Abstract
The orthopoxvirus SPI-3 (K2) and A56 (hemagglutinin, HA) proteins interact and together prevent cell-cell fusion. SPI-3/A56 has been proposed to prevent the superinfection of previously infected cells by reducing virus-cell fusion. Binding of mature virions of vaccinia virus (VV) to VV-infected cells was unaffected by SPI-3 or A56 on the surface of infected cells. Entry of VV into infected cells was assessed using VV-PT7-luc carrying the luciferase reporter under T7 control. Cells infected with VV or cowpox virus (CPV) expressing T7 RNA polymerase and lacking SPI-3 and/or A56 were superinfected with VV-PT7-luc, and luciferase activity measured. Inactivation of SPI-3 or A56 from the pre-infecting virus resulted in greater luciferase expression from the superinfecting VV-PT7-luc. Antibody against SPI-3 present during infection with wild-type CPV-T7 increased luciferase expression from superinfecting VV-PT7-luc. The SPI-3/A56 complex on the infected cell surface therefore appears to reduce the entry of virions into infected cells.
Keywords: Cell-cell fusion, virus entry, superinfection, SPI-3 (K2L), hemagglutinin (A56R), vaccinia virus, orthopoxvirus, luciferase
INTRODUCTION
Vaccinia virus (VV), the prototypic orthopoxvirus, belongs to the same genus as cowpox virus (CPV) and variola virus, the causative agent of smallpox (Moss, 2001). Poxviruses have large, complex virions with dsDNA genomes, but replicate within the cytoplasm of the infected cell. Many details of VV transcription and DNA replication are known, but the lists of virus proteins required for virion morphogenesis (Smith et al., 2002; Condit et al., 2006) and entry into cells (Moss, 2006) are still being compiled.
The most important forms of poxvirus virions are the intracellular form MV (mature virus, formerly IMV) and the extracellular form EV (extracellular virus, formerly EEV). Both forms are membrane-wrapped, but EV contains an additional membrane envelope relative to MV, and the protein compositions of the outer membranes of EV and MV are distinct (Condit et al., 2006). The EV surface carries virus glycoproteins including B5, A33, and A34, together with the non-essential hemagglutinin (HA) encoded by the A56R gene (Payne, 1979), which is associated with the serpin SPI-3 (K2) (Brum et al., 2003; Turner & Moyer, 2006). In contrast the MV surface has no glycoproteins, but MV particles like EV are fully infectious. In nature, the EV form is responsible for virus spread within an infected host (Payne, 1980), and the stable MV form mediates spread between hosts.
Recently a group of highly conserved genes has been identified whose products are required for entry of MV into cells (Moss, 2006), including A28 (Senkevich et al., 2004), H2 (Senkevich & Moss, 2005), A21 (Townsley et al., 2005b), L5 (Townsley et al., 2005a), A16 (Ojeda et al., 2006b), G3 (Izmailyan et al., 2006), G9 (Ojeda et al., 2006a), and J5, all of which are components of an entry-fusion complex (Senkevich et al., 2005). Mutation in any one of these genes (except for J5, which to our knowledge has not yet been tested) results in the synthesis of morphologically normal MV that can bind to cells but cannot enter (Moss, 2006). In addition, a mutation in F9, a sub-stoichiometric component of the complex that resembles the L1 morphogenesis protein, results in a similar phenotype (Brown et al., 2006). A mutant in I2 behaves in the same fashion (Paula Traktman, pers. comm.).
MV is thought to enter cells by fusion with a cellular membrane, either directly at the cell surface at neutral pH, or at low pH within an endosome. Cell-cell fusion can be mediated by virions bound to the cell surface following a brief acid treatment. Low pH-triggered fusion of cells occurs following binding of exogenous purified MV to cells at very high multiplicity (“fusion from without”), or following natural infection with synthesis and deposition of virus particles (presumably EV) at the cell surface (“fusion from within”). Mutant virions lacking any one of the entry proteins tested to date are defective both in fusion from without and fusion from within (Moss, 2006), supporting the close linkage between virus entry and cell-cell fusion triggered by low pH.
The entry of EV into cells starts with the attachment of EV to the cell membrane, then the non-fusogenic dissolution of the EV outer membrane bilayer, which produces a “shroud” covering the resulting MV at the cell surface (Law et al., 2006). From this point the entry process is proposed to be the same as for MV. Two of the components of the EV envelope, the A56 and SPI-3 proteins, inhibit the process of cell-cell fusion. Mutation in either A56 (Ichihashi & Dales, 1971) or SPI-3 (Turner & Moyer, 1992; Law & Smith, 1992; Zhou et al., 1992) results in extensive cell-cell fusion following infection at neutral pH. A56 and SPI-3 are present both on the surface of EV and on the surface of infected cells (Payne, 1979; Brum et al., 2003), and the two proteins colocalize on the cell membrane by confocal microscopy (Brum et al., 2003). Co-immunoprecipitation studies indicate that SPI-3 and A56 interact (Turner & Moyer, 2006). SPI-3, which contains a signal sequence but lacks a transmembrane domain, is only detected on the cell membrane when A56 is present (Brum et al., 2003). Removal of the transmembrane domain from A56 results in cell-cell fusion (Turner & Moyer, 2006), suggesting that the A56/SPI-3 complex must be membrane-localized for fusion inhibition.
Wagenaar and Moss (2007) showed that the proteins of the entry complex co-purified with A56 when TAP (tandem affinity purification) epitope tags were appended to A56, provided that SPI-3 was present. Similarly entry proteins co-purified with TAP-tagged SPI-3 (K2) in the presence of A56; and SPI-3 and A56 were co-purified with TAP-tagged A28, along with entry proteins (Wagenaar & Moss, 2007). However, in infected cells SPI-3/A56 would not normally be expected to come into contact with the entry/fusion complex.
The most likely natural scenario for interaction between SPI-3/A56 and the entry/fusion complex is when virions are about to enter a previously infected cell, i.e. superinfection. The first infecting virus frequently prevents the replication of superinfecting virus by an exclusion mechanism. Precedents for superinfection exclusion include influenza virus, which expresses a neuraminidase that inactivates the cell surface receptor required for virus entry (Palese et al., 1974), vesicular stomatitis virus (Whitaker-Dowling et al., 1983), and herpes virus, whose glycoprotein D at the cell surface interferes with penetration of superinfecting virus (Johnson & Spear, 1989). Superinfection exclusion operates in VV based on experiments involving infection with wt VV and superinfecting with VV-lacZ at different times after the initial infection (Christen et al., 1990). The lacZ reporter was driven either by the early/late TK promoter or the late P11 promoter. By titering lacZ+ virus, the replication of the superinfecting virus was reduced by 90% 4 h after the first infection, and by >99% after 6 h (Christen et al., 1990). Prior infection did not prevent adsorption of the superinfecting virus, but early gene transcription was abolished when superinfecting virus was added 6 h after the initial infection, indicating that the block occurs in between adsorption and early transcription (Christen et al., 1990). Reductions in the rates of virus entry, uncoating or early transcription would be consistent with published data on superinfection exclusion. The phenomenon may involve a combination of these distinct mechanisms.
Our results support a role for SPI-3 and A56 on the surface of infected cells in reducing superinfection, by an apparent slowing down of the entry/fusion process. Binding of SPI-3 and A56 to proteins in the entry complex of incoming virions is a likely mechanism for reduction in the entry rate.
RESULTS
Binding of superinfecting VV-A5-YFP to cells pre-infected with VV-T7 was not affected by the absence of SPI-3 or A56 from the preinfecting virus
Under a normal infection, the entry complex on the surface of MV and the SPI-3/A56 pair on the outer membrane of EV and on the surface of infected cells are probably never directly adjacent. The most likely circumstance for interaction would be when a virion (either MV, or EV with a disrupted outer envelope) has attached to a previously infected cell, and is poised to enter by fusion with the cell or endosome membrane. If SPI-3 and A56 on the surface of infected cells reduce superinfection, as proposed by our laboratory and others (Moss, 2006; Turner & Moyer, 2006), the proteins could operate at the level of virus binding or entry or both.
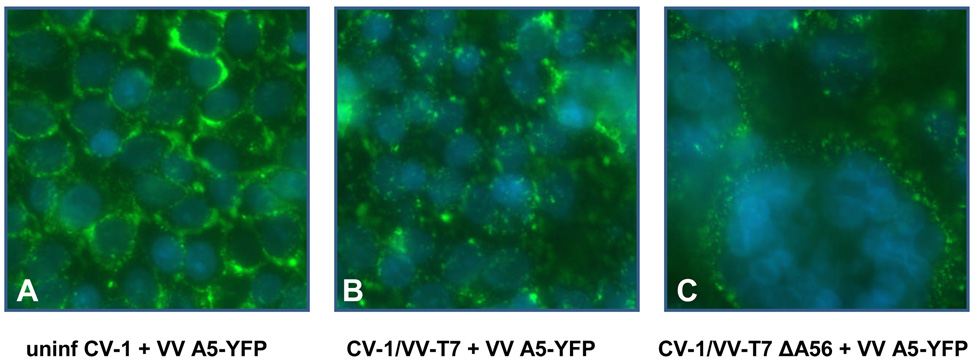
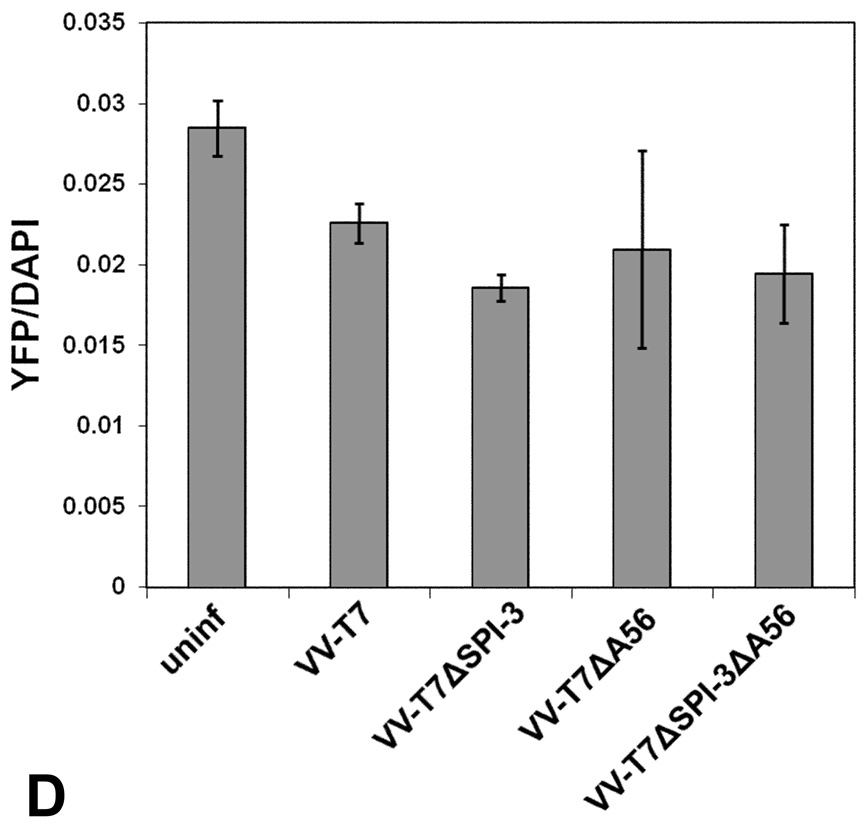
To investigate binding of mature virions to infected cells, we used VV-A5-YFP in which the A5 core protein (A4 in Copenhagen nomenclature) has been fused to yellow fluorescent protein (Katsafanas & Moss, 2007). Virions of VV A5-YFP can be directly detected by fluorescence without immunostaining. CV-1 cells were infected with VV-T7 or with derivatives lacking either SPI-3, A56, or both, and at 16 h p.i. mature virions of VV A5-YFP were adsorbed for 1 h at room temperature. Cell-cell fusion caused by infection with VV-T7 SPI-3 or A56 mutants at the 16 h timepoint was incomplete, with variable degrees of fusion observed for different areas of the same well. At 48 h p.i. fusion triggered by the mutants was complete (data not shown). There is no SPI-3 or A56 present in the VV A5-YFP MV used in this experiment as the intracellular form of virus lacks both proteins (Payne, 1979; Brum et al., 2003). After washing to remove unbound virus, the cells were stained with DAPI. Green virions of VV A5-YFP bound to uninfected cells (Fig. 1A) were readily visible using FITC filters. Although binding to infected cells appeared to be reduced relative to uninfected cells, no obvious differences in the extent of binding were visible by microscopy that depended on the genotype of the first infecting virus (Fig.1ABC). Virion binding to cells preinfected with VV-T7 (Fig. 1B) appeared to be similar to binding to the syncytia resulting from infection of cells with virus lacking A56 (Fig. 1C), as did binding to cells infected with VV-T7ΔSPI-3 or with VV-T7ΔSPI-3ΔA56 (data not shown). The extent of VV A5-YFP bound per cell was compared by harvesting the cells and measuring the ratio of YFP: DAPI fluorescence. The results (Fig. 1D) indicated that there was a slight decrease in the extent of virus binding when VV-T7-infected cells were compared with uninfected cells. However, there was no significant increase or decrease in the amount of virus binding caused by deletion from the pre-infecting virus of SPI-3 or A56 or both (Fig. 1D), suggesting that the presence of SPI-3/A56 on infected cells does not affect binding of superinfecting virus.
Figure 1. Binding of VV A5-YFP MV particles to uninfected and VV-infected CV-1 cells.
(A – C) CV-1 cells were either uninfected (A) or infected with VV-T7 (B) or VV-T7 ΔA56 (C). At 16h p.i., VV A5-YFP was adsorbed for 1 h at room temperature. Unadsorbed virus was removed by washing, and the cells stained with DAPI. The images shown are merges of the DAPI blue channel (nuclei) and the FITC green channel (bound virions).
(D) CV-1 cells in 12-well plates were mock-infected or infected with VV-T7 or with mutant derivatives lacking either SPI-3, A56, or both. At 16 h p.i., purified MV of VV A5-YFP was adsorbed to the cells at room temperature for 1 h. After washing to remove unadsorbed virions, cells were DAPI-stained, harvested, and transferred to a black 96-well plate to measure by fluorescence the amount of YFP (representing bound virus) and DAPI (number of cell nuclei). The results are expressed as the ratio of YFP/DAPI fluorescence. Error bars represent the standard deviation.
Evaluation of a VV recombinant with a T7-driven luciferase gene for measuring virus superinfection
To measure entry of virions into cells pre-infected with VV-T7 derivatives, we constructed a VV recombinant carrying the luciferase reporter under the control of the T7 promoter. Entry of such virions into a cytoplasm containing T7 RNA polymerase should give rapid and robust luciferase expression upon uncoating, and transcription of luciferase should not compete with pre-existing VV genes for transcription. The T7 promoter was used to drive luciferase rather than a poxvirus early promoter to avoid the block to early transcription observed following superinfection (Christen et al., 1990).
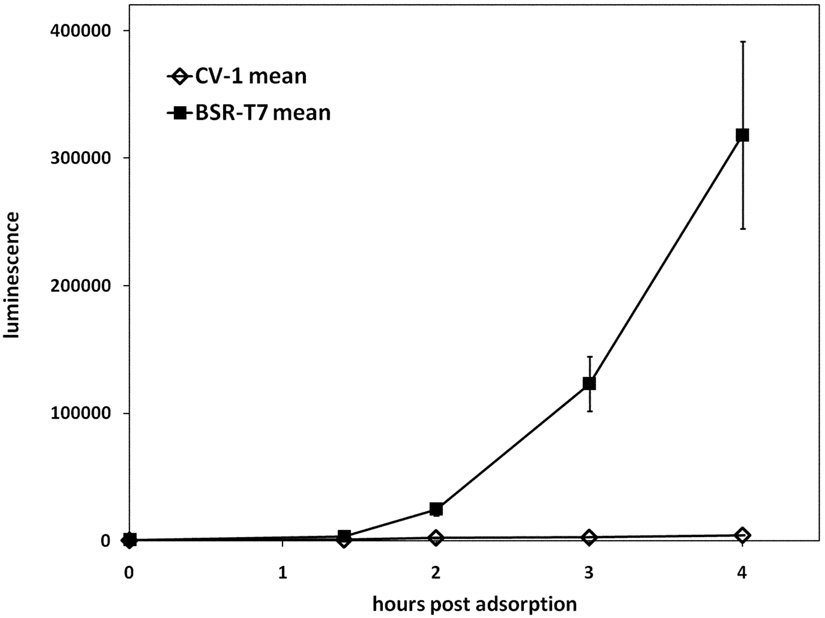
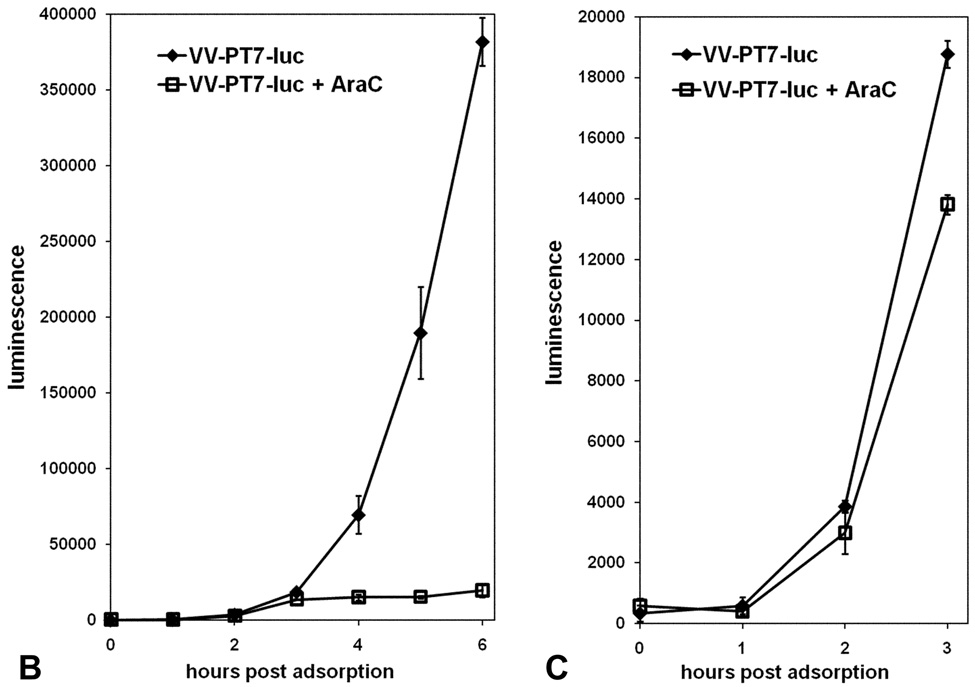
The luciferase gene was inserted into the transfer plasmid pTM1, and the resulting PT7-luciferase cassette was introduced into the VV-WR genome by homologous recombination between the TK flanks. The VV-PT7-luc recombinant gave a very low background of luciferase expression on infection of CV-1 cells lacking the T7 RNA polymerase, but gave substantial luciferase activity after infection of BSR-T7 cells expressing T7 RNA polymerase constitutively (Fig. 2A), indicating that the luciferase gene was tightly regulated. Luciferase synthesis resulting from infection of BSR-T7 cells with VV-PT7-luc was still detectable at early times (<3 h) in the presence of the DNA synthesis inhibitor cytosine arabinoside (AraC), which blocks late gene expression (Fig. 2BC). The sensitivity of the luciferase reporter enabled early detection of luciferase expression from incoming virus genomes in the absence of virus DNA replication. AraC was used in all subsequent experiments to block possible DNA replication of superinfecting virus genomes.
Figure 2. Expression of luciferase in CV-1 and in BSR-T7 cells following infection with VV-PT7-luc, and the effect of AraC on luciferase expression by infected BSR-T7 cells.
(A) CV-1 and BSR-T7 cells were infected with VV-PT7-luc at moi = 10, and luciferase activity determined at intervals. (B, C). Expression of luciferase by VV-PT7-luc in BSR-T7 cells in the presence and absence of 40 µg/ml AraC. The time course from 0 to 6 h p.i. is shown (B), with an enlargement of the time course from 0 to 3h (C).
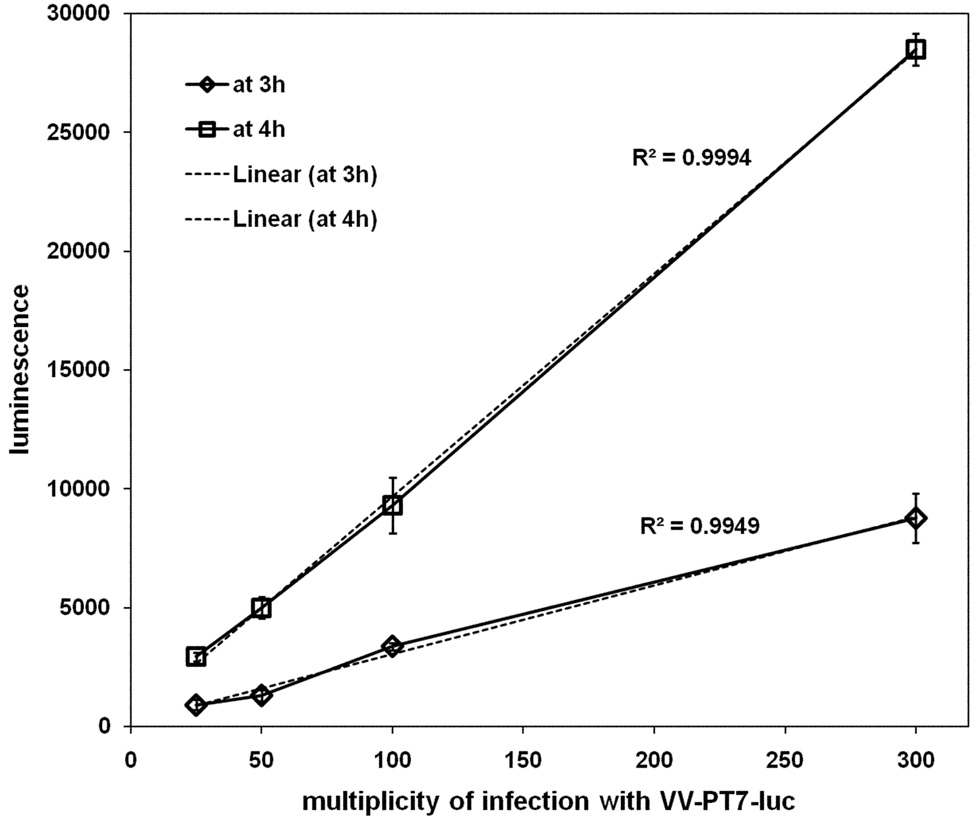
Pilot experiments showed that infection of CV-1 cells with VV-T7 expressing T7 RNA pol followed by superinfection at 16 h p.i. with VV-PT7-luc gave appreciable luciferase expression. The extent of luciferase expression at 3 h p.i. and at 4 h p.i. was found to be related to the multiplicity of superinfection in a linear fashion, over a range of moi from 25 to 300 (Fig. 3). The characteristics of VV-PT7-luc were therefore suitable to assess virus entry into cells pre-infected with VV recombinants expressing T7 RNA pol.
Figure 3. Expression of luciferase from VV-PT7-luc depends on the multiplicity of superinfection.
CV-1 cells infected with VV-T7 at moi = 5 were superinfected at 16 h p.i. with VV-PT7-luc at multiplicities of 25, 50, 100, and 300 in the presence of AraC, and luciferase expression followed at intervals. The data for luciferase expression at 3h and at 4h post adsorption of VV-PT7-luc were plotted as a function of multiplicity, and linear trendlines fitted (dotted lines). The R2 values indicate how closely the values fit a linear trendline.
Entry of superinfecting virus is reduced by the presence of SPI-3 and A56 during the primary infection
The effects of SPI-3 and A56 in the primary infecting virus on subsequent superinfection were evaluated by constructing VV-T7 derivatives lacking either SPI-3 (VV-T7ΔSPI-3), A56 (VV-T7ΔA56) or both (VV-T7ΔSPI-3 ΔA56). CV-1 cells were grown in 96-well plates for 3 days to give dense and tightly-attached monolayers, then infected at moi = 5 with the parental virus VV-T7 or with each of the mutant derivatives. At 16 h p.i., the cells were superinfected with VV-PT7-luc at moi = 100 in the presence of AraC. Luciferase activity was determined at intervals by adding BriteLite luciferase reagent directly to the wells and measuring luminescence. Under these conditions, the extent of cell-cell fusion observed with VV-T7 derivatives lacking SPI-3, A56, or both was minimal (Suppl. Fig. 1). Fusion mediated by VV SPI-3 or A56 mutants at neutral pH is much slower than is seen with CPV mutants (Turner & Moyer, 1992), and complete fusion is not seen until 48 h p.i.
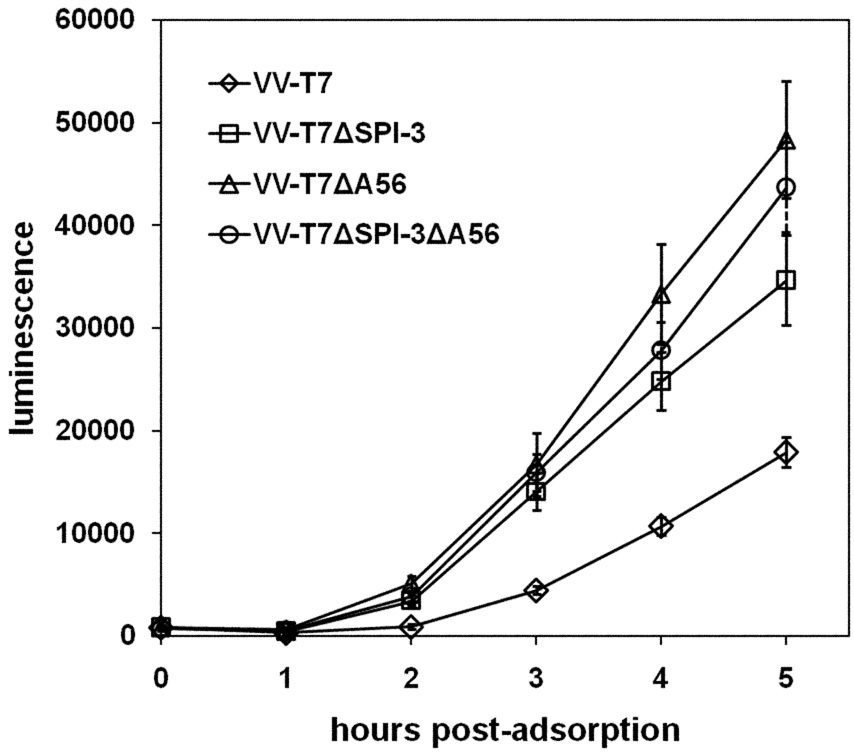
The results (Fig. 4) clearly indicate that luciferase expression from the superinfecting VVPT7- luc virus, and hence virus entry, was more rapid and occurred to a greater extent when SPI-3, A56 or both genes were absent from the primary infecting virus. No appreciable differences were noted between cells infected with VV-T7ΔSPI-3 ΔA56 and cells infected with either single mutant (Fig. 4), suggesting that SPI-3 and A56 act together to reduce the rate of virus entry. Control experiments to measure the level of T7 RNA polymerase expression in infected cells by transfection of pTM1-luc plasmid DNA showed that luciferase levels were identical for VV-T7, VV-T7ΔSPI-3, VV-T7ΔA56, and the double mutant (data not shown), as would be expected since each virus contained the same T7 RNA polymerase gene 1 driven by the same P7.5 early/late promoter in the TK gene (Fuerst et al., 1986). The data of Fig. 4 suggest that both SPI-3 and A56 are required to reduce virion entry into infected cells.
Figure 4. Comparison of luciferase expression after infection with VV-T7 or with derivatives lacking SPI-3 or A56, and superinfection with VV-PT7-luc.
CV-1 cells in 96-well plates were infected with VV-T7, VV-T7ΔSPI-3, VV-T7ΔA56, or VV-T7ΔSPI-3 ΔA56 at moi =5, and at 16 h p.i. superinfected with VV-PT7-luc at moi = 100. Luciferase activity was determined at the indicated time points after adsorption of the superinfecting virus. Each time point shows the mean of 4 wells, plus and minus the standard deviation.
SPI-3 mutants defective in proteinase inhibition are active in reducing luciferase expression by superinfecting virus
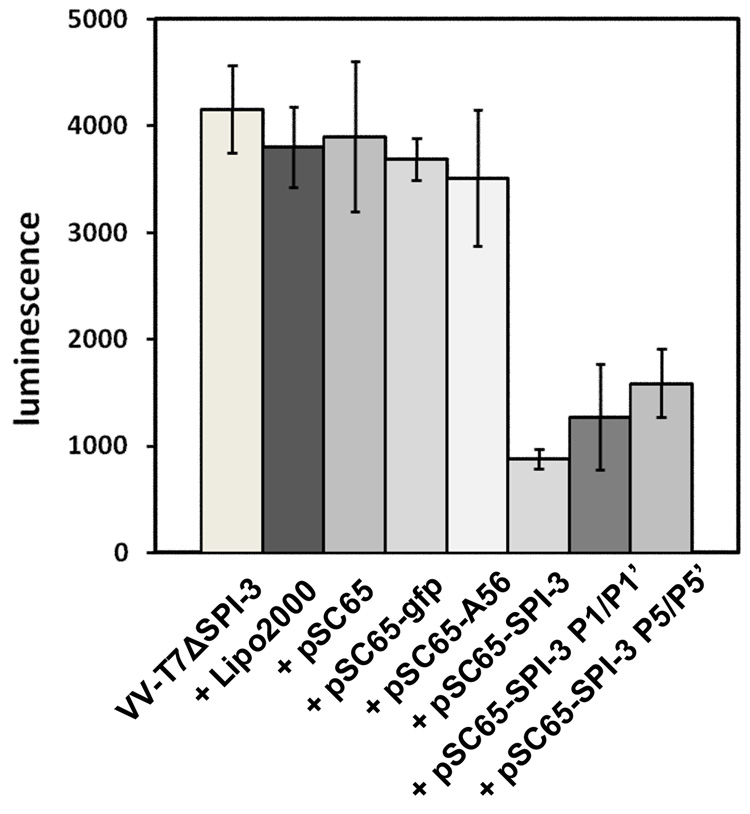
The observed inhibition of cell-cell fusion by SPI-3 and A56 is consistent with SPI-3/A56 slowing the rate at which bound virions fuse with the infected cell membrane. The serpin SPI-3 is known to be a functional inhibitor of the proteinases tissue-type plasminogen activator, urokinase-type plasminogen activator, and plasmin (Turner et al., 2000). However, site-directed CPV SPI-3 mutants in the conserved reactive center loop (RCL) are active in preventing cell-cell fusion, despite being inactive as proteinase inhibitors (Turner & Moyer, 1995). The CPV SPI-3 mutants with substitutions at the P1/P1’ positions and the P5 through P5’ positions in the RCL were tested for their ability to reduce reporter expression from superinfecting virus by a transfection assay. CV-1 cells were infected with VV-T7ΔSPI-3 and immediately transfected with plasmid DNAs derived from pSC65 (Chakrabarti et al., 1997) carrying either CPV wt SPI-3 or the P1/P1’ or P5/P5’ mutant. Control transfections used pSC65 carrying no inserted gene, or with gfp or CPV A56. In all cases the inserted gene was coupled to a synthetic early/late VV promoter (Chakrabarti et al., 1997). Luciferase expression was measured at 2 h after superinfection of infected/transfected cells with VV-PT7-luc. Treatment with the transfection reagent Lipofectamine 2000 alone did not diminish luciferase expression from VV-T7ΔSPI-3 infected cells superinfected with VV-PT7-luc, and likewise transfection with empty vector, pSC65-gfp, or pSC65-A56 had no effect (Fig. 5). However, transient expression of wt SPI-3 from pSC65-SPI-3 did markedly reduce luciferase activity, showing that CPV SPI-3 was able to substitute for VV SPI-3 in this assay. The CPV SPI-3 P1/P1’ and SPI-3 P5/P5’ mutants were also active in reducing luciferase expression following VV-PT7-luc superinfection of cells infected with VV-T7ΔSPI-3 (Fig. 5). The evidence suggested that the P1/P1’ and P5/P5’ SPI-3 mutants were able to reduce entry of VV-PT7-luc into infected cells by virus-cell fusion, in the absence of proteinase inhibition, consistent with their known ability to prevent cell-cell fusion when transfected into cells infected with VV-T7ΔSPI-3 (Turner & Moyer, 1995)
Figure 5. Luciferase expression after infection with VV-T7ΔSPI-3, transfection with plasmid DNAs carrying SPI-3 mutants, and superinfection with VV-PT7-luc.
96-well plates of CV-1 cells were infected with VV-T7ΔSPI-3 at moi = 5, transfected with the indicated plasmid DNAs, and at 16h p.i. superinfected with VV-PT7-luc at moi = 100. Luciferase expression was measured at 2 h after adsorption of the superinfecting virus. Each bar shows the mean of 3 wells, plus and minus the standard deviation.
The presence of SPI-3 mAb during superinfection of CPV-T7-infected cells with VV-PT7-luc increases the rate of superinfecting virus entry
The mAb 4A11-4A3 against cowpox virus SPI-3 has been shown to block the inhibitory effect of SPI-3 upon cell-cell fusion. Cells infected with wild-type CPV in the presence of SPI-3 mAb give cell-cell fusion, in contrast with the lack of fusion seen in the absence of SPI-3 mAb (Turner & Moyer, 2006). By immunofluorescence the SPI-3 mAb is only detected on the surface of cells under these conditions, and the mAb is presumably exerting its effect by binding to SPI-3 protein present on the cell membrane, and not by entering infected cells.
If the SPI-3 protein on the surface of infected cells is reducing superinfection by slowing the rate of fusion of incoming virions with the cell membrane, we might predict that SPI-3 mAb would diminish this effect, and therefore increase the rate of luciferase expression following superinfection with VV-PT7-luc. The SPI-3 mAb reacts much more strongly with CPV SPI-3 than with VV SPI-3 (data not shown), and we therefore tested this hypothesis by constructing CPV-T7, a SPI-3+ A56+ recombinant expressing T7 RNA polymerase, and a derivative CPV-T7ΔSPI-3 lacking SPI-3. The SPI-3 mAb should have no effect on luciferase expression following superinfection of cells infected with CPV-T7ΔSPI-3. CPV-T7 derivatives lacking A56 or both SPI-3 and A56 were also constructed. The amount of cell-cell fusion at 16 h after infection with CPV-T7 lacking SPI-3 and/or A56 (Suppl. Fig. 2) was much greater than with the corresponding VV-T7 mutants (Suppl. Fig. 1).
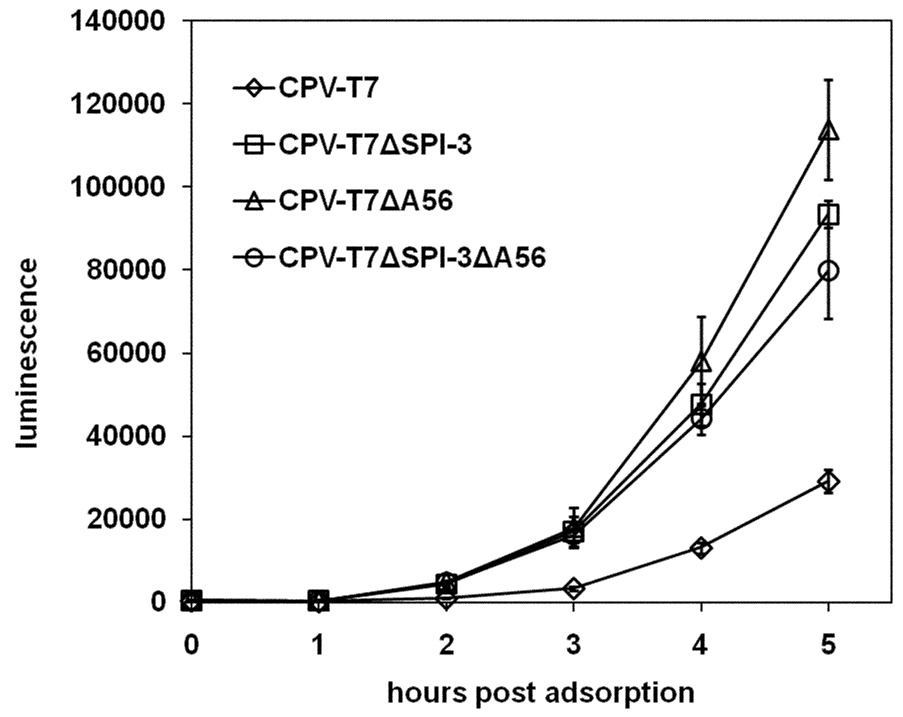
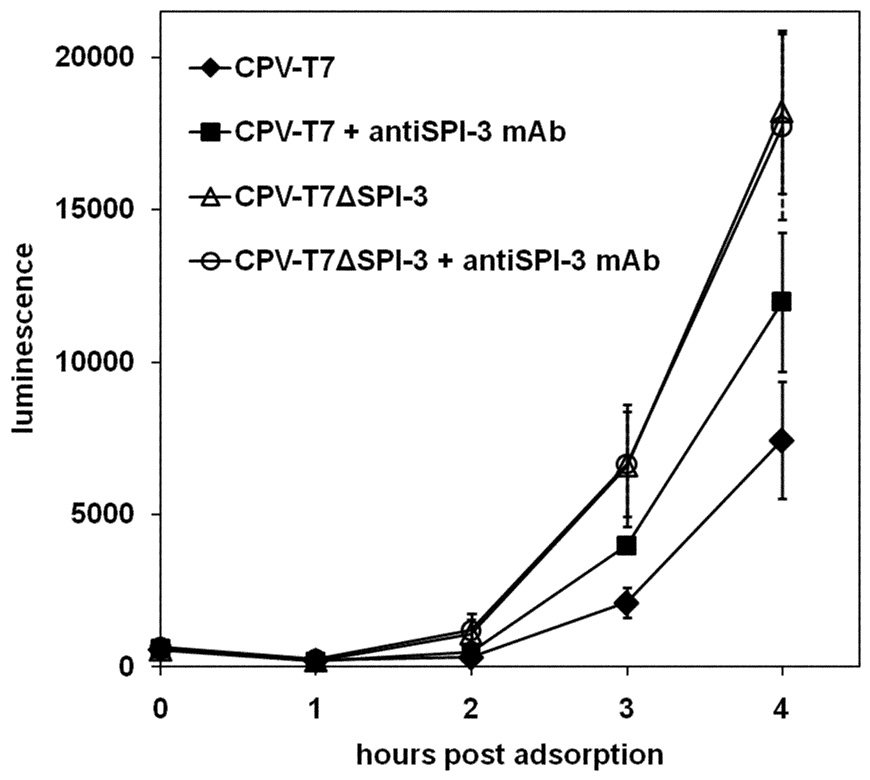
The extent of superinfection of cells first infected with CPV-T7 or CPV-T7ΔSPI-3 was assessed using VV-PT7-luc (Fig. 6A), as in Fig. 4 for the VV-T7 series. The extent of luciferase expression was increased when CPV-T7ΔSPI-3-infected cells were compared with cells infected with CPV-T7 (Fig. 6A). A similar increase was seen with cells pre-infected with CPV-T7ΔA56 or with CPV-T7ΔSPI-3 ΔA56 (Fig.6A). These results indicate that the CPV SPI-3 and A56 appear to reduce superinfection in an identical fashion to their VV counterparts. The magnitude of the increase in luciferase expression following deletion of SPI-3 and/or A56 from CPV-T7 (Fig. 6A) was very similar to that seen after inactivation of SPI-3 and/or A56 in VV-T7 (Fig. 4). The fact that similar increases in reporter gene expression were seen despite the greater cell-cell fusion seen with the CPV-T7 mutants relative to the VV-T7 mutants (Suppl. Fig. 1 and Suppl. Fig. 2) argues that increased luciferase activity reflected increased virus entry, and was not an indirect consequence of cell-cell fusion.
Figure 6. Luciferase expression following superinfection of cells infected with CPV-T7 derivatives.
(A) CV-1 cells were infected with either CPV-T7 expressing the T7 RNA polymerase or with derivatives lacking SPI-3, A56, or both. At 16 h p.i. the cells were superinfected with VV-PT7-luc, and luciferase activity determined at the indicated time points, as for the VV-T7 series in Figure 4. (B) Cells were infected with CPV-T7 or CPV-T7ΔSPI-3 in the absence or presence of 10 µg/ml purified anti-SPI-3 mAb. Superinfecting VV-PT7-luc was added at 16h p.i. and luciferase activity determined at intervals after the end of the adsorption period. SPI-3 mAb was present during adsorption of VV-PT7-luc and during subsequent incubation at 37°C in the treated wells. Error bars indicate the standard deviation.
The SPI-3 mAb was tested for its effect on the rate of luciferase expression following VV-PT7-luc superinfection of cells infected with either CPV-T7 or CPV-T7ΔSPI-3 (Fig. 6B). SPI-3 mAb had no effect on the luciferase expression measured following superinfection of CPV-T7ΔSPI-3-infected cells (Fig. 6B), indicating that the antibody by itself does not affect virus binding or entry. In contrast, the presence of SPI-3 mAb prior to and during superinfection of cells infected with CPV-T7 did increase luciferase expression, to an extent intermediate between the low level for CPV-T7 and the higher level for CPV-T7ΔSPI-3 (Fig. 6B). This result supports the model that SPI-3 present in the infected cell is mediating its effect on superinfection at the cell surface, and not by some indirect mechanism within the cytoplasm.
Low pH treatment accelerates the entry of VV-PT7-luc bound to pre-infected cells, but does not eliminate reduction in luciferase expression caused by SPI-3/A56
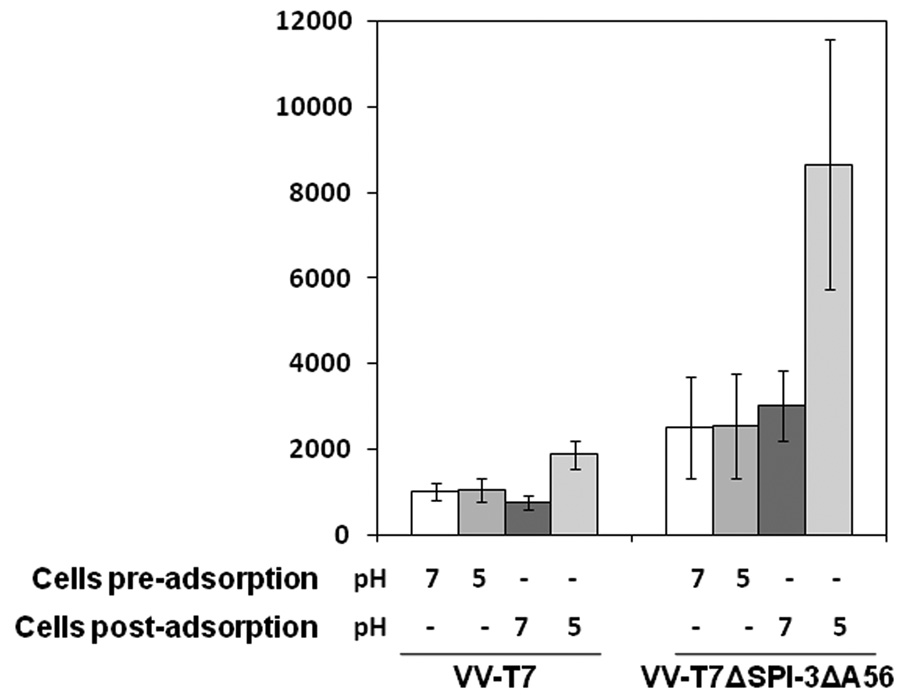
The fact that fusion from within following low pH treatment of infected cells occurs in the presence of SPI-3 and A56 on the cell surface suggests that the apparent inhibitory effects of SPI-3/A56 on virus fusion might be eliminated following exposure to acid conditions. Low pH accelerates the entry of VV into cells (Townsley et al., 2006), apparently both by unmasking the fusion complex on virions, and by stimulating fusion of virions bound at the cell surface (Townsley & Moss, 2007). To investigate the effect of low pH treatment on superinfection, cells were infected with VV-T7, superinfected with VV-PT7-luc at 16hr p.i., and treated for a short period with pH 7 or pH 5 buffer. Low pH treatment of cells was either before the adsorption of the superinfecting virus, or immediately following adsorption. Luciferase expression was measured at 2.5 h after the end of the adsorption. Treatment of VV-T7-infected cells at pH 5 prior to adsorption had no effect on the level of luciferase expression after superinfection (Fig. 7). However, treatment at pH 5 of VV-T7-infected cells to which VV-PT7-luc virions had been bound did increase luciferase expression relative to a pH 7 treated control (Fig. 7), consistent with acceleration of virus entry.
Figure 7. The effect of low pH treatment on cells infected with VV-T7 or VV-T7ΔSPI-3 ΔA56 and superinfected with VV-PT7-luc.
CV-1 cells were infected with VV-T7 or VV-T7ΔSPI-3 ΔA56 and at 16 h p.i. superinfected with VV-PT7-luc. Cells were treated with pH 7 or pH 5 buffer for 3 min at room temperature either immediately before or after adsorption of VV-PT7-luc, as indicated below the axis. Luciferase activity was measured at 2.5 h after the end of the adsorption period.
Cells preinfected with VV-T7ΔSPI-3ΔA56 were similarly superinfected and treated at pH 7 or 5 before or after adsorption of VV-PT7-luc (Fig.7). Low pH treatment of cells infected with the double mutant prior to superinfection did not result in increased luciferase expression relative to the pH 7 control (Fig. 7). Note that the luciferase level was higher for cells treated pre-adsorption at pH 7 or 5 when the first infection was with VV-T7ΔSPI-3ΔA56 compared with VV-T7 (Fig. 9). Low pH treatment of VV-PT7-luc virions bound to cells infected with VV-T7ΔSPI-3ΔA56 gave greater luciferase expression than exposure to pH 7 buffer (Fig. 7). Figure 7 shows that luciferase expression resulting from superinfection of cells infected with VV-T7ΔSPI-3ΔA56 was higher than for VV-T7-infected cells, when cells treated at pH 7 are compared, and when pH 5-treated cells are compared. Both SPI-3 and A56 were detectable by immunofluorescence at normal levels on the surface of infected cells that had been treated at pH 5 (data not shown), indicating that the proteins are still present following low pH treatment. The fact that SPI-3/A56 in VV-T7 reduces luciferase expression from superinfecting virus, even after low pH treatment (Fig. 7), indicates that inhibition of virus fusion by SPI-3/A56 is not abrogated by exposure to pH 5 buffer. The presence of SPI-3/A56 in the membrane surrounding endocytosed virions in superinfected cells would therefore be expected to slow the rate of virion entry into the cytoplasm, even at the acidic conditions within endosomes.
DISCUSSION
The construction of VV-PT7-luc with the luciferase reporter coupled to the T7 promoter allowed us to follow virus entry at early times following superinfection of VV-T7-infected cells. The strategy was similar to previous studies where entry into uninfected cells was followed by using luciferase as a sensitive reporter (Townsley et al., 2006; Townsley & Moss, 2007), except that we used the T7 promoter rather than a strong early/late VV promoter to drive luciferase expression. Under the conditions of our experiments, binding of MV to infected cells was reduced somewhat compared with uninfected cells, by an extent that varied from approximately 30% inhibition for CV-1 cells to 70% for HeLa monolayer cells (Fig. 1D and data not shown). These data are consistent with the earlier observation that the initial adsorption of the superinfecting virus was decreased by up to 40% by prior infection of BSC-40 African green monkey kidney cells (Christen et al., 1990). SPI-3 and A56 did not contribute to this reduction by inhibiting binding of virus, but rather appeared to reduce the rate of MV entry. The most likely mechanism for this reduction is interaction of SPI-3/A56 with proteins in the entry/fusion complex (Wagenaar and Moss, 2007), with resulting inhibition of virus fusion and entry. The results with CPV-T7 derivatives indicated that CPV SPI-3/A56 are active in preventing superinfection by the related orthopoxvirus VV. The entry complex proteins A28, H2, L5, A16, A21, G3, G9, and J5 are between 97.3 and 99.2% conserved between CPV strain Brighton Red and VV strain WR. In comparison the SPI-3 and A56 proteins of the two viruses are 94.9% and 83.2% identical, respectively.
Our experiments do not address potential roles of SPI-3/A56 in the outer envelope of EV. Theoretically, SPI-3/A56 in EV could negatively regulate the activity of the entry/fusion complex until the outer membrane has been removed by a non-fusogenic mechanism after binding of EV to susceptible cells (Law et al., 2006). However, it is difficult to see how the presence of SPI-3/A56 on the outer surface of the EV envelope would allow inhibition of the entry/fusion complex on the surface of the MV, given that the two sets of proteins are physically separated by an intervening membrane. SPI-3/A56 could contribute to the stability of the EV envelope, so that fusogenic MV are released more efficiently at neutral pH in the absence of SPI-3 or A56. Alternatively A56 and/or SPI-3 could play an entirely different role, for example in determining which cells EV binds to, i.e. tissue tropism. Future experiments are aimed at assessing the biological consequences of deletion of SPI-3 and A56 in appropriate animal models.
MATERIALS AND METHODS
Cells and antibodies
CV-1 African green monkey kidney cells (ATCC CCL-70) were propagated in MEM with Earle’s salts (Invitrogen) supplemented with 5% FBS, non-essential amino acids, penicillin/streptomycin, sodium glutamate, and sodium pyruvate (Cellgro). BSR-T7 cells (BSR-T7/5 (Buchholz et al., 1999) were grown in DMEM with 8% FBS in the presence of 10% CO2, and with G418 added at alternate passages.
SPI-3 mAb 4A11-4A3 has been described (Brum et al., 2003).
Plasmids and PCR constructs
The A56 gene of both CPV and VV was replaced with a gfp assette driven by the synthetic PE/L promoter (Chakrabarti et al., 1997). The sequences flanking the A56 gene were amplified by PCR and attached to the PE/L –gfp cassette by ligation. The resulting A55R-gfp-A57R product was obtained by PCR amplification directly from the ligation reaction.
The luciferase gene, originally from pGL3 (Promega), was inserted into pTM1 (Moss et al., 1990) to create pTM1-luc, with luciferase downstream from the T7 promoter and in between the left and right flanks of the TK gene. The wild type A56 and SPI-3 genes and P1/P1’ and P5/P5’ site-directed SPI-3 mutants (Turner & Moyer, 1995) were recloned into pSC65 (Chakrabarti et al., 1997) to allow expression from the synthetic PE/L promoter following transfection.
Viruses
VV-T7 (vTF7-3, (Fuerst et al., 1986)) and VV-T7ΔSPI-3 with the SPI-3 gene replaced by a gpt cassette conferring resistance to mycophenolic acid (Turner & Moyer, 1995) have been described previously. VV-T7ΔSPI-3 ΔA56 was constructed by infecting cells with VV-T7ΔSPI-3, transfecting with A55R-gfp-A57R DNA, and picking and purifying plaques expressing gfp. Similarly VV-T7ΔA56 was derived from VV-T7. The T7 RNA polymerase gene was inserted from the plasmid pTF7-3 (Fuerst et al., 1986) into the TK gene of wtCPV to construct CPV-T7. From CPV-T7, CPV-T7ΔSPI-3 was constructed by insertion of a gpt cassette at the SPI-3 locus (Turner & Moyer, 1992), and CPV-T7ΔA56 was isolated by replacing A56 with gfp. The double mutant CPV-T7ΔSPI-3 ΔA56 was isolated by sequentially inactivating the SPI-3 and A56 genes.
VV-PT7-luc with the luciferase gene under T7 control was isolated following transfection of pTM1-luc DNA into cells infected with wt VV, and selection for TK− recombinants by resistance to 5-bromo-2’-deoxyuridine. Plaques containing the desired insertion were identified by expression of luciferase following infection of BSR-T7 cells.
Virus binding assay for VV A5-YFP
Purified MV of VV A5-YFP (Katsafanas & Moss, 2007) were bound to CV-1 cells in 12-well plates for 1 h at room temperature at moi = 100. After washing to remove unbound virions, PBS containing 0.05% Saponin and 0.5 μg/ml DAPI was added to stain the nuclei. Cells with bound virions were harvested by scraping, pelleted by centifrugation for 3 min at 4,000 g, and the pellets resuspended in 100 μl PBS before transfer to a black 96-well plate. YFP was measured by excitation at 488 nm and emission at 530 nm with a 515 nm cutoff filter, and DAPI by 360 nm excitation and 464 nm emission (420 nm cutoff).
Luciferase assays following infection with VV-PT7-luc
CV-1 cells were grown to confluence over 3 days in 96-well white, clear-bottom plates, and either mock-infected or infected with a VV-T7 or CPV-T7 derivative at a multiplicity of 5. At 16 h p.i., superinfecting VV-PT7-luc in 50 μl serum-free medium with 40 μg/ml AraC was added per well at moi = 100, and adsorbed for 1 h at room temperature on a rocking platform. Following removal of the VV-PT7-luc inoculum, 100 μl medium containing 40 μg/ml AraC was added per well, and the cells incubated at 37°C. At intervals 100 µl BriteLite luciferase reagent (PerkinElmer) was added per well, the well contents mixed by pipetting, and luminescence measured in a Molecular Devices SpectraMax M2 plate reader. For each time point and virus, 4 replicate wells were used.
Supplementary Material
CV-1 cells were mock-infected (A) or infected at moi = 5 with VV-T7 (B), VV-T7ΔSPI-3 (C), VV-T7ΔA56 (D), or VV-T7ΔSPI-3ΔA56 (E). Phase contrast photographs are shown at 16 h p.i.
CV-1 cells were photographed at 16 h after infection at moi = 5 with CPV-T7 (A), CPV-T7ΔSPI-3 (B), CPV-T7ΔA56 (C), or CPV-T7ΔSPI-3ΔA56 (D).
ACKNOWLEDGMENTS
We thank Rafael Blasco for contributing to an early discussion of the likely roles of the SPI-3 and A56 proteins, Bernie Moss and Tatiana Senkevich for providing VV A5-YFP, and Jeff Browning for competent technical assistance. Support was provided by NIH NIAID grant R56 AI-015722.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Peter C. Turner, Email: pcturner@ufl.edu.
Richard W. Moyer, Email: rmoyer@mgm.ufl.edu.
REFERENCES
- Brown E, Senkevich TG, Moss B. Vaccinia virus F9 virion membrane protein is required for entry but not virus assembly, in contrast to the related L1 protein. J Virol. 2006;80:9455–9464. doi: 10.1128/JVI.01149-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum LM, Turner PC, Devick H, Baquero MT, Moyer RW. Plasma membrane localization and fusion inhibitory activity of the cowpox virus serpin SPI-3 require a functional signal sequence and the virus encoded hemagglutinin. Virology. 2003;306:289–302. doi: 10.1016/s0042-6822(02)00017-x. [DOI] [PubMed] [Google Scholar]
- Buchholz UJ, Finke S, Conzelmann KK. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. Journal of Virology. 1999;73:251–259. doi: 10.1128/jvi.73.1.251-259.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Sisler JR, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- Christen L, Seto J, Niles EG. Superinfection exclusion of vaccinia virus in virus-infected cell cultures. Virology. 1990;174:35–42. doi: 10.1016/0042-6822(90)90051-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit RC, Moussatche N, Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv.Virus Res. 2006;66:31–124. doi: 10.1016/S0065-3527(06)66002-8. [DOI] [PubMed] [Google Scholar]
- Fuerst TR, Niles EG, Studier FW, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc.Natl.Acad.Sci.U.S.A. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y, Dales S. Biogenesis of poxviruses: Interrelationship between hemagglutinin production and polykaryocytosis. Virology. 1971;46:533–543. doi: 10.1016/0042-6822(71)90057-2. [DOI] [PubMed] [Google Scholar]
- Izmailyan RA, Huang CY, Mohammad S, Isaacs SN, Chang W. The Envelope G3L Protein Is Essential for Entry of Vaccinia Virus into Host Cells. J Virol. 2006;80:8402–8410. doi: 10.1128/JVI.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RM, Spear PG. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989;63:819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsafanas GC, Moss B. Colocalization of transcription and translation within cytoplasmic poxvirus factories coordinates viral expression and subjugates host functions. Cell Host.Microbe. 2007;2:221–228. doi: 10.1016/j.chom.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law KM, Smith GL. A vaccinia serine protease inhibitor which prevents virus-induced cell fusion. J.Gen.Virol. 1992;73:549–557. doi: 10.1099/0022-1317-73-3-549. [DOI] [PubMed] [Google Scholar]
- Law M, Carter GC, Roberts KL, Hollinshead M, Smith GL. Ligand-induced and nonfusogenic dissolution of a viral membrane. Proc.Natl.Acad.Sci.U.S.A. 2006;103:5989–5994. doi: 10.1073/pnas.0601025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Poxviridae: The viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Virology. New York: Raven Press; 2001. pp. 2849–2883. [Google Scholar]
- Moss B. Poxvirus entry and membrane fusion. Virology. 2006;344:48–54. doi: 10.1016/j.virol.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Moss B, Elroy Stein O, Mizukami T, Alexander WA, Fuerst TR. Product review. New mammalian expression vectors. Nature. 1990;348:91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- Ojeda S, Domi A, Moss B. Vaccinia virus G9 protein is an essential component of the poxvirus entry-fusion complex. J Virol. 2006a;80:9822–9830. doi: 10.1128/JVI.00987-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda S, Senkevich TG, Moss B. Entry of Vaccinia Virus and Cell-Cell Fusion Require a Highly Conserved Cysteine-Rich Membrane Protein Encoded by the A16L Gene. J Virol. 2006b;80:51–61. doi: 10.1128/JVI.80.1.51-61.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P, Tobita K, Ueda M, Compans RW. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974;61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Payne LG. Identification of the vaccinia hemagglutinin polypeptide from a cell system yielding large amounts of extracellular enveloped virus. J.Virol. 1979;31:147–155. doi: 10.1128/jvi.31.1.147-155.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne LG. Significance of Extracellular Enveloped Virus in the Invitro and Invivo Dissemination of Vaccinia. Journal of General Virology. 1980;50:89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- Senkevich TG, Moss B. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion. J Virol. 2005;79:4744–4754. doi: 10.1128/JVI.79.8.4744-4754.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich TG, Ojeda S, Townsley A, Nelson GE, Moss B. Poxvirus multiprotein entry-fusion complex. Proc.Natl.Acad.Sci.U.S.A. 2005;102:18572–18577. doi: 10.1073/pnas.0509239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich TG, Ward BM, Moss B. Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene. Journal of Virology. 2004;78:2357–2366. doi: 10.1128/JVI.78.5.2357-2366.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen.Virol. 2002;83:2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- Townsley AC, Moss B. Two distinct low-pH steps promote entry of vaccinia virus. J Virol. 2007;81:8613–8620. doi: 10.1128/JVI.00606-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley AC, Senkevich TG, Moss B. The product of the vaccinia virus L5R gene is a fourth membrane protein encoded by all poxviruses that is required for cell entry and cell-cell fusion. J Virol. 2005a;79:10988–10998. doi: 10.1128/JVI.79.17.10988-10998.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley AC, Senkevich TG, Moss B. Vaccinia virus A21 virion membrane protein is required for cell entry and fusion. J Virol. 2005b;79:9458–9469. doi: 10.1128/JVI.79.15.9458-9469.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley AC, Weisberg AS, Wagenaar TR, Moss B. Vaccinia virus entry into cells via a low-pH-dependent endosomal pathway. J Virol. 2006;80:8899–8908. doi: 10.1128/JVI.01053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PC, Baquero MT, Yuan S, Thoennes SR, Moyer RW. The Cowpox Virus Serpin SPI-3 Complexes with and Inhibits Urokinase-Type and Tissue-Type Plasminogen Activators and Plasmin. Virology. 2000;272:267–280. doi: 10.1006/viro.2000.0377. [DOI] [PubMed] [Google Scholar]
- Turner PC, Moyer RW. An orthopoxvirus serpinlike gene controls the ability of infected cells to fuse. J.Virol. 1992;66:2076–2085. doi: 10.1128/jvi.66.4.2076-2085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PC, Moyer RW. Orthopoxvirus fusion inhibitor glycoprotein SPI-3 (open reading frame K2L) contains motifs characteristic of serine proteinase inhibitors that are not required for control of cell fusion. J.Virol. 1995;69:5978–5987. doi: 10.1128/jvi.69.10.5978-5987.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PC, Moyer RW. The cowpox virus fusion regulator proteins SPI-3 and hemagglutinin interact in infected and uninfected cells. Virology. 2006;347:88–99. doi: 10.1016/j.virol.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Wagenaar TR, Moss B. Association of vaccinia virus fusion regulatory proteins with the multicomponent entry/fusion complex. J Virol. 2007;81:6286–6293. doi: 10.1128/JVI.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Dowling P, Youngner JS, Widnell CC, Wilcox DK. Superinfection exclusion by vesicular stomatitis virus. Virology. 1983;131:137–143. doi: 10.1016/0042-6822(83)90540-8. [DOI] [PubMed] [Google Scholar]
- Zhou J, Sun XY, Fernando GJ, Frazer IH. The vaccinia virus K2L gene encodes a serine protease inhibitor which inhibits cell-cell fusion. Virology. 1992;189:678–686. doi: 10.1016/0042-6822(92)90591-c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CV-1 cells were mock-infected (A) or infected at moi = 5 with VV-T7 (B), VV-T7ΔSPI-3 (C), VV-T7ΔA56 (D), or VV-T7ΔSPI-3ΔA56 (E). Phase contrast photographs are shown at 16 h p.i.
CV-1 cells were photographed at 16 h after infection at moi = 5 with CPV-T7 (A), CPV-T7ΔSPI-3 (B), CPV-T7ΔA56 (C), or CPV-T7ΔSPI-3ΔA56 (D).