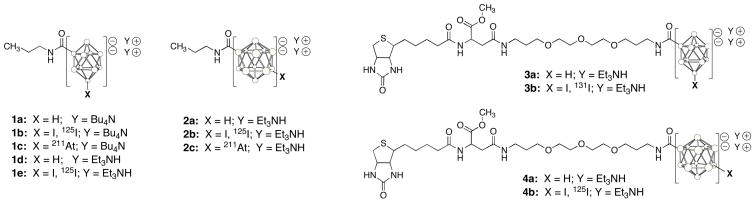
Abstract
In vivo deastatination has been a major problem in the development of reagents for therapeutic applications of the α-particle emitting radionuclide 211At. Our prior studies demonstrated that the use of a closo-decaborate(2-) ([closo-B10H9R]2−) moiety for 211At labeling of biomolecules provides conjugates that are stable to in vivo deastatination. In this investigation, the closo-decaborate(2-) moiety was compared with the structurally similar closo-dodecaborate(2-) ([closo-B12H11R]2−) to determine if one has more favorable properties than the other for use in pendant groups as 211At labeling molecules. To determine the differences, two sets of structurally identical molecules, with the exception that they contained either a closo-decaborate(2-) or a closo-dodecaborate(2-) moiety, were compared with regards to their synthesis, radiohalogenation, stability to in vivo deastatination and tissue distribution. Quite different rates of reaction were noted in the synthetic steps for the two closo-borate(2-) moieties, but ultimately the yields were similar, making these differences of little importance. Differences in radiohalogenation rates were also noted between the two closo-borate(2-) moieties, with the more electrophilic closo-decaborate(2-) reacting more rapidly. This resulted in somewhat higher yields of astatinated closo-decaborate(2-) derivatives (84% vs 53%), but both cage moieties gave good radioiodination yields (e.g. 79–96%). Importantly, both closo-borate(2-) cage moieties were shown to have high stability to in vivo deastatination. The largest differences between pairs of compounds containing the structurally similar boron cage moieties were in their in vivo tissue distributions. For example, [Et3NH]2B12H10I-CONHpropyl, [125I]2b had high concentrations in kidney (1h, 19.8 %ID/g; 4h, 26.5%ID/g), whereas [Et3NH]2B10H8I-CONHpropyl, [125I]1e had much lower concentrations in kidney (1h, 6.6%ID/g; 4h, 0.27%ID/g). Interestingly, when another salt of the closo-decaborate(2-), [nBu4N]2B10H8I-CONHpropyl, [125I]1b, was evaluated, the route of excretion appeared to be hepatobiliary rather than renal. Identical biotin derivatives containing the two closo-borate(2-) cage moieties had similar tissue distributions, except the closo-decaborate(2-) derivative had lower concentrations in kidney (1h, 19.9%ID/g; 4h, 24.4%ID/g vs. 1h, 38.9%ID/g; 4h, 40.6%ID/g). In summary, the higher reactivity, faster tissue clearance, and lower kidney concentrations make the closo-decaborate(2-) more favorable for further studies using them in reactive groups for 211At labeling of biomolecules.
Keywords: Astatination, Radioiodination, Decaborate, Dodecaborate, Biotin Derivatives
INTRODUCTION
The radionuclide, 211At (t1/2 = 7.21 h) is one of only a few α-emitting radionuclides considered useful for targeted radionuclide therapy of cancer (1–4). Although 211At has a relatively short half-life (t1/2 = 7.21 h), 100% of its decays (in a branched chain) emit an α-particle and the decay process does not result in production of α-particle emitting daughters (5). The short pathlengths of α-particle emissions (e.g. 50–80 μm) and the high linear energy deposition (>100 keV/μm) of those emissions make α-particles very effective in cell killing. Most importantly, the cell-killing properties of α-emissions make them potentially useful for killing micrometastatic disease, which is a major impediment to successful therapy of cancer (6).
Coupling 211At with biomolecules that target cancer cells is very important for its application to therapy of cancer. Unfortunately, in vivo deastatination of biomolecules labeled with 211At has been problematic in the development of therapeutic radiopharmaceuticals (7). In fact, the deastatination of labeled compounds has severely limited the in vivo application of 211At. To circumvent this shortcoming, several research groups have evaluated the use of aryl pendant groups to stabilize the 211At-labeled biomolecules in vivo (8–11). However, in many examples astatinated aryl groups are not stable to deastatination when the carrier biomolecule is rapidly metabolized. More recently our research group has focused on using anionic boron cage pendant groups for labeling with 211At. While the in vivo stability was found to be low for 211At-bonded nido-carborane1 pendant groups (12), we have found that the pendant groups containing closo-decaborate(2-) moieties, [B10H9R]2−, can be used to provide high 211At labeling yields and results in high stability in vivo (13).
Other investigators have reported the use of a similar boron cage moiety, closo-dodecaborate(2-), [closo-B12H11R]2− for radioiodine (14, 15) and 211At labeling (16). Our interest in using closo-borate(2-) cage derivatives as pendant groups in 211At labeling led to an investigation to determine which of the two closo-borate(2-) cage moieties has the most favorable properties for in vivo applications. In the investigation, we compared the chemical syntheses, radiolabeling efficiency and in vivo stability of two pairs of identical compounds containing closo-decaborate(2-) and closo-dodecaborate(2-) moieties as depicted in Figure 1 (i.e. 1 vs. 2 & 3 vs. 4). Use of pairs of compounds having the same structure except for the closo-borate(2-) cage permits evaluation of the effect of those moieties on chemical and in vivo properties of the compounds studied. Although the test compounds were identical except for the closo-borate(2-) moiety, it was anticipated that differences would be observed as it is known that the chemistry and properties of closo-decaborate (2-) and closo-dodecaborate(2-) derivatives can be quite different (17–19).
Figure 1.
closo-Decaborate(2-) derivatives (1a–1e, 3a, 3b) and closo-dodecaborate(2-) derivatives (2a–2c, 4a, 4b) prepared and evaluated. For simplicity in the closo-borate(2-) cage depictions, the open circles represent B (where substituted) or BH atoms.
In the first part of the investigation, carbonyl derivatives of the two boron cage moieties were modified by n-propylamine to provide simple closo-borate(2-) derivatives, 1a and 2a, which have a (small) lipophilic propylamide moiety attached. In an initial study, the nBu4N salt of closo-decaborate(2-), 1a, was prepared to facilitate its isolation and characterization. The Et3NH salt was used with the closo-dodecaborate(2-), 2a. The propionamide derivatives 1a and 2a were radioiodinated and astatinated, then each radiolabeled closo-borate(2-) pair (i.e. [125I]1b/[211At]1c & [125I]2b/[211At]2c) was mixed and coinjected into mice to determine their biodistributions and relative in vivo stability of 211At attachment. Due to unexpected in vivo distributions of the [125I]1b/[211At]1c pair of radiolabeled compounds, another biodistribution study was carried out with [125I]1e, in which the counterions were changed from nBu4N to Et3NH. As another part of the investigation, biotin derivatives 3a and 4a were prepared, again incorporating the two closo-borate(2-) cage moieties into otherwise identical chemical structures. To evaluate the differences in their in vivo distributions, the biotin derivatives were radioiodinated to yield [131I]3b and [125I]4b, and that pair of radiolabeled compounds was coinjected into mice. The results of our investigation to compare chemistry and properties of structurally similar compounds containing the closo-decaborate(2-) and closo-dodecaborate(2-) moieties are reported herein.
MATERIALS AND METHODS
Materials
All chemicals obtained from commercial sources were analytical grade or better and were used without further purification. 4,7,10-Trioxa-1,13-tridecanediamine, solvents and most other chemicals were obtained from Aldrich (Sigma-Aldrich, Milwaukee, WI). L-Aspartate-α-methyl ester (H-Asp-OMe) was obtained from Bachem (Bachem California, Inc., Torrance, CA). Biotin N-hydroxysuccinimide ester, 9, was obtained from Quanta BioDesign, Ltd (Powell, OH). Trifluoroacetyl Tetrafluorophenol (TFA-O-TFP) was prepared as previously described (20). Silica gel chromatography was conducted with 70–230 mesh 60 Å silica gel (Aldrich). Decaborane was obtained from Alfa (Alfa Aesar, Ward Hill, MA).
Radioactivity
All radioactive materials were handled according to approved protocols at the University of Washington. Na[125I]I and Na[131I]I were purchased from Perkin-Elmer Life and Analytical Sciences, Inc. (Waltham, MA) as a high concentration solution in 0.1 N NaOH. 211At was produced by irradiation of a thin layer of bismuth metal (99.999%, Aldrich) with a 28 MeV α-particle beam on a Scandatronix MC-50 cyclotron (University of Washington). Isolation of Na[211At]At was conducted in a charcoal-filtered glove box (Innovative Technologies, Inc., radioisotope glove box) by distillation from the irradiated target using the conditions previously described (13, 21). Radiohalogenations were conducted within a charcoal-filtered Plexiglas enclosure (Biodex Medical Systems Inc., Shirley, N.Y.) housed in a radiochemical fume hood. Radiohalogenation reactions were carried out in vials capped with Teflon-coated septa vented through a 10 mL charcoal-filled syringe.
Measurement of 125I, 131I, and 211At was accomplished on the Capintec CRC-15R Radioisotope Calibrator using the manufacturer’s settings for those radionuclides. In the experiment evaluating an admixture of 131I with 125I, the 125I counts were compensated for spillover from the 131I window (32%). Tissue samples were counted in a Wallac 1480 gamma counter with the following window settings: channels 35–102 and 165–185 for 125I and 131I were counted together, and channels 20–2000 (open window) when 211At and 125I were counted together. In experiments containing an admixture of 211At/125I counting was conducted twice, soon after animal sacrifice to obtain total counts and again after samples had decayed for 3–5 days to obtain the 125I counts. 211At counts were obtained by subtracting 125I counts from the total counts. All 211At counts were corrected for decay during the counting process.
Spectral Analyses
1H/11B NMR and mass spectral analyses were obtained on new compounds. The data obtained is provided with the experimental procedures. Spectral data for compounds previously reported are not listed, but were consistent with the reported data. 1H and 11B NMR spectra were obtained on a Bruker AV-300 (300 MHz for 1H; 96.3 MHz for 11B); or a Bruker AV-500 (500 MHz 1H and 160.4 MHz for 11B). 1H NMR data are referenced to tetramethylsilane as an internal standard (δ = 0.0 ppm), and 11B NMR data are referenced to BF3·OEt2 as an external standard. Infrared spectra were obtained on a Bruker Vector 33 Fourier Transform Infrared Spectrophotometer. Mass spectral data (both low and high resolution) were obtained on a VG Analytical (Manchester, England) VG-70 SEQ mass spectrometer with associated 11250J Data System. Although several attempts were made, we were unsuccessful in obtaining mass spectral data for two of the closo-dodecaborate(2-) derivatives, 2b and 4b.
Analytical Chromatography
HPLC separations of the non-radioactive compounds were obtained using a system that contained a Hewlett-Packard quaternary 1050 gradient pump, a variable wavelength UV detector (254 nm), and an ELSD 2000 evaporative light-scattering detector (Alltech, Deerfield, IL). Analysis of the HPLC data was conducted on Hewlett-Packard HPLC ChemStation software. Reversed-phase HPLC chromatography was carried out on an Alltech Altima C-18 column (5 μm, 250 × 4.5 mm) using a gradient solvent system at a flow rate of 1 mL/min. The gradient mixture was composed of MeOH (eluant A) and 0.05 M, pH 5.5 aqueous triethylammonium acetate (eluant B). The gradient started with 20% MeOH and 80% eluant B for two minutes; then the % MeOH was increased linearly to 100% over a 13 min period; following this the elution continued with 100% MeOH for an additional 5 min. The retention time (tR) of each iodinated compound is provided with the experimental procedure.
Preparative Chromatography
Products were purified from crude reaction mixtures using a Biotage SP Flash Purification System (Charlottesville, VA) on a reversed-phase C18 FLASH 25+M column. The purification used a gradient mixture composed of MeOH and 0.05 M Et3NHOAc (TEAA). The gradient started with 80% 0.05 M TEAA, and MeOH was increased linearly to 100% over the next 20 min. Fractions were collected based on UV detection at 215 or 254 nm. Fractions containing pure products were determined by analytical HPLC and were combined, solvent evaporated, and isolated to provide the yields listed.
Radio-HPLC
The product mixtures from radioiodination and astatination reactions were analyzed by HPLC using a C-18 column (Altima C-18, 5 μm, 250 × 4.5 mm; Alltech, Deerfield, IL) eluting at a flow rate of 1 mL/min with a gradient beginning with 20% MeOH/80% of an aqueous Et3NHOAc solution. The HPLC equipment used in the analyses consisted of a Hewlett-Packard quaternary 1050 gradient pump, Waters 601 UV detector, and a Beckman model 170 radioisotope detector. Analysis of the HPLC data was conducted on Hewlett-Packard HPLC ChemStation software. Isolation of radiolabeled compounds was accomplished by collection of part (when broad) or the entire radioactive peak. The retention times (tR) of the radioiodinated and astatinated compounds are provided in the experimental procedures.
[Et3NH]2[closo-B10H10], 5a
This compound was prepared by a modification of the procedure described by Hawthorne and Pilling (22). A 25 g quantity (0.21 mol) of decaborane (B10H14) was dissolved in 400 mL xylene in a 3-neck flask. The flask was flushed with Ar, then 75 mL (0.54 mol) of Et3N was added dropwise over 30 min. Following the addition, the reaction mixture was heated to 105°C for 3 h, then refluxed (140°C) for another 5 h. The reaction mixture was allowed to cool to room temperature, transferred to a round bottom flask and the xylene was removed with heating on a rotary evaporator. Addition of 250 mL of MeOH to the residue gave a colorless precipitate. The precipitate was filtered and set aside. The remaining solution was triturated with EtOAc (750 mL), filtered and washed with EtOAc (1L). The combined precipitate was placed under high vacuum to yield 62.3 g (94%) of 5a. Spectral properties of this compound were as previously reported (22).
[nBu4N]2[closo-B10H10], 5b
A 2.0g (6.20 mmol) quantity of [Et3NH]2[B10H10] was dissolved in water (100 mL), then a solution containing n-Bu4NBr (6.0 g, 18.61 mmol) in water (20 mL) was added slowly with stirring at room temperature. The precipitate was filtered, washed with water (1 L), dried under vacuum for overnight to afford the product as a colorless solid. 3.2 g (86%).
Na2[closo-B10H10], 5c
A 25 × 1.5 cm column was filled with Amberlite IR120 resin (Sigma-Aldrich, St. Louis, MO) in deionized H2O. Then a 200 mL quantity of 3M HCl was run across the column, followed by 750 mL of deionized H2O. After acid conditioning, 250 mL of a NaCl solution (1M) was passed over the column, followed by 750 mL of deionized H2O. After conversion to the Na form, 100 mg sample of 5a (0.31 mmol) was dissolved in 2 mL of a 1:1 mixture of CH3CN:H2O and was loaded on the column. The column was eluted with deionized H2O (100 mL). The eluted aqueous solution was evaporated to a solid residue on a rotoevaporator, then placed under high vacuum overnight to give 51 mg (0.31 mmol, 100%) of 5c.
[Et3NH]2[closo-B12H12], 6
This compound was prepared by a modification of a procedure described by Miller and Muetterties (23). A 240 mL quantity of undecane was placed in a 1L 3-neck flask equipped with a thermometer on one neck and a condenser with an addition funnel on top on the middle neck and a septum on the third neck. The addition funnel was capped with a septum for Ar introduction (with bubbler) and hydrogen release. The system was placed under argon and heated to 190–197°C. In a separate flask, 32.0 g decaborane (262 mmol) was placed in a flask under argon and 84 mL of borane-triethylamine complex (65.3 g, 567 mmol) was added. When the decaborane was dissolved, it was filtered through glass wool into the addition funnel. The mixture was then slowly dripped into the hot undecane, keeping the temperature above 190°C. Large amounts of hydrogen evolved. After addition of the decaborane solution, the mixture was heated for an additional 30 min. The mixture became thick with white/yellow solid and was difficult to stir. After cooling, the precipitate was filtered by gravity and the solid was washed with 200 mL ether to remove residual undecane. The solid was air-dried, then was dissolved in 1.5L boiling water and heated for 30 min. A thick gummy material collected, floating on top of the solution. This was then filtered hot and the resultant solution was allowed to cooled to room temperature, then placed in a refrigerator at 4°C. After 3 hours at 4°C, the colorless solid was collected by vacuum filtration and dried on high vacuum to yield 34.6 g. The filtrate was concentrated to 200 mL, yielding another 15.9 g. A total yield 50.5 g (53.7%) of 6 was obtained. Spectral properties of isolated 6 were as previously reported (23).
[nBu4N][closo-B10H9-CO], 7a
This compound was prepared using a procedure similar to that previously reported (24). A 0.5 mL quantity (5.26 mmol) of oxalyl chloride was added slowly over 5 min to a solution of [nBu4N]2[B10H10] (1 g, 1.66 mmol) in anhydrous CH2Cl2 (15 mL) at 0°C. The mixture was stirred at the 0°C for 30 min, then allowed to warm to room temperature while stirring for additional 30 min. The reaction solution was evaporated to dryness on a rotary evaporator under vacuum. The residue was recrystallized from CH2Cl2/Et2O to yield 0.74 g (71%) of brown tacky solid. IR (cm−1, KBr disk): 2389 (s), 2340 (s), 2308 (s); 11B NMR (DMSO-d6, 160.43 MHz ) δ 5.71 (1B), −4.52 (4B), −15.70 (2B), −21.64 (2B), −25.80 (1B).
[Et3NH][closo-B10H9-CO], 7b (24)
A 1.08 mL (12.4 mmol) quantity of oxalyl chloride was added slowly over 5 min to a solution of [Et3NH]2[B10H10] (2 g, 6.2 mmol) in anhydrous CH3CN (30 mL) at 0°C. The mixture was stirred at 0°C for 30 min, then allowed to warm to room temperature while stirring for additional 30 min. The reaction solution was evaporated to dryness on a rotary evaporator under vacuum. The residue was recrystallized from CH3CN/Et2O to yield 1.8 g (83%) of a brown tacky solid.
[Et3NH][closo-B12H11-CO], 8
A 1 mL (11.46 mmol) quantity of oxalyl chloride was added to a solution of [Et3NH]2[B12H12] (1 g, 2.89 mmol) in anhydrous CH3CN (20 mL) at room temperature, then the mixture was stirred and heated to reflux for 3 h. The solution was allowed to cool to room temperature, then was evaporated to dryness on a rotary evaporator under vacuum. The residue was recrystallized from CH3CN/Et2O to yield 0.84 g (78%) of a brown tacky solid. IR (cm−1, KBr disk): 2392 (s), 2336 (s), 2284 (s); 11B NMR (DMSO-d6, 160.43 MHz ) δ −10.96 (1B), −11.98 (1B), −13.11 (1B), −14.20 (2B), −15.16 (3B), −15.82 (4B).
[nBu4N]2[closo-B10H9-CONHpropyl], 1a
A solution of [nBu4N][closo-B10H9-CO], 7a (400 mg, 1.032 mmol), n-propylamine (0.75 mL, 9.12 mmol), Et3N (0.72 mL, 5.16 mmol) in anhydrous CH3CN (10 mL) was stirred at room temperature for 16 h. EtOAc (15 mL) was added and the resultant solution was stirred at room temperature for 20 min. The solvents were decanted, and the crude residue was redissolved in 4 mL of MeOH/CH3CN (1/1) and purified via preparative reversed-phase chromatography. Purification yielded 410 mg (83%) of 1a. 1H NMR (CD3OD, 500 MHz): δ −0.25–0.75 (m, 9H), 0.86(t, J = 7.4 Hz, 3H), 1.02 (t, J = 7.2 Hz, 24 H), 1.36–1.48 (m, 16 H), 1.65–1.75 (m, 18 H), 2.98 (t, J = 6.8 Hz, 2 H), 3.26–3.29 (m, 16H). 11B NMR (CD3OD, 160.43 MHz): δ −0.62 (1B), −1.39 (1B), −23.90 (1B), −28.26 (7B). LRMS (ES−) calcd for C4H17B10NO (M)−: 205.2. Found: 205.1. HPLC: tR = 11.6 min.
[Et3NH]2B10H8I-CONH-propyl, 1b
A 746 μL aliquot of a 10 mg/mL solution of chloramine-T (32.7 μmol) in H2O was added to a solution containing 20 mg (65.5 μmol) [Et3NH]2B10H9-CONH-Pr, 1a and 4.9 mg NaI (49 μL of 100 mg/mL in H2O, 32.7 μmol;) in 1 mL MeOH/H2O with 5% HOAc (1:1). The reaction mixture was stirred at room temperature for 30 sec, then Na2S2O5 (622 μL, 32.7 μmol, 10 mg/mL in H2O) was added. The product was identified as a new, more lipophilic, species by reversed-phase HPLC (1b had approximately the same peak area as 1a by ELSD detection). LRMS (ES−) calcd for C4H16B10INO (M)−: 331.1. Found: 331.1; HPLC: tR = 4.0 min.
[nBu4N]2B10H8I-CONHpropyl, [125I]1b
To 100 μL of 1 mg/mL 1a in 50/50 water/MeOH with 5% HOAc was added 3 μL of Na[125I]I (980 μCi in 0.1N NaOH) followed by 10 μL of 1 mg/mL chloramine-T in H2O. After 2 min at room temperature, 10 μL of 1 mg/mL Na2S2O5 was added to quench the reaction. Analytical radioHPLC indicated that an 86% labeling efficiency was obtained. The remainder of the radiolabeled material was injected on the HPLC in two fractions and collected in a peak at 4.2 min to give 250 μCi of isolated product (26% isolated from leading portion of product peak). This was concentrated on a rotary evaporator, diluted with PBS and neutralized with NaOH for the animal study.
[nBu4N]2B10H8At-CONHpropyl, [211At]1c
To 100 μL of 1 mg/mL 1a in 50/50 water/MeOH with 5% HOAc was added 100 μL of Na[211At]At (500 μCi in 0.05N NaOH) followed by 10 μL of 1 mg/mL chloramine-T in H2O. After 5 min at room temperature, 10 μL of 1 mg/mL Na2S2O5 in H2O was added to quench the reaction. Analytical radioHPLC indicated that an 84% labeling efficiency was obtained. The remainder of the radiolabeled material was injected on the HPLC and the leading portion of the peak at 4.8 min to give 180 μCi was isolated (36%). This was concentrated on a rotary evaporator, diluted with PBS and neutralized with NaOH for an animal study.
[Et3NH]2[closo-B10H9-CONHpropyl], 1d
This compound was prepared in 79% yield using the same reaction conditions as used to prepare 1a. HPLC: tR = 3.8 min.
[Et3NH]2B10H8I-CONHpropyl, [125I]1e
To 100 μL of 1 mg/mL 1d in 50/50 MeOH/5% HOAc was added 4 μL of Na[125I]I (1.4 mCi) in 0.1N NaOH followed by 20 μL of 1 mg/mL chloramine-T in water. After 1 min at rt, the reaction was quenched with 20 μL of 1 mg/mL Na2S2O5 in H2O. The reaction mixture was injected on the HPLC and the peak at 12 min was collected for an isolated yield of 81% (HPLC indicated 82% reaction efficiency).
[Et3NH]2[closo-B12H11-CONHpropyl], 2a
A 500 mg (1.84 mmol) quantity of [Et3NH][B12H11-CO], 8 was dissolved in 10 mL n-propylamine and was stirred at room temperature for 16 h. Excess n-propylamine was removed under vacuum. The crude residue was dissolved in 6 mL of MeOH/DMF/H2O (1/1/1) and was purified via preparative reversed-phase chromatography. Isolation of the product yielded 510 mg (84%) of 8. 1H NMR (DMSO-d6, 500 MHz): δ 0.39–1.30 (m, 11H), 0.87 (t, J = 7.4 Hz, 3H), 1.16 (t, J = 7.2 Hz, 18H), 1.50–1.58 (m, 2H), 2.73 (t, J = 7.4 Hz, 2H), 3.01 (q, J = 7.3 Hz, 12H), 3.15 (s, 1H). 11B NMR (CD3OD, 160.43 MHz): δ −0.43 (1B), −14.92 (5B), −15.54 (6B). LRMS (ES−) calcd for C4H19B12NO (M)−: 229.2. Found: 229.1. HPLC: tR = 3.7 min.
[Et3NH]2B12H10I-CONH-propyl, 2b
A 692 μL (30.4 μmol) aliquot of a 10 mg/mL solution of chloramine-T in H2O was added to a solution containing 20 mg (60.8 μmol) [Et3NH]2B12H11-CONH-Pr, 2a and 4.56 mg NaI (30.4 μmol; 45.6 μL of a 100 mg/mL solution in H2O) in 1 mL of MeOH/H2O with 5% HOAc (1:1). The solution was stirred at room temperature for 30 sec, then Na2S2O5 (578 μL, 30.4 μmol, 10 mg/mL in H2O) was added. The product was identified as a new, more lipophilic, species by reversed-phase HPLC (2b had approximately the same peak area as 2a by ELSD detection). HPLC: tR = 4.0 min.
[Et3NH]2B12H10I-CONHpropyl, [125I]2b
To 120 μL of 1 mg/mL 1a in 50/50 water/MeOH with 1% HOAc was added 1 μL of Na[125I]I (566 μCi in 0.1 N NaOH) followed by 20 μL of 1 mg/mL chloramine-T in H2O. After 2 min at room temperature, 20 μL of 1 mg/mL Na2S2O5 in H2O was added to quench the reaction. Analytical radioHPLC indicated that a 79% labeling efficiency was obtained. The remainder of the radiolabeled material was injected on the HPLC in two fractions and collected in a broad peak at 10 min to give 306 μCi (68%) of isolated product. This was concentrated on a rotary evaporator, diluted with PBS and neutralized with NaOH for use in the animal study.
[Et3NH]2B12H10At-CONHpropyl, [211At]2c
To 120 μL of 1 mg/mL 1a in 50/50 water/MeOH with 1% HOAc was added 100 μL of Na[211At]At (633 μCi in 0.05N NaOH) followed by 20 μL of 1 mg/mL chloramine-T in H2O. After 10 min at room temperature, 20 μL of 1 mg/mL Na2S2O5 in H2O was added to quench the reaction. Analytical radioHPLC indicated that a 53% labeling efficiency was obtained. A portion of the reaction mixture (250 μCi) was injected on the HPLC and a broad peak at 9 min was collected to give 126 μCi of isolated product (45%). This was concentrated on a rotary evaporator, diluted with PBS and neutralized with NaOH for the animal study.
Biotin-Asp-α-OMe, 11
H-Asp-OMe, 10 (516 mg, 3.51 mmol) was dissolved in a solution of H2O (15 mL) and NaHCO3 (738 mg, 8.78 mmol), then biotin N-hydroxysuccinimide ester, 9 (1.0 g, 2.93 mmol) and acetone (15 mL) were added respectively. The resulting solution was stirred at room temperature for 16 h, then the solution was evaporated to dryness on a rotary evaporator under vacuum. The residue was dissolved in water (50 mL), and the aqueous solution was acidified to pH 2.0 by 1 N HCl. After storage at 5°C for overnight, the white precipitate was filtered, washed with water (500 mL), and dried under vacuum to yield 791 mg (73%) of 11 as a colorless solid. 1H NMR (DMSO-d6, 500 MHz): δ 1.24–1.36 (m, 3H), 1.41–1.53 (m, 4H), 1.57–1.64 (m, 1H), 2.08–2.12 (m, 2H), 2.56–2.61 (m, 2H), 2.70 (dd, J = 5.9, 16.2 Hz, 1H), 2.82 (dd, J = 5.3, 12.3 Hz, 1H), 3.07–3.11 (m, 1H), 3.61 (s, 3H), 4.11–4.14 (m, 1H), 4.30 (dd, J = 5.4, 7.6 Hz, 1H), 4.57 (dd, J = 7.5, 13.4 Hz, 1H), 6.37 (s, 1H), 6.41 (s, 1H), 8.28 (d, J = 7.5 Hz, 1H), 12.44 (br s, 1H). HRMS (ES+) calcd for C15H23N3NaO6S (M+Na)+: 396.1205. Found: 396.1202. HPLC: tR = 4.9 min.
Biotin-Asp-α-OMe Tetrafluorophenyl Ester, 12
TFA-O-TFP (182 mg, 0.70 mmol) was added slowly to a solution of biotin-Asp-OMe, 11 (200 mg, 0.54 mmol), Et3N (0.112mL, 0.80 mmol) and anhydrous DMF (8 mL) at 0°C. After stirring at 0°C for 30 min, the solution was evaporated on a rotary evaporator under vacuum. The crude product was then purified by silica gel column (1.5 cm × 25 cm) eluting with a gradient solution from 50% EtOAc/hexanes to 100% EtOAc. Purification yielded 235 mg (84%) of 12 as a colorless solid. mp 127–128 °C. 1H NMR (DMSO-d6, 500 MHz): δ 1.25–1.38 (m, 3H), 1.42–1.55 (m, 4H), 1.58–1.65 (m, 1H), 2.09–2.15 (m, 2H), 2.57 (d, J = 12.6 Hz, 1H), 2.81 (dd, J = 5.9, 16.4 Hz, 1H), 3.06–3.10 (m, 1H), 3.16 (dd, J = 7.1, 16.5 Hz, 1H), 3.29 (dd, J = 6.2, 16.8 Hz, 1H), 3.66 (s, 3H), 4.11–4.14 (m, 1H), 4.30 (dd, J = 5.0, 7.8 Hz, 1H), 4.76 (dd, J = 7.3, 14.5 Hz, 1H), 6.36 (s, 1H), 6.42 (s, 1H), 7.92–7.99 (m, 1H), 8.52 (d, J = 7.7 Hz, 1H). HRMS (ES+) calcd for C21H23F4N3NaO6S (M+Na)+: 544.1141. Found: 544.1126. HPLC: tR = 11.6 min.
[Et3NH]B10H9-CONH-trioxatridecane-NH2, 13
This compound was prepared in 68% yield by reaction of 7b with 4,7,10-trioxa-1,13,-tridecanediamine at room temperature for 3 days as previously reported (13).
[Et3NH]B12H11-CONH-trioxatridecane-NH2, 14
A solution of [Et3NH][B12H11-CO], 8 (0.30 g, 1.11 mmol) and 4,7,10-trioxa-1,13,-tridecanediamine (6.0 mL) was stirred at room temperature for 16 h. EtOAc (10 mL) was added and the resulting solution was stirred at room temperature for 20 minutes. The solution was decanted, and the residue was washed with EtOAc (3 × 15 mL). The crude product was dissolved in 4 mL of MeOH/DMF/water (1/1/1) and purified via preparative reversed-phase chromatography. Purification yielded 407 mg (75%) of 14. 1H NMR (DMSO-d6, 500 MHz): δ 0.21–1.50 (m, 11H), 1.12 (t, J = 7.2 Hz, 9H), 1.66–1.76 (m, 4H), 1.78 (s, 1H), 2.68–2.75 (m, 4H), 2.94–2.97 (m, 8H), 3.38–3.52 (m, 12H). 11B NMR (DMSO-d6, 160.43 MHz): δ −6.20 (1B), −15.54 (5B), −16.15 (6B). LRMS (ES−) calc for C11H33B12N2O4LiNa (M+Li+Na) 419.4, found 419.4. HPLC: tR = 3.8 min.
[Et3NH]2B10H9-CONH-trioxatridecane-NHCO-Asp-α-OMe-Biotin, 3a
[Et3NH]B10H9-trioxatridecane-NH2, 13 (116 mg, 0.249 mmol) was added to a solution of biotin-Asp-OMe TFP ester, 12 (100 mg, 0.192 mmol), Et3N (0.08 mL, 0.575 mmol) and anhydrous DMF (10 mL). The resulting solution was stirred at room temperature for 1 h, then the volatile materials were removed on a rotary evaporator under vacuum. The residue was dissolved in 5 mL of MeOH/CH3CN (1/1) and purified via preparative reversed-phase chromatography. Purification yielded 108 mg (69%) of 3a. 1H NMR (CD3OD, 500 MHz): δ −0.18–0.79 (m, 9H), 1.22 (t, J =7.3 Hz, 18H), 1.38–1.44 (m, 2H), 1.52–1.73 (m, 6H), 2.19–2.27 (m, 2H), 2.66–2.69 (m, 3H), 2.91 (dd, J = 5.0, 12.8 Hz, 1H), 3.06 (q, J = 7.2 Hz, 12H), 3.17–3.23 (m, 4H), 3.48 (t, J = 6.4 Hz, 3H), 3.53–3.56 (m, 2H), 3.58–3.61 (m, 3H), 3.62–3.67 (m, 16H), 3.69 (s, 3H), 4.29 (dd, J = 4.4, 7.8 Hz, 1H), 4.46 (dd, J = 5.0, 8.0 Hz, 1H), 4.69 (t, J = 6.0 Hz, 1H). 11B NMR (CD3OD, 160.43 MHz): δ −0.91 (2B), −23.79 (2B), −27.99 (6B). LRMS (ES−) calcd for C26H53B10N5O9S (M)−: 721.5, Found: 721.5. HPLC: tR = 12.1 min.
[Et3NH]2B10H8I-CONH-trioxatridecane-NHCO-Asp-α-OMe-Biotin, 3b
A 246 μL aliquot of chloramine-T solution (11 μmol, 10 mg/mL in H2O) was added to a solution of 3a (20 mg, 22 μmol), NaI (16 μL, 11 μmol, 100 mg/mL in water) and 5% HOAc of MeOH/water (50/50, 1 mL). The solution was allowed to stir at room temperature for 1 min, then 205 μL of Na2S2O5 (11 μmol, 10 mg/mL in H2O) was added. The product was identified as a new, more lipophilic, species by reversed-phase HPLC (3b had approximately the same peak area as 3a by ELSD detection). LRMS (ES−) calcd for C26H53B10IN5O9S (M+H+Na): 871.3, Found: 871.3. HPLC: tR = 4.3 min.
[Et3NH]2B10H8[131I]I-CONH-trioxatridecane-NHCO-Asp-OMe-Biotin, [131I]3b
A 20 μL aliquot of a 1 mg/mL solution of chloramine-T was added to a solution containing 3a (100 μg, 0.11 μmol), Na[131I]I (3 μL, 780 μCi, 0.1 N NaOH solution) and 100 μL of 5% HOAc in 1:1 H2O:MeOH. The reaction was quenched with Na2S2O5 (20 μL of 1 mg/mL solution in H2O) after 1 min at room temperature. Analytical radioHPLC indicated that a 96% labeling efficiency was obtained. A portion of the eluted [131I]3b was isolated for the animal study (44%).
[Et3NH]B12H11-CONH-trioxatridecane-NHCO-Asp-OMe-Biotin, 4a
[Et3NH]B12H11-CONH-trioxatridecane-NH2, 14 (122 mg, 0.249 mmol) was added to a solution of biotin-Asp-OMe TFP ester, 12 (100 mg, 0.192 mmol), Et3N (0.08 mL, 0.575 mmol) and anhydrous DMF (10 mL). The resulting solution was stirred at room temperature for 1 hour, then the volatile materials were removed on a rotary evaporator under vacuum. The residue was dissolved in 5 mL of MeOH/CH3CN (1/1) and purified via preparative reversed-phase chromatography. Purification yielded 86 mg (53%) of 4a. 1H NMR (DMSO-d6, 500 MHz): δ 0.22–1.44 (m, 13H), 1.22 (t, J =7.3 Hz, 9H), 1.52–1.73 (m, 6H), 2.19–2.27 (m, 2H), 2.66–2.69 (m, 3H), 2.91 (dd, J = 5.0, 12.8 Hz, 1H), 3.06 (q, J = 7.2 Hz, 6H), 3.17–3.23 (m, 4H), 3.48 (t, J = 6.4 Hz, 3H), 3.53–3.56 (m, 2H), 3.58–3.61 (m, 3H), 3.62–3.67 (m, 16H), 3.59 (s, 3H), 4.13 (dd, J = 4.7, 7.8 Hz, 1H), 4.31 (dd, J = 4.8, 7.7 Hz, 1H), 4.55 (dd, J = 6.9, 13.8 Hz, 1H), 6.35 (s, 1H), 6.40 (s, 1H), 7.89 (t, J = 5.3 Hz, 1H), 8.16 (d, J = 7.9 Hz, 1H). 11B NMR (DMSO-d6, 160.43 MHz) δ −4.55 (1B), −15.88 (5B), −16.55 (6B). LRMS (ES−) calcd for C26H55B12N5O9SNa (M+Na): 768.5, Found: 768.5. HPLC: tR = 3.7 min.
[Et3NH]2B12H10I-CONH-trioxatridecane-NHCO-Asp-α-OMe-Biotin, 4b
A 240 μL aliquot of a chloramine-T solution (11 mmol, 10 mg/mL in water) was added to a solution of [Et3NH]B12H11-trioxa-Asp-OMe-biotin (20 mg, 21 μmol), NaI (16 μL, 11 mmol, 100 mg/mL in water) and 5% HOAc of MeOH/water (50/50, 1 mL). After the solution was stirred at room temperature for 1 min, 200 μL of Na2S2O5 (11 mmol, 10 mg/mL in water) was added. The product was identified as a new, more lipophilic, species by reversed-phase HPLC (4b had approximately the same peak area as 4a by ELSD detection). HPLC: tR = 4.5 min.
[Et3NH]2B12H11[125I]I- CONH-trioxatridecane-NHCO-Asp-OMe-Biotin, [125I]4b
A 20 μL aliquot of a 1 mg/mL solution of chloramine-T was added to a solution containing 4a (100 μg, 0.11 μmol), Na[125I]I (2 μL, 1 mCi, 0.1 N NaOH solution) and 100 μL of 5% HOAc in 1:1 H2O:MeOH. The reaction was quenched with Na2S2O5 (20 μL of 1 mg/mL solution in H2O) after 10 min at room temperature. Analytical radioHPLC indicated that a 86% labeling efficiency was obtained. A portion of the eluted [125I]4b was isolated for the animal study (33%).
Biodistribution Studies
The biodistribution studies were conducted under a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Washington. Procedures were conducted in compliance with US laws relating to the conduct of animal experimentation. Male athymic mice (nu/nu), obtained from Simonson Laboratories (Gilroy, CA), were housed for 1 week in the isolator facility prior to beginning the study. In each experiment, the peaks corresponding to the desired products were isolated from the HPLC, and were mixed in appropriate quantities to prepare an admixture containing predetermined quantities of each astatinated and/or radioiodinated compound. The admixture was diluted with phosphate buffered saline (PBS) to prepare injection quantities of ~100 μL. This quantity was injected into each of 10 athymic mice via the lateral tail vein. The actual amount of injectate each animal received was determined by weighing the administering syringe before and after injection. Groups of 5 mice were sacrificed by cervical dislocation at 1 and 4 h post injection. The tissues were excised, blotted free of blood, weighed, and counted. Blood weight was estimated to be 6% of the total body weight (25). Calculation of the percent injected dose per gram (%ID/g) in the tissues was accomplished using internal standards for the 211At and 125I or 131I counts. The quantities injected and data obtained in the biodistribution studies are provided in Tables S1 – S4 (Supporting Information).
RESULTS
Preparation of closo-borate(2-) derivatives
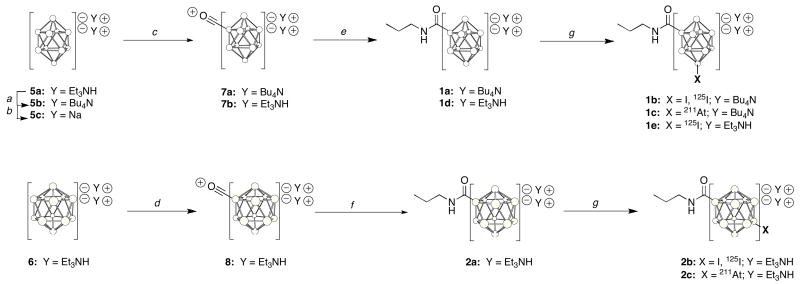
The closo-borate(2-) derivatives, 1a–1e and 2a–2c, were prepared by the reaction sequences shown in Scheme 1. [Et3NH]2[closo-B10H10], 5a was prepared in 94% yield by reaction of decaborane (B10H14) with Et3N in xylene at 105–140°C (22). [nBu4N]2[closo-B10H10], 5b was prepared in 86% yield from 5a by counterion exchange in H2O after mixing with nBu4NBr. The Na salt Na2[closo-B10H10], 5c, was prepared from 5a by elution over an Amberlite IR120 ion exchange resin (Na form). [Et3NH]2[closo-B12H12], 6, was prepared in 54% yield by reaction of decaborane with triethylamine borane complex (Et3N.BH3) at 190°C in undecane (23). [nBu4N]2[closo-B10H10], 5b, was substituted with carbon monoxide by reaction of oxalyl chloride in anhydrous acetonitrile, initially in an ice bath for 30 min, then at room temperature for 30 minutes, to provide [nBu4N][closo-B10H9-CO], 7a, in 71% yield. [Et3NH][closo-B10H9-CO], 7b, was prepared in 83% yield using the same reaction conditions. Similarly, [Et3NH]2[B12H12], 6, was substituted with carbon monoxide using the same reaction at reflux for 3 hours to provide [Et3NH][B12H11-CO], 10, in 78% yield. Reaction of 7a with n-propylamine and triethylamine in anhydrous acetonitrile at room temperature for 16 hours provided the adduct [nBu4N]2[B10H9]-CONHpropyl, 1a, in 83% yield. Reaction of 8 with n-propylamine was slower, requiring it to be used as solvent. However, the reaction was complete when run at room temperature for 16 h, yielding the adduct, [Et3NH]2[B12H11]-CONHpropyl, 2a, in 84%.
Scheme 1.
Synthesis and radiohalogenation of n-propylamide derivatives of closo-decaborate(2-) and closo-dodecaborate(2-). Circles in cage structures represent B (when substituted) or BH atoms.
a(nBu)4NBr, H2O, 86%; b120 IR ion exchange (Na), 100%; coxalyl chloride, anhyd. CH2Cl2, 0°C-rt, 30 min: 7a, 71%, 7b, 83%; doxalyl chloride, anhyd. CH2Cl2, reflux, 3h, 8, 78%; en-propylNH2, Et3N, anhyd. CH3CN, rt, 16h, 1a, 83%, 1d, 79%; fn-propylNH2, rt, 16h, 2a, 84%; fNa[*X], ChT, MeOH/H2O, rt: [125I]1b, 2 min, 86%, [211At]1c, 5 min, 84%, [125I]1e, 1 min, 82%; [125I]2b, 2 min, 68%; [211At]2c, 10 min, 53%.
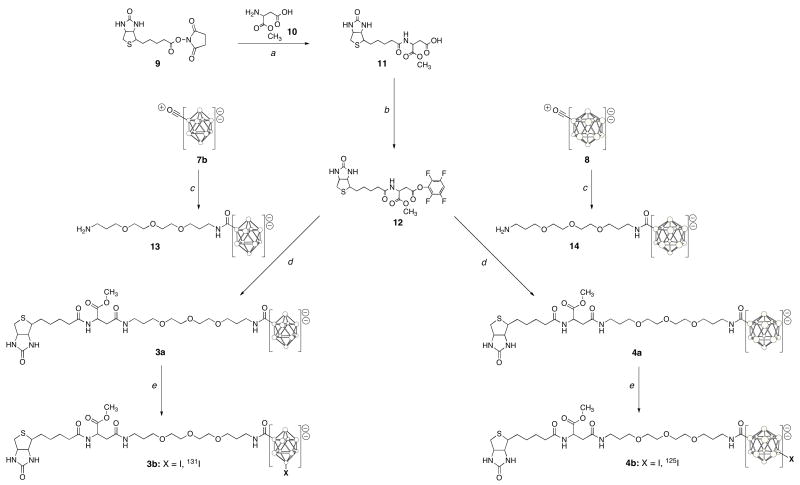
The biotin derivatives 3a and 4a were prepared in multi-step syntheses as depicted in Scheme 2. The reaction sequence began with preparation of a biotinidase-stabilized biotin derivative, the aspartate methyl ester adduct of biotin, 11 (26). Reaction of biotin N-hydroxylsuccinimide ester 9 with the α-methyl ester of aspartic acid, 10, in aqueous sodium bicarbonate provided 11 in 73% yield. Activation of the free carboxylate of 11 was accomplished by reaction with trifluoroacetyl tetrafluorophenol (TFA-O-TFP) with Et3N in DMF at 0°C for 30 minutes to give an 84% yield of the tetrafluorophenyl ester, 12. Reaction of 7b with 4,7,10-trioxa-1,13-tridecanediamine for 3 days at room temperature gave a 68% yield of the adduct, 13. Similarly, reaction of 8 with 4,7,10-trioxa-1,13-tridecanediamine for 16 hours at room temperature gave a 75% yield of 14. Reaction of biotin derivative 12 with 13 for 1 hour at room temperature in anhydrous DMF containing Et3N gave the targeted biotin derivative 3a in 69% yield. Reaction of 12 with 14 under the same reaction conditions gave 4a in 53% yield.
Scheme 2.
Synthesis and radioiodination of biotin derivatives containing decaborate(2-) and dodecaborate(2-) moieties. Circles in cage structures represent B or BH atoms. The Et3NH counterions for closo-borate(2-) derivatives are not shown for simplicity.
aH2O, NaHCO3, acetone, rt, 16h, 73%; b10, TFA-O-TFP, Et3N, anhyd. DMF, 0°C, 30 min, 84%; ctrioxtridecanediamine, rt: 13, 3 d, 68%; 14, 16 h, 75%; d 4a, 53%; eEt3N, anhyd. DMF, rt, 1h: 3a, 69%, ChT, H2O/MeOH/HOAc, rt: 3b, 1 min, 96%; 4b, 10 min, 86%.
Iodination reactions were performed on 1a, 2a, 3a and 4a to provide HPLC reference standards (1b, 2b, 3b and 4b) for use in the radiohalogenation studies. In the iodination reactions, 0.5 equivalent of both NaI and chloramine-T were reacted with each closo-borate(2-) derivative in H2O. This stoichiometry was used to ensure that the reaction did not substitute chlorine or multiple iodine atoms on the closo-borate(2-) moiety, and to distinguish the product from starting material (which had 50% remaining) by HPLC. Both of the closo-borate(2-) moieties are known to add multiple halogen atoms to the same molecule (27) and are reactive with all of the electrophilic halogen species (28, 29). Yields of the iodinated products were measured by HPLC (ELSD detection), and in each case were approximately 50% due to limited quantities of iodination reagent used.2 Iodinated products were isolated from HPLC to obtain a pure sample for use as a HPLC standard. Although attempts were made to obtain mass spectra data for identity of the iodinated standards, this was unsuccessful for some derivatives even though several attempts were made using negative ion electrospray mass spectrometry.
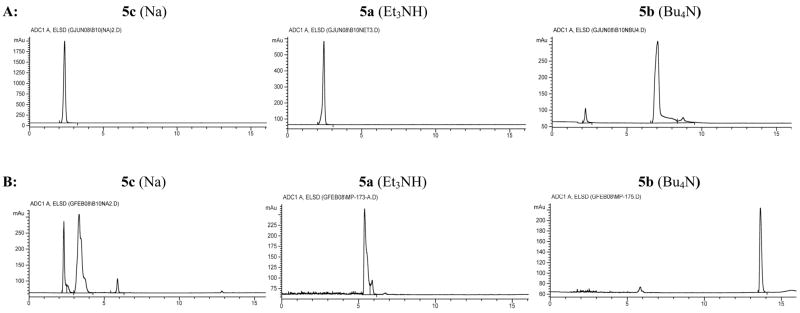
HPLC of closo-decaborate(2-) salts
The Na, Et3N and nBu4N salts of closo-decaborate(2-) (5c, 5a, 5b respectively) were prepared and analyzed by reversed-phase HPLC using two different solvent mixtures under the same gradient conditions. One solvent mixture was composed of [solvent A] MeOH and [solvent B] H2O containing 0.1% HOAc (v/v). The second solvent mixture also employed MeOH as solvent A, but solvent B was 50 mM aqueous Et3NHOAc. The Et3NHOAc modifier was used to determine if the Et3NH ion would displace the counterions on the isolated closo-decaborate(2-) under the elution conditions. Detection of the eluted non-chromophoric ions and salts was accomplished by an evaporative light scattering detector (ELSD). Using the ELSD, Na ions are detected, but organic ammonium salts were not (due to volatility). Therefore, the peaks observed are those of non-volatile Na ions and/or the closo-decaborate(2-) derivative salts. Chromatograms showing the peak retention times (tR) after injecting the same quantity of each salt are shown in Figure 2, panels A and B. The left and middle chromatograms in Panel A (3 chromatograms across) show that the Na and Et3NH salts of closo-decaborate(2-) are highly ionic, eluting with the solvent front. In contrast, it appears from the right chromatogram in Panel A that at least one of the nBu4N ions remains with the closo-decaborate(2-) under the same conditions, as it elutes with a tR of ~7 min. Chromatograms in Panel B are also informative. As expected, the left chromatogram shows that the Na ions in 5c are readily displaced by at least one Et3NH ion. In the case where the isolated compound has two Et3NH counterions (i.e. 5a), as in the middle chromatogram of Panel B both Et3NH counterions appear to elute with the closo-decaborate(2-) ion. Interestingly, it appears from the right chromatogram in Panel B that both of the nBu4N ions also elute with the closo-decaborate(2-) derivative 5b when Et3NHOAc is in the eluting solvent.
Figure 2.
HPLC chromatograms of closo-decaborate(2-) having either Na (5c), Et3NH (5a) or Bu4N (5b) counterions. Identity of the compound and its counterion (in parentheses) are shown above each chromatogram. Panel A chromatograms (3 across) were obtained using MeOH/0.1% aqueous HOAc (v/v) as eluant. Panel B chromatograms (3 across) were obtained using MeOH/50 mM aqueous Et3NHOAc as the eluant. An evaporative light scattering detector (ELSD) was used for detection. (Note that the small peak at tR = ~6 min in chromatograms in Panel B is likely to be an impurity.)
Radiohalogenation Reactions
Radioiodinations (nca) were conducted using Na*I/chloramine T in a mixture of 5% HOAc/MeOH/H2O at room temperature for 1–10 min. Radioiodinations of both closo-decaborate(2-) and closo-dodecaborate(2-) derivatives were facile, giving labeled compounds in 68–96% yields by HPLC analyses, but the closo-dodecaborate(2-) derivatives were slower requiring up to 10 min to complete. Astatination yields were also high for the closo-decaborate(2-) derivative 1c, providing 84% radiochemical yield after a 5 min reaction time. However, the slower reaction of the closo-dodecaborate derivative 2c, gave only 53% yield after 10 min reaction time. No studies were conducted to optimize the reaction conditions, including reaction times. However, radiohalogenations of the closo-decaborate(2-) derivatives appeared to be complete within 1 min and no improvement in labeling yields were observed after 10 min reaction time for the closo-dodecaborate. Previous studies by Orlova et al. (15) indicated that the astatinataion reaction of closo-dodecaborate(2-) was “essentially complete” within 5 min under the conditions they studied. In some examples, isolated radiochemical yields were considerably lower than what the HPLC indicated, as only portions of the prepared radiolabeled compounds were collected from the HPLC effluent. The quantities of radioactivity collected were not optimized as only small quantities of activity were required for the animal studies.
Biodistribution Studies
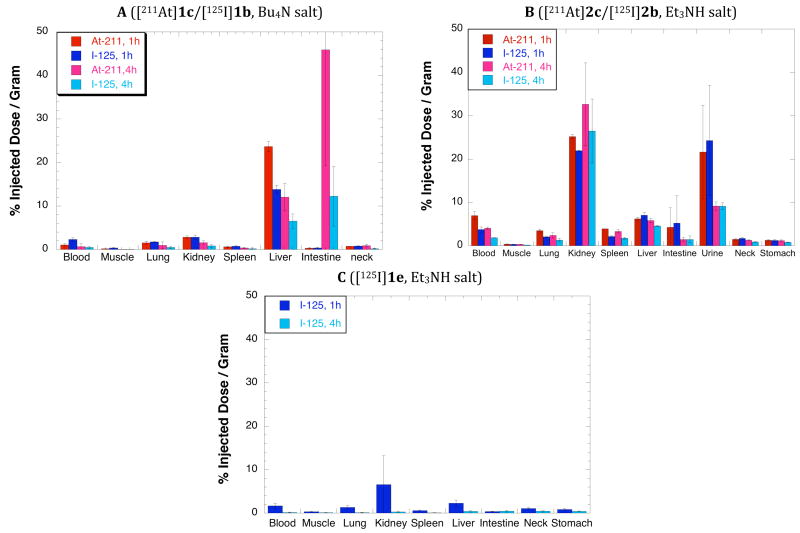
Four biodistribution studies were conducted with radiolabeled closo-borate(2-) derivatives in this investigation. In the first biodistribution study, radiolabeled closo-decaborates [211At]1c and [125I]1b were mixed and coinjected into athymic mice. At 1 and 4 hours post injection (pi), concentrations of radionuclides were evaluated in blood, urine and selected tissues. Tissue concentrations (%ID/g) for selected tissues are plotted in Figure 3, panel A, and all data obtained are provided in Table S1 (Supporting Information). Surprisingly, the highest concentrations of both 211At and 125I were found in the liver and intestines. While most tissues had concentrations of 211At and 125I that are not significantly different (paired Student’s t-test; P < 0.05), the concentrations of the two radionuclides in blood, urine, liver and stomach were significantly different. Interestingly, the concentration of 125I in urine at 1 h pi (174.43 ± 90.33 %ID/g) was much higher than that of the 211At (33.11 ± 14.06 %ID/g), suggesting that there is a difference in the route of excretion of the otherwise identical radiolabeled closo-decaborate(2-) derivatives. In fact, the higher concentration of 211At than 125I observed in liver also supports having different excretion routes. Although 211At concentrations were found to be higher than 125I concentrations in stomach, the fact that there are no significant differences in the concentrations of radionuclides in lung and spleen suggests that only very small amounts, if any, of the free [211At]astatide were present (7).
Figure 3.
Graphed data from tissue biodistributions in mice at 1 and 4 hours after injection of (Panel A) nBu4N salt of 2-propylamido-closo-decaborate(2-) labeled with 211At ([211At]1c) / 125I ([125I]1b); (Panel B) Et3NH salt of 2-propylamido-closo-dodecaborate labeled with 211At ([211At]2c] / 131I ([125I]2b); and (Panel C) Et3NH salt of 2-propylamido-closo-decaborate (2-) labeled with 125I ([125I] 1e). Red bars represent concentration of 211At at 1h post injection (pi), pink bars represent concentration of 211At at 4h pi, dark blue bars represent 125I at 1h pi, and light blue bars represent 125I at 4h pi. Data has been plotted on the same scale for all 3 graphs for comparison purposes.
In the second biodistribution study, coadministered radiolabeled closo-dodecaborates, [211At]2c and [125I]2b, were evaluated at the 1 and 4 hour pi time points. Selected tissue concentrations (%ID/g) are plotted in Figure 3, panel B, and all data obtained are provided in Table S2 (Supporting Information). The highest tissue concentrations of 211At and 125I were found in kidney, indicating that the kidney was likely to be the route of excretion for this pair of radiolabeled closo-dodecaborate(2-) compounds. Unlike the radiolabeled closo-decaborate(2-) derivatives [211At]1c and [125I]1b, there was no significant difference in the concentration of 211At and 125I in urine (at sacrifice). There were signficant differences (paired Student’s t-test; P < 0.05) between the concentrations of 211At and 125I in lung, spleen, neck and stomach suggesting that some free 211At was present. However, the %ID/g concentrations of 211At in those tissues indicates that the amount of deastatination was small.
The surprising differences in routes of excretion for the radiolabeled closo-decaborate(2-) and closo-dodecaborate(2-) led to a third biodistribution study where the tissue distribution of the Et3NH salt of closo-decaborate(2-), [125I]1e, was evaluated at 1 and 4 h pi. Selected tissue concentrations (%ID/g) are plotted in Figure 3, panel C, and all data obtained are provided in Table S3 (Supporting Information). The tissue concentration data from the Et3NH salt, [125I]1e, is strikingly different from that obtained with the nBu4N salt, [125I]1b, of the same compound. The highest concentration of 125I is in kidney after injection of [125I]1e, suggesting that renal clearance is the primary route of excretion. The high concentration in urine at 1 h (320 ± 135 %ID/g) also supports renal clearance. Importantly, all tissue concentrations for the Et3NH salt of [125I]1e-labeled closo-decaborate(2-) (Panel B, blue bars) were much lower than those observed for the nBu4N salt.
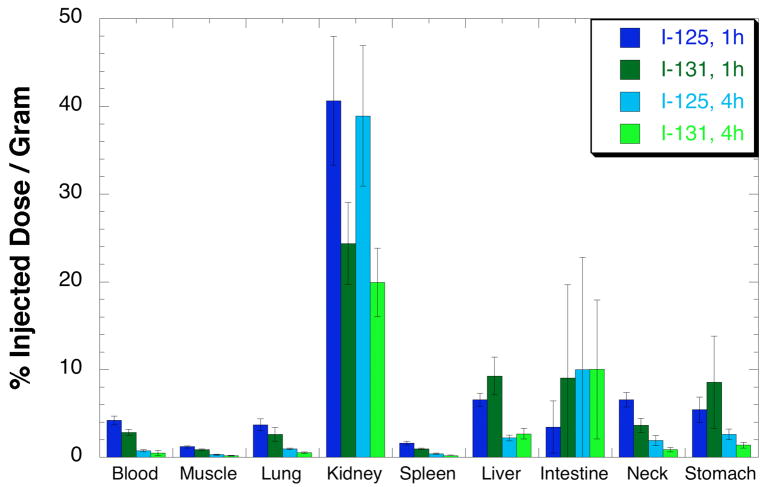
In the fourth biodistribution the biotin derivatives containing the closo-decaborate(2-) moiety, [131I]3b, and the closo-dodecaborate(2-) moiety, [125I]4b, were coinjected into athymic mice and evaluated at 1 and 4 h pi. Concentrations (%ID/g) of radionuclides in selected tissues are plotted in Figure 4, and all data obtained are provided in Table S4 (Supporting Information). The highest concentrations were found in the kidney for both radiolabeled compounds, but the biotin derivative containing the closo-dodecaborate(2-) moiety, [125I]4b, had considerably higher concentrations than the closo-decaborate(2-) derivative, [131I]3b. The high kidney concentrations may be due, at least in part, to the higher lipophilicity of the methyl esters, as other similar biotin derivatives did not show as high kidney concentrations. For example, the biotin derivative having the same structure as [*I]3b without the methyl ester had kidney concentrations of 12.27±1.30 at 1 h pi and 16.24±2.71 at 4 h pi (unpublished data), and in a series of biotin derivatives containing nido-carborane moieties the highest kidney concentrations were < 8% ID/g at 1 h pi and < 4%ID/g at 4 h pi (30).
Figure 4.
Biodistribution of radioiodinated biotin derivatives in mice at 1 and 4 h post injection. Dark blue bars show concentrations of biotin derivative containing the closo-decaborate(2-) moiety ([131I]3b) at 1 h pi and light blue bars show concentrations of that compound at 4 h pi. Dark green bars show concentrations of biotin derivative containing closo-dodecaborate ([125I]4b) at 1h pi and light green bars show concentrations of that compound at 4 h pi.
Significant differences (paired Student’s t test; P < 0.05) were seen between the two radiolabeled biotin derivatives in blood, lung, kidney, spleen, liver and neck at 1 h pi, and in lung, kidney, spleen, neck and stomach at 4 h pi. However, the differences, while significant, were small in most tissues, making the kidney localization/retention the most important factor in deciding between the two closo-borate(2-) moieties.
DISCUSSION
Our previous studies demonstrated that biomolecule-conjugated pendant groups having 211At bonded to an aryl carbon can be quite unstable in vivo, releasing [211At]astatide to localize to lung, spleen, stomach and thyroid (7, 31). Since there are no stable nuclides of At found in nature, it is unlikely that there are specific enzymes to cleave the C-At bond. One possible explanation for the in vivo deastatination is that its low bond energy of the C-At bond (32, 33) makes it possible for non-specific processes to release 211At from metabolized biomolecules. We reasoned that the in vivo stability of 211At-labeled compounds might be increased if the At was coupled to the carrier molecule through a stronger bond. This led us to consider boron-halogen bonds, which are considerably stronger than the corresponding carbon-halogen bonds (34). We further reasoned that 211At bonded to a boron atom on an aromatic boron cage moiety would likely provide the highest bond energy, as is the case with aromatic C-At bonding (33). Thus, we became interested in boron cage moieties as components of pendant (conjugation) groups for 211At labeling.
Our boron-211At labeling studies began with the nido-carborane moiety (12). While that moiety was relatively easy to modify and incorporate into biomolecules, its conjugation appeared to increase the half-life of the biomolecule in blood compared with the same biomolecule astatinated with an astatobenzoate pendant group (12, 30). Importantly, when 211At-labeled molecules containing conjugated nido-carborane groups were metabolized, the 211At was released. Following those studies we evaluated the reactivity of monocarbon carboranes and found that cage system has a low reactivity for radioiodinations, so astatinations were not conducted (35). Subsequently, we evaluated bis-nido-carboranes (also called Venus Flytrap Cluster (36)) and found that they provided high astatine stability in vivo, but dramatically affected the nature of the biomolecule to which they were attached (13). In the later studies, we also found that another boron cage moiety, closo-decaborate(2-), 5, provided high in vivo stability with minimal affect on the biomolecules investigated (13).
Orlova et al. reported that a similar boron cage moiety, the closo-dodecaborate(2-) cage, could be astatinated in high yield (16). The same research group described several modified closo-dodecaborate(2-) derivatives that can be used for conjugation to biomolecules (14, 37–39). The reported closo-dodecaborate(2-) derivatives all had hetero atoms (N,O,S) attached to the closo-borate(2-) cage. We prepared two of the reported derivatives and found that the rate of halogenation was very slow under the conditions studied (unreported results). It was postulated that the heteroatom attached to the boron cage moiety was protonated under the reaction conditions, resulting in an adjacent positive charge that deactivated the cage to halogenation. To circumvent that situation, we sought derivatives of dianionic closo-borate(2-) cage moieties that would not have a protonated heteroatom adjacent to the boron cage. We found that derivatives of closo-borate(2-) moieties, which were conjugated to another moiety through an amide bond retained their high reactivity with halogens. Therefore, our recent studies have focused on derivatives containing amido-bonded closo-borate(2-) moieties for 211At labeling.
This investigation was conducted to assess the differences in chemistry and in vivo properties associated with using closo-decaborate(2-) or closo-dodecaborate(2-) moieties as the labeling components of pendant groups for astatination of biomolecules. To illustrate the differences, 2-n-propylamido-derivatives of two closo-borate(2-) moieties were prepared and evaluated in vivo. As expected based on prior literature reports, the synthetic chemistry and radiolabeling chemistry differed significantly between the two cage systems. First, while the yields of amine reactions with the two closo-borate(2-) carbon monoxide adducts, [closo-B10H9-CO]1−, 7 and [closo-B12H11-CO]1−, 8, were similar (i.e. 78% and 83%), the reaction times for coupling amines with 7 were considerably longer than with 8 (3 days vs. 16 hours). The low reactivity of 7 with amines most likely reflects the higher electron density of the closo-decaborate(2-) cage, which makes the carbon on the carbon monoxide moiety less electrophilic. Importantly, the higher electron density of the closo-decaborate(2-) cage makes it more reactive in the no-carrier-added radiohalogenations. Although the radioiodination yields were similar (1b,1f,3b: 82–96% vs. 2b,4b: 79–86%) the astatination yields (1c, 84% vs. 2c, 53%) yields favor the use of the closo-decaborate(2-) moiety.
An important finding of the investigation was that biodistributions of radiolabeled molecules differing only in the type of closo-borate(2-) cage incorporated were significantly different. Indeed, tissue distributions of the radiolabeled n-propylamido derivatives of closo-decaborate(2-), 1b/1c (Figure 3A), and closo-dodecaborate(2-), 2b/2c (Figure 3B), were surprisingly different. Biodistribution data from the Et3NH salt of radiolabeled closo-dodecaborate(2-) derivatives 2b/2c indicated that it was excreted primarily by the kidney. This was not surprising as renal clearance is the route of excretion that was anticipated for both closo-borate(2-) derivatives. However, the biodistribution data from the nBu4N salt of closo-decaborate(2-) indicated that it is primarily excreted by the hepatobiliary system. This surprising result made us consider the other difference in the molecules, the nature of the ammonium counterion used. In our initial study of the in vivo distributions of the nBu4N salt of closo-decaborate(2-) derivatives [211At]1c and [125I]1b were investigated. The Bu4NH salt was employed with the closo-decaborate(2-) derivatives to aid purification of the radiolabeled compounds by HPLC. Under the elution conditions employed, the Et3NH salt eluted at the solvent front whereas the nBu4N salt eluted at a later time allowing collection of the purified material from the HPLC eluant. The nBu4N salt was also used in the preparation of closo-decaborate(2-) derivatives to assist in isolation and purification of products as the salts have very low solubility in H2O. The Et3NH salts of closo-decaborate(2-) derivatives are fairly water soluble (17). The nBu4N salts were not used with the closo-dodecaborate(2-) derivatives, as their Et3NH salts are fairly insoluble in water, making isolation and purification readily achieved.
We surmised that the observed differences in tissue concentrations between the radiolabeled 2-propylamido derivatives of closo-decaborate(2-) [125I]1b and closo-dodecaborate(2-) [125I]2b could be due the differences in counterion used. We reasoned that if the nBu4N counterion was not readily exchanged by other counterions in the blood, the radiolabeled closo-decaborate(2-) derivative would remain highly lipophilic causing it to bind with proteins in blood, ultimately leading to excretion by the hepatobiliary system. As a cursory assessment of the extent of counterion association with the closo-decaborate(2-) derivatives a simple reversed-phase HPLC comparison was carried out. For the HPLC comparison, the Na, Et3N and nBu4N salts of unsubstituted closo-decaborate(2-) (i.e. 5a, 5b, 5c) were prepared and analyzed by reversed-phase HPLC. To create different challenges to the closo-decaborate(2-) counterions, two solvent mixtures were used for elution. In one solvent mixture, the counterions were challenged by hydronium ion (H3O+) and in the other solvent mixture the counterions were challenged with ammonium ions (Et3NH+). The chromatograms obtained are shown in Figure 2, panels A and B. It appears from the HPLC analyses that the nBu4N counterions of the closo-decaborate(2-) are not readily displaced, even under conditions that would seem to favor their displacement (i.e. high concentrations of H3O+ or Et3NH+). In contrast, both the Na and Et3NH salts appear to be highly ionic as they elute at the solvent front when there is no ammonium ions in the eluant. As expected, the Na and Et3NH ions appear to exchange rapidly with Et3NH when it is present in the eluant.
The HPLC elution data did not prove that the nBu4N counterions remain with the radiolabeled closo-decaborate(2-) derivative in vivo. Thus, the ultimate test was to evaluate the Et3NH salt in vivo and compare the biodistributions of the two identical compounds that only differ in the nature of their counterions. The tissue distributions for the nBu4N salt, [125I]1b, and the Et3NH salt, [125I]1e, at 1 and 4 h pi are very different (Figure 3, panels A and C). While the nBu4N salt had high concentrations in the liver, the Et3NH salt had its highest tissue concentration in the kidney. This finding suggests that the highly lipophilic nBu4N counterion remains associated with the closo-decaborate(2-) derivative in vivo. Importantly, all of the tissue concentrations were substantially lower when the Et3NH salt was used, making this counterion preferred for use when the compounds have in vivo applications. Although the Na salts of closo-decaborate(2-) can be prepared and used in vivo, it does not seem that is required. This is fortunate as we now routinely use Et3NHOAc as a modifier in the HPLC eluant to move the radiolabeled closo-decaborate(2-) derivative away from the solvent front, resulting in isolation of the Et3NH salt.
The fourth biodistribution evaluated the differences in tissue distributions between the closo-decaborate(2-) and closo-dodecaborate(2-) moieties (both as Et3NH salts) in a more complex biomolecule, a biotinidase-stabilized biotin derivative. For in vivo evaluation, stabilization of the biotinamide bond to cleavage from the serum enzyme biotinidase (40–42) is required so that the radiolabel remains with the biotin moiety. Our previous studies have demonstrated that a number of functional groups can be built into the carbon alpha to the biotinamide bond to block enzymatic cleavage of that bond (26, 43, 44). In this study, a carboxylate methyl ester was used in the biotin derivatives. The carboxylate methyl ester is not generally used for biotinidase blocking due to its high lipophilicity. However, for this comparison, optimization of the biotin derivative was not required so the methyl ester was used in the study because it can be readily prepared. The radioiodinated derivatives [131I]3b and [125I]4b were evaluated in mice at 1 and 4 h pi. The biodistribution data (Figure 4) show that the only substantive difference between the two radioiodinated biotin derivatives is in the kidney concentrations. As might be expected based on the prior studies with simple n-propylamide-substituted closo-borate(2-) moieties, the closo-dodecaborate(2-) derivative, [125I]4b, had significantly higher kidney concentrations than the corresponding closo-decaborate(2-) derivative, [131I]3b.
Summary
The goal of this investigation was to compare the synthetic chemistry, radioiodine and 211At labeling efficiencies and in vivo tissue distributions between compounds that were identical except that they contained either a closo-decaborate(2-) or a closo-dodecaborate(2-) moiety. Synthesis of closo-borate(2-) derivatives 1a, 2a, 3a and 4a pointed out differences in reaction rates between the two closo-borate(2-) moieties. However, the differences are not considered important in choosing one closo-borate(2-) moiety over the other as both could be substituted with carbon monoxide and subsequently coupled with amine-containing molecules. While only one comparison was done, it appears that 211At-labeling efficiency is higher in compounds containing the more electron rich closo-decaborate(2-). The tissue distribution data indicated that the 211At-labeled derivatives with both closo-borate(2-) moieties have high stability toward in vivo deastatination, making this parameter unimportant in the decision between the use of two closo-borate(2-) moieties. However, the tissue distribution indicated that the closo-dodecaborate(2-) is highly sequestered and retained in the kidney, whereas the closo-decaborate(2-) is not. An interesting finding was the fact that the counterion can have a large influence on the tissue distribution, with the highly lipophilic nBu4N salt causing the compound to be excreted by the hepatobiliary system whereas the Et3NH salt appears to have much less influence on the distribution. In the final analysis, the low kidney sequestration and rapid tissue clearance of the closo-decaborate(2-) make it the better candidate for use as a pendant group in 211At-labeling (or radioiodination) of biomolecules for in vivo therapy applications.
Supplementary Material
Acknowledgments
We thank , , Holly Nguyen and Dr. Robert Vessella for assistance with the animal studies conducted in this investigation. Funding for this research was provided by NCI, NIH (1RO1 CA11343-01).
Footnotes
Nomenclature of compounds: (a) The designations “nido-“ refers to a polyhedral structure that is open or “nest-like”, and “closo-“ refers to a polyhedral “cage” (from Greek “clovo” (45)) structure that is closed such as an icosahedran; (b) the naming of closo-borate(2-) compounds is simplified herein for brevity. For example, tetrabutylammonium 2-(n-propylamido)-nonahydro-closo-decaborate(2-), 1a, is designated as [nBu4N]2[closo-B10H9-CONHpropyl], and triethylammonium 2-(n-propylamido)-undecahydro-closo-dodecaborate(2-), 2a, is designated as [Et3NH]2[closo-B12H11-CONHpropyl].
Reactions containing one equivalent each of NaI and chloramine-T have provided isolated iodinated products in >80% after separation of the other halogenated products by Biotage Flash Chromatograpy. In these studies, adequate quantities of products for MS analyses and HPLC standards was obtained by using only 0.5 equivalents of reagents.
References
- 1.Couturier O, Supiot S, Degraef-Mougin M, Faivre-Chauvet A, Carlier T, Chatal JF, Davodeau F, Cherel M. Cancer radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med Mol Imaging. 2005;32:601–14. doi: 10.1007/s00259-005-1803-2. [DOI] [PubMed] [Google Scholar]
- 2.Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted α-particle therapy. J Nucl Med. 2005;46 Suppl 1:199S–204S. [PubMed] [Google Scholar]
- 3.Zalutsky MR. Targeted alpha-particle therapy of microscopic disease: Providing a further rationale for clinical investigation. J Nucl Med. 2006;47:1238–40. [PubMed] [Google Scholar]
- 4.Cherel M, Davodeau F, Kraeber-Bodere F, Chatal JF. Current status and perspectives in alpha radioimmunotherapy. Q J Nucl Med Mol Imaging. 2006;50:322–9. [PubMed] [Google Scholar]
- 5.Zalutsky MR, Reardon DA, Pozzi OR, Vaidyanathan G, Bigner DD. Targeted alpha-particle radiotherapy with 211At-labeled monoclonal antibodies. Nucl Med Biol. 2007;34:779–85. doi: 10.1016/j.nucmedbio.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Cancer Society. (2008) Cancer Facts and Figures.
- 7.Wilbur DS. [211At]Astatine-Labeled Compound Stability: Issues with Released [211At]Astatide and Development of Labeling Reagents to Increase Stability. Current Radiopharmaceuticals. 2008;1:144–176. [Google Scholar]
- 8.Zalutsky MR, Narula AS. Astatination of Proteins using an N-Succinimidyl Tri-n-Butylstannyl Benzoate Intermediate. Appl Radiat Isot. 1988;39:227–232. doi: 10.1016/0883-2889(88)90176-1. [DOI] [PubMed] [Google Scholar]
- 9.Wilbur DS. Radiohalogenation of proteins: an overview of radionuclides, labeling methods, and reagents for conjugate labeling. Bioconjugate Chem. 1992;3:433–70. doi: 10.1021/bc00018a001. [DOI] [PubMed] [Google Scholar]
- 10.Vaidyanathan G, Affleck DJ, Bigner DD, Zalutsky MR. N-succinimidyl 3-[211At]astato-4-guanidinomethylbenzoate: an acylation agent for labeling internalizing antibodies with α-particle emitting 211At. Nucl Med Biol. 2003;30:351–359. doi: 10.1016/s0969-8051(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 11.Talanov VS, Yordanov AT, Garmestani K, Milenic DE, Arora HC, Plascjak PS, Eckelman WC, Waldmann TA, Brechbiel MW. Preparation and in vivo evaluation of novel linkers for 211At labeling of proteins. Nucl Med Biol. 2004;31:1061–1071. doi: 10.1016/j.nucmedbio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Wilbur DS, Chyan MK, Hamlin DK, Kegley BB, Risler R, Pathare PM, Quinn J, Vessella RL, Foulon C, Zalutsky M, Wedge TJ, Hawthorne MF. Reagents for astatination of biomolecules: comparison of the in vivo distribution and stability of some radioiodinated/astatinated benzamidyl and nido-carboranyl compounds. Bioconjugate Chem. 2004;15:203–23. doi: 10.1021/bc034175k. [DOI] [PubMed] [Google Scholar]
- 13.Wilbur DS, Chyan MK, Hamlin DK, Vessella RL, Wedge TJ, Hawthorne MF. Reagents for Astatination of Biomolecules. 2 Conjugation of Anionic Boron Cage Pendant Groups to a Protein Provides a Method for Direct Labeling that is Stable to in Vivo Deastatination. Bioconjugate Chem. 2007;18:1226–40. doi: 10.1021/bc060345s. [DOI] [PubMed] [Google Scholar]
- 14.Tolmachev V, Koziorowski J, Sivaev I, Lundqvist H, Carlsson J, Orlova A, Gedda L, Olsson P, Sjoberg S, Sundin A. Closo-Dodecaborate(2-) as a linker for iodination of macromolecules. Aspects On conjugation chemistry and biodistribution. Bioconjugate Chem. 1999;10:338–45. doi: 10.1021/bc980033s. [DOI] [PubMed] [Google Scholar]
- 15.Orlova A, Lebeda O, Tolmachev V, Sjoberg S, Carlsson J, Lundqvist H. Closo-dodecaborate (2-) anion as a potential prosthetic group for attachment of astatine to proteins. Aspects of the labelling chemistry with Chloramine-T. J Labelled Compds Radiopharm. 2000;43:251–260. [Google Scholar]
- 16.Orlova A, Lebeda O, Tolmachev V, Sjoberg S, Carlsson J, Lundqvist H. Astatination of Closo-Dodecaborate(2-) Anion. J Labelled Compd Radiopharm. 1999;42:S735–S737. [Google Scholar]
- 17.Muetterties EL, Balthis JH, Chia YT, Knoth WH, Miller HC. Chemistry of Boranes. VIII Salts and Acids of B10H10−2 and B12H12−2. Inorg Chem. 1964;3:444–451. [Google Scholar]
- 18.Knoth WH, Sauer JC, Balthis JH, Miller HC, Muetterties EL. Chemistry of Boranes. XXX Carbonyl Derivatives of B10H102− and B12H122−. J Am Chem Soc. 1967;89:4842–4850. [Google Scholar]
- 19.Hertler WR, Knoth WH, Muetterties EL. Chemistry of Boranes. XXIV Carbonylation of Derivatives of B10H102− and B12H122− with Oxalyl Chloride. Inorg Chem. 1965;4:288–293. [Google Scholar]
- 20.Gamper HB, Reed MW, Cox T, Virosco JS, Adams AD, Gall AA, Scholler JK, Meyer RB., Jr Facile preparation of nuclease resistant 3′ modified oligodeoxynucleotides. Nucleic Acids Res. 1993;21:145–50. doi: 10.1093/nar/21.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilbur DS, Vessella RL, Stray JE, Goffe DK, Blouke KA, Atcher RW. Preparation and evaluation of para-[211At]astatobenzoyl labeled anti-renal cell carcinoma antibody A6H F(ab′)2. In vivo distribution comparison with para-[125I]iodobenzoyl labeled A6H F(ab′)2. Nucl Med Biol. 1993;20:917–27. doi: 10.1016/0969-8051(93)90092-9. [DOI] [PubMed] [Google Scholar]
- 22.Hawthorne MF, Pilling RL. 5. Bis(Triethylammonium) Decahydrodecaborate (2-) Inorg Synth. 1967;9:16–19. [Google Scholar]
- 23.Miller HC, Muetterties EL. 16. Borane Anions. Inorg Synth. 1967;10:81–91. [Google Scholar]
- 24.Shelly K, Knobler CB, Hawthorne MF. Synthesis of Monosubstituted Derivatives of closo-Decahydrodecaborate(2-). X-ray Crystal Structures of [closo-2-B10H9CO]- and [closo-2-B10H9NCO]2−. Inorg Chem. 1992;31:2889–2892. [Google Scholar]
- 25.Kaplan HM, Brewer NR, Blair WH. Physiology. In: Foster HL, Small JD, Fox JG, editors. The Mouse in Biomedical Research. Academic Press; New York: 1983. pp. 248–292. [Google Scholar]
- 26.Wilbur DS, Hamlin DK, Chyan MK, Kegley BB, Pathare PM. Biotin reagents for antibody pretargeting. 5 Additional studies of biotin conjugate design to provide biotinidase stability. Bioconjugate Chem. 2001;12:616–23. doi: 10.1021/bc0100096. [DOI] [PubMed] [Google Scholar]
- 27.Bührens KG, Preetz W. Isolation of Halohydroborates of Type [B10H10-nXn]2−. Angew Chem Int Ed. 1977;16:173–174. [Google Scholar]
- 28.Knoth WH, Miller HC, Sauer JC, Balthis JH, Chia YT, Muetterties EL. Chemistry of Boranes. IX Halogenation of B10H10−2 and B12H12−2. Inorg Chem. 1964;3:159–167. [Google Scholar]
- 29.Ivanov SV, Ivanova SM, Miller SM, Anderson OP, Solntsev KA, Strauss SH. Fluorination of B10H102− with an N-Fluoro Reagent. A New Way To Transform B–H Bonds into B–F Bonds. Inorg Chem. 1996;35:6914–6915. doi: 10.1021/ic961043c. [DOI] [PubMed] [Google Scholar]
- 30.Wilbur DS, Hamlin DK, Chyan MK, Kegley BB, Quinn J, Vessella RL. Biotin reagents in antibody pretargeting. 6 Synthesis and in vivo evaluation of astatinated and radioiodinated aryl- and nido-carboranyl-biotin derivatives. Bioconjugate Chem. 2004;15:601–16. doi: 10.1021/bc034229q. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton JG, Durbin PW, Parrott M. The Accumulation and Destructive Action of Astatine211 (EKA- Iodine) in the Thyroid Gland of Rats and Monkeys. J Clin Endocrinol Metab. 1954;14:1161–1178. doi: 10.1210/jcem-14-10-1161. [DOI] [PubMed] [Google Scholar]
- 32.Eberle SH. Chemical Behavior and Compounds of Astatine. In: Kugler HK, Keller C, editors. Gmelin Handbook of Chemistry, Astatine. Springer-Verlag; Berlin: 1985. pp. 183–259. [Google Scholar]
- 33.Coenen HH, Moerlein SM, Stöcklin G. No-Carrier-Added Radiohalogenation Methods with Heavy Halogens. Radiochimica Acta. 1983;34:47–68. [Google Scholar]
- 34.Kerr JA. Strengths of Chemical Bonds. In: Lide DR, editor. CRC Handbook of Chemistry and Physics. CRC Press; Boca Raton: 1993. pp. 9–123.pp. 9–145. [Google Scholar]
- 35.Wilbur DS, Hamlin DK, Srivastava RR, Chyan MK. Synthesis, radioiodination, and biodistribution of some nido- and closo-monocarbon carborane derivatives. Nucl Med Biol. 2004;31:523–30. doi: 10.1016/j.nucmedbio.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Paxton RJ, Beatty BG, Hawthorne MF, Varadarajan A, Williams LE, Curtis FL, Knobler CB, Shively JE. A Transition metal complex (Venus flytrap cluster) for radioimmunodetection and radioimmunotherapy. Proc Nat Acad Sci USA. 1991;88:3387–3391. doi: 10.1073/pnas.88.8.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sivaev IB, Sjuberg S, Bregadze VI, Gabel D. Synthesis of Alkoxy Derivatives of Dodecahdro-closo-dodecaborate Anion [B12H12]2−. Tetrahedron Lett. 1999;40:3451–4. [Google Scholar]
- 38.Sivaev IB, Bruskin AB, Nesterov VV, Antipin MY, Bregadze VI, Sjoberg S. Synthesis of Schiff bases derived from the ammoniaundecahydro-closo-dodecaborate(1-) anion, [B12H11NH=CHR]−, and their reduction into monosubstituted amines [B12H11NH2CH2R]−: A new route to water soluble agents for BNCT. Inorg Chem. 1999;38:5887–5893. [Google Scholar]
- 39.Nestor M, Persson M, Cheng J, Tolmachev V, Van Dongen G, Anniko M, Kairemo K. Biodistribution of the Chimeric Monoclonal Antibody U36 Radioiodinated with a closo-Dodecaborate-Containing Linker. Comparison with Other Radioiodination Methods. Bioconjugate Chem. 2003;14:805–810. doi: 10.1021/bc034003n. [DOI] [PubMed] [Google Scholar]
- 40.Pispa J. Animal Biotinidase. Ann Med Exptl Bio Fenniae. 1965;43:3–40. [PubMed] [Google Scholar]
- 41.Chauhan J, Ebrahim H, Bhullar RP, Dakshinamurti K. Human Serum Biotinidase. Ann N Y Acad Sci. 1985;447:386–388. [Google Scholar]
- 42.Hymes J, Fleischhauer K, Wolf B. Biotinidase in Serum and Tissues. Meth Enzymol. 1997;279:422–434. doi: 10.1016/s0076-6879(97)79046-3. [DOI] [PubMed] [Google Scholar]
- 43.Wilbur DS, Hamlin DK, Pathare PM, Weerawarna SA. Biotin reagents for antibody pretargeting. Synthesis, radioiodination and in vitro evaluation of water soluble, biotinidase resistant biotin derivatives. Bioconjugate Chem. 1997;8:572–584. doi: 10.1021/bc9700852. [DOI] [PubMed] [Google Scholar]
- 44.Wilbur DS, Hamlin DK, Chyan MK. Biotin reagents for antibody pretargeting. 7 Investigation of chemically inert biotinidase blocking functionalities for synthetic utility. Bioconjugate Chem. 2006;17:1514–22. doi: 10.1021/bc060084m. [DOI] [PubMed] [Google Scholar]
- 45.Adams R. Cage Borane Nomenclature. Inorg Chem. 1963;2:1087–1088. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.