Abstract

The “reverse polarity” or “umpolung” strategy for the total synthesis of aryl C-glycosides was developed in the context of the antibiotic (−)-griseusin B. Although a key reaction in a model sequence for the total synthesis produced two structurally divergent products, both were converted to the same advanced model intermediate that contains the complete carbon skeleton and (except for the extraneous oxygen substituent in the model series) the functional group pattern of the griseusins.
(−)-Griseusin A (1a) and B (2a),1,2 their more recently isolated 4′-deacetyl derivatives 1b and 2b,3 and the more complex 3′-O-α-D-forosaminyl-(+)-griseusin A (3)4 are aromatic, polyketide derived antibiotics produced by the actinomycete strain Streptomyces griseus (Figure 1).
Figure 1.
(−)-Griseusin A and B, the 4′-Deacetyl Griseusins and 3′-O-α-D-Forosaminyl-(+)-Griseusin A.
The griseusins belong to the growing family of pyrano naphthoquinones that includes the well-known kalafungin, nanaomycins, medermycin, and granaticin.5 Members of this class display a variety of interesting biological activities. Moore and Czerniak proposed that the pyrano naphthoquinones including the griseusins act as bioreductive alkylation agents via quinone methide intermediates in a manner similar to that of the anticancer agent mitomycin C.6 The griseusins are active against gram-positive bacteria; the deacetyl griseusins 1b and 2b and the forosaminyl griseusin A 3 have demonstrated activity against methicillin-resistant Staphylococcus aureus (MRSA), a growing health problem.7
The pyrano naphthoquinones have attracted interest in both the biosynthesis/bioengineering8,9,10 and organic synthesis11 communities. Total syntheses of (+)-griseusin A2a and (+)-9-deoxy griseusin B2b and a synthesis of a mixture of protected (−)-griseusin A isomers have been reported.12
Our own work on the synthesis of the griseusins is one component of a broad program focused on the synthesis of naturally occurring aryl C- glycosides with a variety of substitution patterns on the aromatic aglycone.13 The oxidation pattern of the naphthyl C-glycoside moiety in the griseusins is the same as that in the mederrhodins,8b an arrangement that we have designated the group IV substitution pattern.14 The synthesis of one of these compounds by the “reverse polarity” or “umpolung” strategy would provide a demonstration of its power for the preparation of members of the group IV aryl C-glycosides.
Our retrosynthetic analysis of (−)-grieusin B (2a) suggested the elaboration of quinone glycal 4, envisioned as the Stille coupling product of bromo quinone glycal 5 with an appropriate stannane compound (Scheme 1). On the basis of our previous work, we anticipated that key intermediate 5 would be available from a dienone-phenol type rearrangement15 of quinol intermediate 6. Quinol 6, in turn, should be the product of regioselective addition16 of the lithiated reagent from a 4-deoxy glycal 817 to 2-bromo juglone derivative 7.
Scheme 1.
Retrosynthesis of (−)-Griseusin B (2a).
To test what appeared to be a reasonable but not directly precedented dienone-phenol rearrangement, we examined the behavior of the model quinol 9 (Scheme 2).16 In experiments intended to effect the protection of the tertiary hydroxyl group of this compound (TBDMSOTf, DIPEA, 0 °C, 4.5 hr),18 we had noticed that extended reaction times or higher temperatures led to the appearance of aromatized product 11. Indeed, when the intermediate silyl ether 10 was subjected to the silyl triflate reagent for 2 days at room temperature, the protected, rearranged naphthalenehydroquinone was isolated in 45% yield (not optimized).
Scheme 2.
Dienone-Phenol Rearrangement to the Group 4 Substitution Pattern of Aryl C-Glycosides.
Next, we investigated the 1,2-addition reaction that is the foundation of the “umpolung strategy” with 2-bromo juglone methyl ether (12).19 For these feasibility studies (Scheme 3), we used readily available rhamnal derivative 1320 as a model for the less accessible deoxy glycal 8. Thus, lithiated rhamnal 1421 was added to quinone 12. Quenching of the reaction with water at room temperature22 led to the isolation of an approximately 1:1 mixture23 of the expected diastereomeric mixture of quinol glycal 15 and the surprising but attractive quinone glycal 16. In the context of a synthesis of griseusins, the appearance of this functionalized quinone was potentially advantageous.
Scheme 3.
Lithiated Glycal Addition to 2-Bromo Juglone Methyl Ether.
The mechanism of formation of quinone 16 is not clear. It is presumably the result of air oxidation of the corresponding hydroquinone, formed by quenching the product of conjugate addition or of coupling of a radical anion/radical cation pair.24 In any case, attempts to convert adduct 15 to the rearranged and oxidized 16 under the conditions of the addition reaction were unsucessful.23 Although we were unable to alter the ratio of these two adducts in the product mixture, we found that this 1:1 mixture was consistently produced in combined yields of >80 %. Therefore we set out to discover the chemistry that would convert each of the products to the same advanced (model) intermediate.
We first examined the elaboration of quinone glycal 16. Stille coupling with vinyl stannane 1825 as proposed in our retrosynthetic analysis did not afford the desired compund 19 but, instead, a complex mixture of inseparable products (Scheme 4). This result is not surprising in light of the ease by which vinyl quinones undergo cyclization reactions under thermal and photochemical conditions and the inherent instability of the products of these conversions.26,27
Scheme 4.
Transformation of Quinone Glycal 16 to Advanced Intermediate 22.
Focusing again on progress toward the griseusins, we resorted to an indirect strategy that utilizes protected bromo hydroquinone substrates for the Stille coupling with vinyl stannanes.26 Thus, reduction of quinone glycal 16 yielded hydroquinone 20, which was directly protected as hydroquinone 21. Stille coupling of intermediate 21 with vinyl stannane 18 yielded protected vinyl hydroquinone glycal 22 in good yield. This readily available compound contains the complete carbon skeleton of the griseusins and, except for the extra hydroxyl equivalent on the dihydropyran ring, it is appropriately functionalized for completion of a total synthesis.
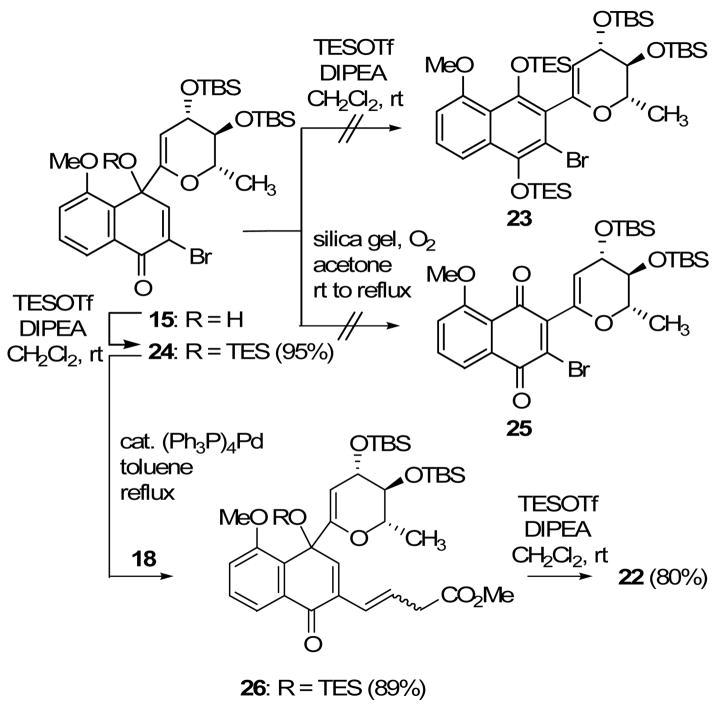
Next we turned our attention to the potential of quinol glycal 15 as a precursor for this same advanced model intermediate 22 (Scheme 5). In an attempt to effect the conversion of quinol 15 to intermediate 23, we subjected it to the TESOTf rearrangement conditions (Hunig’s base at room temperature, Scheme 2). However, this experiment yielded only the TES-protected quinol glycal 24. Next, we attempted the silica gel-promoted rearrangement of quinols as reported by Wigal.28 However, substrate 15 proved to be stable under the applied conditions and no rearranged product 25 was observed. These results are consistent with related observations that suggest that a bromo substituent retards a dienol-phenol rearrangement.29
Scheme 5.
Elaboration of Advanced Intermediate 22 from Quinol Glycal 15 by a Dienone-Phenol-Type Rearrangement.
In order to access a suitable and useful substrate for the dienone-phenol-type rearrangement, we converted TES-protected quinol 24 to protected quinol 26 by Stille coupling with vinyl stannane 18 (Scheme 5). When protected quinol 26 was treated with excess TESOTf and Hunig’s base at room temperature, it was cleanly converted to quinone 22, identical in all respects to the product derived from bromoquinone 16.
With this achievement, both quinol glycal 15 and quinone glycal 16 have been converted to advanced model intermediate 22, each in only three steps and each in good overall yield (approximately 70 %). This result provides an unusual example of the utility of two regioisomeric products, obtained from a single reaction mixture, for the preparation of the same, desired compound.
Despite the additional manipulations associated with processing two intermediates, only seven steps total are required to prepare a complex structure that contains the complete carbon skeleton and (except for the extraneous C-oxygen substituent in the model 22) the functional group pattern of the griseusins from a bromoquinone and a protected glycal.
Application of the divergent reconvergent approach to the total synthesis of (−)-griseusin B (2a) is currently being investigated. The modular assembly nature of the synthetic scheme suggests that it might be efficiently applied to the preparation of griseusin analogs for structure-activity-relationship (SAR) studies.
Supplementary Material
Detailed descriptions of the experimental procedures and complete analytical data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
The work described in this communication was supported by the National Institutes of Health (CA-87503). For a part of his graduate school career, T.L.M. was a Fellow of the Graduate Assistance in Areas of National Need program of the U.S. Department of Education. We thank Dr. Tun-Li Shen for the mass spectroscopic measurements.
References
- 1.(a) Tsuji N, Kobayashi M, Wkisaka Y, Kawamura Y, Mayama M, Matsumoto K. J Antibiot. 1976;29:7. doi: 10.7164/antibiotics.29.7. [DOI] [PubMed] [Google Scholar]; (b) Tsuji N, Kobayashi M, Terui Y, Tori K. Tetrahedron. 1976;32:2207. [Google Scholar]
- 2.For the elucidation of the absolute stereochemistry of (−)-griseusin A (1) and B (2) see Kometani T, Takeuchi Y, Yoshii E. J Org Chem. 1983;48:2311.Tsuji N, Kamigauchi T, Nakai H, Shiro M. Tetrahedron Lett. 1983;24:389.Kometani T, Takeuchi Y, Yoshii E. J Org Chem. 1982;47:4725.
- 3.Igarashi M, Chen W, Tsuchida T, Umekita M, Sawa T, Naganawa H, Hamada M, Takeuchi T. J Antibiot. 1995;48:1502. doi: 10.7164/antibiotics.48.1502. [DOI] [PubMed] [Google Scholar]
- 4.Maruyama M, Nishida C, Takhashi Y, Naganawa H, Hamada M, Takeuchi T. J Antibiot. 1994;47:952. doi: 10.7164/antibiotics.47.952. [DOI] [PubMed] [Google Scholar]
- 5.Brimble MA, Duncalf LJ, Nairn MR. Nat Prod Rep. 1999;16:267. doi: 10.1039/a804287j. [DOI] [PubMed] [Google Scholar]
- 6.Moore HW, Czerniak R. Med Res Rev. 1981;1:249. doi: 10.1002/med.2610010303. [DOI] [PubMed] [Google Scholar]
- 7.Aires de Sousa M, de Lencastre H. FEMS Immunol Med Microbiol. 2004;40:101. doi: 10.1016/S0928-8244(03)00370-5. [DOI] [PubMed] [Google Scholar]
- 8.See, for example, Taguchi T, Kunieda K, Takeda-Shitaka M, Takaya D, Kawano Ni, Kimberley MR, Booker-Milburn KI, Stephenson GR, Umeyama H, Ebizuka Y, Ichinose K. Bioorg & Med Chem. 2004;12:5917. doi: 10.1016/j.bmc.2004.08.026.Ichinose K, Ozawa M, Itou K, Kunieda K, Ebizuka Y. Microbiol. 2003;149:1633. doi: 10.1099/mic.0.26310-0. and references therein.Tang Y, Lee TS, Kobayashi S, Khosla C. Biochemistry. 2003;42:6588. doi: 10.1021/bi0341962.
- 9.For an early example of bioengineering in this series, see: Ômura S, Ikeda H, Malpartida F, Kieser HM, Hopwood DA. Antimicrob Agents Chemother. 1986;29:13. doi: 10.1128/aac.29.1.13.
- 10.Unlike the medermycin family and the granaticins which are glycosylated octaketides, the griseusins are decaketides: see Yu TW, Bibb MJ, Revill WP, Hopwood SA. J Bacteriology. 1994;176:2627. doi: 10.1128/jb.176.9.2627-2634.1994.
- 11.Review: Brimble MA, Nairn MR. Tetrahedron. 2000;56:1937.
- 12.Brimble MA, Nairn MR, Park JSO. Perkin Trans I. 2000:697. [Google Scholar]
- 13.Parker KA, Georges AT. Org Lett. 2000;2:497. doi: 10.1021/ol991346l. and references therein. [DOI] [PubMed] [Google Scholar]
- 14.Parker KA. Pure Appl Chem. 1994;66:2135. [Google Scholar]
- 15.(a) Goodwin S, Witkop B. J Am Chem Soc. 1957;79:179. [Google Scholar]; (b) Dodge JA, Chamberlin AR. Tetrahedron Lett. 1988;29:4827. [Google Scholar]
- 16.Parker KA, Coburn CA, Johnson PD, Aristoff P. J Org Chem. 1992;57:5547. [Google Scholar]
- 17.(a) Moilanen SB, Tan DS. Org Biomol Chem. 2005;3:798. doi: 10.1039/b417429a. [DOI] [PubMed] [Google Scholar]; (b) Wipf P, Graham TH. J Org Chem. 2003;68:8798. doi: 10.1021/jo034813s. [DOI] [PubMed] [Google Scholar]
- 18.Parker KA, Koh Y-h. J Am Chem Soc. 1994;116:11149. [Google Scholar]
- 19.Jung ME, Hagenah JA. J Org Chem. 1987;52:1889. [Google Scholar]
- 20.Paquette LA, Oplinger JA. Tetrahedron. 1989;45:107. [Google Scholar]
- 21.Boeckman RK, Jr, Bruza KJ. Tetrahedron Lett. 1977;48:4187. The success of the lithiation of glycal 13 was verified by quenching samples of the reaction mixture with D2O and NMR analysis of the product. Integration of the diminished signal of the vinyl proton indicated >90 % lithiation of substrate 13
- 22.Addition of water at low temperature (−78 °C) resulted in the formation of a mixture of quinol 15 and quinone 17 in which the bromo substituent had been lost. In this experiment, the isolated yields of 15 and 17 were 35% and 37% respectively.
- 23.The ratio of the two products 15 and 16 could not be influenced by altering the reaction conditions. The same 1:1 mixture of adducts in varying yields was isolated from experiments at different temperatures (−100 °C to 0 °C) and after extended reaction time (warming to rt over 8 h), in different solvents (THF, Et2O, t-BuOMe) and at different concentrations of quinone 12 (0.1 M or 20 mM).
- 24.Wigal CT, Grunwell JR, Hershberger J. J Org Chem. 1991;56:3759. [Google Scholar]
- 25.Presumably a cis/trans mixture; the NMR spectra of this compound are complex because of coupling with the tin nucleus. For the preparation of vinyl stannane 18 see: Collins PW, Kramer SW, Gasiecki AF, Weier RM, Jones PH, Gullikson GW, Bianchi RG. J Med Chem. 1987;30:193. doi: 10.1021/jm00384a032.
- 26.Parker KA, Mindt TL. Org Lett. 2001;3(24):3875. doi: 10.1021/ol0167199. [DOI] [PubMed] [Google Scholar]
- 27.Iwamoto H, Takuwa A, Hamada K, Fujiwara R. J Chem Soc Perkin Trans 1. 1999:575. [Google Scholar]
- 28.Aponick A, Buzdygon RS, Tomko RJ, Fazal AN, Shughart EL, McMaster DM, Myers MC, Pitcock WH, Wigal CT. J Org Chem. 2002;67:242. doi: 10.1021/jo016071d. [DOI] [PubMed] [Google Scholar]
- 29.Bordwell FG, Wellman KM. J Org Chem. 1964;29:509. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed descriptions of the experimental procedures and complete analytical data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.