Abstract
Background
Cellular oxidative stress is an important factor in asthma and is thought to be the principle mechanism by which oxidant pollutants such as ozone and particulates mediate their pro-inflammatory effects. Endogenous Phase II enzymes abrogate oxidative stress through the scavenging of reactive oxygen species and metabolism of reactive chemicals.
Objective
We conducted a placebo-controlled dose escalation trial to investigate the in vivo effects of sulforaphane, a naturally occurring potent inducer of Phase II enzymes, on the expression of glutathione-s-transferase M1 (GSTM1), glutathione-s-transferase P1 (GSTP1), NADPH quinone oxidoreductase (NQO1), and hemoxygenase-1 (HO-1) in the upper airway of human subjects.
Methods
Study subjects consumed oral sulforaphane doses contained in a standardized broccoli sprout homogenate (BSH). RNA expression for selected Phase II enzymes was measured in nasal lavage cells by RT-PCR before and after sulforaphane dosing.
Results
All subjects tolerated oral sulforaphane dosing without significant adverse events. Increased Phase II enzyme expression in nasal lavage cells occurred in a dose-dependent manner with maximal enzyme induction observed at the highest dose of 200 grams broccoli sprouts prepared as BSH. Significant increases were seen in all sentinel Phase II enzymes RNA expression compared to baseline. Phase II enzyme induction was not seen with ingestion of non-sulforaphane containing alfalfa sprouts.
Conclusion
Oral sulforaphane safely and effectively induces mucosal Phase II enzyme expression in the upper airway of human subjects. This study demonstrates the potential of antioxidant Phase II enzymes induction in the human airway as a strategy to reduce the inflammatory effects of oxidative stress.
Clinical Implications
This study demonstrates the potential of enhancement of Phase II enzyme expression as a novel therapeutic strategy for oxidant induced airway disease.
Capsule Summary
A placebo-controlled dose escalation trial demonstrated that naturally occurring sulforaphane from broccoli sprouts can induce a potent increase in antioxidant Phase II enzymes in airway cells.
Keywords: oxidative stress, antioxidants, asthma, allergic inflammation, sulforaphane, Phase II enzymes, air pollution
INTRODUCTION
Recent years have seen a growing appreciation of the role of oxidative stress in the pathogenesis of asthma and allergy.1–2 Certain allergens such as ragweed pollen containing NADPH oxidase can generate reactive oxygen species (ROS) which at least in some murine models have been shown to be critical in the induction of a robust inflammatory response.3 Additionally, ROS generation is thought to be the principal pathways by which certain pollutants such as diesel exhaust particles (DEP) and secondhand tobacco smoke exert their pro-inflammatory and adjuvant effects.4–5
Phase II (PII) enzymes are an important cellular defense mechanism against oxidative stress.6–7 Several studies have demonstrated they have important protective effects against xenobiotics such as DEP, ozone, and tobacco smoke. PII enzymes neutralize reactive oxygen species and metabolize xenobiotics.8–10 Overexpression of PII enzymes will ablate DEP-induced pro-inflammatory cytokine production from airway epithelial cells.11 Thus, PII enzyme induction may be a valuable interventional strategy to prevent the effects of oxidative stress in asthma, especially with regard to OS induced by certain pollens or oxidant pollutants. Sulforaphane (SFN), is a naturally occurring isothiocyanate found in cruciferous vegetables, and is richest in broccoli sprouts.12 It is the most potent inducer of PII enzymes identified to date and is thought to act via activation of the Nrf2 transcription factor and the Anti-oxidant Response Element (ARE).13–14 These studies, however, have been limited to either in vitro or animal models. In these systems SFN abrogates the pro-inflammatory and pro-allergic effects typically observed with cellular DEP exposure.15 SFN has therefore been proposed as a novel therapeutic approach. However, the efficacy of oral SFN in elevating Phase II enzymes in airway cells of humans has yet to be determined.
Previous work employing broccoli sprout preparations administered to human subjects has delineated the basic pharmacokinetics and safety of oral SFN.16–19 Based on that preliminary data, we performed a placebo-controlled human study to investigate the safety, efficacy, and dose-effect of oral SFN for the induction on Phase II enzyme expression in upper airway cells.
METHODS
Subjects
Sixty-five human volunteers were recruited by advertisements and flyers posted at the University of California – Los Angeles (UCLA) campus and surrounding communities. Eligible subjects were clinically healthy nonsmokers ≥18 years of age. The use of inhaled, topical, or systemic corticosteroids or any other immune modulating medications was prohibited during the study period. At initial screening, a cheek scrape was performed to determine genotype for GSTM1 polymorphisms. Subjects were not selected or excluded based on genotypic status, however, subjects homozygous for GSTM1 null were excluded from analysis of GSTM1 expression. The Human Subject Protection Committee at UCLA approved all study activities.
Study Design
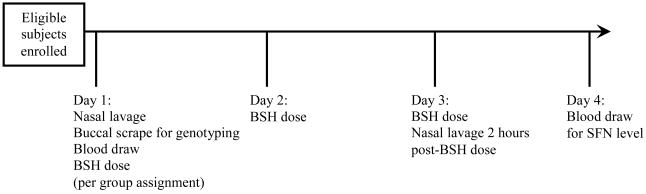
The study protocol schedule is summarized in Figure 1. Prior to starting the single-blind study, subjects were provided a list of sulforaphane-containing foods and instructed to avoid these foods during the study period. Baseline nasal lavage and blood samples were collected from each enrolled subject on day 1 to assess baseline Phase II enzyme expression. Subjects subsequently ingested a measured amount of broccoli sprout homogenate (BSH) once daily on days 1, 2, and 3. An escalating dose block design was employed to monitor for tolerability and toxicity associated with the treatment. A group of five subjects completed each dose level prior to enrolling subjects in the next dose level. New subjects were recruited for each dose block so that individuals completed only one dose regimen during the study, thus avoiding potential interference of previous BSH dosing on the measurement of Phase II enzyme expression. Following this design, doses of BSH prepared from 25, 50, 75, 100, 125, 150, 175, and 200 grams were completed. Once safety and tolerability were established, additional subjects were subsequently enrolled at doses of 125, 150, 175, and 200 grams to examine dose-response effect. Five subjects completed alfalfa sprout homogenate dosing at 200 grams as a control group. Alfalfa sprouts are similar in appearance and taste but do not contain significant amounts of sulforaphane glucosinolates. To assess Phase II enzyme expression following sprout homogenate doses, a repeat nasal lavage sample was collected on day 3 (2 hours after 3rd BSH dose). A blood sample was obtained on day 4 to evaluate serum SFN levels.
Figure 1. Protocol schedule for oral broccoli sprout homogenate dosing study.
Nasal lavage and blood processing
Nasal lavage collection was performed in standard fashion as previously described.20 After collection, cell pellets were isolated from nasal lavage samples, resuspended in 350μl lyses buffer, and stored at −80 degrees C until RT-PCR analysis. A blood sample was collected on day 4 to assess for serum SFN levels 24 ± 4 hours after the final BSH dose. Blood samples were collected, processed, and stored in a manner to avoid contamination with rubber materials known to interfere with the cyclocondensation assay. See additional Methods information in the Online Respository.
Broccoli sprout homogenate preparation
Sulforaphane was administered to subjects using broccoli sprout homogenate. Broccoli sprouts contain high concentrations of sulforaphane glucosinolates (SGS), the inert precursors of sulforaphane.21 Sulforaphane is released when SGS undergoes hydrolysis by myrosinase released from plant tissues during the chewing process. Based on procedures previously described by Talalay et al.,17 Broccosprouts® (Brassica Protection Products LLC, Baltimore) from a single production lot were used for the protocol. Broccosprouts® are a patented strain of broccoli sprout shown to contain > 6 μmol glucosinolates/gram by routine cyclocondensation and HPLC assays conducted by the supplier. BSH was prepared from a single lot of fresh BroccoSprouts® shipped directed from California Sprouts (Tracy, CA) a certified grower for Brassica Protection Products LLC (Baltimore, MD). The basic steps for BSH preparation are depicted in Figure 2. Sprouts were processed in a designated food product preparation area immediately upon arrival and prior to the expiration date printed on the packaging. Proper hand-washing procedures and vinyl gloves were used by staff to prevent contamination during processing. Sprouts were combined with sterile WFI water purchased from Mediatech Inc. (Herndon, VA) in 1:1.2, w: w proportions. This mixture was homogenized in 1 liter aliquots in a clean blender (600-watt Cuisinart Smartpower™ Series Blender Model CBT-500) to eliminate the variable of chewing by subjects. The aliquots of homogenate were pooled and mixed with daikon sprouts purchased fresh from Friends Fields, Inc. (Arvada, CO). This was accomplished by adding 2% daikon (compared to broccoli sprout mixture based on fresh weight) and homogenizing the preparation in the blender once again. The daikon/broccoli sprout mixture was incubated at 37 degrees C for 2 hours. This process added excess myrosinase to maximally convert the free glucosinolates of the broccoli sprouts to sulforaphane, the biologically active compound. After incubation, the isothiocyanate mixture was aliquoted into 50 ml sterile Falcon tubes and stored at −20° C using dedicated freezer storage space. The broccoli sprout homogenate was thawed at 4° C 12–24 hours prior to dosing and was not administered if thawed for greater than 24 hours. Alfalfa sprout homogenate was prepared using the same procedures, substituting locally purchased alfalfa sprouts for BroccoSprouts®.
Figure 2. Steps in Broccoli Sprout Homogenate (BSH) preparation.
Measurement of glucosinolate and isothiocyanate content
Analysis of BSH for glucosinolate (SGS) and isothiocyanate (ITC) content was performed by the Brassica Laboratory at Johns Hopkins University. Previous studies have demonstrated that the primary glucosinolate in broccoli sprouts is sulforaphane glucosinolate or glucoraphanin.19 Glucosinolate levels were determined using HILIC assay.22 Isothiocyanate content of BSH is determined by cyclocondensation assay.23 Plasma sulforaphane levels were also determined using the cyclocondensation procedure described, though for plasma, the procedure was modified slightly.19 See additional Methods information in the Online Respository.
Measurement of Phase II enzyme gene expression
Gene expression was detected and measured by real-time quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) using a method similar to that described by Wan et al.8 See additional Methods information in the Online Respository. For each individual, gene expression at baseline (pre-challenge) was considered the calibrator and was given an arbitrary figure of 1.0 and relative increase in gene expression following DEP challenge was calculated in reference to this value. 0.3–1.0 μg RNA was routinely extracted from a nasal lavage sampling. Typically 20 ng of RNA in a 25 μL reaction volume was used for each PCR and measurements were performed in triplicate.
Statistics
Statistical analysis was performed using Medrio (Oakland, CA) Clinical Study Software and SAS (Cary, NC) Statistical Software. Comparison of PII enzyme induction between BSH 200 g and placebo (Alfalfa 200 g) was performed using Wilcoxon rank sum test. We fit a linear regression model to approximate the BSH Dose-Response relationship. The slope of the linear regression model estimates the amount of increase in response by increasing one unit of dose, and the significance of the slope indicates significant dose dependent increase on the response.
RESULTS
Oral Sulforaphane is well tolerated and produced no serious adverse events
Fifty-seven subjects completed the study protocol. Subject demographics are reported in Table I. Eight subjects discontinued the protocol due to inability to keep the required visits, but none reported dose intolerance or side effects as a factor. No serious adverse events were reported. Five of sixty subjects reported mild events during BSH dosing of which 4 were gastrointestinal events. (Table II)
Table I.
Demographics of Subjects Completing Protocol
| Dose (g) | 25 BSH | 50 BSH | 75 BSH | 100 BSH | 125 BSH | 150 BSH | 175 BSH | 200 BSH | 200 Alfalfa |
|---|---|---|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 34.0 ± 5.0 | 35.3 ± 10.3 | 33.3 ± 4.0 | 28.0 ± 1.7 | 33.0 ± 7.1 | 41.8± 16.7 | 36.4 ± 12.4 | 32.2 ± 10.4 | 31.6 ± 8.0 |
| Gender (F/M) | 4/1 | 4/1 | 2/2 | 2/2 | 4/4 | 8/2 | 7/0 | 3/6 | 2/3 |
| Number of Subjects Discontinued | 0 | 0 | 1 | 1 | 2 | 0 | 3 | 1 | 0 |
Table II.
Safety and Tolerability of Oral Sulforaphane*
| Dose | Description | Medication Required | Duration |
|---|---|---|---|
| 150 grams BSH | Large Bowel Movement | No | 1 day |
| 175 grams BSH | Nasal Congestion | No | 1 day |
| 200 grams BSH | Soft Stool | No | 1 day |
| 200 grams BSH | Increased Urination | No | 3 days |
| 200 grams BSH | Flatulence | Yes | 3 days |
Events reported occurring during the Clinical Study
Oral Sulforaphane induces Nasal Phase II enzyme induction
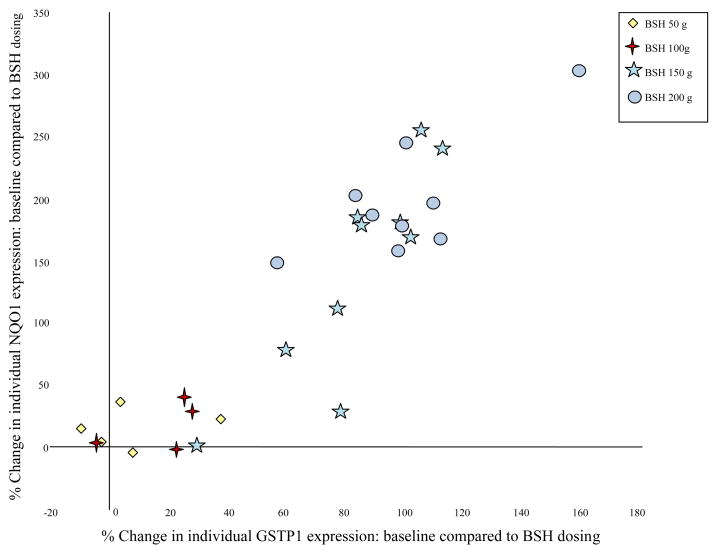
Expression of PII enzymes significantly increased in NLF samples at BSH doses > 100 g daily. Table III shows the NLF data for individual PII enzymes. At BSH doses > 100 g daily, NQO1 consistently showed the greatest induction followed by HO-1. In contrast, no significant change in PII enzyme expression was observed for subjects completing the protocol with oral alfalfa sprouts. Significant differences in PII enzyme expression were evident for BSH 200 g dosing compared to placebo (alfalfa 200 g) by Wilcoxon Rank sum test (p ≤ 0.004). A linear regression model demonstrated the highly significant dose-response effect of BSH on mucosal PII enzyme induction with p-values less than or equal to 0.001 for all measured PII enzymes. A strong positive correlation was evident for the induction of individual Phase II enzymes as demonstrated in Figure 3 for GSTP1 and NQO1. This linear correlation was apparent for all of the measured PII enzymes, consistent with the common pathway of induction by SFN, i.e. Nrf-2 activation.
Table III.
Group Mean Percent Change Mucosal Phase II Enzyme Expression* Compared to Baseline with Oral BSH
| BSH Dose | GSTM1 | GSTP1 | HO-1 | NQO1 |
|---|---|---|---|---|
| 25 g | −5.60 ± 6.29 | 1.53 ± 7.89 | 4.43 ± 7.18 | −2.17 ± 4.97 |
| 100 g | 5.78 ± 12.21 | 14.10 ± 15.07 | 15.32 ± 17.95 | 13.86 ± 19.43 |
| 125 g | 30.53 ± 48.80 | 37.47 ± 27.97 | 37.80 ± 34.21 | 53.32 ± 41.86 |
| 150 g | 74.31 ± 69.83 | 82.83 ± 24.81 | 105.06 ± 33.49 | 142.19 ± 86.30 |
| 175 g | 89.24 ± 63.88 | 86.19 ± 28.30 | 106.56 ± 27.28 | 160.19 ± 65.67 |
| 200 g | 118.81 ± 39.68 | 100.80 ± 27.33 | 120.86 ± 42.66 | 198.81 ± 48.36 |
| Placebo (Alfalfa 200 g) |
28.71 ± 34.01 | 11.00 ± 48.43 | 1.14 ± 18.40 | 1.69 ± 41.21 |
| p-value for BSH 200 g vs. Placebo comparison (Wilcoxon Rank sum test) |
0.002 | 0.004 | 0.001 | 0.001 |
| p-value for Slope of BSH Dose-Response linear | <0.001 | <0.001 | <0.001 | 0.001 |
Gene mRNA expression ± standard deviation relative to pre-treatment baseline, results for GSTM1 for GSTM1+ subjects only.
Figure 3. Induction of individual Phase II enzymes by oral sulforaphane is strongly correlated.
Expression of GSTP1 vs. NQO1 in nasal lavage cells following consumption of oral sulforaphane in each of the subjects.
Analysis of glucosinolate and isothiocyanate levels
Analysis of BSH demonstrated undetectable levels of SGS, supporting effective and complete conversion of glucosinolates to isothiocyanates. Cyclocondensation assay demonstrated SFN content of 0.283 μmol/ml BSH. As the preparation process yielded a mass (g fresh sprouts) to volume (ml BSH) conversion factor of 1.8 (i.e. 25 g sprouts = 45 ml BSH), the 175 mg and 200 mg BSH doses delivered 89 and 102 μmol of SFN per dose respectively.
Oral Sulforaphane is bioavailable as assessed by serum SFN levels
Serum trough SFN levels were measured in selected subjects. Baseline serum SFN levels by cyclocondensation assay were undetectable in all measured samples, but reached levels of 0.0115–0.0121 nmol at 24 hours after the final BSH dose.
DISCUSSION
This is the first clinical study to demonstrate that oral sulforaphane can enhance Phase II antioxidant enzyme expression in human airway cells. The induction of Phase II enzymes by SFN and other Nrf-2 activators has previously been shown using in vitro and animal models, 8, 11, 14, 24–25 however this is the first data to clearly demonstrate this biological effect of SFN in vivo in the human airway. Previous investigation has confirmed that Phase II enzyme mRNA expression as measured by RT-PCR correlates with protein and enzyme activity levels. This has been demonstrated with NQO1 protein expression by Western blot in human lymphocytes8 and GST enzyme activity correlation with GSTM1 mRNA expression in normal human bronchial epithelial cells.11 This evidence supports the potential biologic significance of the marked Phase II enzyme mRNA induction now described with SFN administration in human subjects. The up regulation of endogenous Phase II enzymes was accomplished without apparent toxicity using the administration of oral BSH, a natural readily available source of SFN which was well-tolerated by study subjects. This finding may have important therapeutic implications with regard to asthma and other conditions associated with airway OS.
Cellular OS is believed to be an important underlying pathologic mechanism in the inflammation associated with asthma. Such OS may result from the cellular inflammation seen in asthma, but there is strong evidence that environmental respiratory exposures to air pollutants (i.e. diesel exhaust particles, ozone, NO2) and pollen fragments cause OS in the airways which subsequently leads to tissue inflammation and clinical symptoms.3, 26–28 Cellular exposure to pollutant reactive chemicals or pollens generates reactive oxygen species (ROS) capable of oxidizing proteins, lipids, and DNA. This results in subsequent glutathione depletion and the activation of intrinsic cellular antioxidant defenses via Nrf2 signaling.29 A primary mechanism of this pathway is the activation of the antioxidant response element (ARE) and induction of Phase II metabolizing enzymes.30 This group of enzymes includes glutathione transferases (GSTM, GSTP), heme-oxygenase (HO1), and NAD(P)H:quinone oxidoreductase (NQO1) as well as other enzymes that serve to metabolize xenobiotic chemicals and neutralize ROS.31–32 If this cytoprotective antioxidant response is insufficient or overwhelmed by heavy oxidative burden, glutathione depletion continues resulting in MAPK and NFKB signaling and gene transcription for a cascade of inflammatory cytokines such as IL-4, IL-5, IL-13, TNF-alpha as well as chemokines and cellular adhesion molecules.33 In addition to these pro-inflammatory effects of environmentally-induced OS, there is compelling evidence that OS also serves as a potent adjuvant to enhance Th2-responses to allergens and may initiate immune sensitization to common environmental allergens.34–36
The recognized importance of OS in respiratory inflammation has spurred recent interest in therapeutic measures to prevent or reduce OS. At present, the effects of current allergy and asthma therapy on OS are unclear.37–41 As current standard treatment may not adequately address respiratory OS burden induced by environmental exposures, additional therapies protecting against OS may represent a significant step in reducing the morbidity of allergic respiratory disease.
Our current investigation explores a unique strategy to enhance an important endogenous cytoprotective response designed to prevent cellular damage from ROS. The Nrf-2 signaling pathway is a negative regulator of inflammation through induction of many antioxidant, cytoprotective, and detoxification enzymes.42 These inducible Phase II enzymes represent an early and sensitive response to OS by scavenging ROS and metabolizing xenobiotics such as air pollutants. SFN, as used in our investigation, has a number of attractive qualities with regard to human use. Glucosinolate precursors of SFN are naturally found in cruciferous vegetables with previous studies demonstrating bioavailability and basic pharmacokinetics with human consumption. A number of human studies have demonstrated the safety and tolerability of glucosinolates and SFN, findings further confirmed by our recent study data.16–19 In vitro, animal, and human studies have supported the beneficial biological effects of SFN, with Phase II enzyme induction believed to be an important mechanism of action for these observed effects.43–46 With regard to respiratory inflammation, recent in vitro investigations have shown Phase II enzyme induction with SFN to be effective in blocking the pro-allergic effects of DEP on human B-cells as DEP-induced IgE enhancement is inhibited by preculture with SFN.8 Additional studies using human BEC confirm this strategy to be protective against DEP extract oxidative effects.11 SFN effectively upregulates Phase II enzyme expression and blocks DEP extract induced IL-8, GM-CSF, and IL-1β production by human BEC. Thus, while the protective antioxidant effects of PII enzyme induction have yet to be established in human clinical studies, compelling data exists to support the potent anti-inflammatory effects of this strategy in the setting of DEP-induced OS.
Our placebo-controlled study is the first to examine the in vivo effects of SFN administration on PII enzyme expression in the human airway. At BSH doses > 100 grams (51 μmol SFN) daily, we observed a significant dose-response effect in Phase II enzyme expression as measured by RT-PCR using cells recovered from nasal lavage. Our NL collection yields predominantly respiratory epithelial cells making such findings congruent with SFN-mediated PII enzyme induction seen in previous studies using human BEC. We did not observe SFN-induced changes in NL cell counts or differentials, yet the increase in mean expression of PII enzymes ranged from 101% for GSTP1 to 199% for NQO1 at the highest dose of BSH administration. This represents a doubling to tripling of baseline enzyme expression rates. Also significant is the observation that measured increases in individual sentinel PII enzymes were strongly associated with increases in all other PII enzymes. This positive linear-correlation is consistent with our current understanding of a common mechanism of induction for these enzymes, i.e. Nrf-2 signaling with resultant ARE activity promoting transcription of numerous PII enzymes.
While our study was not designed to examine the pharmacokinetics (pK) of oral SFN, the results appear to confirm previously described human pK results. SFN has an apparent volume of distribution of 59.9 ± 7.0 L, consistent with total body water, a mean half-life of 1.77 ± 0.13 hours, and first-order kinetics.19 Previous dosing of 200 μmol SFN has yielded peak plasma levels of 2.00 ± 0.30 μmol/l, suggesting 60% bioavailability.17 Our results are consistent with these previous observations. Using the aforementioned pK parameters, the 200 gram BSH dose (102 μmol SFN) used in our study would be expected to give a peak serum concentration of 1.02 μmol/l. Expected serum concentration 24 hours later (14 t1/2) would be 0.0622 nmol/l. For samples collected nearer the 28 hour mark, the expected concentration is 0.0155 nmol/l. Our serum samples analyzed for SFN content 24 ± 4 hours after the 200 gram BSH dose are within this range (0.0115–0.0121 nmol).
This study demonstrating the potent biological effects of oral SFN on PII enzyme expression in the human upper airway provides vital information for planning additional clinical trials. Future human studies will be necessary to thoroughly investigate the potential beneficial effects of Phase II enzyme induction on environmentally-induced oxidative stress and associated allergic airway inflammation. Currently, it is unknown whether the observed increase in PII enzyme expression will be sufficient to prevent or reduce respiratory OS in the human airway. Also unknown is whether higher doses of SFN will lead to further increases in PII enzyme expression or whether toxicity will be dose-limiting. Our experience, and those of other investigators, is that the currently reported doses are well-tolerated and non-toxic. Additionally, genetic polymorphisms affecting PII enzymes will be an important consideration in future studies examining this therapeutic strategy. We excluded GSTM1 null subjects from our analysis so as to have a clear picture of the induction effects of SFN on PII enzymes. However, with regard to the clinical value of SFN, the presence or absence of specific functional enzymes may be quite important in the resulting therapeutic or nominal effect. Given the complex network of PII antioxidant enzymes, gene-gene-environment interactions are likely to have a strong influence on the observed responses to specific antioxidant therapies.47–48 Careful selection of susceptible target populations based on genetic polymorphisms will be an important consideration for future interventional studies aimed at reducing pollutant-induced oxidative stress.
In summary, our placebo-controlled human study has demonstrated that oral SFN contained in BSH can significantly induce PII enzyme expression in the human airway. This data allows for future clinical studies to examine the potential benefit of SFN in abrogating allergic respiratory inflammation from oxidant stimuli and demonstrates proof-of-concept for Nrf2-activation as a mechanism of PII enzyme upregulation in the human respiratory tract.
Supplementary Material
Acknowledgments
This work was supported by NIH/NCRR K12 R011 7611, NIH/NIAID P01 AI050495, NIEHS 5P01ES09581-10, and US EPA # RD83186101
The authors wish to thank Dr. Jed Fahey at Johns Hopkins University for performing the SFN and SGS analyses and for his helpful advice regarding sulforaphane.
Abbreviations
- ARE
antioxidant response element
- BEC
bronchial epithelial cells
- BSH
broccoli sprout homogenate
- DEP
diesel exhaust particles
- GSTM1
glutathione-s-transferase M1
- GSTP1
glutathione-s-transferase P1
- HO1
hemeoxygenase-1
- ITC
isothiocyanate
- MAPK
mitogen activated protein kinase
- NF-κB
nuclear factor kappa B
- NLF
nasal lavage fluid
- NQO1
NADPH quinone oxidoreductase 1
- Nrf-2
Nuclear erythroid 2 p45-related factor 2
- OS
oxidative stress
- PII
phase II
- ROS
reactive oxygen species
- RT-PCR
real-time quantitative reverse transcriptase-polymerase chain reaction
- SFN
sulforaphane
- SGS
sulforaphane gluconsinolates
- WFI
water for injection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bowler RP, Crapo JD. Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol. 2002;110:349–56. doi: 10.1067/mai.2002.126780. [DOI] [PubMed] [Google Scholar]
- 2.Riedl MA, Nel AE. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr Opin Allergy Clin Immunol. 2008;8:49–56. doi: 10.1097/ACI.0b013e3282f3d913. [DOI] [PubMed] [Google Scholar]
- 3.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, et al. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–79. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol. 2005;115:221–8. doi: 10.1016/j.jaci.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 5.Diaz-Sanchez D, Rumold R, Gong H., Jr Challenge with environmental tobacco smoke exacerbates allergic airway disease in human beings. J Allergy Clin Immunol. 2006;118:441–6. doi: 10.1016/j.jaci.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 6.Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38:995–1014. doi: 10.1590/s0100-879x2005000700003. [DOI] [PubMed] [Google Scholar]
- 7.Dhakshinamoorthy S, Long DJ, 2nd, Jaiswal AK. Antioxidant regulation of genes encoding enzymes that detoxify xenobiotics and carcinogens. Curr Top Cell Regul. 2000;36:201–16. doi: 10.1016/s0070-2137(01)80009-1. [DOI] [PubMed] [Google Scholar]
- 8.Wan J, Diaz-Sanchez D. Phase II enzymes induction blocks the enhanced IgE production in B cells by diesel exhaust particles. J Immunol. 2006;177:3477–83. doi: 10.4049/jimmunol.177.5.3477. [DOI] [PubMed] [Google Scholar]
- 9.Li N, Alam J, Venkatesan MI, Eiguren-Fernandez A, Schmitz D, Di Stefano E, et al. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J Immunol. 2004;173:3467–81. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- 10.Gilliland FD, Li YF, Gong H, Jr, Diaz-Sanchez D. Glutathione s-transferases M1 and P1 prevent aggravation of allergic responses by secondhand smoke. Am J Respir Crit Care Med. 2006;174:1335–41. doi: 10.1164/rccm.200509-1424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritz SA, Wan J, Diaz-Sanchez D. Sulforaphane-stimulated phase II enzyme induction inhibits cytokine production by airway epithelial cells stimulated with diesel extract. Am J Physiol Lung Cell Mol Physiol. 2007;292:L33–9. doi: 10.1152/ajplung.00170.2006. [DOI] [PubMed] [Google Scholar]
- 12.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94:10367–72. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahey JW, Talalay P. Antioxidant functions of sulforaphane: a potent inducer of Phase II detoxication enzymes. Food Chem Toxicol. 1999;37:973–9. doi: 10.1016/s0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 14.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–203. [PubMed] [Google Scholar]
- 15.Wan J, Diaz-Sanchez D. Antioxidant enzyme induction: a new protective approach against the adverse effects of diesel exhaust particles. Inhal Toxicol. 2007;19 Suppl 1:177–82. doi: 10.1080/08958370701496145. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev. 2001;10:501–8. [PubMed] [Google Scholar]
- 18.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–100. [PubMed] [Google Scholar]
- 19.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 20.Diaz-Sanchez D, Dotson AR, Takenaka H, Saxon A. Diesel exhaust particles induce local IgE production in vivo and alter the pattern of IgE messenger RNA isoforms. J Clin Invest. 1994;94:1417–25. doi: 10.1172/JCI117478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 22.Troyer JK, Stephenson KK, Fahey JW. Analysis of glucosinolates from broccoli and other cruciferous vegetables by hydrophilic interaction liquid chromatography. J Chromatogr A. 2001;919:299–304. doi: 10.1016/s0021-9673(01)00842-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Wade KL, Prestera T, Talalay P. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Anal Biochem. 1996;239:160–7. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Surh YJ. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224:171–84. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 25.Morimitsu Y, Nakagawa Y, Hayashi K, Fujii H, Kumagai T, Nakamura Y, et al. A sulforaphane analogue that potently activates the Nrf2-dependent detoxification pathway. J Biol Chem. 2002;277:3456–63. doi: 10.1074/jbc.M110244200. [DOI] [PubMed] [Google Scholar]
- 26.Sanders SP, Zweier JL, Harrison SJ, Trush MA, Rembish SJ, Liu MC. Spontaneous oxygen radical production at sites of antigen challenge in allergic subjects. Am J Respir Crit Care Med. 1995;151:1725–33. doi: 10.1164/ajrccm.151.6.7767513. [DOI] [PubMed] [Google Scholar]
- 27.Lim HB, Ichinose T, Miyabara Y, Takano H, Kumagai Y, Shimojyo N, et al. Involvement of superoxide and nitric oxide on airway inflammation and hyperresponsiveness induced by diesel exhaust particles in mice. Free Radic Biol Med. 1998;25:635–44. doi: 10.1016/s0891-5849(98)00073-2. [DOI] [PubMed] [Google Scholar]
- 28.Gurgueira SA, Lawrence J, Coull B, Murthy GG, González-Flecha B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002;110:749–55. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- 30.Li N, Venkatesan MI, Miguel A, Kaplan R, Gujuluva C, Alam J, et al. Induction of heme oxygenase-1 expression in macrophages by diesel exhaust particle chemicals and quinones via the antioxidant-responsive element. J Immunol. 2000;165:3393–401. doi: 10.4049/jimmunol.165.6.3393. [DOI] [PubMed] [Google Scholar]
- 31.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–82. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 32.Baulig A, Garlatti M, Bonvallot V, Marchand A, Barouki R, Marano F, et al. Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L671–9. doi: 10.1152/ajplung.00419.2002. [DOI] [PubMed] [Google Scholar]
- 33.Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin Immunol. 2003;109:250–65. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi T. Exposure to diesel exhaust aggravates nasal allergic reaction in guinea pigs. Am J Respir Crit Care Med. 2000;162:352–6. doi: 10.1164/ajrccm.162.2.9809035. [DOI] [PubMed] [Google Scholar]
- 35.Diaz-Sanchez D, Garcia MP, Wang M, Jyrala M, Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J Allergy Clin Immunol. 1999;104:1183–8. doi: 10.1016/s0091-6749(99)70011-4. [DOI] [PubMed] [Google Scholar]
- 36.Fujieda S, Diaz-Sanchez D, Saxon A. Combined nasal challenge with diesel exhaust particles and allergen induces In vivo IgE isotype switching. Am J Respir Cell Mol Biol. 1998;19:507–12. doi: 10.1165/ajrcmb.19.3.3143. [DOI] [PubMed] [Google Scholar]
- 37.Pereira B, Rosa LF, Safi DA, Bechara EJ, Curi R. Hormonal regulation of superoxide dismutase, catalase, and glutathione peroxidase activities in rat macrophages. Biochem Pharmacol. 1995;50:2093–8. doi: 10.1016/0006-2952(95)02116-7. [DOI] [PubMed] [Google Scholar]
- 38.Marwick JA, Kirkham PA, Stevenson CS, Szulakowski P, Biswas SK, Bauter MR, et al. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol. 2004;31:633–42. doi: 10.1165/rcmb.2004-0006OC. [DOI] [PubMed] [Google Scholar]
- 39.Davies RJ, Rusznak C, Calderón MA, Wang JH, Abdelaziz MM, Devalia JL. Allergen-irritant interaction and the role of corticosteroids. Allergy. 1997;52:59–65. doi: 10.1111/j.1398-9995.1997.tb04873.x. [DOI] [PubMed] [Google Scholar]
- 40.Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Effect of topical fluticasone propionate on the mucosal allergic response induced by ragweed allergen and diesel exhaust particle challenge. Clin Immunol. 1999;90:313–22. doi: 10.1006/clim.1998.4676. [DOI] [PubMed] [Google Scholar]
- 41.Sadowska AM, Klebe B, Germonpré P, De Backer WA. Glucocorticosteroids as antioxidants in treatment of asthma and COPD. New application for an old medication? Steroids. 2007;72:1–6. doi: 10.1016/j.steroids.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Li N, Nel AE. Role of the Nrf2-mediated signaling pathway as a negative regulator of inflammation: implications for the impact of particulate pollutants on asthma. Antioxid Redox Signal. 2006;8:88–98. doi: 10.1089/ars.2006.8.88. [DOI] [PubMed] [Google Scholar]
- 43.Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–90. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 44.Munday R, Mhawech-Fauceglia P, Munday CM, Paonessa JD, Tang L, Munday JS, et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008;68:1593–600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- 45.Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, et al. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci U S A. 2007;104:17500–5. doi: 10.1073/pnas.0708710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–13. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 47.London SJ. Gene-air pollution interactions in asthma. Proc Am Thorac Soc. 2007;4:217–20. doi: 10.1513/pats.200701-031AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macías H, Reyes-Ruiz NI, Estela del Río-Navarro B, et al. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax. 2004;59:8–10. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.