Abstract
Individual differences in traits such as impulsivity involve high reward sensitivity and are associated with risk for substance use disorders. The ventral striatum (VS) has been widely implicated in reward processing, and individual differences in its function are linked to these disorders. Dopamine (DA) plays a critical role in reward processing and is a potent neuromodulator of VS reactivity. Moreover, altered DA signaling has been associated with normal and pathological reward-related behaviors. Functional polymorphisms in DA-related genes represent an important source of variability in DA function that may subsequently impact VS reactivity and associated reward-related behaviors. Using an imaging genetics approach, we examined the modulatory effects of common, putatively functional DA-related polymorphisms on reward-related VS reactivity associated with self-reported impulsivity. Genetic variants associated with relatively increased striatal DA release (DRD2 – 141C deletion) and availability (DAT1 9-repeat), as well as diminished inhibitory postsynaptic DA effects (DRD2 – 141C deletion and DRD4 7-repeat), predicted 9–12% of the interindividual variability in reward-related VS reactivity. In contrast, genetic variation directly affecting DA signaling only in the prefrontal cortex (COMT Val158Met) was not associated with variability in VS reactivity. Our results highlight an important role for genetic polymorphisms affecting striatal DA neurotransmission in mediating interindividual differences in reward-related VS reactivity. They further suggest that altered VS reactivity may represent a key neurobiological pathway through which these polymorphisms contribute to variability in behavioral impulsivity and related risk for substance use disorders.
Keywords: dopamine, impulsivity, ventral striatum, fMRI, genetic polymorphisms, reward
Introduction
Interindividual variability in overlapping psychological constructs, such as self-regulation, impulse control, delay of gratification and intertemporal choice,1 has been associated with the likelihood of engaging in addictive behaviors (for example, cigarette smoking) and of developing related psychopathologies, such as pathological gambling, and drug and alcohol abuse.2–5 Thus, such individual tendencies represent important intermediate behavioral phenotypes of predictive utility which offer traction in the search for pathways mediating risk for addiction and related disorders.
Explication of the underlying neural processes that give rise to such interindividual variability will similarly allow for a more comprehensive understanding of the mechanisms leading to not only normal variability in such behaviors but also the pathophysiology of addiction. Through reciprocal cortical and subcortical connections, the nucleus accumbens and, more broadly, the ventral striatum (VS) contribute to the motivational salience of stimuli and abet appetitive or reward-dependent behaviors.6 The magnitude of VS reactivity predicts individual differences in simple laboratory indices of preference for immediate over delayed rewards7 as well as more complex measures of incentive-based decision-making. 8 Moreover, dysregulation of the VS contributes to addiction, perhaps by affecting impulsive decision-making. 9 Therefore, interindividual variability in VS reactivity to reward-related stimuli likely contributes to the emergence of differences in the intermediate behavioral risk factors for, as well as the clinical expression of, addictions.
Dopamine (DA) modulation of neuronal activity, especially in the VS (that is, the mesolimbic system), serves as a nexus for the expression of DA signaling at the level of reward-related behaviors.10,11 Functioning of the DA system has been linked to normal individual differences in reward-related traits,12 and disorders involving enhanced reward-seeking, such as addiction, have been hypothesized to reflect maladaptive alterations of this mesolimbic reward system.13,14 As such, identifying factors that determine interindividual variability in DA signaling and its related impact on the reactivity of the VS will facilitate our understanding of the neurobiological mechanisms governing reward-related behaviors and augment efforts to improve the treatment and even prevention of pathological behaviors such as drug abuse and addiction.
In the current study, we used imaging genetics15 to explore the role of altered DA signaling, resulting from DA-related genetic polymorphisms, in determining interindividual variability in reward-related VS reactivity and correlated variability in behavioral impulsivity. Reward-related VS reactivity was determined via blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) while subjects completed a simple number-guessing game resulting in positive and negative feedback in the context of monetary reward. Importantly, we have successfully employed this challenge paradigm to explore the relationship between interindividual variability in VS reactivity and delay discounting, a behavioral index of relative preference for immediate versus delayed rewards that is often associated with both impulsivity and risk for addictive disorders.7 We focused on four relatively common polymorphisms with demonstrated functional effects on multiple steps of DA neurotransmission, including extracellular reuptake and clearance via the dopamine transporter (DAT), postsynaptic receptor signaling via D2 and D4 dopamine receptor subtypes (DRD2 and DRD4) and autoregulation of midbrain DA neurons via DRD2.
The selection of our candidate polymorphisms was driven by available in vitro and/or in vivo assays demonstrating significant impact of these variants on aspects of biological function related to DA neurotransmission and not on available data from association studies with either behavioral (for example, impulsivity) or clinical (for example, alcoholism) phenotypes. While association studies are necessary for understanding the ultimate contribution of genetic polymorphisms to variability in behavioral and clinical phenomena, they do not readily allow for inferences regarding polymorphic effects on gene or protein function. Such inferences are instrumental for the development of biologically plausible and tractable hypotheses regarding the impact of genetic variation on interindividual variability in brain function and associated behaviors such as those pursued in our current work.15,16
Based on the above strategy, we hypothesized that polymorphisms resulting in relatively increased striatal DA release (DRD2) and synaptic availability (DAT1) as well as decreased postsynaptic inhibition (DRD2 and DRD4) would be associated with relatively greater reward-related VS reactivity. Specifically, we hypothesized that there would be relatively greater VS reactivity associated with the 9-repeat allele of a 40 bp variable number of tandem repeats (VNTR) polymorphism in the 3′ untranslated region of the DAT gene (DAT1), which is linked with reduced DAT expression and presumably greater striatal synaptic DA.17–21 We also hypothesized that there would be relatively greater VS reactivity associated with the deletion variant of a single nucleotide polymorphism (SNP) in the promoter region (–141C insertion/deletion, Ins/Del) of the DRD2 gene, which results in reduced DRD2 expression.22 Similarly, we hypothesized that there would be relatively greater VS reactivity associated with the 7-repeat allele of a 48 bp VNTR in the third exon of the DRD4 gene, which leads to reduced DRD4-mediated inhibitory postsynaptic effects.23,24 In contrast to the polymorphisms above, we hypothesized that a commonly studied polymorphism encoding an amino acid substitution in catechol-O-methyltransferase (COMT Val158Met), which primarily affects DA availability in prefrontal cortex and not in striatum,25 would be unrelated to individual differences in VS reactivity.
Materials and methods
Participants
Candidate genotypes and fMRI data were available in 89 adult volunteers (Table 1), recruited from a larger parent study, the Adult Health and Behavior (AHAB) project, which assesses a wide range of behavioral and biological traits among a community sample of nonpatient, middle-aged volunteers. All participants provided informed consent according to the guidelines of the University of Pittsburgh Institutional Review Board. All participants were in good general health and cleared of the following study exclusions: (1) medical diagnoses of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease, or a lifetime history of psychotic symptoms; (2) use of psychotropic, glucocorticoid, or hypolipidemic medication; (3) conditions affecting cerebral blood flow and metabolism (for example, hypertension) and (4) diagnoses of Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) Axis I psychopathology, including substance use, mood and anxiety disorders, as assessed by the Structured Clinical Interview for DSM-IV, nonpatient edition.26
Table 1.
Sample- and genotype-specific demographics
| Genotype | N | Sex |
Race |
Mean age±s.d. | |||
|---|---|---|---|---|---|---|---|
| Male | Female | White | Non-white | ||||
| DAT1 | Total | 86 | 38 | 48 | 78 | 8 | 44.2±6.8 |
| 9-repeat carriers | 42 | 18 | 24 | 39 | 3 | 44.0±6.9 | |
| 10/10 | 44 | 20 | 24 | 39 | 5 | 44.4±6.8 | |
| DRD4 | Total | 86 | 37 | 49 | 77 | 9 | 44.1±6.8 |
| 7-repeat carriers | 34 | 13 | 21 | 30 | 4 | 45.1±6.7 | |
| Other | 52 | 24 | 28 | 47 | 5 | 43.4±6.8 | |
| DRD2 | Total | 76 | 35 | 41 | 70 | 6 | 43.8±6.9 |
| Ins/Ins | 58 | 23 | 35 | 55 | 3 | 43.7±6.9 | |
| Ins/Del | 18 | 12 | 6 | 15 | 3 | 43.9±7.1 | |
| COMT | Total | 86 | 37 | 49 | 77 | 9 | 44.1±6.8 |
| Val/Val | 26 | 9 | 17 | 21 | 5 | 46.5±6.5 | |
| Val/Met | 44 | 21 | 23 | 41 | 3 | 43.8±6.5 | |
| Met/Met | 16 | 7 | 9 | 15 | 1 | 41.0±7.1 | |
| ALL | — | 89 | 38 | 51 | 79 | 10 | 43.8±6.5 |
There were no significant differences in sex, race or age between any of the genotype groups.
Non-white participants were either African-American or of mixed race.
The age range for the entire sample of 89 subjects was 31–54, and approximately half (44) of the subjects within this sample were aged 45 years and younger (i.e., range 31–45).
Candidate genotyping
High molecular weight DNA was isolated from EDTA anticoagulated whole-blood samples obtained from all participants using the Puregene kit (Gentra Systems, Minneapolis, MN, USA). Each sample was genotyped using allele-specific primers and polymerase chain reaction conditions from published protocols: DAT1 VNTR;27 DRD4 third exon 48 bp VNTR,28 DRD2 –142C Ins/Del29 and COMT Val158Met.30 All genotypes were scored by two independent readers by comparison to sequence-verified standards. Participants were classified by genotype as follows (Table 1). For DAT1, two genotype groups were established: 9-repeat allele carriers and 10-repeat allele homozygotes. For DRD2, two genotype groups were also established: –141C deletion carriers (Ins/Del) and noncarriers (Ins/Ins). Similarly for DRD4, two genotype groups were established: 7-repeat allele carriers and all other allele combinations. For COMT, three genotype groups were established: Val158 allele homozygotes, Val158Met heterozygotes and Met158 homozygotes. All four genotypes were available in 76 of the 89 subjects. DRD2 genotype was unavailable in 10 subjects and DAT1, DRD4 and COMT genotypes were unavailable in 3 subjects.
Impulsivity
As part of their participation in AHAB, subjects completed the Barratt Impulsiveness Scale (BIS), which contains 30 questions regarding subjects’ control of thoughts and behavior, each scored on a four-point Likert scale.31 Typical items assess tendencies to act without thinking (motor impulsivity), to make decisions ‘on the spur of the moment’ (cognitive impulsivity) and to fail to plan ahead (nonplanning impulsiveness). The instrument has high internal consistency (α coefficients = 0.79–0.83),32 and we found the BIS (total) scores of adults to be highly stable over time, with retest reliabilities of 0.88 and 0.85 over testing intervals of 6 months and 3 years, respectively.33,34 High levels of impulsivity, as assessed by the BIS, have also been shown to predict increased vulnerability to addictive disorders such as alcoholism.35
Striatal reactivity paradigm
A modified fMRI paradigm based on the work of Delgado et al.36 was used to probe VS activity in response to positive and negative feedback-associated monetary reward. Our blocked-design paradigm consisted of pseudorandom presentation of trials wherein participants played a card guessing game and received positive or negative (that is, win or loss) feedback for each trial. All participants were told that their performance on the card game would determine a monetary reward to be received at the end of the game. During each trial, participants had 3 s to guess, via button press, whether the value of a visually presented card was higher or lower than 5 (index and middle finger, respectively). After a choice was made, the numerical value of the card was presented for 500ms and followed by appropriate feedback (green upward-facing arrow for positive feedback; red downward- facing arrow for negative feedback) for an additional 500 ms. Upon receiving positive feedback (that is, green arrow), subjects were required to respond via button press (either index or middle finger) to engage consummatory processes that may be necessary to elicit VS activation. No response was required upon negative feedback (that is, red arrow). A crosshair was then presented for 3 s, for a total trial length of 7 s. Each block was comprised of five trials, with three blocks each of predominantly positive feedback (75% correct) and three of predominantly negative feedback (25% correct) interleaved with three control blocks. During control blocks, participants were instructed to simply make alternating button presses during the presentation of an ‘x’ (3 s) which was followed by an asterisk (500 ms) and a yellow circle (500 ms). Each block was preceded by a 2 s instruction of ‘Guess Number’ (for positive or negative feedback blocks) or ‘Press Button’ (for control blocks), resulting in a total block length of 38 s and a total task length of 342 s. Participants were unaware of the fixed outcome probabilities associated with each block and were led to believe that their performance would determine their net monetary gain. Instead, all participants received $10. We included one incongruent trial within each task block (for example, one of four trials during positive feedback blocks was incorrect, resulting in negative feedback) to prevent participants from anticipating the feedback for each trial and to maintain participants’ engagement and motivation to perform well.
BOLD fMRI acquisition, processing and analysis
Each participant was scanned using a Siemens 3T Allegra scanner. BOLD functional images were acquired with a gradient echo planar imaging (EPI) sequence and covered 34 axial slices (3mm thick) beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (TR/TE = 2000/25 ms, FOV= 20 cm, matrix = 64 × 64). All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before the collection of fMRI data for each participant, we acquired a reference EPI scan that we visually inspected for artifacts (for example, ghosting) as well as for good signal across the entire volume of acquisition, including the VS. The fMRI data from all 89 participants included in this study were cleared of such problems.
Whole-brain image analysis was completed using SPM2 (http://www.fil.ion.ucl.ac.uk/spm). For each scan, images for each participant were realigned to the first volume in the time series to correct for head motion. Data sets were then selected for their high quality (scan stability) as demonstrated by small (< 2mm) motion correction. Based on this criterion, data from all participants were included in subsequent analyses. Realigned images were spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) using a 12- parameter affine model. These normalized images were then smoothed to minimize noise and residual differences in gyral anatomy with a Gaussian filter, set at 6mm full-width at half-maximum. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean.
These preprocessed data sets were analyzed using second-level random effects models that account for both scan-to-scan and participant-to-participant variability to determine task-specific regional responses. For each participant and scan, predetermined condition effects at each voxel were calculated using a t-statistic, producing a statistical image for each contrast: (1) positive feedback > control and (2) negative feedback > control. For each individual, these contrast images were in turn used to generate a statistical image for the main effects of reward: ((positive feedback > control) > (negative feedback > control)). These individual contrast images were then used to determine mean reward-related VS reactivity using one-sample t-tests, which were thresholded at a voxel level of P < 0.05, corrected for multiple comparisons across the volume of the VS as defined by the main effects of reward contrast, and an extent of at least 10 contiguous voxels. Our VS region of interest was constructed using the WFU PickAtlas Tool (v1.04) and defined as a sphere of 20mm in radius, centered on the Talairach coordinates of x=0, y=10 and z =−10. After generation of our spherical region of interest (ROI), we visually inspected its position on the canonical structural image used for spatial normalization as well as that used for visualization of task-related activations. Visual inspection confirmed that the ROI encompassed the entire VS as well as adjacent regions of the caudate nucleus (that is, head of caudate) in both right and left hemispheres.
Data analysis
Genotype effects were examined using one-way analysis of variance wherein BOLD signal values for VS clusters reflecting a main effect of reward were entered as dependent variables with DAT1, DRD2, DRD4 or COMT as the independent variable. Exploratory analyses of genotype interaction effects were conducted by entering all genotypes exhibiting a main effect on VS reactivity and their interactions into a single model with reward-related VS cluster BOLD value as the dependent variable. Sex and age were entered as covariates in all analyses. Associations between impulsivity and VS activation were evaluated by Pearson’s r.
Results
Allele and genotype frequencies
Demographic information for all subjects and candidate genotype groups is detailed in Table 1. The allele frequencies for all candidate genotypes (DAT1, DRD2, DRD4 and COMT) were comparable to those reported for Caucasians in larger samples. All resulting genotype frequencies from our cohort of participants did not deviate from Hardy–Weinberg equilibrium (all P-values > 0.10). Genotype groups for all polymorphisms did not differ with regard to sex, age or ethnicity/race (all P-values > 0.05).
Impulsivity
BIS scores averaged 58.39 ± 9.77 (s.d.) across all subjects and did not differ by sex (P = 0.092), age (P = 0.193) or race (P = 0.445). These values are within a normative range and consistent with those of the larger community sample of volunteers from which our subjects were derived.33,34 Surprisingly, given our sample size, there was a significant effect of the DAT1 VNTR on BIS (F(1,89) = 6.02, P = 0.02), with higher scores reported by 9-repeat allele carriers (Mean = 60.89, s.d. = 9.95) in comparison with 10-repeat homozygotes (Mean = 56.00, s.d. = 9.05). There were no significant effects associated with DRD2, DRD4 and COMT genotypes (all P-values > 0.25).
Reward-related VS reactivity
Consistent with our recent study,7 BOLD fMRI showed strong bilateral striatal activity associated with both positive and negative feedback blocks, relative to control blocks. Striatal activity associated with general feedback extended from the VS to the caudate head. This pattern may reflect the presence of unexpected rewards (25% of trials during negative feedback blocks) in our blocked design, which could serve as positive prediction errors that elicit striatal activation or, more generally, may reflect feedback learning associated with the probabilistic nature of our blocked design.37,38 The extension of VS activation to the caudate head during both positive and negative feedback blocks in comparison to the nofeedback control is consistent with this pattern.36,37
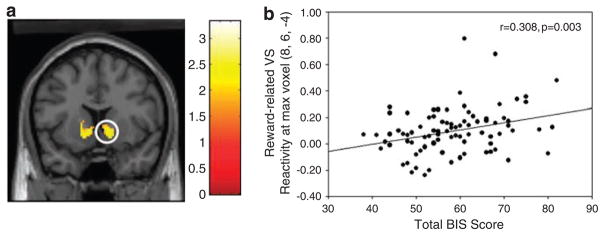
VS activity varied significantly by type of feedback, with greater bilateral activation in response to predominantly positive, compared to negative, feedback blocks (Figure 1a). Differential VS activation associated specifically with positive versus negative feedback, where the probabilistic nature of each block is identical, suggests that it may more closely reflect reward-related processes and not general feedback learning. Also, this differential VS activation did not extend to the caudate head, further supporting its specific role in reward-related processes, and is consistent with prior studies using various other reward incentive paradigms and stimuli (cf. Breiter and Rosen;39 Knutson and Cooper40). Moreover, this reward-related VS reactivity was specifically correlated with interindividual variability in impulsivity derived from the BIS (Figure 1b; F = 9.114; P = 0.003). Therefore, analyses of DA-related genotypes were conducted using reward-related VS activation derived from the differential effects of feedback (that is (positive feedback > control) > (negative feedback > control)).
Figure 1.
(a) Statistical parametric map illustrating differential reward-related ventral striatum (VS) reactivity (P < 0.05, FDR corrected) across all 89 subjects from the contrast of positive > negative feedback blocks. (b) Correlation between rewardrelated VS reactivity (% BOLD signal change) and self-reported impulsivity from the total Barratt Impulsiveness Scale (BIS) score.
Genotype modulation of reward-related VS reactivity
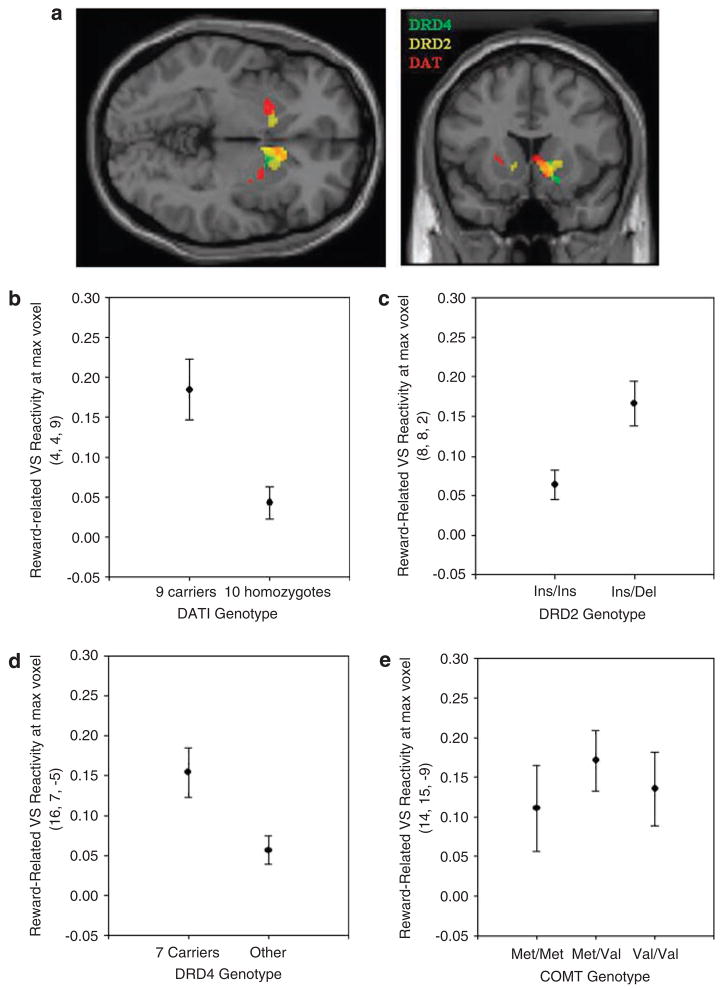
Consistent with our hypotheses, analyses revealed that three of our candidate polymorphisms significantly predicted interindividual variability in rewardrelated VS reactivity: DAT1 (R2 = 0.115, P = 0.001), DRD4 (R2 = 0.092, P = 0.004) and DRD2 (R2 = 0.091, P = 0.008) genotypes. Specifically, relatively greater VS reactivity was exhibited by DAT1 9-repeat allele carriers, DRD2 –141C Del allele carriers and DRD4 7-repeat allele carriers (Table 2; Figures 2b–d). Also consistent with our hypotheses, there was no significant effect of COMT Val158Met genotype on reward-related VS reactivity (Figure 2e).
Table 2.
Genotype effects on reward-related VS reactivity
| Genotype | Talairach coordinates of max voxel in VS cluster |
Cluster size | t-score | Pa | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| DRD2 (Ins/Del > Ins/Ins) | 8 | 8 | −2 | 245 | 3.82 | < 0.000 |
| DAT1 (9-repeat > 10/10) | 4 | 4 | 9 | 161 | 3.32 | < 0.001 |
| DRD4 (7-repeat > Other) | 16 | 7 | −5 | 86 | 2.95 | < 0.002 |
| COMTb | — | — | — | — | — | = 0.65 |
FDR corrected for multiple comparisons over search volume of striatal ROI.
No coordinates listed for COMT because of null effect on VS reactivity.
Figure 2.
(a) Overlay of statistical parametric maps for the main effects of DAT1, DRD2 and DRD4 on reward-related ventral striatum (VS) reactivity (P < 0.05, FDR corrected for all genotypes). (b–e) Mean reward-related VS reactivity (% BOLD signal change±s.e.m.) as a function of each genotype from clusters in (a) as well as catechol-O-methyltransferase (COMT). The BOLD values reported for COMT genotype (e) are derived from the VS voxel exhibiting the largest reward-related activation as there were no specific VS voxels exhibiting a significant effect of COMT genotype.
The largest effect on VS reactivity was associated with DRD2 genotype, with 54% overlap in voxels exhibiting both an effect of DRD2 and a main effect of reward (Figure 2a). There was 35 and 23% overlap between reward-related VS reactivity and DAT1 and DRD4 genotypes, respectively (Figure 2a). A similar pattern emerged when evaluating the relationship between BIS-correlated VS voxels and those exhibiting a genotype effect. The largest overlap was for DRD2 (33%), followed by DAT1 (30%) and DRD4 (7%). Exploratory analysis with a single model including main effects of DAT1, DRD2 and DRD4, as well as all two-way and three-way interactions revealed that the effect of DRD2 genotype on reward-related VS reactivity remained significant when all other factors were included in the model (F(1,70) = 8.33, P < 0.01). All other main effects and interactions were nonsignificant (F-values = 0.05–1.43, all P-values > 0.20).
While we covaried for age in all our analyses, we also explicitly tested for age-related effects given the extensive literature documenting age-related alterations in DA neurotransmission.41 We found neither a main effect of age (F(21,88) = 0.948, P = 0.534) nor any age × genotype interactions (all P-values > 0.1) on reward-related VS reactivity. Moreover, all patterns of genotype-modulated reward-related VS reactivity were similar when the analyses were restricted to subjects 45 years and younger (N= 44) or subjects 46 years and older (N= 45). Thus, we are confident that the observed relationships between the DA-related polymorphisms under investigation and reward-related VS reactivity are not confounded by independent age-related effects on DA neurotransmission.
Discussion
Our current results illustrate that alterations in DA signaling associated with functional polymorphisms in multiple DA-related genes contribute a significant proportion of the interindividual variability in reward- related VS reactivity, which itself covaries with self-reported impulsivity. Specifically, polymorphisms directly associated with relatively increased DA availability (DAT1 9-repeat) and release (DRD2 –141C Del) or decreased postsynaptic inhibition (DRD2 –141C Del and DRD4 7-repeat) in the striatum are associated with relatively greater VS reactivity. In contrast, genetic variation primarily impacting prefrontal DA availability (COMT Val158Met) was not associated with variability in VS reactivity. These findings implicate genetically driven variability in striatal DA neurotransmission and VS reactivity as a key pathway in the emergence of interindividual differences in impulsivity, which represents an important intermediate behavioral phenotype associated with risk for addiction and related disorders.
Although our BOLD fMRI measure of VS reactivity does not represent a direct index of DA neurotransmission, the observed pattern of relatively increased activity in individuals with polymorphisms associated with increased striatal DA is consistent with a recent in vivo human study reporting a direct relationship between striatal DA synthesis, assessed with FDOPA positron emission tomography (PET), and brain activity, assessed with BOLD fMRI.42 Acute increase of DA release via oral amphetamine has also been linked with relatively increased extent of BOLD fMRI-assessed VS activity.43 More generally, acute pharmacologic increase of DA in both healthy volunteers44 and patients with Parkinson’s disease45 results in relatively increased BOLD fMRI-assessed activity in closely related limbic brain regions, namely the amygdala. In contrast, a recent [11C]raclopride PET study reported relatively blunted striatal DA release in response to amphetamine challenge in subjects reporting above-median levels of trait impulsivity. 46 While superficially inconsistent with the direction of our genetically driven effects on VS reactivity, these data may, in fact, be biologically consistent if the relatively blunted drug-induced striatal DA release reflects higher baseline levels of DA neurotransmission, as would be associated with our genetic variants, in subjects with higher levels of impulsivity. Multimodal neuroimaging studies combining PET measures of DA function and BOLD fMRI measures of VS reactivity may offer a unique opportunity to more directly evaluate underlying molecular mechanisms regulating this circuitry. Despite the limitations inherent in attempts to directly compare neurobiological effects associated with constitutive genetic variation, which presumably impacts not only the functional response of neural circuitry but also its development, with those from acute pharmacologic challenge, which impact only the existing functional architecture, our current findings are largely consistent with those linking increased DA neurotransmission and VS reactivity.
The DAT is responsible for the active clearance of synaptic DA and, thus, plays a critical role in regulating the duration of postsynaptic DA signaling, especially in the striatum.47 Accumulating evidence indicates that the DAT1 polymorphism impacts the expression and availability of DAT.17 Although a genotype effect has not been consistently observed across all studies,48–51 several suggest that in comparison to the 9-repeat allele, the 10-repeat is associated with relatively increased levels of DAT both in vivo18,19 and in vitro.20,21 We found that the 9-repeat allele, presumably through lesser DAT expression and increased synaptic DA, is associated with relatively greater VS reactivity in comparison to the 10-repeat allele. This is consistent with a recent imaging genetics study demonstrating relatively increased memory-related midbrain activity in 9-repeat allele carriers.52 Our findings are also biologically consistent with observations that activity of midbrain DA neurons is associated with unconditioned receipt of reward as well as the presentation of conditioned stimuli predicting reward,53 and that subsequent DA release in the VS potentiates reward-related behaviors. 10,11
Concentrations of synaptic DA in the striatum are also affected by acute negative feedback mechanisms consisting of both the inhibition of mesencephalic DA neuron firing rates and blockade of neurotransmitter release through activation of somatodendritic and terminal DRD2 autoreceptors, respectively.54,55 The DRD2 –141C Ins/Del polymorphism may influence striatal DA release by affecting the availability of both types of DRD2 autoreceptors. The –141C Del polymorphism exhibits relatively reduced in vitro gene expression.22 Our current finding of relatively increased VS reactivity in –141C Del allele carriers is consistent with this in vitro effect and may reflect reduced DRD2 autoreceptor-mediated negative feedback and subsequently increased striatal DA release. Our data are also consistent with a recent study reporting relatively increased reward- but not anticipation- related VS reactivity in carriers of the DRD2 TaqIA A1 allele,56 which like the –141C Del is associated with lower DRD2 density.57
DA modulation of striatal circuitry is further mediated by postsynaptic DRD2 and DRD4, both of which exert inhibitory effects via second-messenger signaling cascades.58 Although not definitive, available data suggest that the DRD2 is expressed on local postsynaptic striatal neurons and the DRD4 is expressed on both postsynaptic striatal neurons and presynaptic corticostriatal glutamatergic afferents.59–61 This localization pattern suggests that these DA receptor subtypes can exert either direct (D2 and D4) or indirect (D4) inhibitory effects on striatal neurons. While the –141C Del allele impacts the availability of the DRD2 through reduced gene expression, the 7-repeat allele results in a DRD4 which exhibits reduced cAMP-reduction potency and subsequent postsynaptic inhibition in comparison with the 4-repeat.23,24 Thus, decreased inhibition of striatal neurons through either direct or indirect effects of the DRD2 –141C Del and DRD4 7-repeat alleles, respectively, may underlie their association with relatively increased reward-related VS reactivity. The direction of the DRD2 –141C Del effect on VS reactivity is notably consistent with a recent report demonstrating that in rats, trait behavioral impulsivity predicting cocaine self-administration is associated with reduced striatal DRD2/3 density.62
Finally, to determine the relative specificity of genetically driven variability in striatal DA signaling on VS reactivity we examined the common, functional Val158Met SNP in COMT, an enzyme responsible for the degradation of prefrontal DA.63 The Met158 allele results in relatively diminished enzyme stability and subsequently greater prefrontal DA availability through decreased amine degradation. Although the Val158Met may impact striatal DA through indirect prefrontal feedback onto midbrain DA neurons,64,65 it is unlikely to manifest direct effects on activity-dependent striatal DA release and striatal-dependent behaviors.66 Consistent with our hypothesis, variability in DA associated with the COMT Val158Met did not have a significant impact on reward-related VS reactivity. This finding suggests that genetically driven variation directly impacting striatal DA function is uniquely associated with interindividual variability in VS reactivity related to behavioral impulsivity.
While all three polymorphisms impacted VS clusters associated with the main effects of reward (that is, reward-related voxels) and showed significant overlap with each other (Figure 2a), their spatial extent and distribution were not identical, suggesting that each may impact different aspects of functional VS circuitry. Of the three DA-related polymorphisms significantly impacting VS reactivity, the DRD2 –141C accounted for the largest effect in terms of extent of VS activation, exhibiting 54% overlap with main effects of reward (245 voxels). DAT1 and DRD4 exhibited 35 (161 voxels) and 23% (86 voxels) overlap with reward-related VS activation, respectively. A similar pattern of genetic effects existed in regard to overlap with VS activation that correlated with self-reported impulsivity. The largest proportion of inter-individual variability in reward-related VS reactivity was explained by DAT1 genotype (~12%), followed closely by DRD4 and DRD2 genotypes (~9% each). Consistent with this pattern, the DAT1 genotype alone predicted individual differences in BIS scores, with 9-repeat allele carriers exhibiting both increased VS reactivity and higher BIS scores in comparison with 10-repeat homozygotes. Although this convergence is intriguing we caution against overinterpretation of the current results because, while large for a neuroimaging study, our sample size is far too small to draw meaningful inferences with regard to genetically driven variability in much more distal behavioral phenotypes such as impulsivity. The absence of similar significant behavioral effects for the other genotypes under investigation, although exhibiting robust effects on brain function, speaks to this important limitation.
Despite the similarity of these independent genetic effects, simultaneous modeling of all three genotypes revealed that only the DRD2 –141C significantly impacted reward-related VS reactivity when controlling for the effects of other factors. Although these polymorphisms presumably impact interrelated components of the DA signaling cascade, there was no evidence for statistically significant genetic epistasis on reward-related VS reactivity. However, our sample size, while quite substantial for typical neuroimaging studies, may be underpowered to detect significant epistatic effects, and larger samples are needed to properly evaluate their likelihood. The unique effects of the DRD2 –141C on reward-related VS reactivity may reflect the potential of this polymorphism to impact both striatal DA release, through somatodendritic and terminal DRD2 autoreceptors, and inhibition of striatal neurons, through postsynaptic DRD2 heteroreceptors.
In selecting our candidate polymorphisms, we did not focus on available data from association studies with broad behavioral (for example, impulsivity) or clinical (for example, alcoholism) phenotypes. While such association studies are important in establishing the broader relevance of genetically driven variability in brain function, they are often inconsistent and preclude inferences regarding the effects of polymorphisms at the level of gene or protein function. Regardless, all three genotype effects on reward-related VS reactivity are consistent with some prior association studies implicating the alleles that resulted here in increased reactivity with higher levels of impulsivity67 and risk for addiction.68,69 The existence of convergent findings for positive genetic association with neural, behavioral and clinical phenotypes provides compelling support for the importance of these specific DA-related polymorphisms in the pathways mediating interindividual differences in these processes.
It is important to note, however, that the likely contribution of specific genetic and neural factors may vary as a function of the intermediate steps leading to addiction, such as initiation, persistence and abuse.70 As has been recently demonstrated in genetic studies of liability for mood disorders,71,72 the impact of genetic polymorphisms on complex behavioral and clinical phenotypes is likely to be unmasked in the context of neural responses to specific precipitating environmental factors such as access to drugs of abuse and presence of social stressors.73,74 In fact, drug-induced striatal DA release associated with impulsivity appears to be differentially modulated by the experience of acute and chronic stressors.46,75 Future imaging genetics studies should attempt to dissect the contributions of genetically driven variability in brain function to each component of the addictions pathway and account for potential moderating environmental factors.
Future studies should also examine the unique and shared effects of polymorphisms to anticipation- and reward-related VS reactivity, which may contribute differently to variability in impulsivity and risk for addiction. The pattern of VS activity seen in our prior work7 as well as the present study is generally consistent with that reported using the original version of this task, where each trial was associated with monetary feedback.36 The VS activations we observed are also consistent with prior studies using various other reward incentive paradigms and stimuli (cf. Breiter and Rosen;39 Knutson and Cooper40). However, unlike many previous studies, each trial was not associated with a monetary outcome (that is, gaining or losing money per trial) within our modified blocked design. Rather, each trial occasioned a more general positive or negative feedback reflecting the ‘correctness’ of the subject’s guess. This aspect of our protocol limits our ability to identify VS activity associated specifically with trial-by-trial monetary outcomes,36,76,77 which might be related more closely to differences in reward-related behaviors. It is also possible that our VS activation may more generally reflect feedback learning associated with the probabilistic nature of our blocked design.37,38 Importantly, this potential limitation is largely avoided in our focused analyses on the differential VS activation associated specifically with positive versus negative feedback, where the probabilistic nature of each block is identical. Finally, our blocked design precludes the analysis of VS activity uniquely associated with anticipation and outcome components of each trial. Employing event-related fMRI paradigms that better allow for the dissection of anticipation and outcome may help further parse the relationship between individual variability in reward-related behaviors and VS function.
The candidate polymorphisms in our study were selected based on evidence from studies directly examining the impact of these variants on specific aspects of biological function (for example, in vitro reporter gene assays, mRNA, and protein expression studies and in vivo neuroimaging measures), which are relatively robust and provide basis for specific directional hypotheses regarding genotype effects on DA function and subsequent VS reactivity. Nevertheless, it is possible that the functional effects on VS reactivity associated with the DA polymorphisms in the current study reflect the influence of other variants such as those in linkage disequilibrium (LD) with our candidates. For example, while we have highlighted evidence for a direct functional effect of the DRD2 –141C Del allele on gene expression and DRD2 density, significant neurobiological effects have not always been demonstrated.78,79 Such inconsistency is commonplace in genetic association studies and may, at least in part, result from the effects of occult functional variation for which candidate polymorphisms serve only as indirect markers. The absence of a perfect relationship between such markers and occult functional variants (that is, 100% LD) across study populations can undermine replication of effects. One promising approach to overcome this limitation is through the establishment of informative haplotypes, comprised of ‘tagging’ SNPs distributed throughout the gene, which represent the majority of all possible genetic variation in any given gene. The adoption of haplotypes in future studies of DA-related gene effects on reward-related VS reactivity and correlated behaviors should improve our understanding of the detailed mechanisms leading to interindividual variability in these processes.
In conclusion, our imaging genetics results reveal that DA-related functional polymorphisms resulting in relatively increased striatal DA neurotransmission bias toward increased reward-related VS reactivity. Given the demonstrated relationship between increased VS reactivity and greater self-reported impulsivity, as well as preference for immediate over delayed rewards,7 such genetically mediated effects may contribute to the emergence of behavioral tendencies to favor immediate gratification with little regard for alternative responses or consequences, act without thought, fail to deliberate about decisions and neglect planning. As such, we anticipate that our findings will contribute to the development of a principled framework from which future studies can systematically examine how the dynamic interplay of genes, brain and behavior is sculpted by environmental factors to mediate interindividual variability in these aspects of personality and temperament as well as their contribution to risk for addictions.
Acknowledgments
This work was supported by NIH grants PO1 HL040962 to SBM, K01 MH072837 to ARH, K01 MH074769 to EEF as well as NARSAD Young Investigator Awards to ARH and EEF.
References
- 1.Manuck SB, Flory JD, Muldoon MF, Ferrell RE. A neurobiology of intertemporal choice. In: Loewenstein G, Read D, Baumeister RF, editors. Time and Decision: Economic and Psychological Perspectives on Intertemporal Choice. Sage; New York: 2003. pp. 139–172. [Google Scholar]
- 2.Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav Processes. 2003;64:345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 3.Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- 4.Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- 5.Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- 6.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 7.Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–156. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 10.Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Ann NY Acad Sci. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- 11.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Depue RA, Luciana M, Arbisi P, Collins P, Leon A. Dopamine and the structure of personality: relation of agonist-induced dopamine activity to positive emotionality. J Pers Soc Psychol. 1994;67:485–498. doi: 10.1037//0022-3514.67.3.485. [DOI] [PubMed] [Google Scholar]
- 13.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 14.Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- 15.Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Hariri AR, Weinberger DR. Imaging genomics. Br Med Bull. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- 17.Bannon MJ, Michelhaugh SK, Wang J, Sacchetti P. The human dopamine transporter gene: gene organization, transcriptional regulation, and potential involvement in neuropsychiatric disorders. Eur Neuropsychopharmacol. 2001;11:449–455. doi: 10.1016/s0924-977x(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 18.Cheon KA, Ryu YH, Kim JW, Cho DY. The homozygosity for 10- repeat allele at dopamine transporter gene and dopamine transporter density in Korean children with attention deficit hyperactivity disorder: relating to treatment response to methylphenidate. Eur Neuropsychopharmacol. 2005;15:95–101. doi: 10.1016/j.euroneuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 20.Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′UTR VNTR: evidence from brain and lymphocytes using quantitative RT–PCR. Am J Med Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- 21.VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arinami T, Gao M, Hamaguchi H, Toru M. A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum Mol Genet. 1997;6:577–582. doi: 10.1093/hmg/6.4.577. [DOI] [PubMed] [Google Scholar]
- 23.Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang E, Ding YC, Flodman P, Kidd JR, Kidd KK, Grady DL, et al. The genetic architecture of selection at the human dopamine receptor D4 (DRD4) gene locus. Am J Hum Genet. 2004;74:931–944. doi: 10.1086/420854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, Williams JBM. Structured Clinical Interview for DSM-IV Axis I Disorders, research version, non-patient edition. New York State Psychiatric Institute, Biometrics Research Department; New York: 1996. [Google Scholar]
- 27.Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- 28.Lichter JB, Barr CL, Kennedy JL, Van Tol HH, Kidd KK, Livak KJ. A hypervariable segment in the human dopamine receptor D4 (DRD4) gene. Hum Mol Genet. 1993;2:767–773. doi: 10.1093/hmg/2.6.767. [DOI] [PubMed] [Google Scholar]
- 29.Gelernter J, Kranzler H, Cubells JF, Ichinose H, Nagatsu T. DRD2 allele frequencies and linkage disequilibria, including the –141CIns/Del promoter polymorphism, in European-American, African-American, and Japanese subjects. Genomics. 1998;51:21–26. doi: 10.1006/geno.1998.5264. [DOI] [PubMed] [Google Scholar]
- 30.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Barratt ES. Impulsiveness and aggression. In: Monahan J, Steadman HJ, editors. Violence and Mental Disorder: Developments in Risk Assessment. University of Chicago Press; Chicago: 1994. pp. 61–79. [Google Scholar]
- 32.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- 34.Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF. Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology. 1998;19:287–299. doi: 10.1016/S0893-133X(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 35.Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 37.Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005;24:862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Haruno M, Kuroda T, Doya K, Toyama K, Kimura M, Samejima K, et al. A neural correlate of reward-based behavioral learning in caudate nucleus: a functional magnetic resonance imaging study of a stochastic decision task. J Neurosci. 2004;24:1660–1665. doi: 10.1523/JNEUROSCI.3417-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Ann NYAcad Sci. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- 40.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 41.Reeves S, Bench C, Howard R. Ageing and the nigrostriatal dopaminergic system. Int J Geriatr Psychiatry. 2002;17:359–370. doi: 10.1002/gps.606. [DOI] [PubMed] [Google Scholar]
- 42.Siessmeier T, Kienast T, Wrase J, Larsen JL, Braus DF, Smolka MN, et al. Net influx of plasma 6-[18F]fluoro-l-DOPA (FDOPA) to the ventral striatum correlates with prefrontal processing of affective stimuli. Eur J Neurosci. 2006;24:305–313. doi: 10.1111/j.1460-9568.2006.04903.x. [DOI] [PubMed] [Google Scholar]
- 43.Menon M, Jensen J, Vitcu I, Graff-Guerrero A, Crawley A, Smith MA, et al. Temporal difference modeling of the blood-oxygen level dependent response during aversive conditioning in humans: effects of dopaminergic modulation. Biological Psychiatry. 62:765–772. doi: 10.1016/j.biopsych.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27:1036–1040. doi: 10.1016/S0893-133X(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 45.Tessitore A, Hariri AR, Fera F, Smith WG, Chase TN, Hyde TM, et al. Dopamine modulates the response of the human amygdala: a study in Parkinson’s disease. J Neurosci. 2002;22:9099–9103. doi: 10.1523/JNEUROSCI.22-20-09099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oswald LM, Wong DF, Zhou Y, Kumar A, Brasic J, Alexander M, et al. Impulsivity and chronic stress are associated with amphetamine- induced striatal dopamine release. Neuroimage. 2007;36:153–166. doi: 10.1016/j.neuroimage.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 47.Sesack SR, Hawrylak VA, Guido MA, Levey AI. Cellular and subcellular localization of the dopamine transporter in rat cortex. Adv Pharmacol. 1998;42:171–174. doi: 10.1016/s1054-3589(08)60720-6. [DOI] [PubMed] [Google Scholar]
- 48.Martinez D, Gelernter J, Abi-Dargham A, van Dyck CH, Kegeles L, Innis RB, et al. The variable number of tandem repeats polymorphism of the dopamine transporter gene is not associated with significant change in dopamine transporter phenotype in humans. Neuropsychopharmacology. 2001;24:553–560. doi: 10.1016/S0893-133X(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 49.Michelhaugh SK, Fiskerstrand C, Lovejoy E, Bannon MJ, Quinn JP. The dopamine transporter gene (SLC6A3) variable number of tandem repeats domain enhances transcription in dopamine neurons. J Neurochem. 2001;79:1033–1038. doi: 10.1046/j.1471-4159.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- 50.Mill J, Asherson P, Craig I, D’Souza UM. Transient expression analysis of allelic variants of a VNTR in the dopamine transporter gene (DAT1) BMC Genet. 2005;6:3. doi: 10.1186/1471-2156-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med. 2005;46:745–751. [PubMed] [Google Scholar]
- 52.Schott BH, Seidenbecher CI, Fenker DB, Lauer CJ, Bunzeck N, Bernstein HG, et al. The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. J Neurosci. 2006;26:1407–1417. doi: 10.1523/JNEUROSCI.3463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 54.Hahn J, Kullmann PH, Horn JP, Levitan ES. D2 autoreceptors chronically enhance dopamine neuron pacemaker activity. J Neurosci. 2006;26:5240–5247. doi: 10.1523/JNEUROSCI.4976-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jomphe C, Tiberi M, Trudeau LE. Expression of D2 receptor isoforms in cultured neurons reveals equipotent autoreceptor function. Neuropharmacology. 2006;50:595–605. doi: 10.1016/j.neuropharm.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 56.Cohen MX, Young J, Baek JM, Kessler C, Ranganath C. Individual differences in extraversion and dopamine genetics predict neural reward responses. Brain Res Cogn Brain Res. 2005;25:851–861. doi: 10.1016/j.cogbrainres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 58.Sibley DR, Monsma FJ, Jr, Shen Y. Molecular neurobiology of dopaminergic receptors. Int Rev Neurobiol. 1993;35:391–415. doi: 10.1016/s0074-7742(08)60573-5. [DOI] [PubMed] [Google Scholar]
- 59.Jaber M, Robinson SW, Missale C, Caron MG. Dopamine receptors and brain function. Neuropharmacology. 1996;35:1503–1519. doi: 10.1016/s0028-3908(96)00100-1. [DOI] [PubMed] [Google Scholar]
- 60.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 61.Tarazi FI, Campbell A, Yeghiayan SK, Baldessarini RJ. Localization of dopamine receptor subtypes in corpus striatum and nucleus accumbens septi of rat brain: comparison of D1-, D2-, and D4-like receptors. Neuroscience. 1998;83:169–176. doi: 10.1016/s0306-4522(97)00386-2. [DOI] [PubMed] [Google Scholar]
- 62.Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: catechol-O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- 64.Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23:2008–2013. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- 66.Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 67.Ebstein RP, Zohar AH, Benjamin J, Belmaker RH. An update on molecular genetic studies of human personality traits. Appl Bioinformatics. 2002;1:57–68. [PubMed] [Google Scholar]
- 68.Hurd YL. Perspectives on current directions in the neurobiology of addiction disorders relevant to genetic risk factors. CNS Spectr. 2006;11:855–862. doi: 10.1017/s1092852900015005. [DOI] [PubMed] [Google Scholar]
- 69.Kreek MJ, Nielsen DA, LaForge KS. Genes associated with addiction: alcoholism, opiate, and cocaine addiction. Neuromolecular Med. 2004;5:85–108. doi: 10.1385/NMM:5:1:085. [DOI] [PubMed] [Google Scholar]
- 70.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 71.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 72.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 73.Vanyukov MM, Kirisci L, Tarter RE, Simkevitz HF, Kirillova GP, Maher BS, et al. Liability to substance use disorders: 2. A measurement approach. Neurosci Biobehav Rev. 2003;27:517–526. doi: 10.1016/j.neubiorev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 74.Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neurosci Biobehav Rev. 2003;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, et al. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30:821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- 76.Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ. The role of ventral frontostriatal circuitry in reward-based learning in humans. J Neurosci. 2005;25:8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with eventrelated fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 78.Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- 79.Pohjalainen T, Nagren K, Syvalahti EK, Hietala J. The dopamine D2 receptor 5′-flanking variant, –141C Ins/Del, is not associated with reduced dopamine D2 receptor density in vivo. Pharmacogenetics. 1999;9:505–509. [PubMed] [Google Scholar]