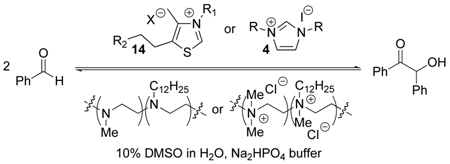
Abstract
Hydrophobic thiazolium and imidazolium coenzyme mimics in the presence of modified-polyethylenimine enzyme mimics catalyze the benzoin condensation 2300–3300 times faster than the coenzyme mimics alone. Polycationic enzyme mimics provide not only a hydrophobic binding domain for coenzyme and substrate, but also electrostatic stabilization of anionic species that arise along the reaction pathway of the benzoin condensation.
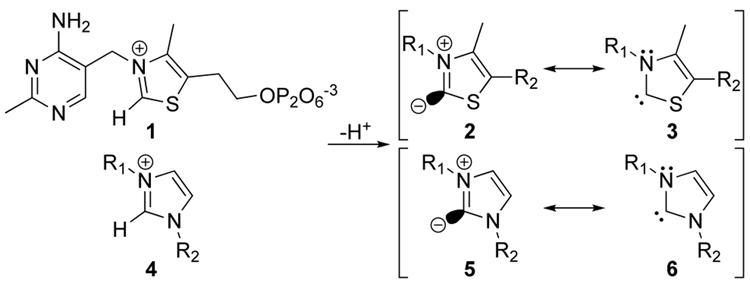
Thiamine diphosphate (Figure 1, 1) is a coenzyme for important enzymatic reactions, including pyruvate decarboxylase that converts pyruvate to acetaldehyde and transketolases that play a critical role in glucose degradation and in the Calvin cycle in photosynthesis.1,2 It also condenses pyruvate to acetolactate in the biosynthetic path to valine.3 In all these reactions, its role is to form a stable equivalent of an acyl anion. In 1957 we showed that the thiazolium ion – the most distinctive part of thiamine – can readily form an anion at C-2, species 2.4 In a full paper in 1958 we pointed out the special stability 2 receives from resonance with a carbene form 3.5 We showed that imidazolium ions 4 can also form such a resonance pair, 5 and 6.
Figure 1.
Thiamine diphosphate (1) and zwitterion/carbene resonance hybrids of thiazolium (2/3) and imidazolium (5/6) ions.
Addition of the 2/3 hybrid to pyruvic acid forms an adduct that can decarboxylate, ultimately releasing acetaldehyde and regenerating the 2/3 hybrid. The other biochemical roles played by thiamine diphosphate can be similarly understood. In 1973, Stetter expanded the synthetic usefulness of the thiazolium anions 2/3.6 As numerous other advances have arisen,7 it has become fashionable to refer to species such as 2/3 and 5/6 as “nucleophilic carbenes”, although of course it is the zwitterion resonance form that makes it clear why these species are nucleophilic.
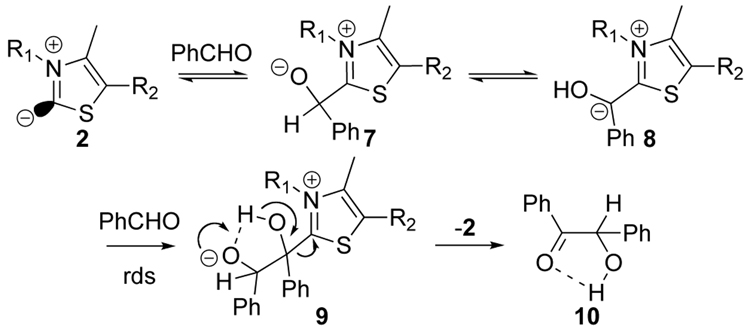
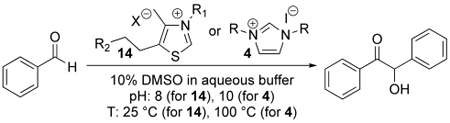
The role that thiazolium ions play in biochemistry is similar to the role that cyanide ion plays in the benzoin condensation, and indeed thiazolium ions can catalyze the benzoin condensation (Scheme 1), as can imidazolium ions. Kinetic studies supported this scheme.8–10 The benzoin condensation was crucial in our earliest studies of thiazolium catalysis, and in many subsequent studies by us and others.
Scheme 1.
Benzoin (10) formation catalyzed by thiazolium (or imidazolium) ions
Previously, we mimicked the binding role played by the enzyme by attaching thiazolium ions to cyclodextrins. With β-cyclodextrin we saw evidence that the intermediate 7 was readily formed in water when benzaldehyde bound into the cyclodextrin cavity, but there was no room for the second benzaldehyde in the overall benzoin-forming mechanism.11 The larger cavity of γ-cyclodextrin, linked to a thiazolium ion, permitted the second benzaldehyde to bind and couple with 7.12 This catalyzed the benzoin condensation with a rate increase of ~7-fold contributed by the cyclodextrin. Pandit attached a thiazolium salt to polystyrene, and saw that this somewhat slowed the benzoin condensation.13
In the present work, we more closely mimic the enzyme/coenzyme system with modified polyethylenimines (PEIs) as enzyme surrogates, and hydrophobic thiamine analogs as corresponding coenzyme mimics. This has resulted in very effective catalysis with greater than 3000-fold rate accelerations in comparison to reactions without PEI.
PEIs were pioneered as enzyme mimics by Klotz.14 We have used them in mimics of transaminase enzymes, where we saw that incorporating a hydrophobic core in the polymers improved both binding and rate constants.15 By contrast with one-directional transaminations by pyridoxamines, which require an additional step to regenerate the pyridoxamine form, thiamine diphosphate-dependent systems are true catalysts.
We have now investigated enzyme mimics in which hydrophobic thiazolium and imidazolium ions reversibly bind to modified PEIs to catalyze the benzoin condensation. The reactions were performed in aqueous solutions, to promote hydrophobic binding of the coenzyme and the substrates into the same polymer region. The benzoin condensation catalyzed by thiazolium and imidazolium ions is effective in polar organic solvents but often more sluggish in water (see Supporting Information). Herein, we show that the modified PEIs accelerated the reactions by accommodating the coenzyme mimics and substrates within the non-polar interior, just as occurs with the hydrophobic cores of natural enzymes.
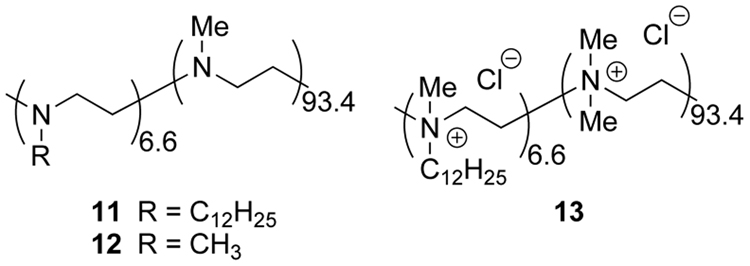
We used a commercial PEI with a number average molecular weight of ca. 10,000, with ca. 230 monomer residues. As previously, we laurylated a portion of the nitrogens of this material with dodecyl iodide in ethanol, then dialyzed and reductively methylated the remaining NH groups with formaldehyde and formic acid to form polymer 11 (Figure 2). 1H-NMR integration indicated that ca. 6.6% of the amines were laurylated. The absence of free NH groups was indicated by the lack of subsequent acetylation by acetic anhydride. In another PEI 12, all alkyl groups were methyl. Some of the laurylated PEI was also permethylated to polycation 13 with dimethyl sulfate and cesium carbonate.
Figure 2.
Neutral (11 and 12) and polycationic (13) PEIs.
We examined the benzoin condensation (Table 1) catalyzed by thiazolium salts 14 and imidazolium salts 4 in which the hydrophobic side chains were varied (see Supporting Information). Thiazolium and imidazolium salts are referred to as coenzyme mimics and the PEI derivatives as enzyme mimics, by analogy with the biochemical species. The thiazolium reactions were run in water with 10 volume % DMSO and 0.5M disodium phosphate buffer (pH 8.0) with 2 mM thiazolium salt, PEI 6 mM in nitrogen atoms, and 40 mM initial [benzaldehyde]. The product [benzoin] was determined by HPLC. When the reaction was carried to only 10% loss of benzaldehyde, benzoin formed linearly with time; the resulting rate constants are listed in Table 1, entries 1 to 11.
Table 1.
Rate constants for the benzoin condensation.a
 | ||||||
|---|---|---|---|---|---|---|
| Thiazolium Coenzyme Mimics (14) | ||||||
| Entry | R1 | R2 | X | PEI | V (×10−9 M/s) | krelb |
| 1c | C12H25 | BnO | Br | - | 4 | 1 |
| 2 | C12H25 | BnO | Br | 11 | 5040 ± 60 | 1260 ± 15 |
| 3 | C12H25 | BnO | Br | 13 | 5136 ± 111 | 1284 ± 28 |
| 4d | C12H25 | BnO | Br | 11 | 5167 ± 222 | 1304 ± 56 |
| 5 | C12H25 | BnO | I | 11 | 4944 ± 111 | 1248 ± 28 |
| 6 | Bn | BnO | Cl | 11 | 24 ± 2 | 6 ± 0.5 |
| 7 | Me | C12H25S | I | 11 | 552 ± 56 | 138 ± 14 |
| 8 | C4H9 | C12H25S | I | 11 | 9424 ± 524 | 2356 ± 131 |
| 9 | C6H13 | C12H25S | I | 11 | 4664 ± 40 | 1166 ± 10 |
| 10 | Bn | C12H25S | Br | 11 | 1552 ± 16 | 388 ± 4 |
| 11 | C4H9 | C12H25S | I | 12 | 596 ± 16 | 149 ± 4 |
| Imidazolium Coenzyme Mimics (4) | ||||||
| Entry | R | X | PEI | V (×10−9 M/s) | krelb | |
| 12 | Me | I | - | 0.36 | 1 | |
| 13 | C4H9 | I | 13 | 13 ± 0.17 | 37 ± 0.5 | |
| 14 | C6H13 | I | 13 | 18.7 ± 0.33 | 52 ± 1 | |
| 15 | C8H17 | I | 13 | 44 ± 2 | 123 ± 6 | |
| 16 | C10H21 | I | 13 | 833 ± 11 | 2314 ± 31 | |
| 17 | C12H25 | I | 13 | 1225 ± 3 | 3402 ± 8 | |
| 18 | C12H25 | I | 11 | 83 ± 5 | 230 ± 14 | |
Coenzyme loading = 10 mol % (14), 40 mol % (4); [PEI] = 6 mM; [PhCHO]initial = 40mM.
Relative to entry 1 or 12, respectively. Errors are the deviations from two runs.
One run.
Buffer concentration = 0.2M Na2HPO4 (pH~8.0).
Calculated from the initial slope of [Benzoin] vs. time. Errors are the deviations from two runs.
As the table shows, catalysts with two hydrophobic chains were the most effective, with ca. 103-fold accelerations relative to the coenzyme mimic alone. The exception was bulky N-3 benzyl substitution (entries 6 and 10). Entry 8 with an n-butyl group was somewhat more active than the n-hexyl group (entry 9), which was on the edge of insolubility. Entries 2 and 3 indicate that a permethylated PEI polycation was, if anything, slightly more effective than was the PEI with titratable amines. This indicates that the nitrogens of the PEIs are furnishing cations for electrostatic stabilization of the negative charges formed in the catalytic processes of Scheme 2, not general acid and general base catalysis as they did in transaminations. The reversible steps of Scheme 1 should not show general acid or general base catalysis. General acid/base catalysis by the buffer is also excluded by comparison of entries 2 ([buffer] = 0.5 M) and 4 ([buffer] = 0.2 M.). Entry 11, without added dodecyl groups on the PEI, has ca. 50% hydrophilic cationic residues and ca. 50% neutral ones, and was less potent than those with added hydrophobic groups.
Imidazolium salts are poorer catalysts, so the study of dialkylimidazolium ions was performed at higher catalyst loading (8 mM), pH (10), and temperature (under reflux, 100 °C). As entries 12 to 17 show, the rates again increased as the alkyl groups were longer, promoting better hydrophobic binding into the PEI, and perhaps with the benzaldehyde. (By contrast, the ions with longer alkyl groups were slower in THF solvent without PEIs, where steric effects were not overridden by hydrophobic binding. See Supporting Information).
The most striking contrast with the thiazolium data was the finding that the polycationic PEI was a much better catalyst (entry 17) than was the polyamine catalyst (entry 18), all relative to the simple dimethylimidazolium ion alone (entry 12). At pH 10 the polyamine 11 has much less positive charge than at pH 8, and these data show that the most important effect of PEI 13 at pH 10, besides binding the catalysts and benzaldehydes into a hydrophobic core, is the presence of a polycationic system that furnished electrostatic stabilization to the anions of the reaction species.
Previously, we showed that modified PEI may act as an artificial binding domain to provide a water-excluded core and important general acid/base reactivity for biomimetic rate enhancement.15 This work shows that the reversible hydrophobic binding of different coenzyme mimics and substrates into modified PEI’s hydrophobic core can lead to very effective true catalysis. Furthermore, PEI can be manipulated to furnish cationic groups that promote electrostatic stabilization of anionic reaction intermediates and transition states.
Supplementary Material
Experimental details, characterization data for new compounds, and non-aqueous benzoin condensation data. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgement
This work was supported by the NSF and the NIH.
References
- 1.(a) Kluger R, Tittmann K. Chem. Rev. 2008;108:1797. doi: 10.1021/cr068444m. [DOI] [PubMed] [Google Scholar]; (b) Jordan F, Patel MS, editors. Thiamine: Catalytic Mechanisms and Role in Normal and Disease States. New York: Marcel Dekker Inc.; 2004. pp. 1–588. [Google Scholar]
- 2.Schenk G, Duggleby RG, Nixon PF. Int. J. Biochem. Cell Biol. 1998;30:1297. doi: 10.1016/s1357-2725(98)00095-8. [DOI] [PubMed] [Google Scholar]
- 3.Bauerle RH, Freundlich M, Størmer FC, Umbarger HE. Biochim. Biophys. Acta. 1964;92:142. [PubMed] [Google Scholar]
- 4.Breslow R. J. Am. Chem. Soc. 1957;79:1762. [Google Scholar]
- 5.Breslow R. J. Am. Chem. Soc. 1958;80:3719. [Google Scholar]
- 6.Stetter H, Schreckenberg M. Angew. Chem. 1973;85:89. [Google Scholar]
- 7.(a) Maki BE, Chan A, Scheidt KA. Synthesis. 2008;8:1306. doi: 10.1055/s-2008-1072516. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606. doi: 10.1021/cr068372z. [DOI] [PubMed] [Google Scholar]; (c) Marion N, Diez-Gonzalez S, Nolan SP. Angew. Chem. Int. Ed. 2007;46:2988. doi: 10.1002/anie.200603380. [DOI] [PubMed] [Google Scholar]; (d) Sohn SS, Rosen EL, Bode JW. J. Am. Chem. Soc. 2004;126:14370. doi: 10.1021/ja044714b. [DOI] [PubMed] [Google Scholar]
- 8.Van den Berg HJ, Challa G, Pandit UK. J. Mol. Catal. 1989;51:1. [Google Scholar]
- 9.Breslow R, Kim R. Tetrahedron Lett. 1994;35:699. [Google Scholar]
- 10.Breslow R, Shmuck C. Tetrahedron Lett. 1996;37:8241. [Google Scholar]
- 11.Hilvert D, Breslow R. Bioorg. Chem. 1984;12:206. [Google Scholar]
- 12.Breslow R, Kool ET. Tetrahedron Lett. 1988;29:1635. [Google Scholar]
- 13.Van den Berg HJ, Challa G, Pandit UK. J. Mol. Catal. 1989;51:13. [Google Scholar]
- 14.Klotz IM, Royer GP, Scarpa IS. Proc. Nat. Acad. Sci. 1971;68:263. doi: 10.1073/pnas.68.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Zhou W, Chruma J, Breslow R. J. Am. Chem. Soc. 2004;126:8136. doi: 10.1021/ja048671a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental details, characterization data for new compounds, and non-aqueous benzoin condensation data. This material is available free of charge via the Internet at http://pubs.acs.org.