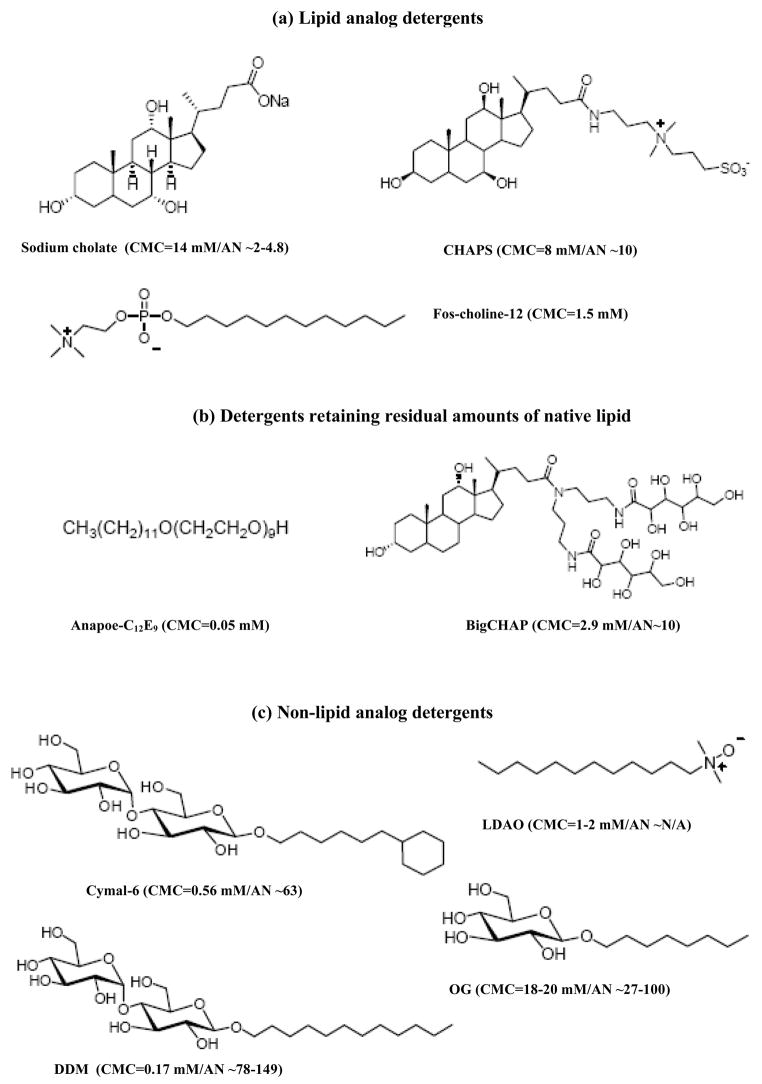
Abstract
The nicotinic acetylcholine receptor (nAChR) of Torpedo electric rays has been extensively characterized over the last three decades. However, the molecular mechanisms by which detergents influence membrane protein stability and function remain poorly understood, and elucidation of the dynamic detergent-lipid-protein interactions of solubilized membrane proteins is a largely unexplored research field. This study examined nine detergents upon nAChR solubilization and purification, to assess receptor lipid composition using GC (Gas Chromatography)-FID (Flame Ionization) and/or GC-MSD (Mass Selective Detectors), stability and aggregation state using A-SEC (Analytical Size-Exclusion Chromatography) and EM (Electron Microscopy), and planar lipid bilayers to measure ion channel function. Detergent solubilization of nAChR-enriched membranes did not result in significant native lipid depletion or destabilization. Upon purification, native lipid depletion occurred in all detergents, with lipid-analog detergents [CHAPS (3-[(3-Cholamidopropyl)-dimethylammonio]-1-propane sulfonate), FC-12 (n-Dodecylphosphocholine) and sodium cholate (3α,7α,12α-Trihydroxy-5β-cholan-24-oic acid)] maintaining stability and supporting ion channel function, while non-lipid analog detergents [Cymal-6 (6-Cyclohexyl-1-hexyl-β-d-maltoside), DDM (n-Dodecyl-β-d-maltopyranoside), LDAO (Lauryldimethylamine-N-oxide) and OG (n-Octyl-β-d-glucopyranoside)] showed decreased stability and significant reduction or loss of ion channel function. Anapoe-C12E9 (Polyoxyethylene-(9)-dodecyl ether) and BigCHAP (N,N′-bis-(3-d-Gluconamidopropyl) cholamide) retained residual amounts of native lipid, maintaining moderate stability and ion channel function when compared to lipid-analog detergents. Overall, these results show that the nAChR can be stable and functional in lipid-analog detergents or in detergents that retain moderate amounts of residual native lipids, while the opposite is true about non-lipid analog detergents. These results highlight the importance of careful biophysical characterization of membrane proteins for future functional or structural studies in the detergent-solubilized state.
Keywords: nAChR, functional characterization, planar lipid bilayers, integral membrane proteins, detergents, membrane lipids, biophysical characterization
Introduction
The nAChR is an essential component in the cholinergic pathways that regulate and control synaptic transmission in brain and muscle tissues. It is a member of the Cys-loop (cysteine loop) or LGIC (ligand-gated ion channel) family of receptors, which includes the neuronal and muscle type nicotinic receptors, Gly-R (glycine receptor), GABAA or GABAC receptors (γ-aminobutyric acid type-A or type-C), 5-HT3 (5-hydroxytryptamine type-3), as well as the invertebrate glutamate and histidine receptors (Karlin, A., 2002). Torpedo and muscle-type nAChRs are five-subunit integral membrane protein complexes with stoichiometry α2βγδ (2-alpha, beta, gamma and delta) in a pentamer arrangement and an internal cation-selective ion channel at the center. Each subunit has four transmembrane segments (M1-M4) and the amino- and carboxy- terminal domains are located extracellularly (Karlin, A., Akabas, M.H., 1995). In vertebrate NMJs (neuromuscular junctions), binding of the endogenous neurotransmitter ACh (acetylcholine) initiates membrane depolarization, generating an action potential that travels across the membrane, which ultimately results in the transmission of nervous impulses.
Recent structural studies have yielded a three-dimensional nAChR EM structure using native Torpedo membranes (Miyazawa, A., et al., 2003 and Unwin, N., 2005), as well as the crystal structure of the recombinant mouse alpha-1 nAChR extracellular domain (Dellisanti, C.D., et al., 2007). High-resolution nAChR structural data in the detergent-solubilized state, which would require a unique knowledge of detergent-lipid-protein interactions, has remained elusive partly as a result of the very few successful nAChR structural studies reported under these conditions (Hertling-Jaweed, S. et al., 1988). Most nAChR detergent-lipid-protein interaction data has been obtained from native or sodium cholate-solubilized Torpedo membranes reconstituted into native lipid vesicles through detergent dialysis. Additionally, the nAChR can be purified by sodium cholate solubilization of Torpedo membranes followed by affinity chromatography to exchange native lipid for exogenous lipids and after detergent dialysis, the receptor is reconstituted into lipid vesicles at defined compositions. Functional data obtained from these preparations suggested that ligand binding was independent of lipid composition but nAChR ion channel function required the presence of both neutral and negatively-charged lipids (Fong, T.M., McNamee, M.G., 1986). Fourier Transform Infrared Spectroscopy (FT-IR) and labeling studies with [125I]-TID (3-(trifluoro-methyl)-3-(m-[125I]iodophenyl)diazirine), a photoreactive probe, suggested that the effect of negatively charged phospholipids is synergistic, rather than additive (daCosta, C.J., et al., 2002 and Hamouda, A.K., et al., 2006) Other studies have suggested that the total phospholipid amount required to support nAChR function is ~45 moles of lipid per-mole, and that complete loss of ion channel function occurred if this ratio fell below ~20 (Jones, O.T., et al., 1988).
Studies probing the effect of detergents on ion channel function suggested that the nAChR solubilized and purified in sodium cholate (Fong, T.M., McNamee, M.G., 1986, Jones, O.T., et al., 1988 and Anholt, R., et al., 1981) or CHAPS (Schürholz, T., et al., 1992) is capable of sustaining ion channel flux, while similar studies in OG remain controversial, with some groups reporting ion channel function (Paraschos, A., et al., 1982) and others inhibition of ion channel function (Anholt, R., et al., 1981). Other studies using [125I]-TID labeling combined with native membrane solubilization in several detergents showed that only sodium cholate maintained a labeling pattern consistent with the native nAChR resting state prior to exposure to its endogenous agonist, ACh (McCarthy, M.P., Moore, M.A., 1992). In OG, Triton X-100 ([α-[4-(1,1,3,3-Tetramethylbutyl)phenyl]-ω-hydroxy-poly(oxy-1,2,-ethanediyl)]) and Tween-20 (Polyoxyethylene(20)sorbitan monolaureate), the receptor showed a labeling pattern consistent with the nAChR desensitized state that occurs upon prolonged exposure to its agonist and slowly reverts to the resting state. Receptor ion channel function has also been probed using planar lipid bilayers in native Torpedo membranes (Schindler, H., Quast, U., 1980), which showed electrophysiological parameters similar to detergent-solubilized and purified receptor in cholate (Nelson, N., et al., 1980) or Triton X-100 exchanged into Tween-80 (Polyoxyethylene(80)sorbitan monolaureate) (Boheim, G., et al., 1981).
Few studies have analyzed the effects of detergent solubilization and purification on Torpedo membrane lipid components, and how lipid depletion can affect nAChR function and stability. Techniques used successfully in previous nAChR studies, including planar lipid bilayers and EM, were combined with GC-FID, GC-MSD and A-SEC to probe ion channel function, total lipid composition and nAChR stability, respectively. This study aims to identify desirable detergent families for nAChR solubilization and purification by probing their effects on the above-described parameters to routinely obtain stable and functional nAChR in its purified state for the benefit of future biophysical and structural studies seeking to further advance current nAChR knowledge.
Materials and methods
Crude membrane isolation, detergent solubilization and nAChR ligand-affinity purification
Sucrose gradient isolation of nAChR-enriched crude membrane fractions was performed as described (Ochoa, E.L., et al., 1983), fractioning the gradient from the bottom up for immediate use or freezing at −20 °C. Membranes were diluted in a 1:3 (mL/mL) ratio with DB-1 (Dialysis Buffer-1): 10 mM MOPS (3-(N-Morpholino)propanesulfonic acid, pH=7.4), 100 mM NaCl, 0.1 mM EDTA (ethylenediamine tetraacetic acid) and 0.02% NaN3. Membrane solubilization was carried out for 1 hr. at 4 °C with a 1–4% final concentration of each detergent, followed by high-speed ultracentrifugation for 1 hr. and application of the supernatant to a 1.5 × 15 cm Econo-column (Bio-Rad Laboratories, Hercules, CA) containing ~12.5 mL Bromoacetylcholine affinity column (BAC), prepared as described (Bhushan, A., McNamee, M.G., 1990). The column was washed with ~ten (10) times the un-diluted crude membrane volume that was originally applied to the column (5 mL of crude membranes=50 mL of wash buffer), using DB-1 buffer supplemented with the detergent of choice at 1.5 times the CMC (critical micelle concentration). The nAChR was eluted with ~50 mL of wash buffer supplemented with 90 mg of Carbamylcholine (Carb.; Sigma, St. Louis, MO) and 292 mg of NaCl, concentrated to ~2.5 mL and applied to a PD-10 desalting column (GE Healthcare, Uppsala, Sweden) to remove the Carb. ligand. The receptor was eluted from the column with ~5 mL of wash buffer and concentrated to ~125–250 μL before SDS-PAGE and BCA™ analysis (Pierce Biotechnology, Inc., Rockford, IL).
Sample preparation for A-SEC
~15–25 μL of purified nAChR was reacted with α-BTX-Alexa Fluor 488 antagonist (Invitrogen-Molecular Probes Inc., Eugene, OR) in a 2.5:1 α-BTX: nAChR molar ratio for ~2 hrs. at 4 °C. 5–10 μL of the complex were injected into a Dionex Ultimate 3000 chromatography system (Dionex Corp., Sunnyvale, CA) with a Shodex KN-803 analytical column (Shodex Inc., Kawasaki, Japan) in a mobile phase of 20 mM HEPES (4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid, pH=7.5), 200 mM NaCl and 0.09% DDM.
Sample preparation for EM
1–2 μL of purified nAChR were layered in glow-discharged, carbon-coated 400-mesh cooper grids which sat undisturbed for 1 min. before ten (10) sequential washes through ~200 uL water drops layered over parafilm, addition of 0.5–1 μL of 1% uranyl acetate for 1 min. and removal of the excess liquid with a fine strand of filter paper. Grids were stored or analyzed with a FEI Tecnai F20 Twin transmission EM with a 4kx4k CCD camera at an accelerating voltage=120 keV. Analysis were performed manually or using a robotic system with the Leginon software (Potter, C.S., et al., 2004 and Suloway, C., et al., 2005) and automatic image acquisition at low magnification (120×), sampling different grid areas for up to nine (9) intact grid squares, chosen by transmission level and spacing. Two to four (2–4) images per square were acquired at low-dose (50,000×), with the camera binned by four [4; pixel size 0.652 nm] and a dose of 3–5 electron/Å. For manual EM grid loading, the same software was used and the operator chose nine (9) imaging squares for automated focusing and final image acquisition.
Sample preparation, analysis and data processing for planar lipid bilayer assays
Purified nAChR detergent micelles were coated with PMAL-C8 (Poly (maleic anhydride-alt-1-decene) substituted with 3-(Dimethylamino) propylamine) detergent (Anatrace), as described (Martínez, K.L., et al., 2002). A Warner Instruments (Hamden, CT) model BC-525D Bilayer Clamp Amplifier, connected to an 8-pole Low-pass Bessel filter (LPF-8) and a PC-based computer through an Axon Instruments Digidata 1322-A interface (Sunnyvale, CA), was used for bilayer analysis. A mixture of Brain PC (L-α-Phosphatidylcholine), PE (L-α-ethanolamine; Avanti Polar Lipids, Alabaster, AL) and cholesterol (Anatrace) on a 3:1:1 ratio at ~75 mg/mL in chloroform was prepared, evaporated under nitrogen and resuspended in decane at ~75 mg/mL. A 1:1 dilution was applied to a 200 μm aperture in a cup-shaped Teflon chamber and allowed to air dry before reopening and fitting into the rear cavity of a rectangular plastic holder, containing electrode apertures which were filled with 1 M KCl. Both the Teflon chamber and front plastic holder cavity were filled with ~1 mL of bilayer buffer solution: 10 mM HEPES (pH=7.2), 1 mM MgCl2 and 150 mM NaI. Agar bridges filled with 1 M KCl and 2% agarose were used to connect the electrodes to the chambers and the Teflon chamber aperture was re-coated with lipid mixture until a seal was detected [membrane capacitance=90–170 pA]. Approximately 1–25 μg of PMAL-coated nAChR vesicles were added to the front chamber and stirred for ~15 min., verifying protein incorporation through a ~5–10 pA change in membrane capacitance. Stirring was stopped for data recording, while the membrane potential was gradually increased to +70 mV for 1 min. and switched to −70 mV for 1 min. to record a baseline signal with protein and no agonist. The membrane potential was turned off before adding 0.5 μM Carb., gradually increasing to +70 mV for 2–5 min. or until steady currents were observed, switching to −70 mV for the same timeframe and continuing these cycles until the membrane broke. Clampfit 10.0 software (Molecular Devices Corporation, Union City, CA) was used to analyze 5–10 min. file segments, manually searching for 100–1000 ion channel opening-closing events, which were integrated and processed with a PC-based computer, generating mean open channel current and mean open channel time histograms from which values for these parameters were obtained for each detergent.
Total phospholipid and cholesterol analysis
Sucrose gradient detergent-solubilized membranes or affinity-purified nAChR samples were subjected to Bligh & Dyer lipid extractions (Bligh, E.G., Dyer, W.J., 1959) containing BHT (butylated hydroxytoluene, 2 × 10−5 M), followed by 3.5 hrs. of reflux with MeOH/HCl for phospholipid hydrolysis. Samples were extracted with PEt (petroleum ether), dried under nitrogen and dissolved in 10% MeOH/DE (diethyl ether) for methylation using 0.5 mL of diazomethane. After 20 min., the reaction mixture was extracted with PEt and applied to rhodamine 6-G stained silica gel TLC (thin-layer chromatography) plates with PEt/DE (98:2 v/v) as the solvent system, excising cholesterol and FAMEs (fatty acid methyl esters) bands for further analysis. Cholesterol was analyzed with the AmplexRed cholesterol assay, as described in the manufacturer instructions (Invitrogen, Carlsbad, CA) and isolated FAMEs were analyzed with a Hewlett-Packard 5890 series II GC/5972A MS equipped with a Supelco SPB-5, 30 m × 0.25 mm i.d. column (St. Louis, Missouri). 1 μL of sample in n-hexane was injected manually, (injector temperature=250 °C/oven temperature=130 °C) and after 5 min., a 3 °C/min. ramp was applied to 200 °C (1 min. hold), resuming the ramp to 220 °C, with a 10 min. hold as the final step. Quantitation was performed with an internal standard (IS) of known concentration absent from the lipids in the Torpedo tissue (20:1Δ11), dividing the total FAME by two (2) and using appropriate control samples to eliminate trace amounts of contaminants found in all solvents tested. Total phospholipid and cholesterol amounts for both sucrose gradient membranes and affinity column samples were normalized using protein concentration values in mg/mL obtained from BCA assay measurements.
Results
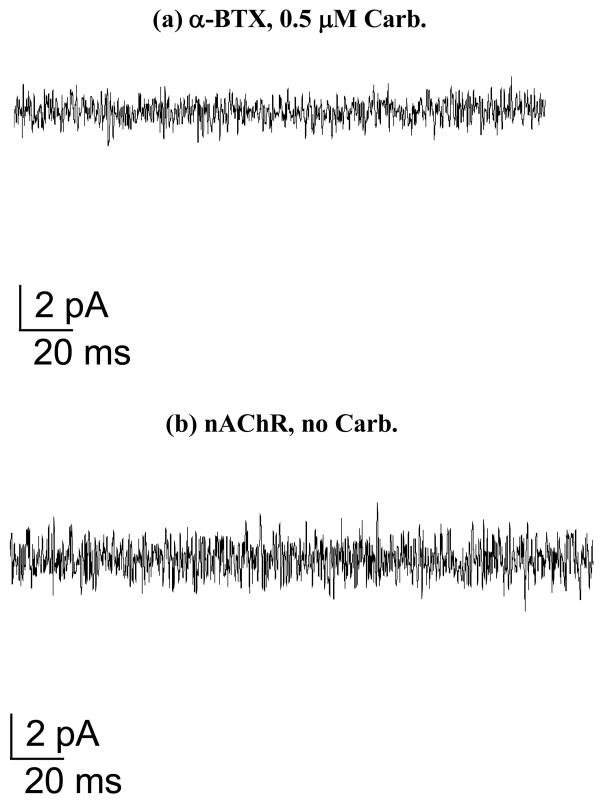
Control experiments for nAChR ion channel function using planar lipid bilayers showed that affinity-purified nAChR in sodium cholate incubated with α-BTX (alpha-bungarotoxin) after purification completely blocked ion channel currents in the presence of 0.5 μM (micromolar) Carb. (Figure 1a), with similar results obtained when incorporating the protein into the bilayer without adding Carb., which resulted in a baseline signal (Figure 1b).
Figure 1. Planar lipid bilayer experimental controls using affinity-purified nAChR in sodium cholate.
Planar lipid bilayer ion channel function assay controls at −70 mV membrane potential: (a) nAChR incubated with α-BTX after affinity column purification in the presence of 0.5 μM Carb. and (b) affinity-purified nAChR with no ligand added.
Preliminary observation of the experimental results revealed significant differences in all biophysical parameters between detergent-solubilized sucrose gradient membranes and affinity-purified nAChR, mainly as a result of alteration of the native lipid composition. Detergent solubilization of sucrose gradient nAChR-enriched membranes did not result in significant native lipid depletion (Table 1). In addition, ligand-affinity column purification and formation of the nAChR-Alexa Fluor 488 α-BTX antagonist complex, a necessary condition for detection in A-SEC stability assays, occurred in all detergents, demonstrating that agonist and antagonist binding were not affected (Figures 2, 3 and 4). Furthermore, nAChR stability was not significantly affected by detergent solubilization, with no aggregation detected in A-SEC assays, which is consistent with the observed minimal lipid depletion, while both nAChR monomer and dimer species were observed in all detergents, which is consistent with data from previous studies (Anholt, R., et al., 1980) (Figures 2, 3 and 4). Affinity column purification, caused significant lipid depletion in all detergents (Table 1) to levels at or below the previously-reported functional thresholds (Jones, O.T., et al., 1988), affecting nAChR stability as evidenced by higher aggregation levels and further altering monomer-dimer ratios, as well as ion channel function, in a detergent-dependent manner (Figures 2, 3 and 4).
Table 1.
Total lipid assays for sucrose gradient detergent-solubilized membranes and ligand-affinity purified nAChR.
| After detergent solubilization of nAChR-enriched sucrose gradient membranes | After ligand-affinity column purification | ||||
|---|---|---|---|---|---|
| Detergent | Total phospholipid (nmoles) ◇ | Total cholesterol (nmoles) ◇ | Total phospholipid (nmoles) ◇ | Total cholesterol (nmoles) ◇ | Molar ratio of lipids per-mole of nAChR◇, * |
| Sodium cholate | 1398 | 1036 | 33 | 14 | 18 |
| CHAPS | 1718 | 334 | 25 | 20 | 16 |
| FC-12 | 1836 | 183 | 53 | 34 | 28 |
| BigCHAP | 2320 | 263 | 53 | 90 | 40 |
| Anapoe-C12E9 | 3001 | 1478 | 166 | 180 | 53 |
| Cymal-6 | 1261 | 150 | 22 | 28 | 9 |
| DDM | 1780 | 814 | 25 | 41 | 20 |
| LDAO | 2557 | 1610 | 5 | 34 | 17 |
| OG | 1875 | 659 | 6 | 17 | 9 |
Total phospholipid and cholesterol amounts for both sucrose gradient membranes and affinity column samples were normalized using protein concentration values in mg/mL obtained from BCA assay measurements.
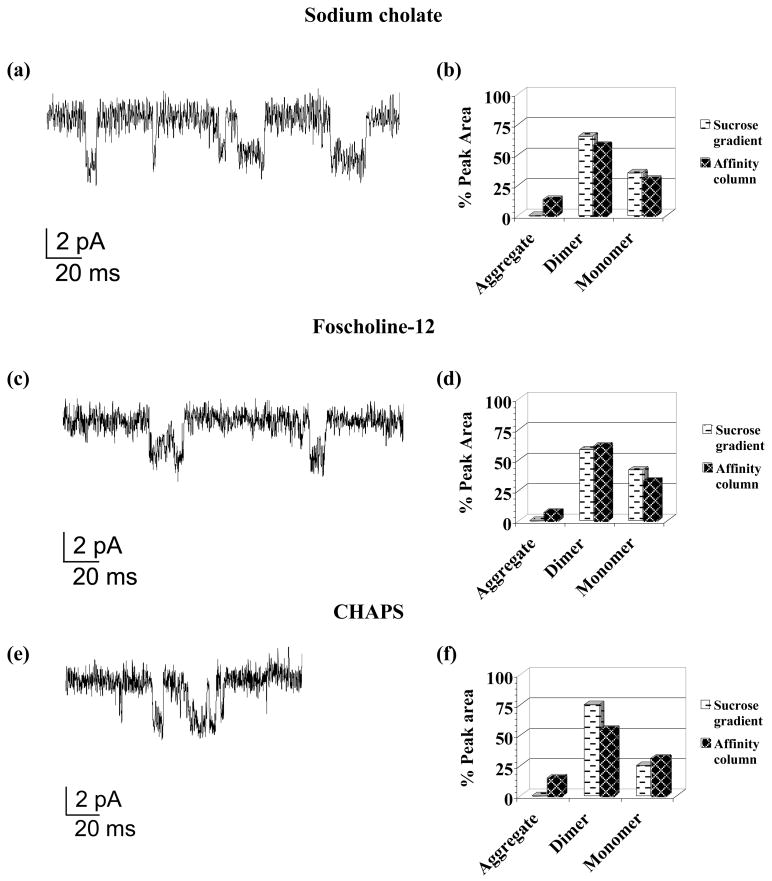
Figure 2. nAChR ion channel function and stability assays for lipid-analog detergents.
Planar bilayer current traces at −70 mV membrane potential in the presence of 0.5 μM Carb. (left panels) for: (a) Sodium cholate, (c) FC-12 and (e) CHAPS. A-SEC stability assay percent (%) peak areas (right panels) for sucrose gradient detergent-solubilized membranes (white background) and ligand affinity-purified nAChR (black background) in (b) Sodium cholate (sucrose gradient % peak areas: 65% dimer/35% monomer; affinity column % peak areas: 13% aggregate/58% dimer/29% monomer) (d) FC-12 (sucrose gradient % peak areas: 58% dimer/42% monomer; affinity column % peak areas: 7% aggregate/61% dimer/32% monomer) and (f) CHAPS (sucrose gradient % peak areas: 75% dimer/25% monomer; affinity column % peak areas: 15% aggregate/55% dimer/31% monomer).
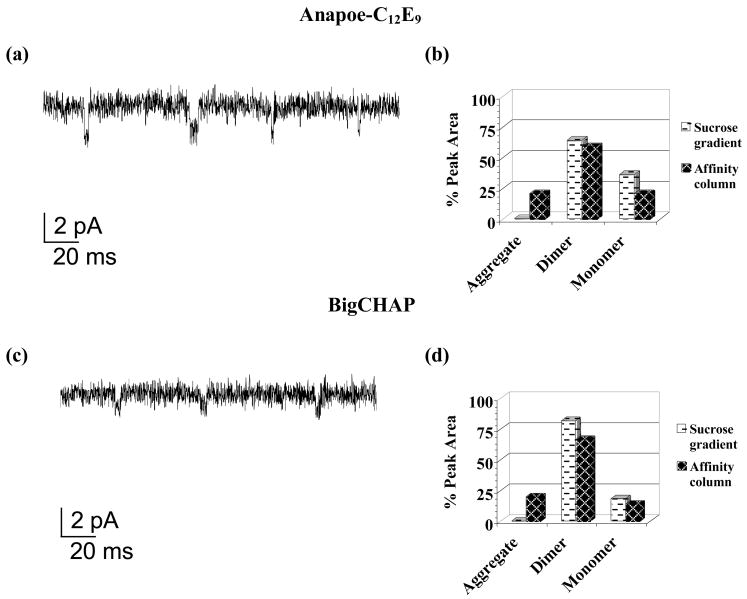
Figure 3. nAChR ion channel function and stability assays for detergents retaining residual amounts of native lipid.
Planar bilayer current traces−70 mV membrane potential in the presence of 0.5 μM Carb. (left panels) for: (a) Anapoe-C12E9 and (c) BigCHAP and A-SEC stability assay percent (%) peak areas (right panels) for sucrose gradient detergent-solubilized membranes (white background) and ligand affinity-purified nAChR (black background) in (b) Anapoe-C12E9 (sucrose gradient % peak areas: 64% dimer/36% monomer; affinity column % peak areas: 20% aggregate/59% dimer/21% monomer) and (d) BigCHAP (sucrose gradient % peak areas: 82% dimer/18% monomer; affinity column % peak areas: 20% aggregate/66% dimer/14% monomer).
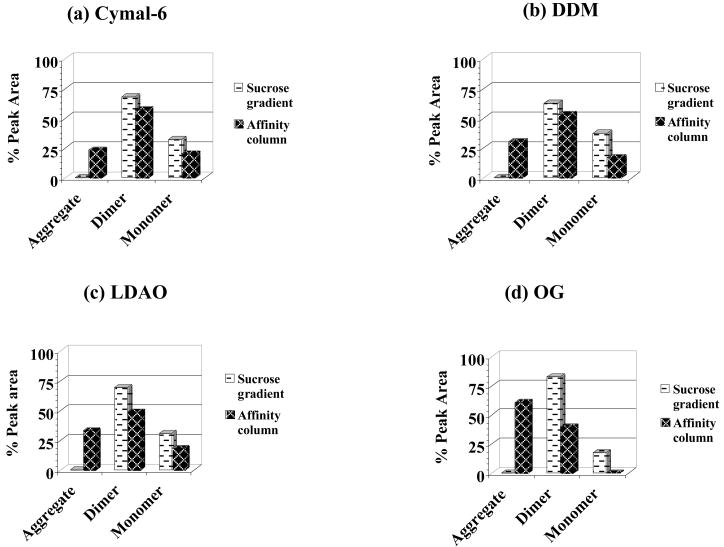
Figure 4. nAChR stability assays for non-lipid analog detergents.
A-SEC stability assay percent (%) peak areas for sucrose gradient detergent-solubilized membranes (white background) and ligand affinity-purified nAChR (black background) in: (a) Cymal-6 (sucrose gradient areas % peak areas: 68% dimer/32% monomer; affinity column % peak areas: 23% aggregate/57% dimer/20% monomer); (b) DDM (sucrose gradient % peak areas: 63% dimer/37% monomer; affinity column % peak areas: 30% aggregate/53% dimer/17% monomer), (c) LDAO (sucrose gradient % peak areas: 69% dimer/31% monomer; affinity column % peak areas: 33% aggregate/49% dimer/18% monomer) and (d) OG (sucrose gradient % peak areas: 83% dimer/17% monomer; affinity column % peak areas: 61% aggregate/39% dimer/0% monomer).
Based on the overall similarity of the observed experimental results, detergents were grouped in three categories: Lipid-analog detergents (Figure 5a) including sodium cholate, CHAPS and FC-12, detergents retaining residual amounts of native lipids (Figure 5b), including Anapoe-C12E9 and BigCHAP and non-lipid analog detergents, including Cymal-6, DDM, LDAO and OG(Figure 5c).
Figure 5. Detergent structures.
This figure shows the structures, CMC values and aggregation numbers obtained from the Anatrace catalog (www.anatrace.com) for the nine (9) detergents used in this study, grouped based on the results obtained in this study: (a) lipid-analog detergents, (b) detergents that retained residual amounts of native lipid and (c) non-lipid analog detergents.
Lipid-analog detergents: sodium cholate, CHAPS and FC-12
Despite their structural differences, these three detergents showed remarkably similar results in all categories. Figures 2a, c and e show planar bilayer ion channel traces for sodium cholate, FC-12 and CHAPS, with clearly-defined ion channel opening-closing events and electrophysiological data comparable to previous studies in native and detergent-solubilized membranes (Schindler, H., Quast, U., 1980, Nelson, N., et al., 1980 and Boheim, G., et al., 1981). These results suggest that despite significant lipid depletion upon affinity purification (Table 1), the receptor is fully functional in lipid-analog detergents (Table 2). A-SEC stability data for these detergents in Figures 2b, d and f showed aggregation following affinity column purification, ranging from a low of 7% observed for FC-12 to a high of 15% for CHAPS, indicating that the receptor was slightly destabilized upon purification, which is consistent with the observed lipid depletion.
Table 2.
Summary of results for planar lipid bilayer nAChR ion channel function assays.
| Detergent | Average number of processed events per- bilayer (N) | Channel current (mean ± SD) pA | Open channel time (mean ± SD) ms |
|---|---|---|---|
| Cholate | 214 | −(2.48 ± 0.03) | (3.0 ± 0.1) |
| CHAPS | 262 | −(2.69 ± 0.04) | (3.51 ± 0.04) |
| FC-12 | 285 | −(2.48 ± 0.02) | (2.52 ± 0.07) |
| Anapoe-C12E9 | 274 | −(1.77 ± 0.02) | (2.40 ± 0.03) |
| BigCHAP | 235 | −(1.76 ± 0.05) | (2.05 ± 0.05) |
| Cymal-6 | N/A | N/A | N/A |
| DDM | N/A | N/A | N/A |
| LDAO | N/A | N/A | N/A |
| OG | N/A | N/A | N/A |
-N/A means that Carb.-induced activity was not observed. For functional detergents, an average of three (3) bilayer experiments with durations of ~30 min. were performed. For detergents that did not display Carb.-induced activity, an average of six (6) bilayer experiments with durations of ~45 min. were performed. Histograms from which mean ion channel current and mean open channel time electrophysiological parameter values were obtained are shown as supplementary materials.
Detergent-dependent variations in the monomer-dimer ratios were observed upon comparison of purified versus detergent-solubilized nAChR-enriched membranes: FC-12 showed a slight increase in the dimer and a ~10% decrease in the monomer, sodium cholate showed a slight decreases in both species and CHAPS showed a ~20% decrease in the dimer and a slight increase in the monomer. EM data (Figures 6a, c and e) was partially consistent with A-SEC, with clearly-visible nAChR molecules in both sodium cholate and FC-12 while considerable aggregation was apparent for CHAPS. SDS-PAGE gels in Figures 6b, d and f show that the nAChR is mostly pure in all three detergents, showing the characteristic four-band pattern with minimal contaminants, especially in sodium cholate.
Figure 6. nAChR EM and SDS-PAGE assays of affinity-purified samples for lipid-analog detergents.
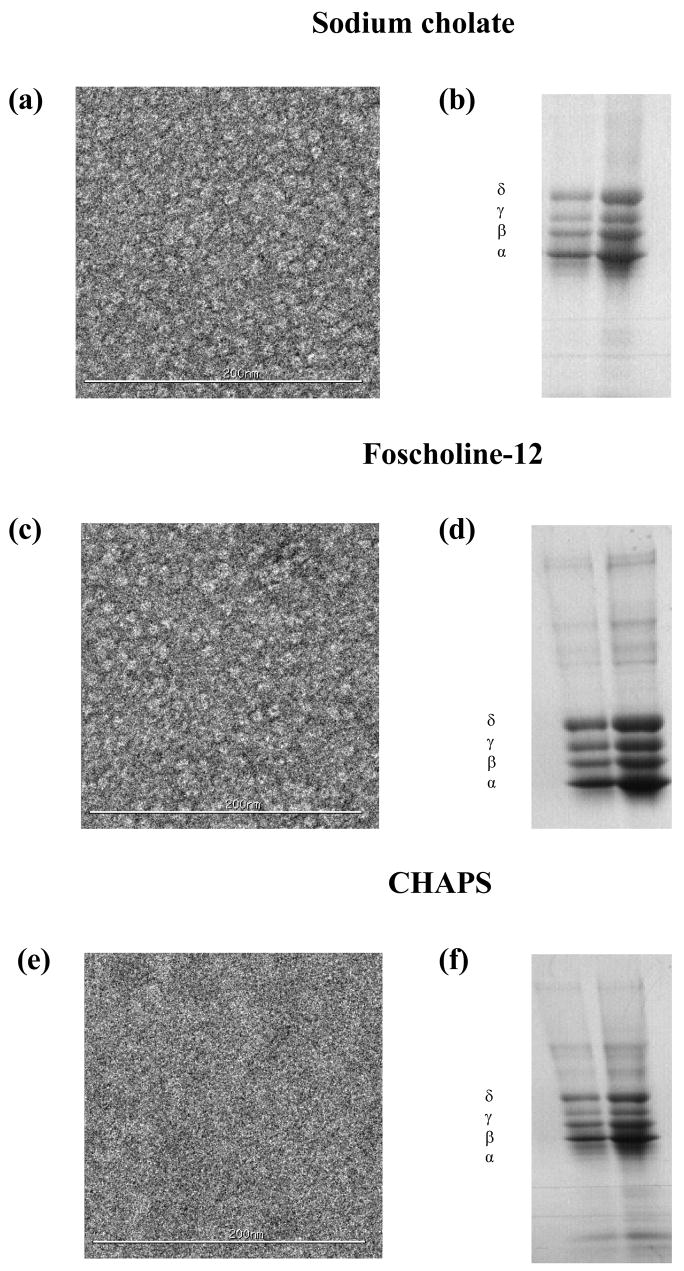
Negative-stain EM (left panels) for: (a) Sodium cholate, (c) FC-12 and (e) CHAPS. SDS-PAGE gels (right panels) for: (b) Sodium cholate, (d) FC-12 and (f) CHAPS.
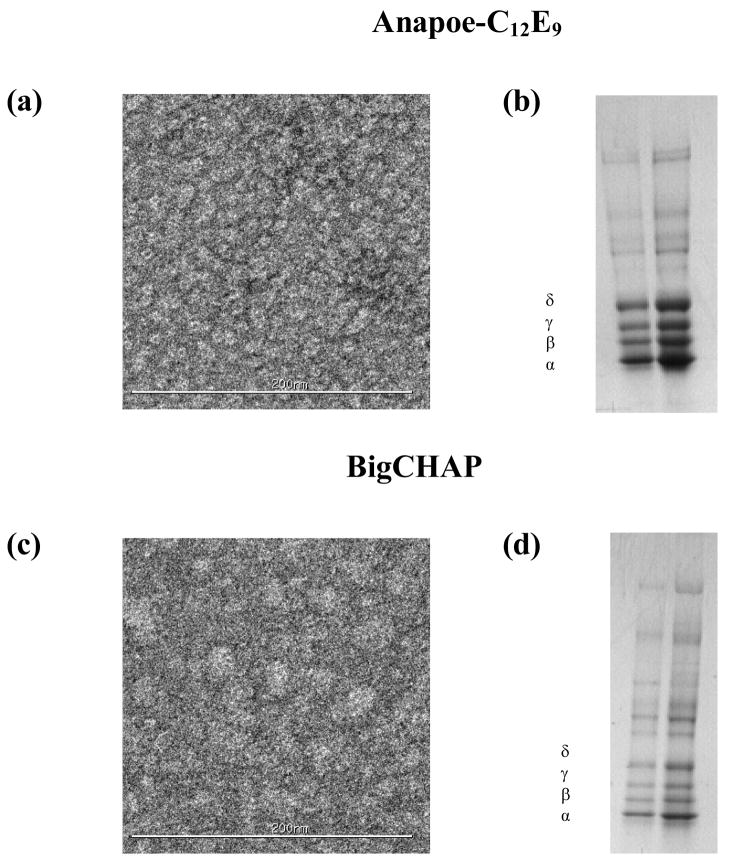
Detergents that retained higher residual native lipid amounts: BigCHAP and Anapoe-C12E9
These detergents retained noticeably higher residual amounts of native lipids after purification when compared to other detergents, as shown in Table 1. Figures 3a and c show single channel currents for BigCHAP and Anapoe-C12E9, with less well-defined ion channel opening-closing events than those for lipid analog detergents. This translated into lower single channel conductance and open channel time values, especially for BigCHAP, while the number of events per-bilayer experiment in both detergents was similar to those for lipid-analog detergents (Table 2). A-SEC stability data showed noticeable increases in the amount of aggregation for both detergents upon purification (Figures 3b and d), which were ~5–13% higher than those observed for lipid-analog detergents, indicating lower stability despite the slightly higher amounts of residual native lipid, which correlates with the observed reduction in ion channel function. Similar to lipid analog detergents, these detergents showed a mixture of monomer-dimer species upon detergent solubilization of the membranes that was altered upon nAChR purification. Anapoe-C12E9 showed a ~5% decrease in the dimer and a ~15% decrease in the monomer while BigCHAP showed the same percentage reductions but with dimer at ~15% and the monomer at ~5%. Figures 7a and c shows EM data for both detergents, with individual nAChR pentamers visible in Anapoe-C12E9, similar to sodium cholate and FC-12, while BigCHAP shows considerably more aggregation, similar to CHAPS. SDS-PAGE gels in Figures 7b and d show noticeably more contaminants than what was observed for lipid-analog detergents, especially for BigCHAP, perhaps as a result of the residual native lipids, but the characteristic nAChR bands are still observable.
Figure 7. nAChR EM and SDS-PAGE assays of affinity-purified samples for detergents retaining residual amounts of native lipid.
Negative-stain EM (left panels) for: (a) Anapoe-C12E9 and (c) BigCHAP. SDS-PAGE gels (right panels) for: (b) Anapoe-C12E9 and (d) BigCHAP.
Non-lipid analog detergents: Cymal-6, DDM, LDAO and OG
Native lipid depletion in these detergents was comparable to lipid-analog detergents (Table 1), but ion channel function was completely suppressed following nAChR purification (Table 2). A-SEC stability assays for these detergents in Figures 4a, b, c and d showed similar effects on monomer-dimer ratios upon detergent solubilization of the membranes as observed for other detergents. Following affinity column purification and native lipid depletion however, significant increases in aggregation were observed, higher than all of the other detergents in the study. Cymal-6 showed the lowest aggregation with ~23%, similar to Anapoe-C12E9 and BigCHAP while DDM, which is structurally similar to Cymal-6, showed ~30%. LDAO showed ~33% aggregation, slightly higher than DDM and OG had the highest aggregation of all detergents at ~60%, nearly double that of the other non-lipid analog detergents. Like the other detergents, alterations in the monomer-dimer ratios were observed upon purification, with Cymal-6, DDM and LDAO showing nearly identical reductions of ~10% in dimer and 20% in the monomer, while OG showed ~40% reduction in the dimer and a total depletion of the monomer. EM data in Figures 8a, c, e and g show clearly visible pentamers in both Cymal-6 and DDM, while significant aggregation was apparent in both LDAO and OG. SDS-PAGE gels in Figures 8b, d, f and h show minimal contaminants with the characteristic nAChR band pattern, comparable to FC-12 and CHAPS and a marked improvement over Anapoe-C12E9 and BigCHAP, but not as clean as that observed for sodium cholate.
Figure 8. nAChR EM and SDS-PAGE assays of affinity-purified non-lipid analog detergents.
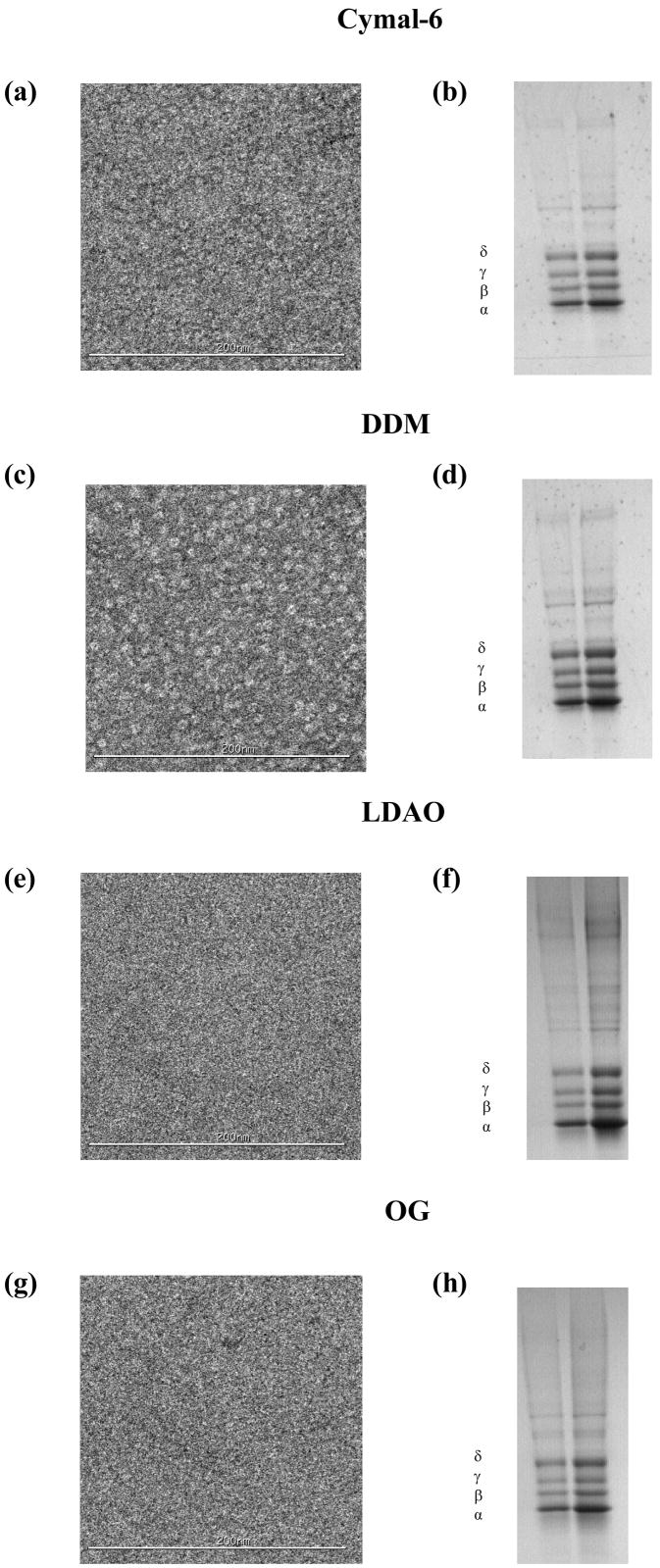
Negative-stain EM (left panels) for: (a) Cymal-6, (c) DDM, (e) LDAO and (g) OG; SDS-PAGE gels (right panels) for: (b) Cymal-6, (d) DDM, (f) LDAO and (h) OG.
Discussion
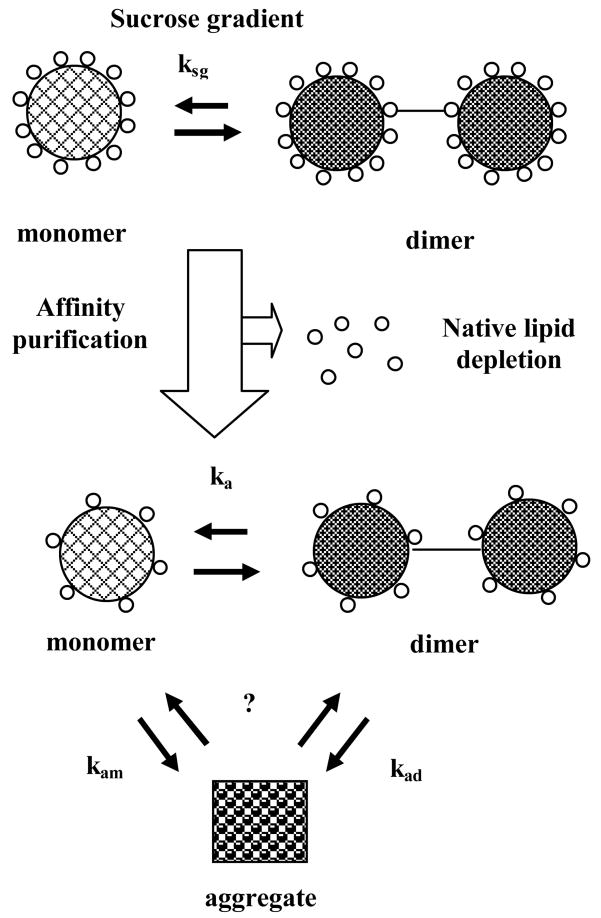
Three possible experimental outcomes were observed upon nAChR purification: (A) stable and functional nAChR in lipid-analog detergents despite native lipid depletion, (B) moderately stable and functional nAChR in detergents that retained residual amounts of native lipids and (C) unstable and non-functional nAChR in non-lipid analog detergents. We propose an equilibrium model to explain these results (Figure 9), where ksg (sucrose gradient constant) governs the monomer-dimer equilibrium for nAChR-enriched sucrose gradient detergent-solubilized membranes. Upon affinity purification and native lipid depletion, ka (overall affinity-purified nAChR constant) governs the monomer-dimer equilibrium, while kam (affinity-purified nAChR monomer constant) and kad (affinity-purified nAChR dimer constant) govern depletion of the monomer and dimer states, respectively, to form the aggregate. The reversibility of this aggregation remains to be established but the observed depletion of the dimer and monomer species in a detergent-dependent manner suggest that both nAChR species could aggregate, perhaps by different mechanisms. It is very likely that to some extent, aggregation could lead to partial or completely irreversible nAChR denaturation, as evidenced by the correlation between loss of ion channel function and increased aggregation.
Figure 9. Proposed nAChR monomer-dimer equilibrium scheme.
The figure describes a possible mechanism to explain the observed experimental results based on how the nAChR monomer-dimer equilibrium could be affected during affinity purification as a result of native lipid depletion.
Native lipid depletion upon receptor purification, a necessary step for structural studies, could expose the nAChR transmembrane domains to the aqueous buffer environment, leading to premature aggregation and loss of ion channel function, as observed for some detergents. The structural features of lipid-analog detergents could have allowed them to interact favorably with nAChR transmembrane domains, perhaps contributing to the ~20–45 moles of lipid per-receptor functional threshold (Jones, O.T., et al., 1988), which could have preserved receptor functionality in spite of lipid depletion. The comparatively low aggregation observed in A-SEC and EM except in CHAPS suggest that kam and kad were small (Figure 9), which indicates that these detergents would clearly be the best choice to maintain both stability and ion channel function upon purification while achieving a reasonably high level of purity, with their only drawback being the nearly total native lipid depletion.
Detergents retaining slightly higher amounts of native lipids showed intermediate A-SEC and functional assay results between the stable, functional nAChR in lipid-analog detergents and the destabilized, non-functional nAChR in non-lipid analog detergents, thus shifting kam and kad in Figure 9 towards the aggregate but not enough to cause loss of ion channel function. The presence of increased amounts of residual native lipids appears to upset the previously-observed structural effects in lipid- and non-lipid analog detergents in the absence of native lipids. Lipid-analog BigCHAP required ~2–4 times more detergent for membrane solubilization, which could cause partial denaturation, while its comparatively bulky functional group could have prevented favorable interactions with the nAChR transmembrane domains. The presence of residual native lipids in Anapoe-C12E9 appeared to allow this non-lipid analog detergent to partially compensate for the potential adverse effects of its non-lipid analog structure on nAChR stability and ion channel function. Anapoe-C12E9 and BigCHAP could also be used for purification, especially since they retain residual amounts of native lipid, but the observed reduction in ion channel function despite the presence of native lipids possibly points to potentially detrimental detergent-lipid-protein interactions, which in the end would make them less suitable than lipid-analog detergents.
Non-lipid analog detergent results showed significant increases in aggregation upon purification and native lipid depletion in both A-SEC (especially in LDAO and OG) and EM (except Cymal-6 and DDM), accompanied by a total loss of ion channel function. These results were consistent with the apparent inability of these detergents to effectively interact with residual lipids and shield potentially exposed nAChR transmembrane domains. This could possibly be due to structural constraints, indicating that kam and kad in Figure 9 were large, which would shift the equilibrium towards the aggregate state.
Previous studies with OG-solubilized crude membranes reconstituted in soybean lipid vesicles showed no ion channel activity (Anholt, R., et al., 1981). Direct functional measurements of detergent-solubilized membranes were not performed in our study due to the significant amount of protein incorporated into the bilayer membrane, which resulted in constant breakage of the membrane. However, the overall results suggest that since detergent-solubilized membranes did not show severe native lipid depletion or decreased stability in A-SEC, the nAChR should be stable and functional under these conditions. However, it is possible that non-lipid detergents could interact with other critical domains to suppress ion channel function, accounting for the previously-reported results.
On the other hand, our results showed that upon purification, native lipid depletion caused destabilization and loss of ion channel function, disagreeing with previously-observed results in OG-solubilized alpha-cobratoxin-purified nAChR reconstituted in native lipid vesicles, which showed ion channel activity (Paraschos, A., et al., 1982). This discrepancy in a sense highlights the potential advantages of our methodologies, which more closely resemble the actual detergent-solubilized environment where most structural studies would be performed. In our study, the protein remains in contact with the detergent at all times, as opposed to the complete detergent removal necessary for reconstitution in lipid vesicles, which may have reversed the detrimental effects of OG. It is clear that non-lipid analog detergents, at least the ones tested in this study, are not suitable for nAChR purification in the detergent-solubilized state because they completely suppress ion channel function upon purification. Certainly, the results for reconstituted OG-solubilized lipid vesicles (Paraschos, A., et al., 1982), as well as the results observed for Anapoe-C12E9 on this study, may suggest that the presence of exogenous or residual native lipids could restore or preserve ion channel function in non-lipid analog detergents. Perhaps addition of native or exogenous lipids during purification to non-lipid analog detergents could restore ion channel function and reduce aggregation in A-SEC, something that could be tested in a future study.
The overall results provide suitable methodologies for the study of detergent-lipid-protein interactions prior to membrane protein structural studies, while perhaps shedding light into some of the problems that have hampered nAChR structural biology over the past few decades. In particular, the fact that all of these techniques can be performed in the detergent-solubilized state could be extremely valuable for future structural studies because they provide a more accurate reflection of the detergent-lipid-protein interactions in the detergent solubilized state, a clear advantage over previous studies performed in native membranes or reconstituted lipid vesicles. The combination of A-SEC as a stability and sample aggregation state prediction tool, and planar lipid bilayers with the PMAL protein delivery system, as well as the more conventional EM, GC-FID and GC-MSD lipid analysis has provided valuable insight into the overall state of the sample in the detergent solubilized state which has not been previously available.
This study shows which detergents are appropriate for receptor purification, along with viable methodologies to allow functional and stability studies to be performed in the detergent-solubilized state. On the other hand, it demonstrates that the nAChR purified under these conditions is by no means in its optimal state for future structural studies and that significant challenges remain in terms of obtaining a homogenous, stable and functional sample. These problems would need to be addressed for future biophysical or structural studies and this study provides an important starting point to a roadmap for future nAChR biophysical or structural studies in the detergent-solubilized state. Through careful optimization of these variables using these techniques as probes, significant improvements could be obtained that may eventually lead to more successful structural studies. Overall, this study highlights the importance of probing detergent-lipid-protein interactions for future membrane protein biophysical and structural studies through the use of these or other methodologies, which could substantially increase current structural and functional knowledge of this important research field in the near future.
Supplementary Material
Acknowledgments
NIH (National Institutes of Health) grants R01GM056371, S06GM008102, SNRP U54NS043011 and UPR Institutional Funds for Research (to José A. Lasalde-Dominicci), the NIH-MBRS (Minority Biomedical Research Support) Research Initiative for Scientific Enhancement (RISE) grant R25GM061151 and the NIH Roadmap grant P50GM073197 (to Raymond C. Stevens) and Alliances for Graduate Education in the Professoriate (AGEP) and RISE fellowships (to Guillermo A. Asmar-Rovira).
References
- Anholt R, Lindstrom J, Montal M. Functional equivalence of monomeric and dimeric forms of purified acetylcholine receptors from Torpedo californica in reconstituted lipid vesicles. Eur J Biochem. 1980;109:481–487. doi: 10.1111/j.1432-1033.1980.tb04819.x. [DOI] [PubMed] [Google Scholar]
- Anholt R, Lindstrom J, Montal M. Stabilization of acetylcholine receptor channels by lipids in cholate solution and during reconstitution in vesicles. J Biol Chem. 1981;256:4377–4387. [PubMed] [Google Scholar]
- Bhushan A, McNamee MG. Differential scanning calorimetry and Fourier transform infrared analysis of lipid-protein interactions involving the nicotinic acetylcholine receptor. Biochim Biophys Acta. 1990;1027:93–101. doi: 10.1016/0005-2736(90)90053-q. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boheim G, Hanke W, Barrantes FJ, Eibl H, Sakmann B, Fels G, Maelicke A. Agonist-activated ionic channels in acetylcholine receptor reconstituted into planar lipid bilayers. Proc Natl Acad Sci USA. 1981;78:3586–3590. doi: 10.1073/pnas.78.6.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa CJ, Ogrel AA, McCardy EA, Blanton MP, Baenziger JE. Lipid-protein interactions at the nicotinic acetylcholine receptor. A functional coupling between nicotinic receptors and phosphatidic acid-containing lipid bilayers. J Biol Chem. 2002;277:201–208. doi: 10.1074/jbc.M108341200. [DOI] [PubMed] [Google Scholar]
- Dellisanti CD, Yao Y, Stroud JC, Wang ZZ, Chen L. Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 Å resolution. Nat Neurosci. 2007;10:953–962. doi: 10.1038/nn1942. Erratum in: Nat Neurosci 10:1222. [DOI] [PubMed] [Google Scholar]
- Fong TM, McNamee MG. Correlation between acetylcholine receptor function and structural properties of membranes. Biochemistry. 1986;25:830–840. doi: 10.1021/bi00352a015. [DOI] [PubMed] [Google Scholar]
- Hamouda AK, Sanghvi M, Sauls D, Machu TK, Blanton MP. Assessing the lipid requirements of the Torpedo californica nicotinic acetylcholine receptor. Biochemistry. 2006;45:4327–4337. doi: 10.1021/bi052281z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertling-Jaweed S, Bandini G, Muller-Fahrnow A, Dommes V, Hucho F. Rapid preparation of the nicotinic acetylcholine receptor for crystallization in detergent solution. FEBS Lett. 1988;241:29–32. doi: 10.1016/0014-5793(88)81024-x. [DOI] [PubMed] [Google Scholar]
- Jones OT, Eubanks JH, Earnest JP, McNamee MG. A minimum number of lipids are required to support the functional properties of the nicotinic acetylcholine receptor. Biochemistry. 1988;27:3733–3742. doi: 10.1021/bi00410a032. [DOI] [PubMed] [Google Scholar]
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- Karlin A, Akabas MH. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron. 1995;15:1231–1244. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Martínez KL, Gohon Y, Corringer PJ, Tribet C, Mérola F, Changeaux JP, Popot JL. Allosteric transitions of Torpedo acetylcholine receptor in lipids, detergent and amphipols: molecular interactions vs. physical constraints. FEBS Lett. 2002;528:251–256. doi: 10.1016/s0014-5793(02)03306-9. [DOI] [PubMed] [Google Scholar]
- McCarthy MP, Moore MA. Effects of lipids and detergents on the conformation of the nicotinic acetylcholine receptor from Torpedo californica. J Biol Chem. 1992;267:7655–7663. [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- Nelson N, Anholt R, Lindstrom J, Montal M. Reconstitution of purified acetylcholine receptors with functional ion channels in planar lipid bilayers. Proc Natl Acad Sci USA. 1980;77:3057–3061. doi: 10.1073/pnas.77.5.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa EL, Dalziel AW, McNamee MG. Reconstitution of acetylcholine receptor function in lipid vesicles of defined composition. Biochim Biophys Acta. 1983;727:151–162. doi: 10.1016/0005-2736(83)90379-6. [DOI] [PubMed] [Google Scholar]
- Paraschos A, González-Ros JM, Martínez-Carrión M. Acetylcholine receptor from Torpedo. Preferential solubilization and efficient reintegration into lipid vesicles. Biochim Biophys Acta. 1982;691:249–260. doi: 10.1016/0005-2736(82)90414-x. [DOI] [PubMed] [Google Scholar]
- Potter CS, Pulokas J, Smith P, Suloway C, Carragher B. Robotic grid loading system for a transmission electron microscope. J Struct Biol. 2004;146:431–440. doi: 10.1016/j.jsb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Schindler H, Quast U. Functional acetylcholine receptor from Torpedo marmorata in planar membranes. Proc Natl Acad Sci USA. 1980;77:3052–3056. doi: 10.1073/pnas.77.5.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürholz T, Kehne J, Gieselmann A, Neumann E. Functional reconstitution of the nicotinic acetylcholine receptor by CHAPS dialysis depends on the concentrations of salt, lipid, and protein. Biochemistry. 1992;31:5067–5077. doi: 10.1021/bi00136a020. [DOI] [PubMed] [Google Scholar]
- Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, Carragher B. Automated molecular microscopy: the new Leginon system. J Struct Biol. 2005;151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.