INTRODUCTION
Decreased sense of smell is a common problem affecting approximately 61-69% of patients with chronic rhinosinusitis (CRS) and is one of the four signs and symptoms used to diagnose CRS.1 Olfactory impairment negatively impacts patients' quality of life and ability to function safely in day to day life. 2
Despite being a common complaint in the setting of sinusitis, relatively little objective data is available regarding the impact of endoscopic sinus surgery (ESS) on olfactory function. Much of the olfactory literature is based on subjective reports of olfactory function, which do not accurately assess objective olfactory impairment. 3-4 A small number of prospective studies with objective olfactory outcomes have been performed with mixed results. 3, 5-10 The use of different olfactory measures and definitions of improvement have added confusion to the interpretation of results. Additionally, the majority of studies report short-term follow up of 6 months or less and do not account for long-term changes that may occur in the post-operative period.
In this multi-institutional, prospective cohort study, the impact of ESS on olfactory impairment in patients with CRS was objectively examined over 6 month and 12 month follow up. We hypothesized that patients with mild olfactory impairment (hyposmia) would benefit from ESS whereas patients with severe olfactory impairment (anosmia) would not.
MATERIALS & METHODS
Study Population and Data Collection
Study subjects were recruited from three tertiary care centers over a three-year period as part of a multi-institutional prospective cohort study. All patients had a diagnosis of CRS based on the Rhinosinusitis Task Force criteria and endorsed by the American Academy of Otolaryngology - Head and Neck Surgery. 11 Adult (≥18 yrs old) patients were enrolled at the time they had failed maximum medical management and had elected to undergo ESS. Maximum medical management was defined as a prolonged course of broad-spectrum or culture-directed antibiotics ≥ 4 weeks and a trial of topical nasal corticosteroid spray. 12, 21 Patients with immunodeficiency (n=5), autoimmune disease (n=8), and/or cystic fibrosis (n=12) were excluded.
Demographic and clinical data were collected, including: age, gender, current tobacco use, history of prior sinus surgery, nasal polyposis, asthma, allergic rhinitis confirmed by allergy testing, acetylsalicylic acid (ASA) intolerance, topical and systemic corticosteroid use. Patients on systemic corticosteroids were being treated for significant comorbid disease, including poorly controlled asthma and severe ASA intolerance. Preoperative computed tomography (CT) scan and preoperative endoscopy exam were scored using the Lund-MacKay and Lund-Kennedy scoring systems. 13,14 Olfactory function was measured using the Smell Identification Test (SIT). Clinicians scoring the CT scan and endoscopy exam were blinded to the olfactory test results. The extent of surgery was tailored to the patient's disease process as defined by signs, symptoms, CT scan, and clinical judgment. Patients were followed for one-year postoperatively. Patients underwent olfactory testing and nasal endoscopy exam at 6 and 12 month postoperative follow up. A total of 111 patients with one year post-operative follow up were available for analysis, including 34 patients from Oregon Health & Science University, 38 patients from the Medical College of Wisconsin, and 39 patients from Stanford University. All study protocols and informed consent were collected and approved by the Institutional Review Boards at each study site.
Measurement of Olfactory Function
The SIT from Sensonics, Inc., an objective measure of olfactory function, was administered to patients. 15 The SIT is a validated 40 question forced-choice test (total score: 0-40) with high test-retest reliability (r > 0.90) and is highly correlated with more sophisticated measures of olfactory dysfunction (r> 0.80). 16-17 Absolute SIT scores were categorized into olfactory severity categories based on robust gender-adjusted, normative data (normosmics: men with SIT scores 34-40 and women with SIT scores 35-40; microsmics/hyposmics: men with SIT scores 19-33 and women with SIT scores 19-34; anosmics: men and women with SIT scores 6-18). 15 Patients with SIT scores 0-5 were categorized as malingering and removed from the analyses (n=4). Age was not part of the classification system and was included as a covariate and adjusted for in the analyses.
Statistical Analyses
All statistical analyses were performed using SPSS v.16.0 statistical software (SPSS Inc., Chicago, IL). Baseline demographics, clinical factors, and changes in mean 12-month postoperative SIT scores were compared across preoperative olfactory diagnostic categories using the Kruskal-Wallis test and Chi-square analyses where appropriate. A two-tailed p-value ≤ 0.05 was considered statistically significant. A subset of patients (n=80) also had 6 month postoperative data available for analysis. There was no difference in baseline characteristics of patients with and without 6 month post-operative follow up. The Wilcoxon Signed Rank test was used to examine differences in SIT scores at different time points. Means and standard deviations (± SD) were reported. Olfactory outcomes were stratified by clinical cofactors to assess for effect modification.
The percentage of patients with an absolute change in SIT score of ≥ 4 points was also examined; this corresponded to an individual test-retest change in SIT score outside the 95% confidence interval.22 Chi-square analysis was used to test significant differences in proportions.
Multivariate linear regression modeling was performed to adjust for all significant cofactors associated with improvement in olfactory function. The main outcome of interest was 12- month postoperative change (postoperative minus preoperative) in SIT score. Preliminary models included demographic and patient variables with univariate significance at the p ≤ 0.25 level. Interactions between variables were examined; an interaction variable was retained in the preliminary model if it was significant at the p ≤ 0.10 level. The final model was chosen using manual forward selection and backwards elimination stepwise procedures based on p = 0.05 and p = 0.10 levels of significance. Baseline differences in olfactory function were accounted for by retaining the preoperative SIT score in the final model. Effect modification between baseline olfactory category and nasal polyposis was noted and retained in the model. Diagnostic analyses were performed to verify linearity assumptions and model accuracy.
RESULTS
Demographic Data and Baseline Characteristics of Patients with Olfactory Impairment
Of the 111 patients with 12 month postoperative follow up in this study, 32.4% (n=36) were normosmic, 50.5% (n=56) were hyposmic, and 17.1% (n=19) were anosmic. Baseline demographic characteristics, clinical characteristics, and comorbidity data are described in Table I. On average, patients with olfactory impairment were older (p=0.028), more likely to have nasal polyposis (p<0.001), asthma (p=0.004), and/or aspirin intolerance (p=0.012), and had higher (worse) CT scores (p<0.001) and endoscopy scores (p=0.006).
Table I.
Patients' baseline demographic data and clinical characteristics by olfactory status (n=111).
| Normosmics (n=36) | Hyposmics (n=56) | Anosmics (n=19) | p-value | |
|---|---|---|---|---|
| Age (mean ± SD) | 42.2 ± 13.2 | 50.2 ± 15.3 | 50.9 ± 13.1 | 0.028 |
| Gender %(n) | ||||
| Male | 44.4% (16) | 46.4% (26) | 47.4% (9) | |
| Female | 55.6% (20) | 53.6% (30) | 52.6% (10) | 0.974 |
| Clinical characteristics %(n) | ||||
| Nasal polyposis | 27.8% (10) | 28.6% (16) | 73.7% (14) | 0.001 |
| Asthma | 38.9% (14) | 30.4% (17) | 73.7% (14) | 0.004 |
| Aspirin intolerance | 2.8% (1) | 7.1% (4) | 26.3% (5) | 0.012 |
| Allergic rhinitis | 30.6% (11) | 26.8% (15) | 5.3% (1) | 0.096 |
| Septal deviation | 33.3% (12) | 30.4% (17) | 10.5% (2) | 0.170 |
| Hypertrophy turbinate | 8.3% (3) | 8.9% (5) | 5.3% (1) | 0.878 |
| Prior sinus surgery | 52.8% (19) | 66.1% (37) | 63.2% (12) | 0.435 |
| Smoker | 5.6% (2) | 5.4% (3) | 0(0) | 0.582 |
| Medication Usage %(n) | ||||
| Systemic | 19.4% (7) | 23.2% (13) | 31.6% (6) | 0.599 |
| corticosteroids | ||||
| Disease Severity Measures (mean ± SD) | ||||
| Lund-MacKay CT scores | 10.1 ± 6.0 | 11.8 ± 6.4 | 18.4 ± 5.0 | <0.001 |
| Lund-Kennedy endoscopy score | 5.1 ± 5.0 | 6.1 ± 4.4 | 9.4 ± 5.0 | 0.006 |
Postoperative Results by Olfactory Status
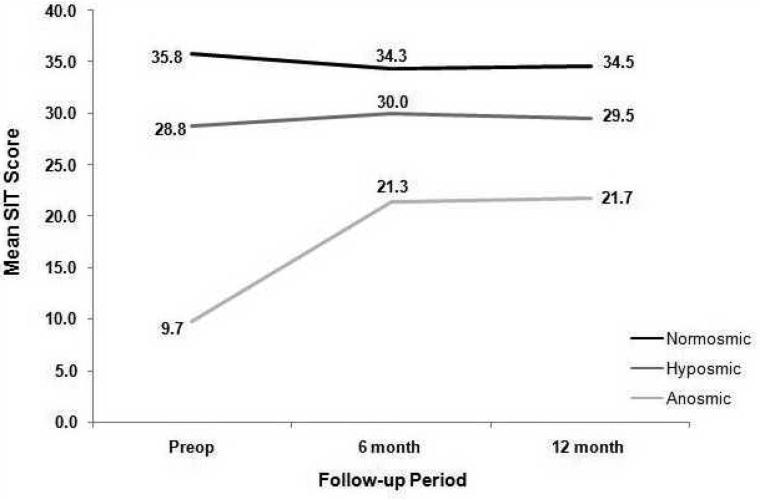
SIT scores in anosmic patients significantly improved after sinus surgery at 6 month follow up (preoperative mean score: 9.7 (± 2.0); 6 month postoperative mean score: 21.3 (±11.2); p=0.001) whereas they did not improve in hyposmic patients (28.8 (±4.5) to 30.0 (±5.5); p=0.113) (Figure 1). Anosmic patients sustained improvement at 12-month follow up (mean SIT score 21.7 (±10.7); p=0.001). There was no change in hyposmics' mean SIT score at 12-month follow up (29.5 (±6.2); p=0.656). In normosmic patients, there was no difference between preoperative and 6 month postoperative scores (35.8 (±1.3) vs 34.3(±5.3), p= 0.273). Furthermore, there was no difference between 6 and 12-month postoperative scores (34.3 (±5.3) vs 34.5 (±3.8); p=0.834).
Figure I.
When change was defined as ≥ 4 point difference between the preoperative SIT score and 12 month postoperative SIT score, olfaction improved in 73.7% of anosmic patients, showed no change in 15.8%, and worsened in 10.5%. In contrast, only 26.8% of hyposmic patients improved after surgery, 53.6% showed no change and 19.6% worsened. Amongst normosmic patients, 88.9% reported no change after surgery, whereas 11.1% reported worse olfaction. The proportion of patients with anosmia who improved after surgery was significantly greater than the proportion of patients with hyposmia who improved after surgery (p<0.001). No preoperative normosmic patient was anosmic at 12-month postoperative follow up.
Role of Nasal Polyposis in Postoperative Improvement
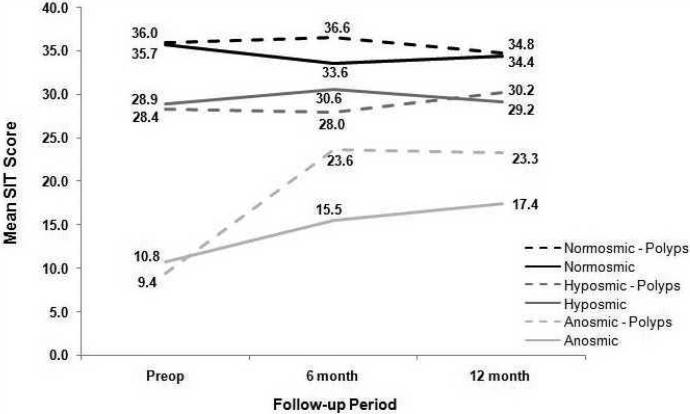
An interaction between nasal polyposis and olfactory function was identified (p=0.007) (Figure 2). In patients with anosmia, those with nasal polyposis had similar preoperative SIT scores as compared to those patients without nasal polyposis (9.4 ± 1.9 vs 10.8 ± 1.9; p=0.224). Postoperatively, anosmic patients with nasal polyposis showed significant improvement in 12-month olfactory change score (13.9 ± 10.2; p=0.002) where as non-polyp patients did not (6.6 ± 12.8; p=0.461). SIT scores did not differ in normosmic and hyposmic patients by nasal polyp status.
Figure II.
Topical and Systemic Corticosteroid Use and Olfaction
Sixty-three percent of patients (n=70) were on topical corticosteroids prior to surgery and 78.4% were using topical corticosteroids one year after surgery (n=87). The preoperative mean SIT score was not statistically different between those taking topical corticosteroids (27.6 ± 9.4) and those not taking topical corticosteroids (28.1 ± 9.6) (p=0.800). The mean SIT scores were not statistically different in each olfactory group by topical corticosteroid use preoperatively or at one-year follow up (Table II).
Table II.
Olfactory scores in patients with and without topical corticosteroid use
| Topical corticosteroid use | No topical corticosteroid use | p-value | |
|---|---|---|---|
| Normosmic (mean ± SD) | |||
| Preoperative | 35.8 ± 1.5 (n=23) | 35.7 ± 1.1 (n=13) | 0.974 |
| 12 month postoperative | 34.8 ± 3.2 (n=27) | 34.0 ± 4.8 (n=9) | 0.886 |
| Hyposmic (mean ± SD) | |||
| Preoperative | 28.3 ± 4.8 (n=35) | 29.7 ± 3.9 (n=21) | 0.336 |
| 12 month postoperative | 29.3 ± 6.1 (n=43) | 29.8 ± 6.5 (n=13) | 0.460 |
| Anosmic (mean ± SD) | |||
| Preoperative | 10.0 ± 2.1(n=12) | 9.1 ± 1.8 (n=7) | 0.384 |
| 12 month postoperative | 22.6 ± 10.2 (n=17) | 20.3 ± 12.2 (n=2) | 0.421 |
Twenty three percent of patients (n=26) were on systemic corticosteroids prior to surgery and 13.5% were taking systemic corticosteroids one year after surgery (n=15). The preoperative mean SIT score was not statistically different between those taking systemic corticosteroids (25.6 ± 10.1) and those not taking systemic corticosteroids (28.4 ± 9.2) (p=0.191). The mean SIT scores were not statistically different in each olfactory group by systemic corticosteroid use preoperatively or at one-year follow up (Table III).
Table III.
Olfactory scores in patients with and without systemic corticosteroids use.
| Systemic corticosteroid use | No systemic corticosteroid use | p-value | |
|---|---|---|---|
| Normosmic (mean ± SD) | |||
| Preoperative | 35.6 ± 1.3 (n=7) | 35.8 ± 1.4 (n=29) | 0.835 |
| 12 month postoperative | 33.0 ± 6.8 (n=4) | 34.7 ± 3.4 (n=32) | 0.959 |
| Hyposmic (mean ± SD) | |||
| Preoperative | 27.5 ± 5.2 (n=13) | 29.1 ± 4.3 (n=43) | 0.369 |
| 12 month postoperative | 26.3 ± 6.4 (n=6) | 29.8 ± 6.1 (n=50) | 0.155 |
| Anosmic (mean ± SD) | |||
| Preoperative | 10.0 ± 2.5 (n=6) | 9.6 ± 1.8 (n=13) | 0.859 |
| 12 month postoperative | 24.0 ± 13.1 (n=5) | 20.9 ± 10.2 (n=14) | 0.516 |
Multivariate Linear Regression Analysis
Finally, a multivariate linear regression analysis was performed with 12 month postoperative change in absolute SIT score as the primary outcome of interest. Baseline olfactory status (namely, anosmia) (p=0.035) and nasal polyposis (p=0.002) were both significant predictors of improvement in SIT score at 12 month postoperative follow up. Furthermore, an interaction, or effect modification, between baseline olfactory status and nasal polyposis was noted both graphically (Figure 2) and statistically in the multivariate linear regression analysis and remained in the final model (p=0.007). Other variables, including age, gender, pre and postoperative topical and systemic corticosteroid use, asthma, allergic rhinitis confirmed by allergy testing, acetylsalicylic acid (ASA) intolerance, current tobacco use, history of prior sinus surgery, preoperative CT scan and preoperative endoscopy score were not significant variables in the model (all p≥ 0.592). Thirty three percent of the variability in the change in olfactory score was explained by the model where:
Such that Y = ß0 - 2.77 (Baseline olfactory status) + 18.84 (Nasal polyposis) - 5.11 (Baseline olfactory status x nasal polyps) + ε, where Y = 12 month change in SIT score, β0 = constant, and ε = error value.
DISCUSSION
In this multi-institutional prospective cohort study, we found that, contrary to our hypotheses, olfactory impairment improved in patients with anosmia after ESS but not in patients with hyposmia. This improvement in anosmic patients' objective olfactory scores was sustained at one-year follow up. Both baseline olfactory status and nasal polyposis were significant predictors of improvement in 12 month postoperative olfactory scores. Finally, no patient who was normosmic prior to surgery was anosmic at one year follow up.
Some believe that nasal polyposis patients find only brief and temporary resolution of olfactory impairment.18 In contrast, we found significant improvement in olfactory scores in anosmic patients with nasal polyposis six months after surgery and this improvement was sustained at 12 month follow up. In fact, nasal polyposis was a significant predictor of better postoperative olfactory outcomes at 12 month follow up. These findings are consistent with other studies in the literature. Pade et al. found nasal polyposis to be the most important factor in determining olfactory improvement 4 months after ESS.7 Minovi et al. found patients with nasal polyposis had a higher success rate of olfactory improvement at six month follow up than other patients.6 Although patients with nasal polyposis are at risk for recurrent disease, the removal of mechanical obstruction from the olfactory cleft and consequent increase in intranasal volume likely improve olfactory function over the one-year postoperative period.
In contrast to our initial hypothesis, we found that anosmic patients improved after ESS whereas hyposmic patients did not. We had suspected that hyposmics had anatomic obstruction secondary to their disease that would benefit from surgical removal whereas anosmic patients would have underlying multifactorial olfactory impairment including both physical obstruction of the olfactory cleft and direct inflammation of the neuroepithelium that would not benefit from surgical treatment. Given our findings that anosmics improved whereas hyposmics did not, we hypothesize that this difference in olfactory outcomes occurred, at least in part, as a result of nasal polyposis causing anosmia. Since patients with nasal polyps and anosmia likely have complete obstruction of the olfactory cleft, surgical resection of this local inflammatory process improved olfaction. In fact, anosmic patients with nasal polyposis improved significantly more than patients without nasal polyposis. Interestingly, this interaction between nasal polyposis and other variables in CRS and olfactory outcomes studies has been noted by us and others in prior studies.6,19 The potential for nasal polyposis to interact with other variables should be addressed in the analyses of outcomes data and should be investigated in future studies.
Several pertinent negative findings were also identified. Age, gender, allergy status, ASA intolerance, and a history of prior sinus surgery were not significant predictors of postoperative olfactory improvement. Some of these findings have been noted in short-term olfactory outcome studies.5-7 ASA intolerance has been found to be a significant predictor in some studies, 6 but not others. 7 We suspect that ASA intolerance was not a significant predictor because it was accounted for in the regression analysis by nasal polyposis. Smoking is a known risk factor for olfactory impairment, but was not a significant predictor of poor olfactory outcomes. Smoking was an uncommon comorbidity in this cohort (n=5) and will likely require a larger sample size to better determine its relationship to olfactory outcomes.
Some studies suggest that use of corticosteroids improves olfaction in patients with sinus disease.20 Reflecting real-life conditions of CRS disease management, a subset of patients took topical and/or systemic steroids to help manage underlying chronic inflammation of the nasal cavity and sinuses. Rather than eliminating them from the dataset, these patients were accounted for in the analyses. Interestingly, there was no difference in the olfactory scores of patients on topical or systemic corticosteroids nor was topical or systemic corticosteroid use a predictor of olfactory improvement at one year follow up.
Importantly, no preoperative normosmic patient became anosmic as a consequence of surgery. A 1% risk of anosmia from nasal surgery is frequently quoted in the literature. 5 However, it is important to note that the patient in the referenced study underwent a septoplasty under local anesthetic, not ESS. While patients should be counseled preoperatively that there is a hypothetical risk of iatrogenic anosmia after ESS, it appears to be low (less than 1%) in the hands of experienced surgeons.
Preoperative CT and endoscopy scores were significantly worse in patients with olfactory impairment. However, neither measure predicted postoperative olfactory improvement; it is likely they were accounted for by patients' nasal polyposis status in the multivariate linear regression analysis. By adjusting for this potential confounder in the model, it was observed that nasal polyposis, not CT score or endoscopy score, predicted who had improved olfactory function after surgery.
Olfactory outcomes following ESS for CRS have been difficult to interpret for several reasons. First, many studies report subjective olfactory results, which do not necessarily correlate with objective olfactory assessment. 4 In fact, patients' self-reported assessment of olfactory dysfunction has poor sensitivity 40% and specificity 30% for determining objective olfactory impairment.3 Consequently, olfactory outcomes studies require the use of objective olfactory measures. Second, several older studies were performed retrospectively, leading to several study limitations and potential biases. More recently, more prospective olfactory outcome studies have performed. However, the heterogeneity of olfactory tests, outcomes, and post-operative follow up have added additional challenges to the comparison of results from various studies, making the interpretation of data difficult, and leading to different outcomes (Table 4). Several different types of objective olfactory measures have been reported in the literature including odor discrimination, threshold, and/or identification testing. Different definitions of improvement have been reported including a minimum change, mean change, and median change in olfactory scores. 5,7-10, Doty
Table IV.
Review of prospective studies that examine olfactory outcomes of endoscopic sinus surgery for chronic rhinosinusitis.
| Study | N | Study Design | Olfactory Measure | Postoperative Follow-up Period | Definition of Improvement | Outcome Measure | Relevant Issues |
|---|---|---|---|---|---|---|---|
| Delank, et al. 19983 | 115 | Prospective | Olfactory threshold and discrimination | 67 days | % of patients who improved | 70% improved; 8% worse | Primary surgery patients only; no revisions |
| Eichel, 199423 | 10 | Prospective case series | UPSIT | ≥12 months | % of patients who improved | 70% (7/10) improved initially, but one patient worsened long-term | Nasal polyp patients only; All patients used topical corticosteroid spray postoperatively; No statistical comparisons |
| Jiang, et al. 20088 | 70 | Prospective | UPSIT; STT; OMT | 6 month | Median change | No significant change in UPSIT, OMT; small but significant change on STT from -2.25 preoperatively to -2.75 postoperatively (p=0.049) | No power analysis to determine adequate sample size; Patients with prior sinus surgery were excluded; No systemic steroids, 44% on nasal steroids postoperatively |
| Kimmelman, et al. 19945 | 93 | Prospective | UPSIT | 2-4 weeks | Mean change | 66% improved or unchanged; 34% worse | Heterogeneous group of nasal surgeries |
| Klimek, et al. 199718 | 31 | Prospective | CCCRC | 6 months | Mean change | Significant change in all 3 qualities (p<0.0001) | All patients had nasal polyps |
| Lund, et al. 19949 | 50 | Prospective | UPSIT; olfactory threshold determination test | 1 year | Mean change | Significant improvement in UPSIT (p<0.05) and olfactory threshold (p<0.05) | Excluded nasal polyp patients |
| Min, et al. 199510 | 80 | Prospective | BTT | 1 year | Mean change; % of patients who improved | Significantly reduced BTT score (p<0.01); % of patients with impaired olfaction decreased from 78% prior to surgery to 64% postoperatively | Excluded patients with asthma, ASA intolerance, history of prior sinus surgery |
| Minovi, et al. 20086 | 64 | Prospective | Custom-built odor ID test | 6 months | Mean change | Chemosensory function improved across all patients (p<0.001) | All patients underwent Type III Draf procedure; unvalidated olfaction instrument |
| Pade, et al. 20087 | 206* | Prospective | “Sniffin' Sticks” | 4 months | 3+ points on a 16 point scale | 23% improved; 68% no change; 9% worsened | Excluded patients on systemic corticosteroids; Topical corticosteroid use unknown |
| Sugiyama, et al. 200624 | 100 | Prospective | T&T3, T&T5, UPSIT12, UPSIT40 | 6 months | Rank order | Significant change in all 4 scores (p<0.001) | 33% of patients smoked |
| Yamagishi, et al. 198925 | 20 | Prospective case series | T&T, Alinamine intravenous admin. test | 6 months | Individual change | 80% improved on T&T |
BTT = Butanol Threshold Test; CCCRC = Connecticut Chemosensory Clinical Research Center (Odor identification test, threshold test, and discrimination test); OMT= odor memory/discrimination test; “Sniffin' Sticks” (Odor identification test); STT (single staircase phenyl ethyl alcohol odor detection threshold test); T&T3 olfactometer = 3 odorant recognition threshold test; T&T5 olfactometer = 5 odorant recognition threshold test; UPSIT40 = University of Pennsylvania Smell Identification Test (Odor identification test); UPSIT12 = Brief University of Pennsylvania Smell Identification Test (Odor identification test);
206 patients underwent sinus surgery and had postoperative follow up.
Finally, many prospective studies are limited by short-term (≤ 6 month) follow up. Long-term follow up is important for several reasons. Patients assessed within the first couple of months after surgery may still be healing from surgery. Edema and granulation tissue may interfere with results and lead to negative studies. Conversely, patients with initial recovery and significant improvement in the early post operative period may later suffer from scar formation, recurrence of disease, and new polyp formation in the region of the olfactory cleft that is not captured in short term data.
In this study, we prospectively evaluate the olfactory outcomes of ESS using an objective olfactory measure, reporting both the means and proportion of patients with improvement, and using intermediate and long-term olfactory outcomes to examine both short-term trends and long-term consequences of ESS. Our findings regarding one year postoperative improvement are consistent with the limited number of other studies in the literature with at least one year follow up where patients' olfactory function improved, but did not return to normal, following surgical intervention. 9,10
Our study size may have been a limitation in that we were unable to detect a difference in patients with uncommon comorbidities, such as smoking. However, we had appropriate power to detect olfactory improvement, differences in subgroups of patients, and the ability to adjust for potential confounders, such as corticosteroid use, in our analysis. Furthermore, our 12-month findings are consistent with the few other published reports in the literature.
CONCLUSION
In conclusion, olfactory impairment is common in patients with CRS. In this multi-institutional prospective cohort study, olfactory dysfunction improved in patients with anosmia after ESS and was sustained at one-year follow up. In contrast, patients with hyposmia did not improve after surgery. While anosmics experienced significant improvement, olfactory function did not return to normal in most patients. No normosmic became anosmic after surgery. Olfactory impairment is an important patient safety and quality of life issue for patients with CRS and one that requires continued research.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would wish to thank Peter Hwang, MD at Stanford University and Todd Loehrl, MD at the Medical College of Wisconsin for their efforts with participant enrollment and study involvement.
This publication was made possible with support from NIH/NIDCD R01 DC005805 (PI: TL Smith) and the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
All study protocols and informed consent were approved by the Institutional Review Board.
Study findings were orally presented at the American Academy of Otolaryngology-Head & Neck Surgery Annual Meeting, Chicago, Illinois, September 22, 2008.
REFERENCES
- 1.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guidelines: Adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(suppl):S1–S31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 2.Temmel AFP, Quint C, Schickinger-Fischer B, et al. Characteristics of Olfactory Disorders in Relation to Major Causes of Olfactory Loss. Arch Otolaryngol Head Neck Surg. 2002;128:635–641. doi: 10.1001/archotol.128.6.635. [DOI] [PubMed] [Google Scholar]
- 3.Delank KW, Stoll W. Olfactory Function after Functional Endoscopic Sinus Surgery for Chronic Rhinosinusitis. Rhinology. 1998;36:15–19. [PubMed] [Google Scholar]
- 4.Landis BN, Hummel T, Hugentobler M, et al. Ratings of overall olfactory function. Chem Senses. 2003;28:691–694. doi: 10.1093/chemse/bjg061. [DOI] [PubMed] [Google Scholar]
- 5.Kimmelman C. The Risk to Olfaction From Nasal Surgery. Laryngoscope. 1994;104:981–988. doi: 10.1288/00005537-199408000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Minovi A, Hummel T, Ural A, et al. Predictors of the outcome of nasal surgery in terms of olfactory function. Eur Arch Otorhinolaryngol. 2008;265:57–61. doi: 10.1007/s00405-007-0409-7. [DOI] [PubMed] [Google Scholar]
- 7.Pade J, Hummel T. Olfactory function following nasal surgery. Laryngoscope. 2008;118:1260–1264. doi: 10.1097/MLG.0b013e318170b5cb. [DOI] [PubMed] [Google Scholar]
- 8.Jiang RS, Lu FJ, Liang KL, et al. Olfactory function in patients with chronic rhinosinusitis before and after functional endoscopic sinus surgery. Am J Rhinol. 2008;22:445–448. doi: 10.2500/ajr.2008.22.3195. [DOI] [PubMed] [Google Scholar]
- 9.Lund VJ, Scadding GK. Objective assessment of endoscopic sinus surgery in the management of chronic rhinosinustis: an update. J Laryngol Otol. 1994;108:749–753. doi: 10.1017/s0022215100128014. [DOI] [PubMed] [Google Scholar]
- 10.Min Y-G, Yun K-S, Song BH, et al. Recovery of nasal physiology after functional endoscopic sinus surgery: olfaction and mucociliary transport. ORL. 1995;57:264–268. doi: 10.1159/000276755. [DOI] [PubMed] [Google Scholar]
- 11.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surgery. 2003;129(suppl):S1–S32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 12.Anand VK, Osguthorpe JD, Rice D. Surgical management of adult rhinosinusitis. Otolaryngology-Head & Neck Surgery. 1997;117:S50–S52. doi: 10.1016/S0194-59989770007-X. [DOI] [PubMed] [Google Scholar]
- 13.Lund VJ, Mackay IS. Staging in rhinosinusitis. Rhinology. 1993;107:183–184. [PubMed] [Google Scholar]
- 14.Lund VJ, Kennedy DW. Quantification for staging sinusitis. International Conference on Sinus Disease: terminology, staging, therapy. Ann Oto Rhinol Laryngol. 1995;104(Suppl):17–21. [PubMed] [Google Scholar]
- 15.Doty RL. Haddon Heights. 3rd ed. Sensonics, Inc.; New Jersey: 1995. The Smell Identification Test™ Administration Manual; pp. 1–17. [Google Scholar]
- 16.Doty RL, Newhouse MG, Azzalina JD. Internal consistency and short-term test-retest reliability of the University of Pennsylvania Smell Identification Test. Chem Senses. 1985;10:297–300. [Google Scholar]
- 17.Doty RL, McKeown DA, Lee WW, Shaman P. A study of the test-retest reliability of ten olfactory tests. Chem Senses. 1995;20:645–56. doi: 10.1093/chemse/20.6.645. [DOI] [PubMed] [Google Scholar]
- 18.Klimek L, Moll B, Amedee RG, Mann WJ. Olfactory function after microscopic endonasal surgery in patients with nasal polyps. Am J Rhinol. 1997;11:251–55. doi: 10.2500/105065897781446621. [DOI] [PubMed] [Google Scholar]
- 19.Litvack JR, Griest S, James KE, Smith TL. Endoscopic and Quality-of-Life Outcomes After Revision Endoscopic Sinus Surgery. Laryngoscope. 2007;117:2233–38. doi: 10.1097/MLG.0b013e31814539e8. [DOI] [PubMed] [Google Scholar]
- 20.Doty RL, Mishra A. Olfaction and Its Alteration by Nasal Obstruction, Rhinitis, and Rhinosinusitis. Laryngoscope. 2001;111:409–423. doi: 10.1097/00005537-200103000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benninger MS, Anon J, Mabry RL. The medical management of rhinosinusitis. Otolaryngology-Head & Neck Surgery. 1997;117:S41–S49. doi: 10.1016/S0194-59989770006-8. [DOI] [PubMed] [Google Scholar]
- 22.Doty RL, Yousem DM, Pham LT, et al. Olfactory Dysfunction in Patients with Head Trauma. Archives of Neurology. 1997;54:1131–1140. doi: 10.1001/archneur.1997.00550210061014. [DOI] [PubMed] [Google Scholar]
- 23.Eichel BS. Improvement of olfaction following pansinus surgery. ENT. 1994;73:248–250. 253. [PubMed] [Google Scholar]
- 24.Sugiyama K, Hasegawa Y, Sugiyama N, et al. Smoking-induced olfactory dysfunction in chronic sinusitis and assessment of brief University of Pennsylvania Smell Identification Test and T&T methods. Am J Rhinol. 2006;20:439–44. doi: 10.2500/ajr.2006.20.2924. [DOI] [PubMed] [Google Scholar]
- 25.Yamagishi M, Hasegawa S, Suzuki S, et al. Effect of surgical treatment of olfactory disturbance caused by localized ethmoiditis. Clin Otolaryngol Allied Sci. 1989;14:405–409. doi: 10.1111/j.1365-2273.1989.tb00394.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.