Abstract
Rift Valley fever virus (RVFV) continues to cause large outbreaks of acute febrile and often fatal illness among humans and domesticated animals in Africa, Saudi Arabia, and Yemen. The high pathogenicity of this bunyavirus is mainly due to the viral protein NSs, which was shown to prevent transcriptional induction of the antivirally active type I interferons (alpha/beta interferon [IFN-α/β]). Viruses lacking the NSs gene induce synthesis of IFNs and are therefore attenuated, whereas the noninducing wild-type RVFV strains can only be inhibited by pretreatment with IFN. We demonstrate here in vitro and in vivo that a substantial part of the antiviral activity of IFN against RVFV is due to a double-stranded RNA-dependent protein kinase (PKR). PKR-mediated virus inhibition, however, was much more pronounced for the strain Clone 13 with NSs deleted than for the NSs-expressing strain ZH548. In vivo, Clone 13 was nonpathogenic for wild-type (wt) mice but could regain pathogenicity if mice lacked the PKR gene. ZH548, in contrast, killed both wt and PKR knockout mice indiscriminately. ZH548 was largely resistant to the antiviral properties of PKR because RVFV NSs triggered the specific degradation of PKR via the proteasome. The NSs proteins of the related but less virulent sandfly fever Sicilian virus and La Crosse virus, in contrast, had no such anti-PKR activity despite being efficient suppressors of IFN induction. Our data suggest that RVFV NSs has gained an additional anti-IFN function that may explain the extraordinary pathogenicity of this virus.
Rift Valley fever virus (RVFV) is a serious emerging pathogen for humans and domestic ruminants in sub-Saharan Africa, Egypt, Yemen, and Saudi Arabia. Since the first description of an outbreak in Kenya in 1931 (19), recurrent epidemics have killed hundreds of thousands of animals, more than a thousand humans, and caused significant economic losses (4). For animals, RVFV is mainly transmitted by mosquitoes, whereas humans are often infected by close contact with sick animals (47, 72). The disease in humans is associated with symptoms ranging from uncomplicated acute febrile illness to retinitis, hepatitis, renal failure, meningoencephalitis, severe hemorrhagic disease, and death (4). The severity of RVFV zoonosis, as well as the capability to cause major epidemics in livestock and humans, has prompted authorities to list RVF as a notifiable disease and the virus as a potential biological weapon (12).
RVFV belongs to the genus Phlebovirus, family Bunyaviridae (21). Bunyaviruses are enveloped and have a trisegmented single-stranded RNA (ssRNA) genome of negative or ambisense polarity. Transcription and replication take place in the cytoplasm. The genome segments of bunyaviruses encode four structural proteins: the viral polymerase (L) on the large (L) segment, two glycoproteins (Gn and Gc) on the medium (M) segment, and the viral nucleocapsid protein (N) on the smallest (S) segment (63). RVFV additionally expresses a 78-kDa protein from the M segment and two nonstructural proteins, encoded on the M segment (termed NSm) and the S segment (termed NSs). The nonstructural proteins are dispensable for viral multiplication in cell culture but play important roles for pathogenesis in vivo (8, 25, 65, 73). In particular, the NSs protein was found to suppress induction of the antiviral type I interferon (alpha/beta interferon [IFN-α/β]) genes (7, 13, 40, 41). Consequently, the natural RVFV isolate Clone 13, which has a large deletion in the NSs gene, is a strong inducer of IFN-α/β, whereas the virulent wild-type (wt) strain ZH548, bearing an intact NSs, suppresses IFN induction (7, 13, 33). Clone 13 is strongly attenuated and immunogenic in wt mice but highly virulent in IFN-α/β-nonresponsive mice (13, 65), demonstrating that the IFN system is required to control RVFV replication in vivo and in vitro.
IFNs are an important part of the innate immune system. These cytokines are synthesized and secreted by infected cells and prime neighboring cells to express antiviral factors that limit virus spread (53, 57, 58, 68). The best-characterized antiviral factors are the Mx proteins (28), the double-stranded RNA (dsRNA)-dependent protein kinase (PKR) (24), and the 2′-5′ oligoadenylate synthetase/RNase L system (61), which interfere with viral transcription, translation, or genome replication.
Exogenous activation of the type I IFN system enables animals to survive RVFV infection (13, 45, 46, 52). This is partially mediated by Mx proteins that are located in the cytoplasm, the site of RVFV replication (22, 59). However, mice that lack cytoplasmic Mx proteins still restrict RVFV replication if treated with IFN-stimulating agents (13), suggesting that additional antiviral factors are inhibiting RVFV. In line with this, we showed previously that PKR reduces replication of Bunyamwera virus (BUNV), which is related to RVFV (62).
PKR is constitutively expressed in a latent, inactive form but its expression is upregulated by treatment with IFN-α/β. PKR is activated by viral dsRNA or ssRNA containing a 5′ triphosphate group and a short stem-loop structure (24, 48). PKR is a serine-threonine kinase that phosphorylates the α subunit of eukaryotic translation initiation factor 2 (eIF-2), thereby leading to a translational arrest of cellular and viral mRNA (24, 57).
Type I IFNs are (i) regarded as potential RVFV therapy (45, 52), and (ii) the crucial host factor mediating the attenuation of Clone 13, a promising live vaccine candidate (13). We therefore deemed it important to further study the inhibitory effect of IFN on RVFV and to identify antiviral factors limiting viral replication. We demonstrate here that PKR has a marked inhibitory effect on RVFV, but only in the absence of the viral NSs protein. wt RVFV is resistant to the antiviral properties mediated by PKR because the NSs protein induces the specific degradation of PKR. Recombinant RVFV expressing the NSs proteins of related but less-virulent bunyaviruses have no such activity, suggesting that the high pathogenicity of RVFV is promoted by the additional ability to degrade PKR. Thus, RVFV uses an unusual mechanism to evade the antiviral actions of IFN. Moreover, these data suggest that PKR is a critical factor for the in vivo attenuation of RVFV live vaccine candidates with the NSs gene deleted.
MATERIALS AND METHODS
Cells, viruses, plasmids, and chemicals.
Vero E6, BHK-21, HEK293, 293T, and cells derived from IFNAR−/− mice, PKR−/− mice, and their corresponding wt counterparts were cultivated in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS; Gibco). PKR-deficient (PKR−/−) cells and corresponding wt cells were isolated from kidneys of newborn C57BL/6 (wt) and PKR−/− mice (74) and immortalized using a retroviral vector expressing the simian virus 40 Large T antigen (kindly provided by Tilo Materna, University of Vienna, Vienna, Austria). Vector-infected cells were selected on resistance to 3 μg of puromycin/ml. Cells were transfected using either Nanofectin transfection reagent (PAA Laboratories; for virus rescues) or Metafectene [for poly(I:C) transfections] according to the protocols provided by the manufacturers. cDNA constructs used to rescue recombinant RVFV (helper plasmids pI.18-RVFV-L and pI.18-RVFV-N, rescue plasmids pHH21-RVFV-vL, pHH21-RVFV-vM, pHH21-RVFV-vS, and pHH21-RVFV-vN_TCS) were described previously (27). RVFV strains ZH548 and Clone 13, as well as all recombinant virus strains (see below) were propagated in Vero E6 cells under BSL-3 conditions. α-Amanitine was from AppliChem; doxycycline, poly(I:C), MG132, clasto-lactacystin β-lactone (CLBL), zVAD-fmk, and cycloheximide were purchased from Sigma.
Doxycycline-inducible Flp-In T-Rex PKR cells.
cDNA for the human PKR gene was cloned by proofreading-PCR amplification using Gateway (Invitrogen) compatible primers for human PKR (5′-GGG GAC AAG TTT GTA CAA AAA AGC AGG CTC CGG CTG GTG ATC TTT CAG CAG-3′ and 5′-GGG GAC CAC TTT GTA CAA GAA AGC TGG GTC TAA CAT GTG TGT CGT TCA T-3′) and plasmid pUNO-hPKR (Invivogen) as a template. The PCR product was extracted from an agarose gel and cloned through LR recombination (Invitrogen) into the target vector pcDNA5/FRT/TO/SH/GW (25a) containing a streptavidin-binding peptide with a hemagglutinin epitope tag (SH-tag). Flp-In T-Rex HEK293 cells (Invitrogen) were cotransfected with pCDNA5/FRT/TO/PKR and pOG44 plasmids (Invitrogen) for coexpression of the Flp-recombinase using Lipofectamine 2000 (Invitrogen). At 2 days after transfection, cells were selected with hygromycin-containing medium (200 μg/ml) for 2 to 3 weeks. To induce PKR expression, doxycycline was added to the medium with a final concentration of 2 μg/ml and incubated for 36 h.
Plaque assay.
Vero E6 or IFNAR−/− mouse embryo fibroblasts (MEFs) grown to 90% confluence in six-well plates were inoculated with 10-fold serial dilutions of supernatants from infected cells in DMEM with 2% FCS and 20 mM HEPES (pH 7.3). After 1 h of incubation at 37°C, the inoculum was removed, and cells were overlaid with 3 ml of DMEM containing 2% FCS, 0.02% DEAE-dextran, and 0.4% Agar Noble (Difco) and further incubated for 72 h at 37°C. Cells were fixed and stained with 1% crystal violet, 3.6% formaldehyde, 1% methanol, and 20% ethanol, and titers were calculated from the plaque numbers and the dilution factor.
Test for antiviral activity of cytokines.
Vero E6, PKR−/−, and corresponding wt cells were seeded at 106 cells per six-well cavity and treated with 1,000 U of IFN-α A/D (BglII; PBL Biomedical Laboratories) or IFN-β (Betaferon; Schering)/ml, 500 U of IFN-γ (Sigma)/ml, or 500 ng of TNF-α (Sigma)/ml for 16 h and then infected with viruses at a multiplicity of infection (MOI) of 0.01. After incubation for 1 h, the infection inoculum was replaced with fresh medium containing either fresh cytokines or, to avoid interference by virally induced IFN, 2.5 μg of neutralizing anti-mouse IFNAR-1 antibodies (clone MAR1-5A3; BioLegend). At 24 h postinfection, the amount of newly generated virus was measured by plaque assay on IFNAR−/− MEFs.
Poly(I:C) transfection.
For transfection of cells with synthetic dsRNA, 5 μg of poly(I:C) was prepared with 5 μl of Metafectene (Biontex) in 200 μl of serum-free medium according to the manufacturer's instructions. After 15 min of incubation, the dsRNA-liposome mixture was dropped onto cells using the same medium.
Western blot analyses.
Cells were lysed in radioimmunoprecipitation assay buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 0.05% sodium dodecyl sulfate) containing protease inhibitors (Complete Protease Inhibitor; Roche) and phosphatase inhibitors (Phosphatase Inhibitor Cocktail II; Calbiochem). A total of 10 μg of protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to an Immobilon-P polyvinylidene difluoride membrane (Millipore), followed by incubation in saturation buffer (phosphate-buffered saline [PBS] containing 5% nonfat dry milk and 0.05% Tween). The membrane was first incubated for 1 h with primary antibodies and then washed three times with 0.05% PBS-Tween, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Pierce). After three additional washing steps, detection was performed by using the SuperSignal West Femto Kit (Pierce). Primary antibodies used were directed against PKR (monoclonal antibody PKR B-10 [Santa Cruz], diluted 1:1,000), RVFV N protein (mouse polyclonal anti-RVFV N, diluted 1:1,000), phosphorylated eIF-2α (rabbit polyclonal anti-Ser51 [Biosource], diluted 1:200), actin (rabbit polyclonal anti-actin [Sigma], diluted 1:5,000) and GCN2 (rabbit polyclonal anti-GCN2 [Cell Signaling], diluted 1:1,000). Polyclonal mouse antiserum recognizing RVFV N protein was produced by inoculating mice with recombinant N protein (A. Pichlmair and F. Weber, unpublished data).
RT-PCR analyses.
Total cellular RNA was extracted by using an RNeasy minikit (Qiagen) and treated with DNase I. Reverse transcription (RT) was performed with 200 U of RevertAid H Minus M-MuLV (Fermentas) and 200 ng of random hexanucleotides in 20 μl of M-MuLV RT reaction buffer supplied with 1 mM concentrations of each deoxynucleotide triphosphate and 40 U of RNase inhibitor (Fermentas). The resulting cDNA was amplified by 32 to 35 cycles of PCR, with each cycle consisting of 30 s at 95°C, 30 s at 54°C, and 90 s at 68°C, followed by a final elongation step for 10 min at 68°C. Primer sequences for diagnostic RT-PCR analyses were as follows: human IFN-β, 5′-GAC GCC GCA TTG ACC ATC TA-3′ and 5′-CCT TAG GAT TTC CAC TCT GAC T-3′; human γ-actin, 5′-GCC GGT CGC AAT GGA AGA AGA-3′ and 5′-CAT GGC CGG GGT GTT GAA GGT C-3′; RVFV NSs, 5′-GAG AGG ATC CGA TTA CTT TCC TGT GAT ATC TGT TGA TTT GC-3′ and 5′-GAG ACT CGA GCT AAT CAA CCT CAA CAA ATC CAT CAT CAT CAC TCT CC-3′; LACV NSs, 5′-GAG AGG ATC CTC GCA TCA ACA GGT GCA AAT GGA-3′ and 5′-GAC ACT CGA GCT AAA TAC CCA GAT AAT CTG TGG AT-3′; and SFSV NSs, 5′-GAC AGA CGT CTC ACA TGA TGA ACA GCC AGT ACA TG-3′ and 5′-GAC AGA CCG TCT CTT CGA TCA AAA GTC AGA GTC AGA CG-3′.
Real-time RT-PCR.
Total cellular RNA was isolated with the NucleoSpin RNA II kit (Macherey-Nagel) and eluted in 50 μl of double-distilled H2O. Then, 1 μg of RNA was then used as a template for cDNA synthesis, which was performed by using the QuantiTect reverse transcription kit (Qiagen) according to the manufacturer's instructions. mRNA levels of murine PKR and IFN-β were detected with QuantiTect primers QT00162715 and QT00249662, using the QuantiTect SYBR green RT-PCR kit (Qiagen) and a LightCycler II (Roche). RVFV RNA was detected as described previously (9) using the QuantiFast probe PCR kit (Qiagen).
Immunofluorescence analyses.
IFNAR−/− MEFs were grown on coverslips to 30 to 50% confluence and infected at an MOI of 5. Cells were fixed with 3% paraformaldehyde and permeabilized with 0.1% Triton X-100 in PBS. After three washes with PBS containing 1% FCS, the cells were incubated with the primary antibodies, polyclonal rabbit anti-Flag (Sigma) and mouse monoclonal anti-PKR (B-10; Santa Cruz) diluted 1:1,500 and 1:125 in PBS containing 1% FCS, respectively. After incubation at room temperature for 1 h, the coverslips were washed three times in PBS containing 1% FCS and then treated with the secondary antibodies at a dilution of 1:200 each. Cells were again washed three times in PBS and mounted by using Fluorsave solution (Calbiochem). Stained cell samples were examined by using an ApoTome-equipped Axioplan 2 microscope connected to an AxioCam MR digital camera (Carl Zeiss MicroImaging, Inc.) with a ×40 objective lens.
Generation of recombinant viruses.
For recovery of recombinant RVFV, we made use of our strain ZH548 pol I/pol II-based rescue system, which is described in detail elsewhere (27). Generation of the NSs deletion mutant rZH548ΔNSs was achieved by using a modified S-segment rescue plasmid (pHH21-RVFV-vN_TCS), where the NSs gene has been replaced by a cloning cassette containing two AarI restriction sites (27). Linearization of this rescue construct with AarI allows exact in-frame insertion of genes via NcoI/XhoI-compatible ends. The reading frames of Clone 13 NSs and ZH548 NSs containing a C-terminal Flag sequence, as well as the NSs reading frames from the phlebovirus sandfly fever Sicilian virus (SFSV) strain Sabin (GenBank database entry EF201822) and the orthobunyavirus La Crosse virus (LACV; GenBank database entry NP_671971), were amplified from cDNA of infected cells using specific oligonucleotides (the sequences are available on request). PCR fragments were inserted into the cloning cassette of the modified S-segment rescue plasmid pHH21-RVFV-vN_TCS. These constructs were verified by DNA sequencing and used for generation of rZH-F-NSsC13, rZH-F-NSsZH, rZHΔNSs::NSsSFSV, and rZHΔNSs::NSsLACV, respectively.
Recombinant viruses were generated by transfecting cocultures of 293T and BHK-21 cells in six-well plates with 0.5 μg of helper plasmids (pI.18-RVFV-L and pI.18-RVFV-N), together with 1 μg each of the L, M, and S segment rescue plasmids (pHH21-RVFV-vL, pHH21-RVFV-vM, and pHH21-RVFV-vN_TCS or variants thereof) using Nanofectin transfection reagent (PAA Laboratories). Supernatants containing recombinant viruses were collected 5 days posttransfection and used to grow virus stocks. The plaque phenotype of all viruses was determined on Vero E6 cells.
In vivo experiments.
PKR−/− mice (74) (C57BL/6 background) were bred in the specific-pathogen-free unit of BIAT (Biomodels Austria). C57BL/6 mice were purchased from Charles River. Mice at the age of 6 to 8 weeks were infected intraperitoneally with the indicated viruses and monitored daily for weight loss and symptoms of disease. Mice were euthanized when body weight dropped for more than 20% from the weight at the time of infection or when they showed signs of severe illness and neurological disorders (such as hind leg paralysis and lethargy).
To determine the virus titer in the liver, mice were infected intraperitoneally with 105 PFU of Clone 13 and liver tissue was isolated 48 h later. Livers were shock frozen, homogenized by using a sterile mortar, and resuspended in an equal volume (vol/wt) of sterile PBS. Virus titers were determined by plaque assay.
RESULTS
Antiviral effect of IFN and PKR against RVFV.
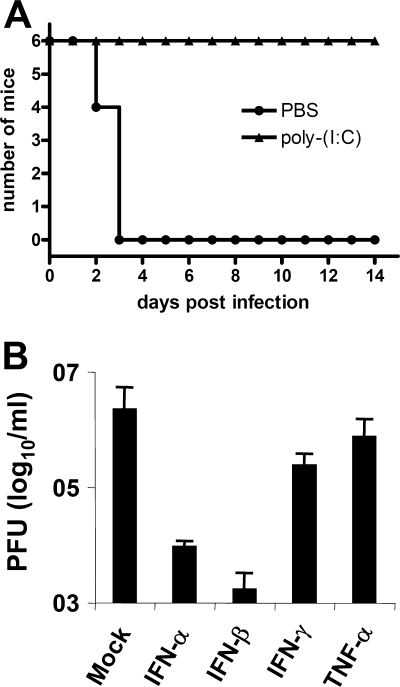
Treatment of mice with the immune-stimulating agent poly(I:C), a synthetic dsRNA, is known to protect from severe virus infections (20). We wanted to confirm this for the highly virulent RVFV strain ZH548, which was isolated during a 1977 epizootic in Egypt (43). Mice were treated overnight with 100 μg of poly(I:C) and then infected with the lethal dose of 103 PFU of ZH548. In line with previous results obtained for other strains (35, 52), C57BL/6 mice pretreated with poly(I:C) survived infection with ZH548, whereas control mice not receiving poly(I:C) died within 3 days (Fig. 1A). dsRNA applied in vivo not only activates the synthesis of type I IFNs but also a range of other cytokines with antiviral and proinflammatory activity (23). To find out which poly(I:C)-induced cytokine could be responsible for the protection in vivo, we pretreated Vero cells with various cytokines and tested whether they affect RVFV replication in vitro (Fig. 1B). The type I IFNs (IFN-α and IFN-β) suppressed replication of RVFV 100- to 1,000-fold, respectively, whereas the type II IFN (IFN-γ) and the proinflammatory cytokine TNF-α each reduced the viral load only 10-fold. This suggests that of the cytokines tested IFN-α/β may largely be responsible for the in vivo protection from RVFV infection by poly(I:C).
FIG. 1.
Effect of poly (I:C) and type I IFNs on RVFV replication. (A) Survival of mice infected with wt RVFV in vivo. C57BL/6 mice (age 6 to 8 weeks, n = 6 per group) were injected intraperitoneally with PBS (control) or 100 μg of poly(I:C) for 12 h and then infected intraperitoneally with 103 PFU wt RVFV (strain ZH548). Uninfected control mice injected with PBS did not display any signs of disease (data not shown). (B) Type I IFNs act as antiviral cytokine against RVFV in vitro. Vero cells were treated with IFN-α (1,000 U/ml), IFN-β (1,000 U/ml), IFN-γ (500 U/ml), or TNF-α (500 ng/ml) and then infected with wt RVFV (MOI = 0.01). Cytokines were replenished for the incubation period of 24 h, after which the amount of virus in the supernatant was determined by plaque assay on IFNAR−/− cells.
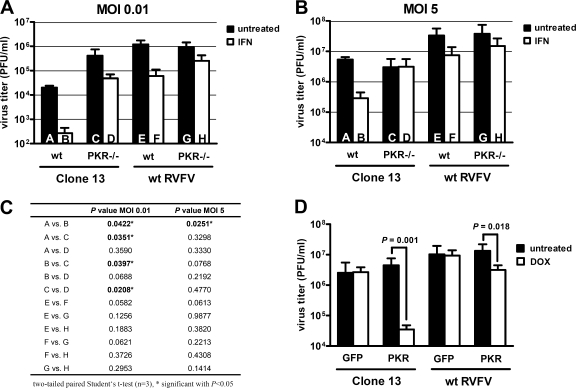
One of the best-understood IFN-regulated antiviral gene products is the double-stranded RNA-dependent protein kinase (PKR). PKR is constitutively expressed at a low level and upregulated by type I IFNs (57). It gains its antiviral activity on binding to viral RNA. Since both type I IFNs and dsRNA displayed a profound activity against RVFV, we tested the effects of IFN and PKR on RVFV growth in vitro using cells isolated from newborn wt and PKR-deficient (PKR−/−) mice. Cells were either left untreated or pretreated with IFN-α for 16 h and infected at an MOI of 0.01, and virus yields were measured by plaque assay after overnight incubation. The NSs-deficient RVFV mutant Clone 13 was used in parallel to wt RVFV. Average virus titers (Fig. 2A) and their pairwise statistical evaluation (Fig. 2C) showed that PKR−/− cells generated significantly higher amounts of Clone 13 than wt cells did (Fig. 2, columns A versus C). wt RVFV, in contrast, did not grow to higher titers in PKR−/− cells than wt cells (Fig. 2, columns E versus G). Moreover, when wt cells were used which had been treated with IFN-α before infection, Clone 13 (Fig. 2, columns A versus B) was more affected than wt RVFV (Fig. 2, columns E versus F). In PKR−/− cells, the IFN effect against both Clone 13 (Fig. 2, columns C versus D) and wt RVFV (columns G versus H) was smaller than in wt cells. We repeated the whole experiment at an increased input MOI of 5 (Fig. 2B and C). In this case, Clone 13 was still IFN sensitive in wt cells but not in PKR−/− cells, whereas for wt RVFV no such apparent difference between cell types was observed.
FIG. 2.
Effect of PKR on RVFV replication in cell culture. (A and B) Virus growth on a kidney cell line derived from C57BL/6 and PKR−/− mice (C57BL/6 background). Approximately 106 cells were seeded per six-well cavity and then treated with 1,000 U of IFN-α/ml or left untreated. After 16 h, the cells were infected with either Clone 13 or wt RVFV at an MOI of 0.01 (A) or an MOI of 5 (B), and IFN-α treatment was continued. To exclude unwanted effects mediated by virus-induced IFN, all untreated cells were supplemented with medium containing saturating amounts of a neutralizing antibody against the murine IFNAR1 receptor. The graph shows the virus titers in the supernatant at 24 h postinfection as determined by plaque assay on IFNAR−/− cells. Columns A to H show means ± the standard deviations (n = 3). (C) Summary of the statistical analysis of the data presented in panels A and B. (D) Virus growth on doxycycline-inducible Flp-In T-Rex cells expressing either GFP or PKR. Cells were treated with doxycycline or left untreated for 36 h, infected at an MOI of 0.01, and evaluated 24 h later as described above. Columns show means ± the standard deviations (n = 3). A two-tailed paired Student t test was performed to compare titers of untreated and doxycycline-treated cells. P values are indicated in the figure where P is <0.05.
To further investigate a possible involvement of PKR in the antiviral response against RVFV, we established a doxycycline-inducible HEK293 cell line (Flp-In T-Rex). Treatment of these cells with doxycycline for 36 h induced PKR expression, whereas untreated cells did not produce detectable amounts of PKR (data not shown). Cells which inducibly express green fluorescent protein (GFP) were used as controls. Figure 2D shows that doxycycline treatment of the control cells had no apparent effect on either virus strain. In the PKR cells, however, induction of transgene expression reduced titers of Clone 13 more than 100-fold, but titers of wt RVFV less than 10-fold.
Collectively, these data suggest that PKR restricts the replication of RVFV and that the mutant virus Clone 13 is more susceptible to PKR than is the NSs-expressing wt RVFV.
PKR blocks Clone 13 replication in vivo.
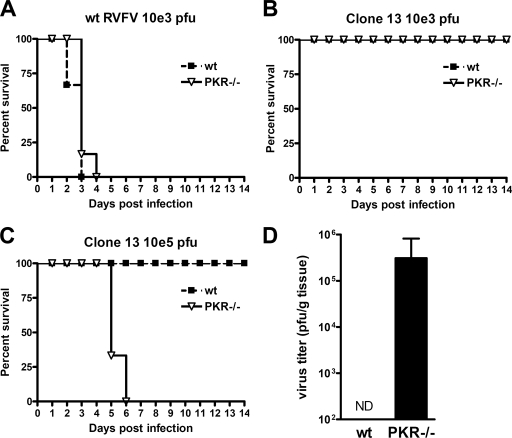
Next, we wanted to investigate the in vivo contribution of PKR to RVFV replication. To this aim, we intraperitoneally infected wt and PKR−/− mice with wt RVFV and Clone 13. All mice infected with 103 PFU of wt RVFV died within 4 days irrespective of whether PKR was present or not (Fig. 3A). This correlates with the in vitro data suggesting that the presence of PKR only mildly affects wt RVFV replication. In contrast, Clone 13 did not invoke any obvious disease in both mouse strains when administered at 103 PFU per mouse (Fig. 3B). However, when mice were infected with a higher dose of Clone 13 (105 PFU per mouse) a striking difference between the wt and PKR−/− mice was unraveled: whereas wt mice did not show any signs of disease until the end of the experiment at day 14, all PKR−/− mice have succumbed to virus infection by day 6 after infection (Fig. 3C). To follow up on these observations, we infected mice with 105 PFU of Clone 13 and determined the virus titers in the liver, a preferred target organ of RVFV, at 48 h after infection. No Clone 13 could be detected in liver homogenates of wt mice, whereas the livers of PKR−/− mice contained more than 105 PFU per g of liver tissue (Fig. 3D). This finding indicates a strong replication of Clone 13 in PKR −/− mice and correlates with the survival data and the cell culture data (see Fig. 2). Thus, the NSs deficiency of RVFV can be complemented by higher infection doses and a PKR deficiency of the host. We therefore concluded that PKR is an important factor restricting RVFV growth both in vitro and in vivo. The effect of PKR, however, becomes only evident in mice infected by a strain with NSs deleted.
FIG. 3.
Effect of PKR on RVFV in vivo. (A to C) Survival of wt and PKR−/− mice (n = 6 per group) infected intraperitoneally either with 103 PFU of wt RVFV (A) or Clone 13 (B) or with 105 PFU of Clone 13 (C). (D) Clone 13 titers in the liver at 48 h after infection of wt or PKR−/− mice (n = 3 per group). Livers from infected mice were homogenized, and the virus titers were determined by plaque assay on IFNAR−/− MEFs. ND, not detectable.
RVFV induces the loss of PKR in an NSs-dependent manner.
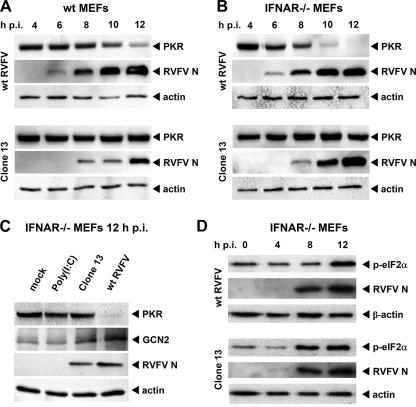
NSs appeared to influence the sensitivity of RVFV to PKR. We therefore evaluated the state of PKR in RVFV-infected cells. MEFs derived from C57BL/6 mice were infected with wt RVFV and Clone 13 and subjected to Western blot analysis with an anti-PKR monoclonal antibody. To our surprise, MEFs infected with wt RVFV showed a decrease in PKR levels within the first 12 h after infection (Fig. 4A). At later time points, PKR disappeared entirely in wt RVFV-infected cells, whereas Clone 13 infection even increased PKR levels (data not shown). Being a strong inducer of IFN-α/β, the unchanged levels of PKR in Clone 13-infected MEFs at 12 h p.i. could simply reflect a balance between IFN-stimulated de novo synthesis and virally induced loss of PKR. Further, Clone 13 replicated in wt MEFs to lower levels than wt RVFV (see Fig. 4A, RVFV N). We therefore infected MEFs which are unresponsive to type I IFN due to a lack of the type I IFN receptor (IFNAR−/− MEFs). These cells allowed a better replication of Clone 13 (Fig. 4B, RVFV N). However, these cells displayed an even more pronounced decrease in PKR expression after infection with wt RVFV, whereas the cellular amount of PKR remained unchanged when infected with Clone 13 (Fig. 4B). As additional controls, we show that treatment of IFNAR−/− MEFs with poly(I:C) had no detrimental effect on PKR and that the expression of GCN2, another antiviral eIF-2α kinase, was not affected by NSs but even increased to some extent after RVFV infection (Fig. 4C). Similarly, RVFV NSs expression had no impact on the levels of actin (Fig. 4A, B, and C) or RIG-I (data not shown). Thus, apparently, the NSs-expressing wt RVFV causes a specific disappearance of PKR independent of IFN or a particular cell type.
FIG. 4.
Influence of RVFV NSs on PKR levels and phosphorylation of eIF-2α. (A to C) Presence of PKR protein in wt RVFV- and Clone 13-infected cells. MEFs from wt mice (A) or IFNAR−/− mice (B) were seeded at a density of 5 × 105 cells and infected at an MOI of 5 with wt RVFV or Clone 13. At the indicated time points postinfection cells were lysed and subjected to Western blot analysis for PKR, RVFV N protein, and actin. (C) IFNAR−/− MEFs (5 × 105 cells per six-well cavity) were left untreated (mock), transfected with 5 μg of poly(I:C), or infected with the indicated viruses at an MOI of 5. After 12 h of incubation, cell lysates were prepared and subjected to Western blot analysis as indicated. (D) IFNAR−/− MEFs were infected with wt RVFV and Clone 13 for the indicated time points, and the abundance of phosphorylated eIF-2α was tested by Western blot analysis.
We hypothesized that the changes in PKR expression should result in altered downstream signaling. Indeed, phosphorylation of the PKR substrate eIF-2α was delayed in wt RVFV-infected IFNAR−/− MEFs compared to Clone 13-infected cells (Fig. 4D), suggesting that the loss of PKR has a functional consequence for the cell. However, eIF-2α phosphorylation was detected at 12 h after infection, indicating that additional eIF-2α kinases such as GCN2 or PERK (71) were activated during infection with wt RVFV.
Taken together, our data led us to conclude that NSs expression by wt RVFV results in a loss of PKR with the consequence of a delayed onset of eIF-2α phosphorylation.
NSs induces the degradation PKR.
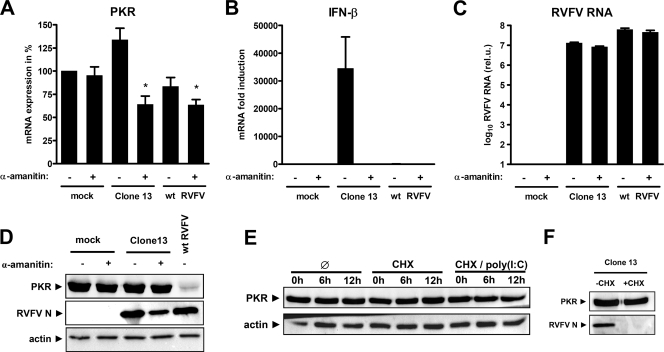
The NSs protein of RVFV is a strong inhibitor of transcription by the cellular RNA polymerase II (40). Thus, the disappearance of PKR could be due to reduced synthesis of PKR mRNA during wt RVFV infection. To investigate this, we compared the PKR mRNA levels of infected cells to those of cells treated with the RNA polymerase II inhibitor α-amanitin, using real-time RT-PCR. As shown in Fig. 5A, a 12-h treatment with α-amanitin alone had no apparent impact on the abundance of PKR mRNAs. Infection with Clone 13 slightly elevated PKR mRNA levels, whereas the combination of Clone 13 and α-amanitin reduced them by ca. 40%. This combination was supposed to mimic the effect of wt RVFV expressing an NSs, which blocks RNA polymerase II. In confirmation of this, the controls show that Clone 13-induced IFN-β mRNA expression was completely suppressed by α-amanitin (Fig. 5B) and that α-amanitin had no measurable effect on RVFV RNA synthesis (Fig. 5C). Infection of cells with wt RVFV also reduced PKR mRNA levels, but to a lesser extent (ca. 20%) than the combination of Clone 13 and α-amanitin (Fig. 5A). Nonetheless, PKR protein levels were only decreased by wt RVFV, but neither by α-amanitin alone nor by the combination of α-amanitin and Clone 13 (Fig. 5D). Thus, in wt RVFV-infected cells PKR protein is absent, but sufficient amounts of its mRNA remain present during the full course of the experiment. This indicates that NSs reduces the protein levels of PKR and that this effect is unrelated to the well-characterized inhibitory effect of NSs on RNA polymerase II.
FIG. 5.
PKR mRNA and protein levels in infected cells. (A to C) Quantification of mRNA levels after virus infection or inhibition of cellular transcription. IFNAR−/− MEFs were seeded at a density of 5 × 105 cells per six-well cavity and left uninfected (mock) or infected at an MOI of 5 with Clone 13 or wt RVFV. Cells were then incubated for 12 h either in the presence or absence of 50 μg of α-amanitin/ml, as indicated. mRNAs of PKR (A), IFN-β (B), and RNA of RVFV (C) were measured by real-time RT-PCR. Cycle threshold values were normalized to mock-infected cells. Each column and vertical bar represents the means ± the standard errors of the mean of three replicate experiments. Statistical analysis for PKR mRNA expression was done by using one-way analysis of variance, followed by Dunnett's post hoc comparison to untreated cells (mock). *, P < 0.05. (D) Protein levels in infected cells after inhibition of cellular transcription. IFNAR−/− MEFs were left uninfected or infected (MOI = 5, 12 h) with Clone 13 or wt RVFV in the presence or absence of 50 μg of α-amanitin/ml. The results of Western blot analysis for PKR, RVFV N, and actin at 12 h after infection are shown. (E) PKR levels in IFNAR−/− MEFs after poly(I:C) treatment and inhibition of cellular translation. The results of Western blot analysis for PKR and actin at 0, 6, and 12 h after treatment with 50 μg of cycloheximide (CHX)/ml and transfection of 5 μg of poly(I:C) are shown. (F) Cycloheximide efficiency control. IFNAR−/− MEFs were infected with Clone 13 (MOI = 5, 12 h) in the presence or absence of 50 μg of cycloheximide/ml.
Virus infection and poly(I:C) treatment are known to shorten the half-life of IFN-induced PKR (32). Our control experiments using the NSs-deleted mutant Clone 13, however, argue against a nonspecific decrease triggered by infection (see Fig. 4A, B, C and Fig. 5D). Moreover, when we transfected cells with poly(I:C) and cut off the supply of newly translated PKR by treatment with the translational inhibitor cycloheximide, the PKR levels were not detectably reduced for 12 h (Fig. 5E). To control for cycloheximide efficiency, we show that Clone 13 multiplication is inhibited (Fig. 5F), since bunyaviruses need ongoing translation for their transcription (50, 66). Taken together, these data led us to conclude that protein degradation, and not mRNA depletion or a block in translation (i.e., viral host cell shutoff), is the cause for reduced PKR levels in wt RVFV-infected cells.
Involvement of the proteasome.
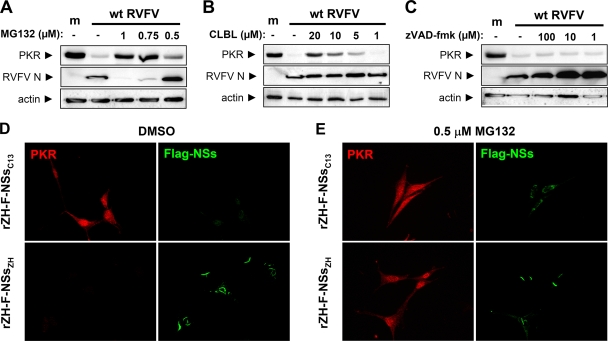
The proteasome is often hijacked by viruses to manipulate the abundance of host proteins (5, 16). To test whether this degradation system could be involved, we treated cells with specific inhibitors and monitored the effect of NSs on PKR. Inhibition of the proteasomal system by MG132 had a negative effect on RVFV growth, similar to what was observed for other RNA viruses (29, 49, 67). Nonetheless, dose-response experiments using different concentrations show that MG132 partly restored PKR levels in RVFV-infected cells (Fig. 6A). MG132 inhibits lysosomal cysteine proteases besides the proteasome (39), possibly explaining its inhibitory effect on RVFV growth. Indeed, when we used the more specific proteasomal inhibitor CLBL, RVFV was much less affected, while PKR levels were restored to almost control levels (Fig. 6B). A broad-spectrum caspase inhibitor, in contrast, had no such effect, although it even increased levels of RVFV N (Fig. 6C). To follow up on this, we used relatively low levels (0.5 μM) of MG132 and investigated the interplay of NSs and PKR on the single-cell level. To demonstrate NSs expression, we generated recombinant ZH548 viruses expressing the NSs genes of Clone 13 (rZH-F-NSsC13) or ZH548 (rZH-F-NSsZH) endowed with a C-terminal Flag epitope tag. The immunofluorescence analysis shown in Fig. 6D confirms that the C-terminal Flag tag does not affect the functionality of wt NSs, as PKR is not detectable in rZH-F-NSsZH-infected cells (lower panel). Moreover, Flag-NSs is also able to suppress IFN induction (data not shown) and forms the same characteristic nuclear filaments as untagged wt NSs does (41). The truncated NSs of Clone 13, in contrast, was only present at very low levels and located in the cytoplasm. When cells were kept under MG132 treatment, levels of Clone 13 NSs were stabilized (Fig. 6E), as shown before (65). Importantly, MG132 treatment allowed detection of PKR in cells infected with rZH-F-NSsZH. Similar results were obtained under CLBL treatment (data not shown). Since the immunofluorescence signals for wt NSs and PKR in nontreated cells are mutually exclusive, the most likely explanation for these observations is that NSs induces the degradation of PKR via the proteasome.
FIG. 6.
Involvement of the proteasomal pathway. (A to C) Western blot analysis. IFNAR−/− MEFs were seeded in six-well dishes and left uninfected (m) or infected with wt RVFV at an MOI of 5. After 1 h of infection, fresh medium with the indicated concentrations of MG132 (A), CLBL (B), or the pan-caspase inhibitor zVAD-fmk (C) was added directly onto the cells. As a control, the medium in untreated cells was supplemented with equivalent amounts of dimethyl sulfoxide (DMSO). After 12 h of incubation, cell lysates were prepared and subjected to Western blot analysis as described for Fig. 4. (D and E) Immunofluorescence analysis. IFNAR−/− MEFs grown on coverslips were infected (MOI of 5) with recombinant RVF viruses expressing the NSs proteins of either Clone 13 or the wt strain ZH548, each fused to a C-terminal Flag tag. During the incubation period of 16 h, cells were either treated with DMSO (D) or with 0.5 μM MG132 dissolved in DMSO (E). Then, cells were fixed and analyzed by indirect immunofluorescence with antibodies directed against PKR (red) or the Flag portion of the fusion proteins (green).
Degradation of PKR is a specific property of RVFV NSs.
The virus family Bunyaviridae contains four different genera which infect mammals, and two of these genera (Orthobunyavirus and Phlebovirus) consistently encode an NSs protein. However, the NSs proteins of these two genera differ tremendously in size, expression strategy, and sequence (21). Nonetheless, all bunyavirus NSs proteins characterized thus far are able to block transcriptional induction of IFN (7, 11, 34, 51, 64). We wanted to know whether NSs proteins from other members of the Bunyaviridae family would degrade PKR similarly to the effect seen for RVFV NSs. To address this, we generated recombinant ZH548 viruses with the NSs gene replaced by the NSs genes of the less-pathogenic SFSV or LACV. SFSV belongs to the genus Phlebovirus and encodes an NSs protein of approximately the same length and with a sequence identity of ca. 28% to RVFV NSs (determined by CLUSTAL W, data not shown). In contrast, LACV belongs to the genus Orthobunyavirus and encodes an NSs protein that is much shorter and has no sequence similarity to RVFV NSs. For an initial phenotypic characterization, Vero cells were infected with the RVFV recombinants expressing RVFV NSs (rZH548), SFSV NSs (rZHΔNSs::NSsSFSV), or LACV NSs (rZHΔNSs::NSsLACV) or with an RVFV recombinant virus with NSs deleted (rZHΔNSs). All recombinant viruses expressing bunyaviral NSs proteins produced clear plaques, whereas the mutant with NSs deleted had a turbid-plaque phenotype (Fig. 7A), indicating that the NSs of RVFV can be functionally replaced by homologous, as well as heterologous NSs proteins from bunyaviruses. Furthermore, when IFN-competent 293T cells were infected, none of the NSs-expressing recombinants induced a significant expression of the IFN-β gene, whereas both the natural (Clone 13) and the artificial (rZHΔNSs) NSs deletion mutants strongly activated IFN induction (Fig. 7B). These results confirm previous reports on the anti-IFN function of RVFV NSs and LACV NSs and demonstrate that SFSV NSs is similarly able to suppress IFN induction. Strikingly, when cellular protein levels were investigated in Vero cells (Fig. 7C), only the recombinant virus expressing the RVFV NSs gene caused the disappearance of PKR. Cells infected with viruses expressing heterologous NSs proteins showed PKR levels that were similar to those of mock-, Clone 13-, or rZHΔNSs-infected cells. Since all NSs proteins were active in suppressing IFN induction, we concluded that RVFV is unique among bunyaviruses by expressing an NSs with the ability to reduce PKR levels.
FIG. 7.
Phenotype of RVFV recombinants expressing homologous and heterologous NSs proteins. (A) Comparison of plaques produced by recombinant RVFV (strain ZH548) expressing the NSs proteins of wt RVFV (rZH548), Sandfly fever Sicilian virus (rZHΔ::NSsSFSV), or La Crosse virus (rZHΔ::NSsLACV) or not expressing an NSs protein (rZHΔNSs). (B) RT-PCR analysis of the indicated genes in 293T cells infected with recombinant viruses and Clone 13 for 16 h. (C) Western blot detection of PKR, RVFV N, and actin in IFNAR−/− cells 16 h after infection.
DISCUSSION
RVFV is of increasing concern because of its enormous potential to cause epidemics among livestock and humans. RVFV strongly suppresses induction of the IFN response by means of its NSs protein (7, 13). The natural isolate Clone 13 expresses a nonfunctional truncated NSs gene and is therefore considered as being highly attenuated and a promising candidate for a live vaccine. It has previously been shown that Clone 13 is lethal to mice that lack type I IFN signaling (13). Our data here demonstrate that part of the IFN-mediated resistance of wt mice to Clone 13 is mediated by the antiviral action of PKR. However, IFN-deficient IFNAR−/− mice are killed by Clone 13 within 36 h (13), whereas for PKR−/− mice it takes several days. This indicates that the high levels of IFN induced by Clone 13 can delay infection independent of PKR and that there must be other IFN-stimulated genes that contribute to the antiviral effect.
Negative-strand RNA viruses, including bunyaviruses, do not produce significant amounts of the PKR ligand dsRNA during the course of infection (70). Nonetheless, many of them are sensitive to PKR (24, 58). A recent report showing that PKR can be activated by ssRNAs containing a short stem-loop structure and a 5′ triphosphate group (48) may explain this discrepancy, since the genomes of most negative-strand RNA viruses have a structured and triphosphorylated 5′ end (26, 31, 54, 55). However, we have recently found that some of those viruses remove the 5′ triphosphate group as a strategy to avoid IFN induction by the RNA helicase RIG-I (26). Among the Bunyaviridae, members of the genera Hantavirus and Nairovirus cleave the triphosphate group off their genome 5′ end, whereas RVFV (genus Phlebovirus) and LACV (genus Orthobunyavirus) still have it. Interestingly, BUNV (genus Orthobunyavirus) was shown to be sensitive to PKR in a manner similar to RVFV (62), whereas the related Dugbe virus (genus Nairovirus) was resistant (14). Thus, among the members of the large family of Bunyaviridae, two unusual ways of PKR inhibition appear to be realized. RVFV induces degradation of PKR, and hantaviruses (e.g., Hantaan virus) and nairoviruses (e.g., Crimean-Congo hemorrhagic fever virus) remove the potentially PKR-activating triphosphate group from their genome 5′ end (26).
Being a highly efficient IFN effector protein, PKR is targeted by viruses in many ways. Well-known strategies are expression of dsRNA-binding proteins or small RNAs which compete with PKR for its ligand, direct binding of a viral protein to PKR, expression of a decoy substrate, or dephosphorylation of eIF-2α (reviewed in references 24, 38, 56, and 69). To our knowledge, poliovirus is the only other example which triggers degradation of PKR in a manner similar to RVFV (10), but neither the responsible viral factor nor the importance for virus replication in vivo have been clarified (60). Destruction of PKR in infected cells removes not only a potent translational inhibitor but also a factor that is involved in a wide variety of antiviral signaling pathways (24, 57).
All bunyaviral NSs proteins investigated thus far were shown to block the transcriptional induction of IFN genes (7, 11, 34, 51, 64). In line with this, we demonstrate that the hitherto-uncharacterized NSs of SFSV is no exception since it also blocks IFN induction. Moreover, both SFSV NSs and LACV NSs can replace RVFV NSs as an IFN induction antagonist, despite a complete lack of sequence similarity for the latter. This strongly suggests that inhibition of IFN induction is a primary and conserved function of bunyavirus NSs proteins. RVFV NSs, however, is unique among the bunyavirus NSs proteins since it has gained the additional ability to degrade PKR. The multifunctionality of its NSs protein may in part be responsible for the exceptionally high virulence of RVFV.
Our data suggest that PKR degradation by RVFV NSs occurs in a specific manner and not by a nonspecific host cell shutoff. Neither an RNA polymerase II inhibitor, nor a translational inhibitor, nor the combination with the PKR activator poly(I:C) had an apparent effect on PKR levels in our experimental system. Also, many negative-strand RNA viruses such as BUNV, influenza A virus, or vesicular stomatitis virus impose a strong host cell shutoff (15, 17, 75) without altering the level of PKR (3, 6, 44, 62). Moreover, our inhibitor experiments strongly suggest an involvement of the proteasome. We therefore postulate that RVFV NSs actively and specifically induces the degradation of PKR via the proteasomal system. Experiments to further characterize the degradation mechanism are currently under way.
RVFV NSs is notoriously difficult to study as it forms large filamentous aggregates and blocks RNA polymerase II, thus limiting its expression from cDNA plasmids in an autocrine manner. Recombinant RVFV containing genes for NSs variants and mutants may be the preferred means for a systematic investigation of this powerful virulence factor. A further advantage in comparison to plasmid-based overexpression studies is that NSs levels are physiological, and the function is studied in the viral context. Using this experimental approach, sequence comparisons with related NSs genes, domain swapping, and mutational analysis may allow to identify the residues relevant for PKR degradation and cellular interaction partners. It is expected that RVFV NSs either activates the cellular E3 ubiquitin ligase targeting PKR or is assembling a novel E3 complex itself. Since the turnover mechanism of PKR in IFN-treated cells is thus far unknown (32), these studies may have implications beyond RVFV-host cell interactions.
Promoter polymorphisms of the PKR gene have been described (18, 36, 37). Moreover, direct loss of PKR function (30, 42) or resistance of eIF-2α to PKR-mediated phosphorylation (1, 2) are common occurrences in tumorigenic cells. Therefore, the partial dependence on PKR to control Clone 13 suggests caution in using this RVFV mutant as a live vaccine and implies the introduction of additional debilitating mutations into the viral genome in order to safely attenuate the virus in animals and humans with a perturbed innate immune system.
Acknowledgments
We thank Otto Haller for support and helpful comments; Georg Kochs, Martin Schwemmle, and Peter Staeheli for critically reading the manuscript; and Tilo Materna for providing important reagents.
Work in the authors' laboratories is supported by the grants We 2616/5-1 from the Deutsche Forschungsgemeinschaft (F.W.), by an EMBO long-term fellowship (ATLF 463-2008) (A.P.), and by the Pinguin Stiftung (A.P. and F.W.).
Footnotes
Published ahead of print on 11 February 2009.
REFERENCES
- 1.Balachandran, S., and G. N. Barber. 2004. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 551-65. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran, S., and G. N. Barber. 2007. PKR in innate immunity, cancer, and viral oncolysis. Methods Mol. Biol. 383277-301. [DOI] [PubMed] [Google Scholar]
- 3.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13129-141. [DOI] [PubMed] [Google Scholar]
- 4.Balkhy, H. H., and Z. A. Memish. 2003. Rift Valley fever: an uninvited zoonosis in the Arabian peninsula. Int. J. Antimicrob. Agents 21153-157. [DOI] [PubMed] [Google Scholar]
- 5.Barry, M., and K. Fruh. 2006. Viral modulators of cullin RING ubiquitin ligases: culling the host defense. Sci. STKE 2006pe21. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann, M., A. Garcia-Sastre, E. Carnero, H. Pehamberger, K. Wolff, P. Palese, and T. Muster. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 746203-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billecocq, A., M. Spiegel, P. Vialat, A. Kohl, F. Weber, M. Bouloy, and O. Haller. 2004. NSs protein of Rift Valley Fever Virus blocks interferon production by inhibiting host gene transcription. J. Virol. 789798-9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird, B. H., C. G. Albarino, and S. T. Nichol. 2007. Rift Valley fever virus lacking NSm proteins retains high virulence in vivo and may provide a model of human delayed onset neurologic disease. Virology 36210-15. [DOI] [PubMed] [Google Scholar]
- 9.Bird, B. H., D. A. Bawiec, T. G. Ksiazek, T. R. Shoemaker, and S. T. Nichol. 2007. Highly sensitive and broadly reactive quantitative reverse transcription-PCR assay for high-throughput detection of Rift Valley fever virus. J. Clin. Microbiol. 453506-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black, T. L., B. Safer, A. Hovanessian, and M. G. Katze. 1989. The cellular 68,000-Mr protein kinase is highly autophosphorylated and activated yet significantly degraded during poliovirus infection: implications for translational regulation. J. Virol. 632244-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blakqori, G., S. Delhaye, M. Habjan, C. D. Blair, I. Sanchez-Vargas, K. E. Olson, G. Attarzadeh-Yazdi, R. Fragkoudis, A. Kohl, U. Kalinke, S. Weiss, T. Michiels, P. Staeheli, and F. Weber. 2007. La Crosse bunyavirus nonstructural protein NSs serves to suppress the type I interferon system of mammalian hosts. J. Virol. 814991-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borio, L., T. Inglesby, C. J. Peters, A. L. Schmaljohn, J. M. Hughes, P. B. Jahrling, T. Ksiazek, K. M. Johnson, A. Meyerhoff, T. O'Toole, M. S. Ascher, J. Bartlett, J. G. Breman, E. M. Eitzen, Jr., M. Hamburg, J. Hauer, D. A. Henderson, R. T. Johnson, G. Kwik, M. Layton, S. Lillibridge, G. J. Nabel, M. T. Osterholm, T. M. Perl, P. Russell, and K. Tonat. 2002. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA 2872391-2405. [DOI] [PubMed] [Google Scholar]
- 13.Bouloy, M., C. Janzen, P. Vialat, H. Khun, J. Pavlovic, M. Huerre, and O. Haller. 2001. Genetic evidence for an interferon-antagonistic function of rift valley fever virus nonstructural protein NSs. J. Virol. 751371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd, A., J. K. Fazakerley, and A. Bridgen. 2006. Pathogenesis of Dugbe virus infection in wild-type and interferon-deficient mice. J. Gen. Virol. 872005-2009. [DOI] [PubMed] [Google Scholar]
- 15.Bridgen, A., F. Weber, J. K. Fazakerley, and R. M. Elliott. 2001. Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis. Proc. Natl. Acad. Sci. USA 98664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, M., and D. Gerlier. 2006. Viral hijacking of cellular ubiquitination pathways as an anti-innate immunity strategy. Viral Immunol. 19349-362. [DOI] [PubMed] [Google Scholar]
- 17.Connor, J. H., and D. S. Lyles. 2005. Inhibition of host and viral translation during vesicular stomatitis virus infection: eIF2 is responsible for the inhibition of viral but not host translation. J. Biol. Chem. 28013512-13519. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham, S., C. Graham, M. Hutchinson, A. Droogan, K. O'Rourke, C. Patterson, G. McDonnell, S. Hawkins, and K. Vandenbroeck. 2005. Pharmacogenomics of responsiveness to interferon IFN-beta treatment in multiple sclerosis: a genetic screen of 100 type I interferon-inducible genes. Clin. Pharmacol. Ther. 78635-646. [DOI] [PubMed] [Google Scholar]
- 19.Daubney, R., J. R. Hudson, and P. C. Gamham. 1931. Enzootic hepatitis of Rift Valley fever: an undescribed virus disease of sheep, cattle and man from East Africa. J. Pathol. Bacteriol. 34545-549. [Google Scholar]
- 20.De Clercq, E. 2006. Interferon and its inducers—a never-ending story: “old” and “new” data in a new perspective. J. Infect. Dis. 194(Suppl. 1)S19-S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott, R. M. 1997. Emerging viruses: the Bunyaviridae. Mol. Med. 3572-577. [PMC free article] [PubMed] [Google Scholar]
- 22.Frese, M., G. Kochs, H. Feldmann, C. Hertkorn, and O. Haller. 1996. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein. J. Virol. 70915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gantier, M. P., and B. R. Williams. 2007. The response of mammalian cells to double-stranded RNA. Cytokine Growth Factor Rev. 18363-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia, M. A., E. F. Meurs, and M. Esteban. 2007. The dsRNA protein kinase PKR: virus and cell control. Biochimie 89799-811. [DOI] [PubMed] [Google Scholar]
- 25.Gerrard, S. R., B. H. Bird, C. G. Albarino, and S. T. Nichol. 2007. The NSm proteins of Rift Valley fever virus are dispensable for maturation, replication and infection. Virology 359459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Glatter, T., A. Wepf, R. Aebersold, and M. Gstaiger. 20 January 2009, posting date. An integrated workflow for charting the human interaction proteome: insights into the PP2A system. Mol. Syst. Biol. 5237. doi: 10.1038/msb.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habjan, M., I. Andersson, J. Klingstrom, M. Schumann, A. Martin, P. Zimmermann, V. Wagner, A. Pichlmair, U. Schneider, E. Muhlberger, A. Mirazimi, and F. Weber. 2008. Processing of genome 5′ termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PLoS ONE 3e2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habjan, M., N. Penski, M. Spiegel, and F. Weber. 2008. T7 RNA polymerase-dependent and -independent systems for cDNA-based rescue of Rift Valley fever virus. J. Gen. Virol. 892157-2166. [DOI] [PubMed] [Google Scholar]
- 28.Haller, O., G. Kochs, and F. Weber. 2007. Interferon, Mx, and viral countermeasures. Cytokine Growth Factor Rev. 18425-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harty, R. N., M. E. Brown, J. P. McGettigan, G. Wang, H. R. Jayakar, J. M. Huibregtse, M. A. Whitt, and M. J. Schnell. 2001. Rhabdoviruses and the cellular ubiquitin-proteasome system: a budding interaction. J. Virol. 7510623-10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hii, S. I., L. Hardy, T. Crough, E. J. Payne, K. Grimmett, D. Gill, and N. A. McMillan. 2004. Loss of PKR activity in chronic lymphocytic leukemia. Int. J. Cancer 109329-335. [DOI] [PubMed] [Google Scholar]
- 31.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314994-997. [DOI] [PubMed] [Google Scholar]
- 32.Hovanessian, A. G., J. Galabru, E. Meurs, C. Buffet-Janvresse, J. Svab, and N. Robert. 1987. Rapid decrease in the levels of the double-stranded RNA-dependent protein kinase during virus infections. Virology 159126-136. [DOI] [PubMed] [Google Scholar]
- 33.Ikegami, T., S. Won, C. J. Peters, and S. Makino. 2006. Rescue of infectious rift valley fever virus entirely from cDNA, analysis of virus lacking the NSs gene, and expression of a foreign gene. J. Virol. 802933-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaaskelainen, K. M., P. Kaukinen, E. S. Minskaya, A. Plyusnina, O. Vapalahti, R. M. Elliott, F. Weber, A. Vaheri, and A. Plyusnin. 2007. Tula and Puumala hantavirus NSs ORFs are functional and the products inhibit activation of the interferon-beta promoter. J. Med. Virol. 791527-1536. [DOI] [PubMed] [Google Scholar]
- 35.Kende, M. 1985. Prophylactic and therapeutic efficacy of poly(I,C)-LC against Rift Valley fever virus infection in mice. J. Biol. Response Mod. 4503-511. [PubMed] [Google Scholar]
- 36.King, J. K., S. H. Yeh, M. W. Lin, C. J. Liu, M. Y. Lai, J. H. Kao, D. S. Chen, and P. J. Chen. 2002. Genetic polymorphisms in interferon pathway and response to interferon treatment in hepatitis B patients: a pilot study. Hepatology 361416-1424. [DOI] [PubMed] [Google Scholar]
- 37.Knapp, S., L. J. Yee, A. J. Frodsham, B. J. Hennig, S. Hellier, L. Zhang, M. Wright, M. Chiaramonte, M. Graves, H. C. Thomas, A. V. Hill, and M. R. Thursz. 2003. Polymorphisms in interferon-induced genes and the outcome of hepatitis C virus infection: roles of MxA, OAS-1, and PKR. Genes Immun. 4411-419. [DOI] [PubMed] [Google Scholar]
- 38.Langland, J. O., J. M. Cameron, M. C. Heck, J. K. Jancovich, and B. L. Jacobs. 2006. Inhibition of PKR by RNA and DNA viruses. Virus Res. 119100-110. [DOI] [PubMed] [Google Scholar]
- 39.Lee, D. H., and A. L. Goldberg. 1998. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 8397-403. [DOI] [PubMed] [Google Scholar]
- 40.Le May, N., S. Dubaele, L. P. De Santis, A. Billecocq, M. Bouloy, and J. M. Egly. 2004. TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell 116541-550. [DOI] [PubMed] [Google Scholar]
- 41.Le May, N., Z. Mansuroglu, P. Leger, T. Josse, G. Blot, A. Billecocq, R. Flick, Y. Jacob, E. Bonnefoy, and M. Bouloy. 2008. A SAP30 complex inhibits IFN-beta expression in Rift Valley fever virus-infected cells. PLoS Pathog. 4e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li, S., and A. E. Koromilas. 2001. Dominant negative function by an alternatively spliced form of the interferon-inducible protein kinase PKR. J. Biol. Chem. 27613881-13890. [DOI] [PubMed] [Google Scholar]
- 43.Meegan, J. M., H. Hoogstraal, and M. I. Moussa. 1979. An epizootic of Rift Valley fever in Egypt in 1977. Vet. Rec. 105124-125. [DOI] [PubMed] [Google Scholar]
- 44.Min, J. Y., S. Li, G. C. Sen, and R. M. Krug. 2007. A site on the influenza A virus NS1 protein mediates both inhibition of PKR activation and temporal regulation of viral RNA synthesis. Virology 363236-243. [DOI] [PubMed] [Google Scholar]
- 45.Morrill, J. C., G. B. Jennings, T. M. Cosgriff, P. H. Gibbs, and C. J. Peters. 1989. Prevention of Rift Valley fever in rhesus monkeys with interferon-alpha. Rev. Infect. Dis. 11(Suppl. 4)S815-S825. [DOI] [PubMed] [Google Scholar]
- 46.Morrill, J. C., G. B. Jennings, A. J. Johnson, T. M. Cosgriff, P. H. Gibbs, and C. J. Peters. 1990. Pathogenesis of Rift Valley fever in rhesus monkeys: role of interferon response. Arch. Virol. 110195-212. [DOI] [PubMed] [Google Scholar]
- 47.Morrill, J. C., and D. J. McClain. 1996. Epidemiology and pathogenesis of Rift Valley fever and other phleboviruses, p. 281-293. In R. M. Elliott (ed.), The Bunyaviridae. Plenum Press, Inc., New York.
- 48.Nallagatla, S. R., J. Hwang, R. Toroney, X. Zheng, C. E. Cameron, and P. C. Bevilacqua. 2007. 5′-triphosphate-dependent activation of PKR by RNAs with short stem-loops. Science 3181455-1458. [DOI] [PubMed] [Google Scholar]
- 49.Neznanov, N., E. M. Dragunsky, K. M. Chumakov, L. Neznanova, R. C. Wek, A. V. Gudkov, and A. K. Banerjee. 2008. Different effect of proteasome inhibition on vesicular stomatitis virus and poliovirus replication. PLoS ONE 3e1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patterson, J. L., and D. Kolakofsky. 1984. Characterization of La Crosse virus small-genome transcripts. J. Virol. 49680-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perrone, L. A., K. Narayanan, M. Worthy, and C. J. Peters. 2007. The S segment of Punta Toro virus (Bunyaviridae, Phlebovirus) is a major determinant of lethality in the Syrian hamster and codes for a type I interferon antagonist. J. Virol. 81884-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters, C. J., J. A. Reynolds, T. W. Slone, D. E. Jones, and E. L. Stephen. 1986. Prophylaxis of Rift Valley fever with antiviral drugs, immune serum, an interferon inducer, and a macrophage activator. Antivir. Res. 6285-297. [DOI] [PubMed] [Google Scholar]
- 53.Pichlmair, A., and C. Reis e Sousa. 2007. Innate recognition of viruses. Immunity 27370-383. [DOI] [PubMed] [Google Scholar]
- 54.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314997-1001. [DOI] [PubMed] [Google Scholar]
- 55.Plumet, S., F. Herschke, J. M. Bourhis, H. Valentin, S. Longhi, and D. Gerlier. 2007. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS ONE. 2e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 891-47. [DOI] [PubMed] [Google Scholar]
- 57.Sadler, A. J., and B. R. Williams. 2008. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8559-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sandrock, M., M. Frese, O. Haller, and G. Kochs. 2001. Interferon-induced rat Mx proteins confer resistance to Rift Valley fever virus and other arthropod-borne viruses. J. Interferon Cytokine Res. 21663-668. [DOI] [PubMed] [Google Scholar]
- 60.Scheuner, D., M. Gromeier, M. V. Davies, A. J. Dorner, B. Song, R. V. Patel, E. J. Wimmer, R. E. McLendon, and R. J. Kaufman. 2003. The double-stranded RNA-activated protein kinase mediates viral-induced encephalitis. Virology 317263-274. [DOI] [PubMed] [Google Scholar]
- 61.Silverman, R. H. 2007. Viral encounters with 2′,5′-oligoadenylate synthetase and RNase L during the interferon antiviral response. J. Virol. 8112720-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Streitenfeld, H., A. Boyd, J. K. Fazakerley, A. Bridgen, R. M. Elliott, and F. Weber. 2003. Activation of PKR by Bunyamwera virus is independent of the viral interferon antagonist NSs. J. Virol. 775507-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Struthers, J. K., R. Swanepoel, and S. P. Shepherd. 1984. Protein synthesis in Rift Valley fever virus-infected cells. Virology 134118-124. [DOI] [PubMed] [Google Scholar]
- 64.Thomas, D., G. Blakqori, V. Wagner, M. Banholzer, N. Kessler, R. M. Elliott, O. Haller, and F. Weber. 2004. Inhibition of RNA polymerase II phosphorylation by a viral interferon antagonist. J. Biol. Chem. 27931471-31477. [DOI] [PubMed] [Google Scholar]
- 65.Vialat, P., A. Billecocq, A. Kohl, and M. Bouloy. 2000. The S segment of rift valley fever phlebovirus (Bunyaviridae) carries determinants for attenuation and virulence in mice. J. Virol. 741538-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vialat, P., and M. Bouloy. 1992. Germiston virus transcriptase requires active 40S ribosomal subunits and utilizes capped cellular RNAs. J. Virol. 66685-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe, H., Y. Tanaka, Y. Shimazu, F. Sugahara, M. Kuwayama, A. Hiramatsu, K. Kiyotani, T. Yoshida, and T. Sakaguchi. 2005. Cell-specific inhibition of paramyxovirus maturation by proteasome inhibitors. Microbiol. Immunol. 49835-844. [DOI] [PubMed] [Google Scholar]
- 68.Weber, F., and O. Haller. 2007. Viral suppression of the interferon system. Biochimie 89836-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weber, F., G. Kochs, and O. Haller. 2004. Inverse interference: how viruses fight the interferon system. Viral Immunol. 17498-515. [DOI] [PubMed] [Google Scholar]
- 70.Weber, F., V. Wagner, S. B. Rasmussen, R. Hartmann, and S. R. Paludan. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 805059-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wek, R. C., H. Y. Jiang, and T. G. Anthony. 2006. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 347-11. [DOI] [PubMed] [Google Scholar]
- 72.Wilson, M. L., L. E. Chapman, D. B. Hall, E. A. Dykstra, K. Ba, H. G. Zeller, M. Traore-Lamizana, J. P. Hervy, K. J. Linthicum, and C. J. Peters. 1994. Rift Valley fever in rural northern Senegal: human risk factors and potential vectors. Am. J. Trop. Med. Hyg. 50663-675. [DOI] [PubMed] [Google Scholar]
- 73.Won, S., T. Ikegami, C. J. Peters, and S. Makino. 2006. NSm and 78-kilodalton proteins of Rift Valley fever virus are nonessential for viral replication in cell culture. J. Virol. 808274-8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 146095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zurcher, T., R. M. Marion, and J. Ortin. 2000. Protein synthesis shut-off induced by influenza virus infection is independent of PKR activity. J. Virol. 748781-8784. [DOI] [PMC free article] [PubMed] [Google Scholar]