and cholinergic fibers innervate secretory cells in the vertebrate islet of Langerhans (16, 31). Consistent with the suggestion that muscarinic inputs increase C. elegans insulin-like signaling, mammalian autonomic cholinergic fibers enhance insulin secretion (29, 31). Pharmacological stimulation with acetylcholine or carbachol induces insulin release both in vivo and in vitro. This induction is completely abolished by atropine, showing that it is mediated by activation of muscarinic receptors on the beta cells (16, 32, 33). In mammalian systems, binding of acetylcholine to the beta cell muscarinic receptor causes activation of sodium channels, which, in turn, leads to a change in membrane potential to induce insulin release (ref. 33; Fig. 3).
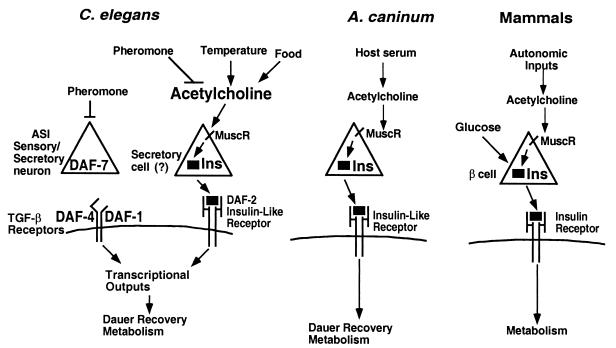
Figure 3.
A model for cholinergic input induction of dauer recovery. In dauer pheromone or in a daf-7 mutant, the DAF-7 TGF-β ligand is not produced by the ASI sensory/secretory neuron. Therefore, there is no activation of the DAF-1 and DAF-4 TGF-β receptors or downstream DAF-8 and DAF-14 Smad proteins, and this results in high DAF-3 Smad activity in signaling cells or target tissues. In pheromone without muscarinic agonists, no insulin-like signal is released, causing less insulin receptor signal transduction to the transcription factor DAF-16, which, in combination with unregulated DAF-3, induces dauer arrest. Under pheromone-induced or daf-7 mutation-induced dauer induction conditions, muscarinic stimulation causes release of an insulin-like DAF-2 ligand, which stimulates the DAF-2/AGE-1-signaling pathway to DAF-16 inactivation. Because daf-7 mutants can recover in muscarinic agonists, the TGF-β signaling pathway is not required for dauer recovery. Under normal conditions of dauer recovery upon release from pheromone and addition of food and low temperature, we suggest that acetylcholine released via temperature or food pathways binds to the muscarinic receptor on the insulin-like signaling cell to cause an increase in insulin release. We suggest that temperature is coupled via the interneurons AIY and AIZ to the DAF-2 insulin-like signaling pathway rather than the TGF-β signaling pathway because mutations in the thermoregulatory gene ttx-3 can suppress mutations in daf-7 and not mutations in daf-2 (ref. 11; O. Hobert and G.R., unpublished observations).
These data suggest the model shown in Fig. 3 for dauer recovery in C. elegans. When pheromone levels decrease and food levels increase, acetylcholine is secreted from an as yet unidentified neuron and binds to the muscarinic receptor on an insulin-like secreting neuron or other cell. This induces secretion of an insulin-like signal, in turn, to induce dauer recovery. Insulin-secreting pancreatic beta cells have many neuronal features and are thought to be specialized “ganglia” related to the enteric nervous system of lower vertebrates (34). In addition, proteins related to insulin are produced by neurons that regulate metabolism in Limulus (35). Distant relatives of insulin are found in the C. elegans genome database (ref. 8; S. Pierce and G.R., unpublished observations). We suggest that the secretory cells expressing such an insulin-like gene will also express muscarinic receptors and have connections to food, pheromone, and temperature sensory neurons (Fig. 3).
Temperature acts as a modulator for dauer recovery (refs. 2 and 14; Fig. 2). The thermoregulatory circuit for temperature sensation and output of that information to motor and endocrine pathways has been identified (11, 12, 36). This pathway consists of the thermosensory neuron AFD coupled to the interneurons AIY and AIZ (11, 12, 14, 36). ttx-3, a gene that affects AIY function, is expressed exclusively in the AIY interneurons (11, 12). Mutations in ttx-3 decouple this thermoregulatory pathway from the dauer pathway: daf-7; ttx-3 double mutant animals form dauer larvae that recover at high temperature, unlike daf-7 single mutants (12). However, daf-2; ttx-3 double mutant dauer larvae do not recover at high temperature, like the daf-2 mutant alone (O. Hobert and G.R., unpublished observation). We suggest that thermosensory signals through the thermoregulatory AIY and AIZ interneurons couple to insulin-like secretory neurons (Fig. 3). Given that rates of growth and metabolism are intimately connected to cultivation temperature in invertebrates, the coupling of thermosensation to metabolic control is reasonable. Such a coupling of thermosensory input to metabolic control by the daf-2 insulin-like signaling pathway may be analogous (or even homologous) to the hypothalamic modulation of autonomic input to the pancreatic beta cells (31, 33, 34).
A muscarinic signaling pathway also induces recovery of the hookworm infective L3 from their arrested “dauer” state. Recovery from dauer arrest in hookworm occurs in the host in response to an undefined host-specific signal. We suggest that up-regulation of an insulin-like molecule by a cholinergic pathway also causes dauer recovery upon entry into the host in A. caninum. Known muscarinic signaling drugs may constitute novel chemotherapeutic strategies to perturb the dauer maintenance process in invertebrate hosts as well as the recovery process in human hosts.
Acknowledgments
We thank Jim Thomas for providing some of the strains used in this study and Ann Sluder for the minimal media protocol. Some of the strains were obtained from the Caenorhabditis Genetics Center, which is supported by the National Institutes of Health National Center for Research Resources. We thank members of the Ruvkun and Kaplan labs and Allan Dines for helpful discussions, suggestions, and critical reading of the manuscript. This work was funded by a grant from Hoechst and National Institutes of Health Grant R01AG14161 to G.R. Part of this work was completed while H.A.T. was supported by the Helen Hay Whitney Foundation.
Abbreviation
- GSM
S-methyl-glutathione
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tauber M J, Tauber C A, Masaki S. Seasonal Adaptation of Insects. New York: Oxford Univ. Press; 1986. [Google Scholar]
- 2.Riddle D L, Albert P S. In: C. elegans II. Riddle D L, Blumenthal T, Meyer B J, Priess J R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 739–768. [Google Scholar]
- 3.Riddle D L, Bird A F. J Nematol. 1985;17:165–168. [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt G D, Roberts L S. Foundations of Parasitology. St. Louis: Times Mirror/Mosby; 1985. [Google Scholar]
- 5.Hawdon J M, Schad G A. In: Developmental Adaptations in Nematodes. Toft C A, editor. Oxford: Oxford Univ. Press; 1991. pp. 274–298. [Google Scholar]
- 6.Patterson G I, Koweek A, Wong A, Liu Y, Ruvkun G. Genes Dev. 1997;11:2679–2690. doi: 10.1101/gad.11.20.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogg S, Paradis S, Gottlieb S, Patterson G I, Lee L, Tissenbaum H A, Ruvkun G. Nature (London) 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 8.Duret L, Guex N, Peitsch M C, Bairoch A. Genome Res. 1998;8:348–353. doi: 10.1101/gr.8.4.348. [DOI] [PubMed] [Google Scholar]
- 9.Kimura K, Tissenbaum H A, Liu Y, Ruvkun G. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 10.Malone E A, Thomas J H. Genetics. 1994;136:879–886. doi: 10.1093/genetics/136.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobert O, D'Alberti T, Liu Y, Ruvkun G. J Neurosci. 1998;18:2084–2096. doi: 10.1523/JNEUROSCI.18-06-02084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobert O, Mori I, Yamashita U, Honda H, Ohshima Y, Liu Y, Ruvkun G. Neuron. 1997;19:345–357. doi: 10.1016/s0896-6273(00)80944-7. [DOI] [PubMed] [Google Scholar]
- 13.Rand J B, Nonet M L. In: in C. elegans II. Riddle D L, Blumenthal T, Meyer B J, Priess J R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 611–643. [Google Scholar]
- 14.Bargmann C I, Mori I. In: C. elegans II. Riddle D L, Blumenthal T, Meyer B J, Priess J R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 717–737. [PubMed] [Google Scholar]
- 15.Bargmann C I. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- 16.Ahren B, Taborsky G J, Jr, Porte D., Jr Diabetologia. 1986;29:827–836. doi: 10.1007/BF00870137. [DOI] [PubMed] [Google Scholar]
- 17.Miller R E. Endocr Rev. 1981;2:471–494. doi: 10.1210/edrv-2-4-471. [DOI] [PubMed] [Google Scholar]
- 18.Hawdon J M, Schad G A. Exp Parasitol. 1993;77:489–491. doi: 10.1006/expr.1993.1110. [DOI] [PubMed] [Google Scholar]
- 19.Sulston J, Hodgkin J. In: The Nematode Caenorhabditis elegans. Wood W B, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. pp. 587–606. [Google Scholar]
- 20.Gottlieb S, Ruvkun G. Genetics. 1994;137:107–120. doi: 10.1093/genetics/137.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawdon J M, Schad G A. J Helm Soc Wash. 1991;58:140–142. [Google Scholar]
- 22.Hawdon J M, Jones B F, Perregaux M A, Hotez P J. Exp Parasitol. 1995;80:205–211. doi: 10.1006/expr.1995.1025. [DOI] [PubMed] [Google Scholar]
- 23.Hawdon J M, Schad G A. J Parasitol. 1990;76:394–398. [PubMed] [Google Scholar]
- 24.Avery L, Bargmann C I, Horvitz H R. Genetics. 1993;134:455–464. doi: 10.1093/genetics/134.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefkowitz R J, Hoffman B B, Taylor P. In: The Pharmacological Basis of Therapeutics. Hardman J G, Limbird L E, editors. New York: McGraw–Hill; 1996. pp. 105–139. [Google Scholar]
- 26.Brown J H, Taylor P. In: The Pharmacological Basis of Therapeutics. Hardman J G, Limbird L E, editors. New York: McGraw–Hill; 1996. pp. 141–160. [Google Scholar]
- 27.Haim N, Nahum S, Dudai Y. J Neurochem. 1979;32:543–552. doi: 10.1111/j.1471-4159.1979.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y S, Park Y S, Chang D J, Hwang J M, Min C K, Kaang B K, Cho N J. J Neurochem. 1999;72:58–65. doi: 10.1046/j.1471-4159.1999.0720058.x. [DOI] [PubMed] [Google Scholar]
- 29.Yamamura H I, Snyder S H. Proc. Natl. Acad. Sci. USA. 1974. 1725–1729. [Google Scholar]
- 30.Burgermeister W, Klein W L, Nirenberg M, Witkop B. Mol Pharmacol. 1978;14:240–256. [PubMed] [Google Scholar]
- 31.Woods S C, Porte D., Jr Physiol Rev. 1974;54:596–619. doi: 10.1152/physrev.1974.54.3.596. [DOI] [PubMed] [Google Scholar]
- 32.Latifpour J, Gousse A, Yoshida M, Weiss R M. J Urol. 1992;147:760–763. doi: 10.1016/s0022-5347(17)37374-3. [DOI] [PubMed] [Google Scholar]
- 33.Henquin J-C. In: Joslin's Diabetes Mellitus. Kahn C R, Weir G C, editors. Malvern, PA: Lea & Febiger; 1994. pp. 56–80. [Google Scholar]
- 34.Bonner Weir S, Smith F E. In: Joslin's Diabetes Mellitus. Kahn C R, Weir G C, editors. Malvern, PA: Lea & Febiger; 1994. pp. 15–28. [Google Scholar]
- 35.Roovers E, Vincent M E, van Kesteren E, Geraets P M, Planta R J, Vreugdenhil E, van Heerikhuizen H. Gene. 1995;162:181–188. doi: 10.1016/0378-1119(95)00323-x. [DOI] [PubMed] [Google Scholar]
- 36.Mori I, Ohshima Y. Nature (London) 1995;376:344–348. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]