Abstract
The genus Echinacea is a popular herbal immunomodulator. Recent reports indicate that Echinacea products inhibit nitric oxide (NO) production in activated macrophages. In the present study we determined the inhibitory effects of alcohol extracts and individual fractions of alcohol extracts of Echinacea on NO production, and explored the mechanism underlying the pharmacological anti-inflammatory activity. The alcohol extracts of three medicinal Echinacea species, E. angustifolia, E. pallida and E. purpurea, significantly inhibited NO production by lipopolysaccharide (LPS)-activated the RAW 264.7 macrophage cell line, among them E. pallida was the most active. The Echinacea-mediated decrease in NO production was unlikely due to a direct scavenging of NO because the extracts did not directly inhibit NO released from an NO donor, sodium nitroprusside. An immunoblotting assay demonstrated that the extract of E. pallida inhibited inducible nitric oxide synthase (iNOS) protein expression in LPS-treated macrophages. The enzymes iNOS and arginase metabolize a common substrate, L-arginine, but produce distinct biological effects. While iNOS is involved in inflammatory response and host defense, arginase participates actively in anti-inflammatory activation. Arginase activity of RAW 264.7 cells stimulated with 8-bromo-cAMP was significantly increased by alcohol extracts of all three Echinacea species. The polar fraction containing caffeic acid derivatives enhanced arginase activity, while the lipophilic fraction containing alkamides exhibited a potential of inhibiting NO production and iNOS expression. These results suggest that the anti-inflammatory activity of Echinacea might be due to multiple active metabolites, which work together to switch macrophage activation from classical activation towards alternative activation.
Keywords: Echinacea, Alcohol extract, Anti-inflammatory, Macrophage, Nitric oxide, Inducible nitric oxide synthase, Arginase
1. Introduction
The genus Echinacea, one of the top-selling botanical supplements, has been widely used for centuries in North America and later in Europe for many therapeutic purposes. Although there are nine known species of Echinacea, three of them, E. angustifolia (EA), E. pallida (EPA) and E. purpurea (EP), are of similar and important medicinal values in the modulation of the immune system (Borchers et al., 2000). These Echinacea species are rich in bioactive metabolites of which lipophilic alkamides, water-soluble phenolic compounds (mainly caffeic acid derivatives) and polysaccharides are the most recognized for their immunomodulatory properties (Barnes et al., 2005). Although historically polysaccharides were considered critical for stimulation of nonspecific immune responses (Borchers et al., 2000; Percival, 2000), recent research focuses on the alkamides and caffeic acid derivatives. Caffeic acid derivatives are good antioxidants in cell-free free radical generation systems (Hu and Kitts, 2000; Pellati et al., 2004; Dalby-Brown et al., 2005) and echinacoside, the main caffeic acid derivative in EA and EPA, has been functionally linked to anti-inflammatory and wound healing properties of Echinacea when applied topically (Speroni et al., 2002; Rousseau et al., 2006). Pharmacokinetic studies demonstrate that natural or synthesized caffeic acid derivatives (e.g. caftaric acid, chlorogenic acid and echinacoside) are quickly absorbed in the rat stomach, but rapidly eliminated from circulation (Jia et al., 2006; Lafay et al., 2006; Vanzo et al., 2007). Matthias et al. (2004) suggests a low bioavailability for caffeic acid derivatives of Echinacea based on their poor permeability through Caco-2 monolayers, a model for the intestinal epithelial barrier. These data indicate that there may be a limited pharmacological role for caffeic acid derivatives when consumed orally. In this regard, lipophilic alkamides, which are considered orally bioavailable, may be more important phytochemicals (Matthias et al., 2005; Woelkart et al., 2005a).
Echinacea-derived alkamides appear to have immunomodulatory and anti-inflammatory activity (for review see Woelkart and Bauer, 2007). Both alkamide-containing Echinacea extracts and purified alkamides inhibit production of proinflammatory tumor necrosis factor-alpha (TNF-α) and nitric oxide (NO) in an activated murine macrophage cell line (Chen et al., 2005; Matthias et al., 2007) with low cytotoxicity in vitro (Zhai et al., 2007a). Orally administered alcohol extracts of Echinacea significantly decreased production of TNF-α and interleukin (IL)-1βby activated splenocytes (Zhai et al., 2007b). Although Echinacea extracts containing alkamides exhibit suppressive effects on these inflammatory mediators, the underlying cellular mechanism is unclear.
Upon induction by certain inflammatory stimuli, e.g., bacterial lipopolysaccharide (LPS), the NF-κB signal pathway activates and regulates the expression of a wide variety of genes involved in inflammatory responses, e.g., the cytokines TNF-α and IL-1β, and the enzymes inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (McKay and Cidlowski, 1999; Yamamoto and Gaynor, 2001). The enzyme iNOS catalyzes the conversion of amino acid L-arginine to NO, which is cytotoxic and cytostatic (Meurs et al., 2003). Although NO as an active free radical and inflammatory mediator is part of the host defense, excessive production of NO and its reactive nitrogen intermediates is involved in the pathogenesis of many chronic diseases, such as inflammatory bowel disease, arthritis, atherosclerosis and tumors (Chan et al., 2000; Yamamoto and Gaynor, 2001). Selective inhibition of the iNOS pathway is an important strategy for control of many chronic inflammatory diseases (Chan et al., 2000).
L-arginine metabolism can be redirected toward the synthesis of L-ornithine catalyzed by the enzyme arginase. The subsequent products from L-ornithine, such as polyamines and L-proline, are involved in cell growth, collagen synthesis and wound healing (Meurs et al., 2003). Thus, arginase and iNOS are the two enzymes that catalyze a common substrate but produce divergent biological effects. They are generally recognized to be functionally competitive because the stimulation of one enzyme will negatively regulate the other one due to the limited availability of the same substrate (Wang et al., 1995; Gotoh and Mori, 1999). These two cross-regulated metabolic processes may reflect opposite functional states of macrophages. It is well known that macrophages can be either classically activated or alternatively activated, and are therefore termed as inflammatory or anti-inflammatory cells (Munder et al., 1998; Porcheray et al., 2005). A given macrophage may switch from one activated state to another upon a specific signal (Porcheray et al., 2005). Inflammatory mediators and cytokines induce classical activation involving iNOS expression while anti-inflammatory cytokines induce alternative activation with upregulated arginase activity (Modolell et al., 1995; Munder et al., 1998; Yamamoto et al., 1998). Oral administration of alcohol extracts of Echinacea to mice increases production of anti-inflammatory cytokines IL-4 and IL-10, but decreases production of TNF-α and IL-1β in activated spleen cells (Zhai et al., 2007b), suggesting that Echinacea may modulate macrophage function to be anti-inflammatory via a decreased proinflammatory/anti-inflammatory cytokine ratio. Thus, we hypothesized that Echinacea can perturb the iNOS/arginase balance in macrophages in favor of increasing arginase activity for alternative anti-inflammatory activation.
In this study, we examined the effects of Echinacea extracts and their fractions on the production of NO in activated macrophage cell line as well as the cellular mode of action underlying the biological activity. We found that alcohol extracts of Echinacea had opposite effects on NO production and arginase activity, and these differential effects were induced by different fractions (or chemical constituents), which could work coordinately to drive macrophages to alternative activation.
2. Materials and methods
2.1. Preparation of alcohol extracts
Dried powdered roots of EA, EPA and EP harvested in 2003 were provided by Iowa State University Botanical Supplements Research Center; and by the USDA North Central Regional Plant Introduction Station in Ames, Iowa, with accession numbers PI 631285, PI 631293, and PI 631307, respectively. Alcohol extracts from the root powders of these plants were prepared as previously described (Zhai et al., 2007b). The extracts were diluted to 20 mg/ml in PBS containing 5% ethanol. Aliquots of the dilutions were stored at −80°C for up to 2.5 years during which no obvious herbal efficacy was reduced.
2.2. Fractionation of alcohol extracts
For fractionation, the dried powdered roots of the plants harvested in 2005 (EA) or 2006 (EPA and EP) were used to prepare new alcohol extracts using the Soxhlet extraction. The dry residues were redissolved in 75% ethanol to obtain ≤ 0.6 g/ml. Five milliliters were injected into a semi-preparative high performance liquid chromatography (HPLC). The starting conditions of the semi-preparative HPLC was 90% Pump A, which was 0.1% acetic acid, and 10% Pump B, which was acetonitrile at a flow rate of 3 ml/min. Eluent was collected over time with increasing solvent B as outlined in Table 1A. The gap of 1–2 minutes between fractions indicated the time of stopping the flow of the mobile phase for the change of the collection flasks. The fractions were evaporated to remove the mobile phase and lyophilized. Each lyophilized fraction was dissolved and diluted to 4 mg/ml in PBS containing 25% ethanol, and aliquots were kept at −80°C and used within one year.
Table 1.
| A. Fractionation procedure using semi-preparative HPLC | |||
|---|---|---|---|
| Retention time (min) | Solvent Aa | Solvent Bb | Fraction |
| 0–31 | 90–70% | 10–30% | 1 |
| 33–42 | 70–60% | 30–40% | 2 |
| 43–83 | 60–20% | 40–80% | 3 |
| 84–94 | 20–0% | 80–100% | 4 |
| 95–115 | 0% | 100% | 5 |
| B. Echinacea fractions and their chemical constituents | |||
| Fraction |
Species |
||
| E. angustifolia | E. pallida | E. purpurea | |
|
| |||
| 1 | Caffeic acid derivatives | Caffeic acid derivatives | Caffeic acid derivatives |
| 2 | Unknown | Unknown | Unknown |
| 3 | Alkamides | Alkamides and ketones | Alkamides |
| 4 | Alkamide 11 and other very hydrophobic compounds | Very Hydrophobic compounds | Very hydrophobic compounds |
| 5 | Most hydrophobic compounds | Most hydrophobic compounds | Most hydrophobic compounds |
Solvent A: 0.1% acetic acid
Solvent B: acetonitrile
2.3. Phytochemical analysis
The phytochemical analysis was performed to detect bioactive metabolites of alkamides and caffeic acid derivatives in the crude alcohol extracts with the use of HPLC as described previously (Wu et al., 2004). We did not identify ketones as no internal standards for ketones were used. However, ketones are expected to be present as lipophilic compounds and concomitantly appear in alkamide fractions of EPA (Chicca et al., 2008).
Fractions were identified as described by Liu and Murphy (2007). The mobile phase used for the analytical HPLC method was, Pump A: 0.1% acetic acid; and Pump B, acetonitrile. At a flow rate of 1 ml/min, a UV scan was collected in the range of 190–600 nm. Alkamides were viewed at 260 nm, while caffeic acid derivatives were viewed at 330 nm.
Peak identification was based on relative retention time, similarity of UV spectra, internal standards, spiking and chromatogram fingerprinting (Wu et al., 2004; Liu and Murphy, 2007). Individual standards were run to check for relative retention times and UV spectra. An EP sample with identified peaks was also run to obtain the finger print of the chromatogram, the relative retention times of the alkamides and caffeic acid derivatives, and to obtain the UV spectra of identified compounds.
2.4. Determination of endotoxin in herbal preparations
All glassware used in the extraction and fractionation procedure was baked at 185°C overnight prior to use in order to minimize endotoxin contamination. Endotoxin-free Nanopure water was used for preparation and dilution of crude alcohol extracts and fractions. The endotoxin levels were evaluated in aliquots of the herbal preparations using the Limulus Amebocyte Lysate Test (Cambrex Bio Science Walkersville, Inc., Walkersville, MD, USA) according to the manufacturer’s specifications for a microplate assay, and were found to be below the limit of detection (< 0.1 EU/ml).
2.5. Macrophage cell line
The murine peritoneal macrophage cell line RAW 264.7 cells were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA) and grown in RPMI 1640 medium (GIBCO, Invitrogen Corporation, Grand island, NY, USA) supplemented with 2 mM glutamine, 25 mM Hepes, 50 μg/ml gentamicin and 10% heated-inactivated iron-fortified bovine calf serum (JRH Biosciences, Lenexa, KS, USA) at 37°C in a 7% CO2 incubator. Cells between passage 5 and 20 (Kiemer et al., 2002) were used in this study.
2.6. NO and TNF-αassays
RAW 264.7 cells (8×104 cells/well) were seeded in flat bottom 96-well tissue culture plates (Corning Inc., Corning, NY, USA) in the presence or absence of various concentrations of Echinacea preparations (as indicated in each graph) and/or LPS (E. coli 055:B5; Sigma, St. Louis, MO, USA). Final concentration of ethanol used as a solvent of lipophilic metabolites in each well was 0.25% (v/v). Ethanol (0.25%) control wells were tested in parallel. After 23 h incubation, culture supernates were collected for NO and TNF-α assays, and the attached cells were evaluated for viability (see below).
In a second set of experiments, Echinacea extracts (100 μg/ml) were either added simultaneously with LPS (1 μg/ml) or up to 6 h after LPS addition (Kiemer et al., 2002). Culture supernates from cells activated with LPS for 22 h were collected for NO assay.
Nitrite, a stable NO metabolite, was determined by the method of Griess reaction (Park et al., 2005). A trace amount of nitrite present in cell-free culture medium was subtracted from each value obtained with cells. Sodium nitrite (0.39–100 μM) was used as a standard. It was confirmed that Echinacea extracts at 200 μg/ml did not interact with nitrite and interfere with its detection (data not shown).
TNF-α was assayed by BD OptEIA ELISA set (BD Biosciences, San Diego, CA, USA). The cytokine levels were calculated by using a purified recombinant mouse cytokine as a standard.
2.7. Scavenging of NO production from NO donor
Sodium nitroprusside (SNP), an NO donor, in aqueous solution at physiological pH spontaneously liberates NO, which rapidly interacts with oxygen to produce nitrite (Sreejayan and Rao, 1997; Chan et al., 2000; Kiemer et al., 2002). In order to determine if Echinacea metabolites directly interact with NO, SNP (final concentration 2.5 mM; Sigma) was mixed with the Griess reagent (Park et al., 2005) in the presence of 0.25% ethanol or various concentrations of alcohol extracts, and then incubated at room temperature for a set of time points. NO released from SNP in this reaction system was immediately captured by Griess reagent via nitrite, which was sequentially monitored on a plate reader (Bio-Tek Instruments, Winooski, VT, USA). A series of sodium nitrite standard concentrations instead of SNP was set up and measured at the same time. The reading of the standard curve remained stable throughout the observational period.
2.8. Determination of arginase activity
Arginase activity was determined according to a microplate method (Corraliza et al., 1994; Munder et al., 1998) with slight changes. RAW 264.7 cells (4×105 cells/ml) were incubated in 96-well tissue culture plates in the presence or absence of LPS (1 μg/ml), 8-bromo-cAMP (0.25 mM; Sigma) and/or Echinacea preparations. After 23–24 h incubation, supernates were collected for NO assay, the cells rinsed with PBS, and then lysed with a CelLytic M lysis solution (Sigma). Following centrifugation at 2,500 × g for 5 min at 4°C, 50 μl of the lysate was mixed with 40 μl of 25 mM Tris-HCl (pH 7.4) and 10 μl of 10 mM MnCl2. The arginase was activated by heating for 10 min at 56°C. Aginine hydrolysis to urea was conducted by addition of 100 μl of 0.5 M L-arginine (Sigma), pH 9.7, with incubation at 37°C for 60 min. The reaction was stopped with 800 μl of H2SO4 (96%)/H3PO4 (85%)/H2O (1/3/7, v/v/v). The urea concentration was measured at 550 nm after addition of 40 μl of 9% (w/v) α-isonitrosopropiophenone (Sigma) dissolved in 100% ethanol and heating at 95°C for 90 min. A standard curve was performed with 2-fold dilutions of urea (0.03–4 mM) followed by mixing with the stop reagent and heating. One unit of arginase activity is defined as the amount of enzyme that catalyzes the formation of 1 μmol urea per min at 37°C. Protein concentrations in cell lysate were determined using a micro BCA (Bicinchoninic Acid) protein assay (Pierce Labs, Rockford, IL, USA).
2.9. Western blotting analysis
RAW 264.7 cells (5×105 cells/well) were seeded in 24-well plates in the presence or absence of LPS (1 μg/ml) and various Echinacea preparations. After 24 h incubation, cells were rinsed with ice-cold PBS and lysed with the CelLytic M lysis solution containing 1% (v/v) protease inhibitor cocktail (Sigma). Cell debris was removed by centrifugation (12,000 × g for 15 min), and the resultant supernates were stored at −80°C untill use. The thawed cell lysates were mixed with 4× NuPAGE SDS sample loading buffer (Invitrogen). Equal amounts of cellular protein (25 μg/lane) were separated on 10% Tris-HCl ready gel (Bio-Rad Laboratories, Hercules, CA, USA), followed by electrotransfer onto a PVDF plus membrane. The immunoblot was performed by incubation with rabbit polyclonal anti-iNOS (1:500 dilution) (sc-8310, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) in 5% non-fat milk in PBS/0.1% Tween-20 (PBST) overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated donkey anti-rabbit IgG (1:2500 dilution) (Santa Cruz Biotechnology Inc.) for 1 h at room temperature. The bands were visualized using an amplified Opti-4CN Substrate kit (Bio-Rad Laboratories). The blots were photographed and the band density quantified with Kodak Image Station 440 CF and Kodak 1D image analysis software (Eastman Kodak Company, Rochester, NY, USA). To assess the changes of iNOS expression and to normalize protein loading, membranes run in parallel were incubated with mouse monoclonal antibody against β-actin (1:5000 dilution) (Santa Cruz Biotechnology Inc.).
2.10. Cell viability assay
Mitochondrial reduction of MTS, a tetrazolium compound, to a colored formazan was used as an indicator of cell viability (Cory et al., 1991). Briefly, after removing of 100 μl of culture supernates (i.e. for NO and TNF-α assays), 15 μl of MTS (CellTiter 96 Aqueous One Solution Cell Proliferation Assay, Promega Corporation, Madison, WI, USA) was added to the remaining 100 μl of culture medium incubation continued for 1 h. The extent of formazan formation was determined photometrically at absorbance 490 nm on a plate reader. The absorbance of cell-free culture medium with or without vehicle or Echinacea preparations was used as a blank (self-control) and subtracted from the value of the corresponding treatment groups.
2.11. Statistical analysis
Statistix software (version 8.0, Analytical Software, Tallahasee, FL, USA) was used for the statistical analysis. Differences between the vehicle and other groups were tested by two-way analysis of variance (group × experiment). A value P<0.05 was considered significant.
3. Results
3.1. Phytochemical analysis
Table 2 lists the amounts of total alkamides and several important caffeic acid derivatives in crude alcohol extracts of three Echinacea species.
Table 2.
Concentrations of known metabolites in alcohol extracts of Echinacea
| Metabolites | Species |
||
|---|---|---|---|
| E. angustifolia | E. pallida | E. purpurea | |
| Amides | 1.71a | 1.04 | 2.61 |
| Caftaric acid | ND | 0.06 | 0.06 |
| Chlorogenic acid | 0.09 | 0.21 | ND |
| Cichloric acid | NDb | 0.06 | 0.46 |
| Echinacoside | 0.39 | 1.26 | ND |
% of dried extract
ND = not detectable
The chemical constituents in the fractions are shown in Table 1B. Fraction 1 was rich in polar caffeic acid derivatives. Fraction 3 contained lipophilic constituents including alkamides (in all three species) and ketones (in EPA). Other hydrophobic compounds were found in fractions 4 and 5. For fractions 3–5, the later fractions contained compounds which are more hydrophobic than the earlier ones.
3.2. Inhibition of TNF-αproduction by alcohol extracts
As TNF-α production was regulated by Echinacea in vivo (Zhai et al., 2007b), we measured this cytokine in Echinacea-treated RAW 264.7 cells. In the absence of LPS, very low amounts (or lower than limit of detection, 15 pg/ml) of TNF-α were measured in the supernates from RAW 264.7 cells incubated with or without alcohol extracts of Echinacea overnight. In the presence of LPS, the cells secrete high levels of TNF-α. Vehicle (0.25% ethanol) alone had no effect on the cytokine production, however, all three alcohol extracts at a concentration of 100 μg/ml significantly inhibited TNF-α production (P values <0.001, Fig. 1A). The inhibitory effects of Echinacea extracts in the presence of 0.1 μg/ml LPS were dose-dependent as indicated by a significant linear effect of dose (P values <0.05) (Fig. 1B). Alcohol extracts of Echinacea at the test concentration range of 10–200 μg/ml had little effect on cell viability based on the MTS assay (Fig. 1B), indicating that the inhibitory effect of Echinacea extracts on TNF-α is unlikely due to cytotoxicity.
Fig. 1.
Alcohol extracts of Echinacea inhibit production of TNF-α by LPS-stimulated macrophages. RAW 264.7 cells were incubated in vitro for 23 h in the presence of indicated Echinacea extracts 100 μg/ml plus different concentrations of LPS (A), or incubated with different concentrations of Echinacea extracts (0–200 μg/ml) in the presence of 0.1μg/ml LPS (B). Culture supernates were assayed for TNF-α by ELISA(A and B (solid lines)). The attached cells were evaluated for cell viability by MTS assay (B (dashed lines)). The data are expressed as mean ± standard error of the mean of 3 independent experiments performed in triplicate. *P<0.001 vs the vehicle control at the same concentration of LPS.
3.3. Inhibition of NO production by alcohol extracts
In the absence of LPS, less than 0.5 μM of NO was detectable in culture medium of RAW 264.7 cells incubated in vitro for 23 h. In the presence of increasing concentrations of LPS, cells produce NO, which reached a maximum with LPS at 1 μg/ml (Fig. 2A). Exposure to vehicle had no effect on NO production, however, the three Echinacea extracts at 100 μg/ml reduced NO production. The inhibitory effect of Echinacea extracts on NO production was dose-dependent as indicated by a significant linear effect of dose (P values <0.008) (Fig. 2B). At 100 μg/ml, the inhibitory potential of three species extracts in the presence of 1 μg/ml LPS is generally EPA > EP > EA. All three extracts at tested concentrations had no effect on cell viability (Fig. 2B).
Fig. 2.
Alcohol extracts of Echinacea inhibit production of NO by LPS-stimulated macrophages. Cells were incubated in vitro for 23 h in the presence of Echinacea extracts 100 μg/ml plus different concentrations of LPS (A), or incubated overnight with different concentrations of indicated Echinacea extracts (0–200 μg/ml) in the presence of 1 μg/ml LPS (B). After removing supernates for NO assay (A and B (solid lines)), cell viability was evaluated by addition of MTS (B (dashed lines)). The data are expressed as mean ± standard error of the mean of 3 independent experiments performed in triplicate.
3.4. Direct scavenging of NO generated by alcohol extracts
NO production was estimated by measuring nitrite using the Griess reaction system. NO derived from SNP increased in a linear time-dependent manner (Fig. 3A). Vehicle alone had no any effect on the NO production, but alcohol extracts of EA and EPA at a final concentration of 50 μg/ml significantly inhibited nitrite accumulation beginning 120 min after initiating of the reaction (Fig. 3A) (P values <0.018 at 120-min time points). However, the inhibitory rates of NO production by these two extracts throughout the 3-h observation period were small (<15%), and were not concentration-dependent (Fig. 3B). EP extracts at lower concentrations of 5–25 μg/ml, but not at higher concentrations (50–200 μg/ml), significantly reduced nitrite accumulation (P values <0.015).
Fig. 3.
Scavenging of NO derived from SNP by alcohol extracts of Echinacea. A. SNP was mixed with Griess reagent in the presence of vehicle (0.25% ethanol) or 50 μg/ml of Echinacea extracts and incubated for 6 h. Nitrite accumulation was monitored at 550nm at various time points and calculated based on the absorbance of sodium nitrite standards manipulated in the same way with Griess reagent. B. SNP was mixed with Griess reagents containing various concentrations of Echinacea extracts and then incubated for 150 min. The data are expressed as mean ± standard error of the mean of 3 independent experiments performed in triplicate.
3.5. Inhibition of iNOS induction by alcohol extracts
Effects of alcohol extracts on iNOS induction were measured by an indirect method described by Kiemer et al. (2002). Once macrophages are activated, iNOS gene transcription is initiated about 1 h after activation, whereas its protein expression occurs some 3–4 h later. If the Echinacea-mediated decrease in NO production is through interference with the transcriptional induction process, earlier treatment (i.e. 0.5 h after LPS stimulation) with Echinacea extracts should have a more profound effect on NO production than adding the extracts later. Therefore, in these experiments, alcohol extracts (100 μg/ml) were either added simultaneously with LPS (1 μg/ml) or up to 6 h after addition of LPS. The results showed that after LPS activation, the earlier addition of alcohol extracts resulted in more inhibition of NO production (Fig. 4), indicating that Echinacea inhibits the transcriptional induction of iNOS. Of three alcohol extracts, EPA extract was the most active, in agreement with its strongest inhibitory potential of TNF-α and NO production.
Fig. 4.
Time course of inhibition of NO production by alcohol extracts of Echinacea. Echinacea extracts (100 μg/ml) were either added simultaneously with LPS (1 μg/ml) (0 h) or up to 6 h after addition of LPS. The nitrite accumulation by RAW 264.7 cells was determined 22 h after LPS stimulation by the Griess reaction. The data are expressed as mean ± standard error of the mean of 4 experiments performed in triplicate. *P<0.05 and **P<0.001 vs 0.25% ethanol in the presence of LPS (control). +P<0.05 and ++P<0.001 vs respective group in the presence of LPS at time point 0 h.
3.6. Opposite regulation of NO production and arginase activity by alcohol extracts
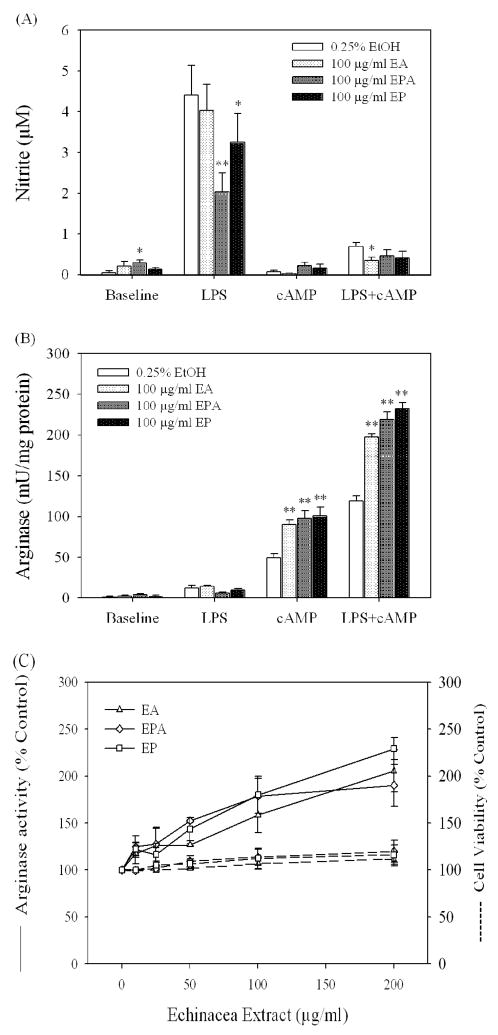
Raw 264.7 cells were incubated with alcohol extracts in the presence or absence of LPS to stimulate iNOS and/or 8-bromo-cAMP to induce arginase activity (Morris et al., 1998). The induction of arginase activity by 8-bromo-cAMP was further enhanced by the addition of LPS, which itself is a weak stimulant of arginase. In the presence of LPS alone, alcohol extracts of EP and EPA at 100 μg/ml significantly inhibited NO production (P values <0.05) (Fig. 5A), but their influence on arginase activity did not reach statistical significance. Note that 8-bromo-cAMP alone did not induce NO production, but decreased the NO production when added with LPS to RAW 264.7 cells. Moreover, all three alcohol extracts induced additional suppression of NO production in conjunction with 8-bromo-cAMP. In the presence of 8-bromo-cAMP, the three Echinacea extracts at 100 μg/ml significantly increased arginase activity up to 2-fold (P values <0.001) (Fig. 5B). The enhancing effect of Echinacea extracts on arginase activity was also seen when both 8-bromo-cAMP and LPS were present in the culture medium (Fig. 5B). When the concentrations of Echinacea extracts were reduced to 10 μg/ml, an enhancing effect was still seen when cells were exposed to Echinacea extracts (Fig. 5C). The test dose range (10–200 μg/ml) of Echinacea extracts had no effect on cell viability (Fig. 5C).
Fig. 5.
Regulation of NO production and arginase activity of activated RAW 264.7cells by alcohol extracts of Echinacea. Cells were incubated in the presence or absence of indicated concentrations of Echinacea extracts, LPS (1 μg/ml) and/or 8-bromo-cAMP (abbreviated cAMP, 0.25 mM) for 23–24 h, then supernates were collected for NO assay (A). The cell lysates were assayed for arginase activity (B and C (solid lines)) as described in Materials and Methods. Cell viability was determined by MTS assay (C (dashed lines)). Data are mean ± standard error of the mean of 4–5 experiments performed in triplicate (for NO and cell viability) or duplicate (arginase). *P<0.05 and **P<0.001 vs 0.25% ethanol as control in respective treatment group.
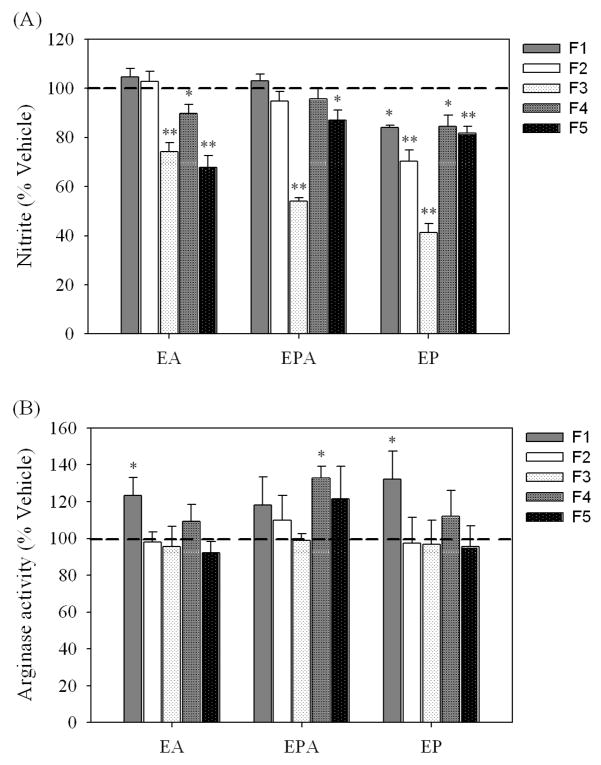
3.7. Differential regulation of NO production and arginase activity by fractions
In order to understand if the same fractions that inhibited NO production are responsible for increased arginase activity, five fractions of alcohol extracts were prepared (Table 1). Generally, the HPLC fractions of the three Echinacea extracts exhibited very similar patterns of effects on NO production and arginase actvity. Note that the opposite effects of Echinacea extracts on NO production and arginase activity resulted from different fractions. For all three species of Echinacea, fraction 3 containing abundant alkamides had robust inhibition of NO production. For EA, NO was also suppressed by fraction 4 with greater suppression by fraction 5, and for EP, there is some NO suppression by all fractions with the greatest suppression by fractions 2, 3, and 5 (Fig. 6A). On the other hand, the polar fractions (fractions 1) induced increased arginase activity (Fig. 6B). However for EPA, the greatest enhancement of arginase activity was seen with fraction 4 which contains unidentified hydrophobic compounds. The HPLC fractions of alcohol extracts showed no cytotoxicity as indicated by the MTS assay (data not shown).
Fig. 6.
Regulation of NO production and arginase activity of activated RAW 264.7 cells by fractions of alcohol extracts of Echinacea. NO production was determined in supernates of cells exposed to LPS (1 μg/ml) plus individual fraction (25 μg/ml) for 24 h (A). Arginase activity was assayed in cells treated with LPS (1 μg/ml), 8-bromo-cAMP (0.25 mM) and individual fraction (25 μg/ml) for 24 h (B). Data are expressed as mean ± standard error of the mean of 4–5 experiments performed in triplicate (NO) or duplicate (arginase). *P<0.05 and **P<0.001 vs 0.25% ethanol as vehicle as in respective treatment group.
3.8. Inhibition of iNOS protein expression
Decreased NO production might be relative to decreased iNOS protein expression as indirectly indicated in Figure 4. To that end, we carried out Western blot analysis of lysates of cells exposed to alcohol extracts of three Echinacea species at 100 μg/ml or their fractions at 25 μg/ml for 24 h in the presence of LPS. For EA, iNOS expression was significantly inhibited by fractions 3, 4 and 5 (P values <0.05). For EPA, the unfractionated alcohol extract and its fraction 3 had a robust inhibiting activity (P values <0.05). The unfractionated alcohol extract of EP and its fractions all showed, to some extent, an inhibiting effect, but they did not reach statistical significance except for fraction 5 (P = 0.046).
4. Discussion
NO and TNF-α are two key mediators in host defence and inflammatory response. They positively regulate each other (Yamamoto and Gaynor, 2001; Wu et al., 2003) and therefore amplify inflammatory signals. However, improper upregulation of these inflammatory players are implicated in a pathological role in inflammatory processes (Yamamoto and Gaynor, 2001; Kiemer et al., 2002). Our results demonstrated the suppressive effects of Echinacea extracts and certain individual fractions on production of NO and TNF-α in an activated macrophage cell line, in accordance with previous in vitro observations (Chen et al., 2005; Matthias et al., 2007; Zhai et al., 2007a). In order to understand the cellular mechanism as well as the biological implication underlying the herbal anti-inflammatory activity, we carried out additional experiments. We focused our interest on the mechanistic basis for decreased NO production because NO is an important effector molecule in the classical activation of macrophages (MacMicking et al., 1997; Munder et al., 1998).
Macrophages metabolize arginine to produce either NO through iNOS or ornithine through arginase. These two metabolic processes have important yet divergent roles in the cellular function. NO functions primarily with cytotoxic and cytostatic activity while the products of arginase pathway promote cell proliferation (Meurs et al., 2003). The predominance of one pathway over the other may rest on the presence of desirable inducers in the microenvironment. Macrophages express two arginase isoforms with distinct subcellular localizations: cytosolic type I and mitochondrial type II (Louis et al., 1999). LPS co-induces iNOS and arginase II, but the arginase II expression lags behind iNOS expression (Wang et al., 1995; Morris et al., 1998; Gotoh and Mori, 1999). The delayed expression of arginase II helps control NO production, and prevent NO-induced cell apoptosis (Munder et al., 1998; Gotoh and Mori, 1999). We hypothesized that an Echinacea-mediated decrease in NO production implies an increased activity of arginase in activated macrophages. As expected, Echinacea extracts oppositely regulated NO production and arginase activity even though the determinations of these two parameters were performed with different inducers (LPS or 8-bromo-cAMP). This differential regulation of two cross-regulated metabolic processes by Echinacea species may have important implications in the anti-inflammatory activity.
Although we predicted that one fraction responsible for decreased NO production would also be a strong stimulant of arginase, we observed the opposite regulatory effects on NO production and arginase activity were due to different fractions. Lipophilic fraction 3 was found to be the most effective in suppressing NO production while the polar fraction containing caffeic acid derivatives was an inducer of arginase activity, though the polar metabolites from EPA only showed an increasing trend in arginase activity. It is not surprising that the lipophilic fractions mediated a decrease in NO production, since an anti-inflammatory effect of alkamides has been reported (Chen et al., 2005; Matthias et al., 2007). Rather, it is noteworthy that caffeic acid derivatives (fraction 1) from EA and EPA have no effects on NO production considering that they are well demonstrated antioxidants (Hu and Kitts, 2000; Pellati et al., 2004; Dalby-Brown et al., 2005). Some natural antioxidants (e.g., flavonoids, resveratrol and curcumin) have been found to have strong inhibitory effects on iNOS expression and NO production in activated macrophages (Surh et al., 2001; Yamamoto and Gaynor, 2001).
Although caffeic acid derivatives have been determined to penetrate poorly across Caco-2 monolayers (Matthias et al., 2004), in vitro they enter into cells to accumulate high enough concentrations and consequently affect arginase activity. Cyclic AMP-stimulated arginase expression is through a complicated signal pathway involving intracellular activation of protein kinase A (Chang et al., 2000), and it has been proposed that Th2 cytokine-induced increase in arginase activity is cAMP-dependent (Corraliza et al., 1997; Wei et al., 2000). Although we can not answer based on the present data if caffeic acid derivatives directly mediate the activation of protein kinase A pathway, they may affect the signal pathway by upregulating Th2 cytokines, such as IL-4 and IL-10, as oral administration of Echinacea-mediates an increase in Th2 cytokines (Zhai et al., 2007b).
The lipophilic fraction of EPA includes two classes of phytochemicals, alkamides and ketones. While alkamides are widely investigated, ketones are almost completely neglected. Ketones are, in fact, the main lipophilic constituents in EPA roots, and ketone 23 is recognized as the diagnostic marker for hydrophobic metabolites (Kraus et al., 2005). The biological activity of ketones from EPA is little known. It has been suggested that ketones have antifungal activity (Binns et al., 2000). Obviously, like other bioactive metabolites of Echinacea (Awang, 1999), the antifungal activity of ketones may be through modulation of immune cell function rather than through a direct fungal killing. For lipophilic fraction 3 of EPA, it is possible that in addition to alkamides, ketones play a role in the regulation of NO production.
NO reacts rapidly with oxygen to form nitrogen dioxide, a gas capable of inducing cell damage (Meurs et al., 2003). It has been suggested that the polar caffeic acid derivatives of Echinacea possess an antioxidant and radical scavenging nature (Hu and Kitt, 2000; Pellati et al., 2004; Dalby-Brown et al., 2005), which could be enhanced by lipophilic alkamides (Dalby-Brown et al., 2005). However, Echinacea extracts (a mixture of caffeic acid derivatives and alkamides) did not scavenge or interact with NO and nitrogen dioxide species directly. Moreover, Echinacea extracts did not strongly inhibit nitrite accumulation by competing with oxygen to react with NO generated from SNP. Additionally, Echinacea extracts could not effectively protect RAW 264.7 cells from SNP-induced cell damage or cytotoxicity via the release of high levels of NO (data not shown). Based on these collected data, we could conclude that Echinacea-mediated decrease in NO production by macrophages is mainly via the modulation of the production yield of NO, but not direct scavenging of NO.
Echinacea present at the onset of LPS-induced iNOS expression resulted in a stronger inhibitory effect on NO production, suggesting that Echinacea could block the LPS-initiated upstream signaling pathway. Sharma et al. (2006) demonstrated that an alcohol tincture of EP roots significantly inhibited the nuclear expression of multiple pro-inflammatory transcription factors including NF-κB and STATs in a viral infection model. Others showed that in stimulated macrophages, certain individual alkamides could inhibit NF-κB activity (Matthias et al., 2007). Down-regulation of NF-κB expression will lead to decreased iNOS expression and NO production. Our results suggested that the Echinacea-mediated decrease in NO production was generally associated with decreased protein expression of iNOS as indicated by Western blotting assay. The control protein β-actin was not affected under the same conditions suggesting that Echinacea-mediated inhibition of iNOS expression did not result simply from a broad decrease in protein expression. Note that several Echinacea preparations showed relatively weak inhibition of iNOS protein expression when compared with their effects on NO production, this may suggest other mechanisms in the context of decreased NO production, i.e. a direct inhibition of iNOS enzyme activity. However, NO production correlates with iNOS activity and is commonly used as an indirect indicator of iNOS activity (Wang et al., 1995; Munder et al., 1998). In addition, decreased NO production might be in part due to a switch of macrophage function to alternative activation, leading to less availability of the substrate for the iNOS pathway.
Echinacea-derived alkamides have been found recently to regulate cellular signaling and immune responses involving the endocannabinoid system. Interestingly, alkamides have a structural similarity with anandamide, an endogenous cannabinoid (CB) receptor ligand. Alkamides showed anandamide-like activities by binding to CB2 receptors highly expressed on immune cells and CB1 receptors predominantly expressed in the brain (Gertsch et al., 2004; Woelkart et al., 2005b; Raduner et al., 2006). This is the first molecular target identified for Echinacea so far. The endocannabinoid system involves many pathophysiological activities including anti-inflammation and analgesic action. The finding of the interaction of alkamides with CB receptors may help explain the traditional use of Echinacea in phytotherapy for wound healing, pain relief and alleviation of cold symptoms. Naturally occurring alkamides may have other therapeutic targets besides the endocannabinoid system (Gertsch et al., 2008; Raduner et al., 2006).
Taken together, Echinacea affects macrophage immune function at multiple levels, resulting in inhibition of NO production and increased arginase activity. Observations on fractions of alcohol extracts of Echinacea indicate that Echinacea-mediated decrease in NO production is due to bioactive alkamides. Such in vitro effects may be directly applicable to in vivo administration as alkamides are bioavailable. Moreover, the anti-inflammatory activity of Echinacea might be dependent on a synergistic action of both alkamides and caffeic acid derivatives, which act together to drive macrophages to alternative activation. These noteworthy findings will help define the mechanisms behind the success of traditional use of Echinacea in phytotherapy for inflammatory diseases.
Fig. 7.

Regulation of iNOS expession by alcohol extracts and fractions of Echinacea. Cells were incubated with or without LPS (1 μg/ml) plus Echinacea extract (100 μg/ml) or individual fraction (25 μg/ml) for 24 h. The iNOS expression was analyzed by Western blotting and digitally quantitated using image analysis software. The data represent the mean of three independent experiments with different cell preparations. Representative bands are shown. The net intensity of iNOS bands was compared to that of corresponding β-actin with the same treatment, and the ratio was expressed as a percent of the ratio for the vehicle- and LPS-treated cells. A. EA; B. EPA; C. EP. *P<0.05 vs vehicle plus LPS.
Acknowledgments
This publication or project was made possible by the National Institute of Environmental Health Sciences (P01ES012020), the Office of Dietary Supplements, and the National Center for Complementary and Alternative Medicine (9 P50 AT004155-06), at the National Institutes of Health, and was performed as part of the Center for Research on Dietary Botanical Supplements at Iowa State University and the University of Iowa. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIEHS, ODS, NCCAM or the NIH. We thank Dr. Manuel Modolell (Max-Planck-Institut für Immunbiologie, Germany), Dr. Nair Sreejayan (University of Wyoming), and Dr. Jodi Mckay (Iowa State University) for technical help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awang DVC. Immune stimulants and antiviral botanicals: Echinacea and ginseng. In: Janick J, editor. Perspectives on new crops and new uses. ASHS Press; Alexandria, VA: 1999. pp. 450–456. [Google Scholar]
- Barnes J, Anderson LA, Gibbons S, Phillipson JD. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): a review of their chemistry, pharmacology and clinical properties. The Journal of Pharmacy and Pharmacology. 2005;57:929–954. doi: 10.1211/0022357056127. [DOI] [PubMed] [Google Scholar]
- Binns SE, Purgina B, Bergeron C, Smith ML, Ball L, Baum BR, Arnason JT. Light-mediated antifungal activity of Echinacea extracts. Planta Medica. 2000;66:241–244. doi: 10.1055/s-2000-8573. [DOI] [PubMed] [Google Scholar]
- Borchers AT, Keen CL, Stern JS, Gershwin ME. Inflammation and Native American medicine: the role of botanicals. American Journal of Clinical Nutrition. 2000;72:339–347. doi: 10.1093/ajcn/72.2.339. [DOI] [PubMed] [Google Scholar]
- Chan MM, Mattiacci JA, Hwang HS, Shah A, Fong D. Synergy between ethanol and grape polyphenols, quercetin, and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochemical Pharmacology. 2000;60:1539–1548. doi: 10.1016/s0006-2952(00)00471-8. [DOI] [PubMed] [Google Scholar]
- Chang CI, Zoghi B, Liao JC, Kuo L. The involvement of tyrosine kinases, cyclic AMP/protein kinase A, and p38 mitogen-activated protein kinase in IL-13-mediated arginase I induction in macrophages: its implications in IL-13-inhibited nitric oxide production. The Journal of Immunology. 2000;165:2134–2141. doi: 10.4049/jimmunol.165.4.2134. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fu T, Tao T, Yang J, Chang Y, Wang M, Kim L, Qu L, Cassady J, Scalzo R, Wang X. Macrophage activating effects of new alkamides from the roots of Echinacea species. Journal of Natural Products. 2005;68:773–776. doi: 10.1021/np040245f. [DOI] [PubMed] [Google Scholar]
- Chicca A, Pellati F, Adinolfi B, Matthias A, Massarelli I, Benvenuti S, Martinotti E, Bianucci AM, Bone K, Lehmann R, Nieri P. Cytotoxic activity of polyacetylenes and polyenes isolated from roots of Echinacea pallida. British Journal of Pharmacology. 2008;153:879–885. doi: 10.1038/sj.bjp.0707639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. Journal of Immunological Methods. 1994;174:231–235. doi: 10.1016/0022-1759(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Corraliza IM, Modolell M, Ferber E, Soler G. Involvement of protein kinase A in the induction of arginase in murine bone marrow-derived macrophages. Biochimica et Biophysica Acta. 1997;1334:123–128. doi: 10.1016/s0304-4165(96)00081-5. [DOI] [PubMed] [Google Scholar]
- Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Communications. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- Dalby-Brown L, Barsett H, Landbo AK, Meyer AS, Molgaard P. Synergistic antioxidative effects of alkamides, caffeic acid derivatives, and polysaccharide fractions from Echinacea purpurea on in vitro oxidation of human low-density lipoproteins. Journal of Agricultural and Food Chemistry. 2005;53:9413–9423. doi: 10.1021/jf0502395. [DOI] [PubMed] [Google Scholar]
- Gertsch J. Immunomodulatory lipids in plants: plant fatty acid amides and the human endocannabinoid system. Planta Medica. 2008;74:638–650. doi: 10.1055/s-2008-1034302. [DOI] [PubMed] [Google Scholar]
- Gertsch J, Schoop R, Kuenzle U, Suter A. Echinacea alkylamides modulate TNF-alpha gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Letters. 2004;577:563–569. doi: 10.1016/j.febslet.2004.10.064. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Mori M. Arginase II downregulates nitric oxide (NO) production and prevents NO-mediated apoptosis in murine macrophage-derived RAW 264.7 cells. The Journal of Cell Biology. 1999;144:427–434. doi: 10.1083/jcb.144.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Kitts DD. Studies on the antioxidant activity of Echinacea root extract. Journal of Agricultural and Food Chemistry. 2000;48:1466–1472. doi: 10.1021/jf990677+. [DOI] [PubMed] [Google Scholar]
- Jia C, Shi H, Wu X, Li Y, Chen J, Tu P. Determination of echinacoside in rat serum by reversed-phase high-performance liquid chromatography with ultraviolet detection and its application to pharmacokinetics and bioavailability. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2006;844:308–313. doi: 10.1016/j.jchromb.2006.07.040. [DOI] [PubMed] [Google Scholar]
- Kiemer AK, Muller C, Vollmar AM. Inhibition of LPS-induced nitric oxide and TNF-α by α-lipoic acid in rat Kupffer cells and in RAW 264.7 cells. Immunology and Cell Biology. 2002;80:550–557. doi: 10.1046/j.1440-1711.2002.01124.x. [DOI] [PubMed] [Google Scholar]
- Kraus GA, Bae J, Schuster J. The first synthesis of a diynone from Echinacea pallida. Synthesis. 2005;2005:3502–3504. doi: 10.1055/s-2005-918418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay S, Gil-Izquierdo A, Manach C, Morand C, Besson C, Scalbert A. Chlorogenic acid is absorbed in its intact form in the stomach of rats. The Journal of Nutrition. 2006;136:1192–1197. doi: 10.1093/jn/136.5.1192. [DOI] [PubMed] [Google Scholar]
- Liu Y, Murphy PA. Alkamide stability in Echinacea purpurea extracts with and without phenolic acids in dry films and in solution. Journal of Agricultural and Food Chemistry. 2007;55:120–126. doi: 10.1021/jf0619481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis CA, Mody V, Henry WL, Jr, Reichner JS, Albina JE. Regulation of arginase isoforms I and II by IL-4 in cultured murine peritoneal macrophages. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 1999;276:R237–R242. doi: 10.1152/ajpregu.1999.276.1.R237. [DOI] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annual Review of Immunology. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocrine Reviews. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- Matthias A, Addison RS, Penman KG, Dickinson RG, Bone KM, Lehmann RP. Echinacea alkamide disposition and pharmacokinetics in humans after tablet ingestion. Life Sciences. 2005;77:2018–2029. doi: 10.1016/j.lfs.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Matthias A, Banbury L, Stevenson LM, Bone KM, Leach DN, Lehmann RP. Alkylamides from Echinacea modulate induced immune responses in macrophages. Immunological Investigations. 2007;36:117–130. doi: 10.1080/08820130600745786. [DOI] [PubMed] [Google Scholar]
- Matthias A, Blanchfield JT, Penman KG, Toth I, Lang CS, De Voss JJ, Lehmann RP. Permeability studies of alkylamides and caffeic acid conjugates from Echinacea using a Caco-2 cell monolayer model. Journal of Clinical Pharmacy and Therapeutics. 2004;29:7–13. doi: 10.1046/j.1365-2710.2003.00530.x. [DOI] [PubMed] [Google Scholar]
- Meurs H, Maarsingh H, Zaagsma J. Arginase and asthma: novel insights into nitric oxide homeostasis and airway hyperresponsiveness. Trends in Pharmacological Sciences. 2003;24:450–455. doi: 10.1016/S0165-6147(03)00227-X. [DOI] [PubMed] [Google Scholar]
- Modolell M, Corraliza IM, Link F, Soler G, Eichmann K. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. European Journal of Immunology. 1995;25:1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- Morris SM, Jr, Kepka-Lenhart D, Chen LC. Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. The American Journal of Physiology. 1998;275:E740–E747. doi: 10.1152/ajpendo.1998.275.5.E740. [DOI] [PubMed] [Google Scholar]
- Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. The Journal of Immunology. 1998;160:5347–5354. [PubMed] [Google Scholar]
- Park E, Kum S, Wang C, Park SY, Kim BS, Schuller-Levis G. Anti-inflammatory activity of herbal medicines: inhibition of nitric oxide production and tumor necrosis factor-alpha secretion in an activated macrophage-like cell line. The American Journal of Chinese Medicine. 2005;33:415–424. doi: 10.1142/S0192415X05003028. [DOI] [PubMed] [Google Scholar]
- Pellati F, Benvenuti S, Magro L, Melegari M, Soragni F. Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. Journal of Pharmaceutical and Biomedical Analysis. 2004;35:289–301. doi: 10.1016/S0731-7085(03)00645-9. [DOI] [PubMed] [Google Scholar]
- Percival SS. Use of Echinacea in medicine. Biochemical Pharmacology. 2000;60:155–158. doi: 10.1016/s0006-2952(99)00413-x. [DOI] [PubMed] [Google Scholar]
- Porcheray F, Viaud S, Rimaniol AC, Léone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clinical and Experimental Immunology. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raduner S, Majewska A, Chen JZ, Xie XQ, Hamon J, Faller B, Altmann KH, Gertsch J. Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects. Journal of Biological Chemistry. 2006;281:14192–14206. doi: 10.1074/jbc.M601074200. [DOI] [PubMed] [Google Scholar]
- Rousseau B, Tateya I, Lim X, Munoz-del-Rio A, Bless DM. Investigation of anti-hyaluronidase treatment on vocal fold wound healing. Journal of Voice. 2006;20:443–451. doi: 10.1016/j.jvoice.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Sharma M, Arnason JT, Hudson JB. Echinacea extracts modulate the production of multiple transcription factors in uninfected cells and rhinovirus-infected cells. Phytotherapy Research. 2006;20:1074–1079. doi: 10.1002/ptr.1998. [DOI] [PubMed] [Google Scholar]
- Speroni E, Govoni P, Guizzardi S, Renzulli C, Guerra MC. Anti-inflammatory and cicatrizing activity of Echinacea pallida Nutt. root extract. Journal of Ethnopharmacology. 2002;79:265–272. doi: 10.1016/s0378-8741(01)00391-9. [DOI] [PubMed] [Google Scholar]
- Sreejayan Rao MN. Nitric oxide scavenging by curcuminoids. The Journal of Pharmacy and Pharmacology. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutation Research. 2001;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- Vanzo A, Cecotti R, Vrhovsek U, Torres AM, Mattivi F, Passamonti S. The fate of trans-caftaric acid administered into the rat stomach. Journal of Agricultural and Food Chemistry. 2007;55:1604–1611. doi: 10.1021/jf0626819. [DOI] [PubMed] [Google Scholar]
- Wang WW, Jenkinson CP, Griscavage JM, Kern RM, Arabolos NS, Byrns RE, Cederbaum SD, Ignarro LJ. Co-induction of arginase and nitric oxide synthase in murine macrophages activated by lipopolysaccharide. Biochemical and Biophysical Research Communications. 1995;210:1009–1016. doi: 10.1006/bbrc.1995.1757. [DOI] [PubMed] [Google Scholar]
- Wei LH, Morris SM, Jr, Cederbaum SD, Mori M, Ignarro LJ. Induction of arginase II in human Caco-2 tumor cells by cyclic AMP. Archives of Biochemistry and Biophysics. 2000;374:255–260. doi: 10.1006/abbi.1999.1563. [DOI] [PubMed] [Google Scholar]
- Woelkart K, Bauer R. The role of alkamides as an active principle of Echinacea. Planta Medica. 2007;73:615–623. doi: 10.1055/s-2007-981531. [DOI] [PubMed] [Google Scholar]
- Woelkart K, Koidl C, Grisold A, Gangemi JD, Turner RB, Marth E, Bauer R. Bioavailability and pharmacokinetics of alkamides from the roots of Echinacea angustifolia in humans. Journal of Clinical Pharmacology. 2005a;45:683–689. doi: 10.1177/0091270004273493. [DOI] [PubMed] [Google Scholar]
- Woelkart K, Xu W, Pei Y, Makriyannis A, Picone RP, Bauer R. The endocannabinoid system as a target for alkamides from Echinacea angustifolia roots. Planta Medica. 2005b;71:70–705. doi: 10.1055/s-2005-871290. [DOI] [PubMed] [Google Scholar]
- Wu CH, Chen TL, Chen TG, Ho WP, Chiu WT, Chen RM. Nitric oxide modulates pro- and anti-inflammatory cytokines in lipopolysaccharide-activated macrophages. The Journal of Trauma. 2003;55:540–545. doi: 10.1097/01.TA.0000033496.62796.3B. [DOI] [PubMed] [Google Scholar]
- Wu L, Bae J, Kraus G, Wurtele ES. Diacetylenic isobutylamides of Echinacea: synthesis and natural distribution. Phytochemistry. 2004;65:2477–2484. doi: 10.1016/j.phytochem.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sawada Y, Katayama I, Nishioka K. Increased production of nitric oxide stimulated by interleukin-1beta in peripheral blood mononuclear cells in patients with systemic sclerosis. British Journal of Rheumatology. 1998;37:1123–1125. doi: 10.1093/rheumatology/37.10.1123. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. The Journal of Clinical Investigation. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z, Haney D, Wu L, Solco A, Murphy PA, Wurtele ES, Kohut ML, Cunnick JE. Alcohol extracts of Echinacea inhibit production of nitric oxide and tumor necrosis factor-alpha by macrophages in vitro. Food and Agricultural Immunology. 2007a;18:221–236. doi: 10.1080/09540100701797363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z, Liu Y, Wu L, Senchina DS, Wurtele ES, Murphy PA, Kohut ML, Cunnick JE. Enhancement of innate and adaptive immune functions by multiple Echinacea species. Journal of Medicinal Food. 2007b;10:423–434. doi: 10.1089/jmf.2006.257. [DOI] [PMC free article] [PubMed] [Google Scholar]