Abstract
As antifungal agents are frequently used in hematology and oncology, economic data on the empirical therapy of suspected systemic fungal infection are pivotal. Data were analyzed according to: (1) the rate of nephrotoxicity related to treatment with caspofungin in comparison to liposomal amphotericin B (L-AmB) from a randomized clinical trial, (2) the effect of nephrotoxicity on length of hospital stay from a European observational study, and (3) an example of total bottom-up cost in a department of hematology in Germany. All estimates include 95% confidence intervals (CI) using two-stage Monte Carlo simulation on binominal and Gaussian random variables from separate studies with comparable populations. Overall, 8.9 (95% CI 5.9–12.1) fewer patients (of 100 randomized) experienced worsening of renal function with caspofungin vs L-AmB, giving a number needed to treat for one patient to be harmed by L-AmB of 12 (95% CI 8–17). This was estimated to translate into 5.3 extra days in hospital (95% CI 1.6–9.1) per event or 0.48 days (95% CI 0.14–0.88) worth €298 (95% CI 89–554) per patient receiving L-AmB rather than caspofungin. From the hospital perspective, use of caspofungin was estimated to be cost-neutral compared to L-AmB at a per diem total hospital cost of €428 with, and €1284 without, consideration of supplementary reimbursement (Zusatzentgelt) of both L-AmB and caspofungin. The data presented in this scenario show that use of caspofungin in hematology–oncology departments in Germany results in shorter hospital stays and is at least cost-neutral compared to use of L-AmB.
Keywords: Economic evaluation, Caspofungin, Liposomal amphotericin B, Bottom-up study, Nephrotoxicity
Introduction
Patients with hematological disorders are at considerable risk for systemic fungal infections [1–3]. In particular, infections caused by organisms of the genera Aspergillus and Candida are an increasingly recognized threat to immunocompromised patients. This results in a high burden to the European healthcare system [1–4].
Early clinical diagnosis of invasive fungal infection is difficult due to the non-specific clinical picture and the plethora of risk factors [3]. Microbiological challenges include availability of tissue, cytology and cultures, and/or non-specific microbiological culture results [1]. Radiological confirmation of diagnosis can be delayed due to the limitations of chest radiographs, and the specificity of CT scans can be limited [5, 6].
To overcome these issues, the concept of empirical therapy of suspected fungal infection has been established for patients at risk of infection who show persistent fever of unknown origin despite broad-spectrum antibiotic therapy [3].
At present, liposomal amphotericin B (L-AmB) and caspofungin are approved for empirical therapy of suspected fungal infection. In a randomized controlled trial (RCT) in patients with persistent fever and neutropenia, the efficacy of caspofungin was recently found to be non-inferior to that of L-AmB [7]. In addition, caspofungin was found to be far better tolerated than L-AmB in terms of nephrotoxicity (2.6 vs 11.5%; p < 0.001) [7]. The occurrence of nephrotoxicity in association with liposomal and other formulations of AmB has been shown to prolong the length of hospital stay in Europe by an average of 5.3 days [8]. No concrete economic data are available as yet on the financial impact of this on hospitals that treat these patients [4]. However, now that reimbursement of services in different countries is increasingly being based on diagnostic-related groups (DRGs), the economic impact on the economics of less tolerable agents on hematology–oncology departments could be considerable.
The objective of the study was to evaluate the bottom-up costs and the clinical consequences of empirical therapy of suspected systemic fungal infection from the perspective of a department of hematology of a German tertiary care hospital.
Materials and methods
Summary of clinical trials
Head-to-head trial caspofungin vs L-AmB
In a recently published double-blind RCT performed in hematology–oncology patients with persistent fever and neutropenia, the efficacy of caspofungin was found to be non-inferior to that of L-AmB [7]. In this trial, 556 patients received caspofungin and 539 received L-AmB (mean duration, 13 and 12.5 days, respectively). In most of the patients, the underlying condition was acute myeloid leukemia (AML), diagnosed in 364 caspofungin patients (65.5%) and 339 L-AmB patients (62.9%). The primary efficacy endpoint, a favorable overall response, was achieved by 33.9% of patients in the caspofungin group and 33.7% in the L-AmB group (95.2% CI −5.6, 6.0) [7]. Nephrotoxicity [defined as doubling of the serum creatinine level or, if the serum creatinine level was elevated at enrolment, an increase of at least 1 mg per deciliter (88 μmol per liter)] formed part of the safety analyses. There was less nephrotoxicity (2.6% of patients treated with caspofungin vs 11.5% of patients treated with L-AmB, p < 0.001) [7].
European study on use of various formulations of amphotericin B
Very recently, data of a prospective longitudinal evaluation of antifungal drugs (LEAD) study performed in 418 European adults with suspected, probable, or proven invasive fungal infection with various formulations of AmB have been published [8]. In the majority of cases, the underlying disease was leukemia or lymphoma (81%). Length of hospital stay was significantly prolonged (5.3 days) in patients who developed nephrotoxicity (creatinine value in the peripheral blood ≥1.5 times greater than baseline) while on AmB formulations [8].
Direct cost of hospital stay in a department of hematology in Germany
The background for the economic analysis was a bottom-up study to investigate hospital care (including day clinic) costs in all patients aged 16 to 60 years with AML receiving standard chemotherapy at the hematology unit, First Department of Medicine, Bonn University, a German tertiary care hospital in 2002. Demographic variables, information on disease status and length of hospital stay, and direct medical and non-medical costs were documented retrospectively by chart review. Components of the per diem costs relating to housing and general charges were combined with more precise calculation of the medical treatment costs associated with individual patients [9, 10]. The cost of a day of institutional care was calculated on the basis of the annual case-load of the entire institution or of the individual department, where applicable. The following depleted resources were identified, measured, and valued in descriptive fashion (Table 1) [11]: (1) medical care by healthcare professionals and its associated cost; (2) medication and blood product cost, comprising individual drugs, other medication, and transfusions; and (3) other costs incurred by transportation, materials, medical infrastructure, diagnostic procedures in microbiology, and housing. The direct bottom-up cost per day of hospital stay was calculated by dividing the total direct medical cost (in €) by mean total length of hospital stay (in days). For certain direct medical and non-medical resource consumption items, proxies were estimated based on German medical flat rates, as no bottom-up cost data were available [11]. For the present analysis, patients were censored in the event of treatment change due to transfer, treatment failure, or change in AML therapy.
Table 1.
Breakdown of direct costs (€) from a bottom-up study of 71 hospital stays (20 patients with acute myeloid leukemia) in a hematology–oncology tertiary care hospital ward in 2002
| (All cost in €) | Mean | SE | 95% CI | Median |
|---|---|---|---|---|
| Staff | ||||
| Medical carea | 1,386 | 60 | 1,266–1,506 | 1,299 |
| Nursing carea | 3,153 | 147 | 2,860–3,446 | 2,843 |
| Medication/blood products | ||||
| Individual drugsb | 5,999 | 503 | 4,995–7,002 | 4,067 |
| Other drugsc | 449 | 20 | 410–489 | 421 |
| Transfusiond | 1,666 | 131 | 1,405–1,927 | 1,092 |
| Transplantation | 1,331 | 224 | 883–1,778 | 0 |
| Other | ||||
| Materialse | 720 | 37 | 647–793 | 660 |
| Medical infrastructuref | 1376 | 60 | 1,257–1,496 | 1,290 |
| Microbiology | 589 | 24 | 542–636 | 572 |
| Base costg | 1,806 | 79 | 1,649–1,963 | 1,692 |
| Total | 19,039 | 998 | 17,050–21,029 | 16,067 |
SE Standard error; 95% CI 95% confidence interval; calculated with SAS PROC SURVEYMEANS
aIncluding ward stand-by duty, temporary help, and extra hours
bDrugs ordered for the individual patients ≥1€ based on ward total pharmacy consumption
cFlat rate for “ward requirement” drugs and drugs <1€
dIncluding blood substitutes
eAll patient-related medical ward supplies
fAll non-patient-related medical ward supplies
gHousing and general service
Cost of antifungal agents in the German hospital setting in 2007
Drug costs [including 19% value added tax (VAT)] for a patient with a weight of 70 kg were calculated based on official list prices and high-user hospital pharmacy drug acquisition cost for caspofungin and L-AmB, respectively. The calculations were based on the dosing scheme used in the RCT [7], i.e., caspofungin 70 mg on day 1 followed by caspofungin 50 mg for 12 days, or L-AmB 3 mg/ kg bodyweight for 12.5 days.
Reimbursement of antifungal agents in the German hospital setting in 2007
In 2005, two types of reimbursement were introduced into the German hospital reimbursement system: Besides DRG-based reimbursement and independently of clinical complexity, certain drugs that cannot be allocated to a single DRG but typically used in a tertiary care hospital setting are covered by the system. The actual reimbursement depends on the total amount of the respective medicine used for the individual patient. This ‘supplementary reimbursement’ (Zusatzentgelt) scheme, based on the average high-user hospital pharmacy drug acquisition costs (including VAT), is adjusted annually. Each year, the individual hospital has to negotiate the amount of this reimbursement with the statutory health insurance funds (Krankenkassen). As this supplementary reimbursement forms part of the overall budget of the hospital, its value to the individual hospital is not yet clear. On the other hand, individual departments may benefit greatly from the scheme, as medications covered are reimbursed in addition to DRGs and an increase in overall budget is therefore possible. In 2007, both caspofungin and L-AmB were included in this scheme [12]. In 2007, the reimbursement per stay in hospital for a total dose of L-AmB of between 2,150 and 3,150 mg is €4,744.70, while that for a total dose of caspofungin of between 600 and 700 mg is €5,150.90.
Number needed to treat for one patient to be harmed
Laupacis et al. [13] introduced the concept of the number needed to treat (NNT) to observe one less adverse event. NNT is the reciprocal of the absolute risk reduction. Based on the outcome of the original RCT [7], we calculated the NNT for one patient to be harmed for nephrotoxicity. To assess uncertainty of the estimation, a 95% confidence interval was obtained by inverting and exchanging the confidence limits for the absolute risk reduction as described by Bender according to Wilson [14].
Health economic evaluation
Perspective
The analysis assessed the direct medical and non-medical costs of resource utilization attributable to prolongation of hospital stay due to L-AmB-related nephrotoxicity. Responsibility for managing hematology–oncology patients with invasive fungal infections lies primarily with the tertiary care sector. Therefore, the perspective of the tertiary care sector, rather than that of German statutory health insurance or society, was chosen for the present analysis.
Economic analysis framework
Our evaluation of costs and its consequences is based on (1) difference in nephrotoxicity rates in the RCT [7]; (2) data on prolongation of hospital stay due to AmB and lipid formulation-related nephrotoxicity in hematology–oncology patients in Europe [8]; (3) cost of antifungal agents [15]; and (4) bottom-up data on direct cost of hospital stay per day in hematology–oncology in Germany. All costs were adjusted for inflation and calculated in 2007 euros [16]. As the evaluation focuses on short-term cost and consequences with immediate benefits, economic analyses were undiscounted. Calculations (for a 70-kg patient; see Table 2) were based on patient-individualized doses per treatment episode per treatment arm of the RCT [7], the official German price list [15], and German high-user hospital pharmacy drug acquisition cost including 19% VAT (provided by German university hospital pharmacists).
Table 2.
Data input for the calculations
| Parameter | Caspofungin group | Liposomal amphotericin B groupa |
|---|---|---|
| Nephrotoxicity riskb | 2.6%c (95% CI 1.55–4.24; n = 547) | 11.5%c (95% CI 9.05–14.53; n = 522) |
| Absolute risk differencec | −8.9% (95% CI 5.9–12.0) | |
| Treatment days per stayc | 13 | 12.5 |
| Drug cost according to official German price list (2007, incl. 19% VAT) | ||
| Per bottle, 70 mg | €799.57d | – |
| Per bottle, 50 mg | €630.68e | €209.43 |
| Per day of treatment | (see above) | €879.61 |
| Total | €8,368 | €10,995 |
| Drug cost according to high-user hospital pharmacy drug acquisition cost (2007, incl. 19% VAT) | ||
| Per bottle, 70 mg | €559.30 (day 1) | – |
| Per bottle, 50 mg | €428.40 (days 2–13) | €96.99 |
| Per day of treatment | (see above) | €407.34 |
| Total | €5,700 | €5,092 |
| Supplementary reimbursement (Zusatzentgelt, 2007) | ||
| Treatment-related code (mg range applicable) | 39.12 (600 mg–<700 mg) | 43.16 (2,150 mg–<3,150 mg) |
| Supplementary reimbursement (ZE) | €5,151 | €4,745 |
ZE Zusatzentgelt, a treatment-related extra reimbursement scheme that is paid besides DRG for medicines and devices that cannot be allocated to a single DRG. In 2007, L-AmB as well as caspofungin are covered under this scheme.
aLiposomal amphotericin B, 3 mg per kg bodyweight per day, 70-kg patient
bCaspofungin vs liposomal amphotericin B
cData from the original publication
dDay 1
eDays 2–13
Probabilistic sensitivity analysis for health economic evaluation
Modifying the work done by Spiegelhalter et al. [17], a two-stage Monte Carlo simulation on binominal and Gaussian random variables from separate studies with comparable populations was performed. Stage 1 included (1) simulation of the binominal distribution of the initial risk of nephrotoxicity due to L-AmB and caspofungin use, respectively [7], followed by simulation of absolute risk reduction and (2) simulation of the density of another normally distributed random variable ‘prolonged length of hospital stay due to L-AmB use’ [8]. In stage 2, ‘cost of hospital stay per day in hematology–oncology’ (gamma distributed random variable) and ‘high-user hospital pharmacy drug acquisition cost of the respective antifungal agent’ (constant) were added to the model. Freely available WinBUGS 1.4.1 software based on SAS 9.1.3 was used to calculate mean, standard error, median, confidence intervals [18, 19], and the probability of cost savings [20]. The per diem total hospital costs at which the use of caspofungin were estimated to be cost-neutral compared to L-AmB were obtained from the inverse of the extra days in hospital due to nephrotoxicity multiplied by the difference in cost for caspofungin and L-AmB before and after accounting for their supplementary reimbursement [19]. Results obtained by WinBUGS were imported into SAS and formatted and processed into SAS data sets to produce tables [21].
Results
Number needed to treat for one patient to be harmed
The NNTs for one patient to be harmed by nephrotoxicity for L-AmB vs caspofungin from the RCT [7] was 12 (95% CI 8–17).
Direct cost of hematology–oncology stay in a tertiary hospital in Germany
Seventy-one hospital stays (mean, 34 days; minimum, 1 day; and maximum, 96 days) of 20 patients with AML treated between 2001 and 2003 were included. Overall staff times and associated costs, medication and blood product costs, and other costs are shown in Table 1. Treatment cost for AML at Bonn university also included costs for induction and consolidation therapy. Patients undergoing auto-SCT are included. The direct bottom-up cost per hospital stay per day in the hematology unit at Bonn University Hospital, Germany was estimated at €626 (95% CI 550–702), equivalent to €587 in 2002 (95% CI €516–€658).
Overall medical care cost consisted of one physician (one full time equivalent/FTE), two interns (two FTE), and two consultants (0.45 and 0.05 FTE). Night duties and extra hours were included. Cost of nursing care, in Germany traditionally characterized by cost per nursing staff regulation minute, was €0.51/min. Total direct cost of autologous SCT was €20,679 (2002). This includes materials (€4,154), stem cell mobilization and separation (€10,744), preparatory steps before re-infusion (€5,239), and quality assurance measures (€542). In 2002, the total number of hospitalizations in this hematology ward at Bonn university was 338, and the total number of myelosuppressive chemotherapy cycles was 154, respectively.
As access to the cost of some medical service units of the hospital was limited, the following costs were estimated based on German medical flat rates [11]: (1) medical services, including electrocardiograms and X-rays, mean €265 (95% CI 230–301); (2) consultancy fees (internal calculation), mean €25 (95% CI 20–31); (3) hematology lab, mean €185 (95% CI 169–201); (4) immunology lab, mean €40 (95% CI 27–52); and (5) other lab services, mean €323 (95% CI 275–370).
Incremental cost due to nephrotoxicity
Based on the data from the RCT [7] and the LEAD study [8], the nephrotoxicity-related prolongation of hospital stay per patient was 0.48 days (95% CI 0.14–0.88). Based on the bottom-up cost data presented in this paper, this results in an additional cost of €298 (2007; 95% CI 89–554) for each patient treated with L-AmB.
Based on the official German price list (2007; including 19% VAT) [15], the difference in cost of medication is €2,627 in favor of caspofungin (see Table 2).
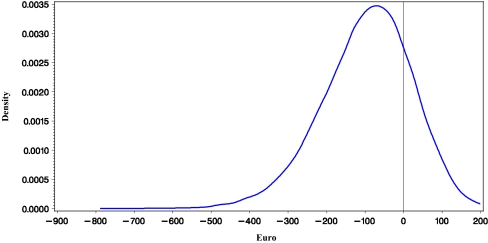
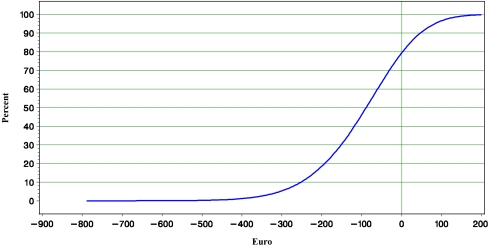
Based on high-user hospital pharmacy drug acquisition cost (2007; including 19% VAT), the difference in cost of medication is €608 in favor of L-AmB. With consideration of supplementary reimbursement (Zusatzentgelt), at a direct cost per day of hospital stay of €428 (95% CI €162; €809) or more the incremental cost due to L-AmB-related nephrotoxicity [7] offsets the higher frequent user acquisition cost of caspofungin. Without consideration of supplementary reimbursement (Zusatzentgelt) of both L-AmB and caspofungin, respectively, this figure was ≥€1,284 (95% CI €479; €2,449). Based on the point estimates and variances of the costing study presented in this paper, the cost savings per patient treated with caspofungin compared to L-AmB is estimated at €96, ranging from savings of €352 up to incremental cost of €113, with 95% confidence (see Table 3). The probability of savings was 79% (Figs. 1 and 2).
Table 3.
Bayesian model outputs
| Parameter | Caspofungin | Liposomal amphotericin B | Differencea |
|---|---|---|---|
| Analysis of the original head-to-head trial | |||
| NNT for one patient to be harmed | – | 12 (95% CI 9–17) | n. a. |
| Additional stay per treated patient per NNT for one patient to be harmed | |||
| Bootstrap calculation; days | – | 0.48 (95% CI 0.14–0.88) | n. a. |
| Cost per additional stay per patient per day due to amphotericin B-related nephrotoxicity | |||
| At direct cost of €626 per day | – | €298 (95% CI €89–€554) | n. a. |
| Total cost at cost per day of hospital stay of €626 | |||
| Drug cost (incl. 19% VAT) | €5,700 | €5,092 | €608 |
| Incremental cost due to nephrotoxicity | - | €298 (95% CI €89– €554) | −€298 (95% CI −€554–−€89) |
| ./. Supplementary reimbursement | €5,151b | €4,745c | −€406 |
| Cost | €549 | €645 (95% CI €436–€901)* | −€96 (95% CI −352–113)d |
aCost of caspofingin./.cost of L-AmB
bSupplementary reimbursement ZE 39.12
cSupplementary reimbursement ZE 43.16
dIn favor of caspofungin. Probability that total difference is ≤€0 is 79% (see Fig. 2).
Fig. 1.
Density function of the cost difference between using caspofungin and L-AmB in hematology–oncology in Germany
Fig. 2.
Cumulative probability of the cost-difference between using caspofungin and L-AmB in hematology–oncology in Germany
Discussion
Systemic fungal infections are a major cause of mortality and morbidity in patients with hematologic diseases and persistent neutropenia [1–3]. Infections caused by organisms of the genera Aspergillus and Candida constitute a challenge for physicians responsible for the care of these patients [2–4]. The studies from which our calculations are based [7, 8] and the cost study presented in this paper were performed in comparable populations at high risk for systemic fungal infections. Both the products under discussion are approved for use in the indication under consideration.
The RCT upon which the calculations are based [7] showed the efficacy of caspofungin to be non-inferior to that of L-AmB. As caspofungin was better tolerated than L-AmB [7], the present analysis focuses on the most clinically relevant tolerability endpoint, which, according to the literature, is the nephrotoxicity associated with antifungal agents [7, 8, 22, 23].
According to the international literature on this subject, the cost of AmB-associated nephrotoxicity in hematology–oncology patients amounts to US$24,756 to 41,083 with projected cost of treating L-AmB associated impaired renal function per patient at risk of US$3,173 [24]. On the other hand, in a single-center study conducted in the USA, Harbarth et al. [25] were unable to identify any increase in costs or any prolongation of hospital stay despite increased mortality. In contrast to the data presented in this paper, all the studies referred to use ‘top-down’ approaches [24–26], which allocate a total budget to specific services and thus provide less precise estimates [9]. Moreover, the findings of these studies are based on tariffs and fees applicable in the various countries concerned and therefore cannot be extrapolated to the German health system. We deliberately undertook a micro-costing (‘bottom-up’) approach, evaluating the situation for all the costs of individual items related to the treatment and care of AML. This approach provides a more specific insight into the relationships between characteristics of activities and their costs and the relative importance of separate activities [9]. The published literature contains only one article that specifies the ‘bottom-up’ costs per day of hospital stay in Germany [27], albeit for intensive care. To our knowledge, the microeconomic analysis presented in this paper is the first of its kind in hematology–oncology that refers to the German health system.
The results of the present analysis are based upon data derived from the year 2002 (before the introduction of DRGs in Germany). We have adjusted cost for inflation. The transition from per diem charges to diagnosis related charges—which started in 2003 and will be completed nationwide in 2010—may have initiated efforts to optimize the costing structure in German hospitals.
We believe that our analyses are conservative. Firstly, the proportion of patients who developed L-AmB related nephrotoxicity defined as a twofold increase in serum creatinine compared to baseline was similar in the randomized controlled trial—which gave rise to the number needed to harm of L-AmB vs caspofungin—and the LEAD study [7, 8]. The LEAD study, however, found already a 1.5-fold increase in serum creatinine compared to baseline—which comprises a far higher proportion of patients [8, 23]—to be associated with longer stays in hospital [8]. In addition, no account was taken of the other tolerability advantages of caspofungin [7], e.g., fewer side effects during the infusion. On the other hand, L-AmB associated nephrotoxicity may also lead to dose reductions and thus lower cost of some antibiotics and other medications.
Secondly, the data from Bonn University Hospital presented in this paper exclude the costs of allogeneic stem cell transplantation, as patients were transferred to another tertiary care hospital to undergo that procedure. The cost of transplantation as a component of direct medical costs may therefore have been considerably underestimated in the present analysis. In two Norwegian studies, the mean cost of autologous and allogeneic stem cell transplantation was found to be US$32,160 (2001) [28] and US$106,825 (1999) [29], respectively.
In the year 2002, the mean direct cost of treating AML at Bonn University Hospital was found to be €19,039. Hospital funding in Germany is a case-mix system based on diagnosis-related groups (DRGs) in which the basic DRG indicates the underlying condition. Secondary diagnoses are indicated by patient clinical complexity levels (PCCL). Discrepancies between DRG reimbursement and real cost are possible and have been identified by the German competence network ‘Acute and chronic leukaemias’ [4]. Thus, where the underlying condition is AML and the clinical complexity level is increased due to the presence of systemic fungal infection, a hospital stay of 34 days in the year 2007 is to be reimbursed by €9,669, [10, 30].
The present analysis refers to the costs and reimbursement of systemic antifungal agents in tertiary care hospitals. The recent introduction of supplementary reimbursement (Zusatzentgelt) makes such reimbursement possible [11] when a definable service of no fixed assignment occurs sporadically across a number of DRGs and causes significant costs to the system as a whole. As such supplementary reimbursements are paid besides DRG payments but within the total budget of the individual hospital, this form of reimbursement spares the base rate of tertiary care hospitals and makes these hospitals competitive compared to lower levels of service provision at a high level of quality care. Thus, the supplementary reimbursement (Zusatzentgelt) in Germany helps to maintain the budget of the individual hospital, and it helps the department caring for an individual patient to get the expenses covered.
The development of nephrotoxicity during treatment with liposomal and other formulations of AmB prolongs hospital stays [8]. From the perspective of the hospital operator, the additional costs due to this nephrotoxicity are therefore directly dependent on the mean direct medical cost per day of hospital stay. Based on the better tolerability of caspofungin, high-user hospital pharmacy drug acquisition cost including 19% VAT and the outcome of the costing study presented in this paper, we estimated that with a probability of 79%, use of this substance is more cost-effective for German hospitals than use of L-AmB. In general terms, caspofungin was found to be cost-saving compared to L-AmB at any direct medical cost greater than €428 per day of hospital stay. If supplementary reimbursement (Zusatzentgelt, 2007) is excluded from the calculation, which is usually not done, this break-even point is €1,284 per day of hospital stay. On the other hand, these figures could be considerably lower if other efficacy and safety endpoints [7] were included in the calculation.
The present analysis of the risk and costs of nephrotoxicity for tertiary care hospitals with hematology–oncology departments is based on the best available evidence. The present system of DRG-based reimbursement for the treatment of AML per se does not cover all costs. The better renal tolerability of caspofungin compared to L-AmB results in shorter hospital stays and therefore more economical use of health care resources. The data presented in this paper show that on the basis of nephrotoxicity alone, use of caspofungin in hematology–oncology departments in Germany is at least cost-neutral compared to use of L-AmB.
Acknowledgments
The authors are grateful to Dr. Peter Lütkes, Medical Controlling, Essen University Hospital, Germany, for fruitful discussions and valuable contributions to the manuscript.
Transparency declaration P.K. and A.W. are employees of MSD Sharp and Dohme GmbH. S.T. has no conflicts to declare. C.B. has received honoraria from Pfizer. O.A.C. has received research funding from Astellas, Basilea, Gilead, Pfizer, MSD Sharp and Dohme/Merck&Co., Schering-Plough, and Vicuron; is a consultant to Astellas, Basilea, Gilead, Pfizer, MSD Sharp and Dohme/Merck&Co., Nektar, Schering-Plough, and Zeneus; and served at the speaker’s bureau of Astellas, Gilead, MSD Sharp and Dohme/Merck&Co., and Schering-Plough. A.G. has received research funding from Janssen-Cilag/Ortho Biotech, MSD Sharp and Dohme/Merck&Co., and Pfizer, is a consultant to Janssen-Cilag/Ortho Biotech, MSD Sharp and Dohme, Pfizer and Schering-Plough/ Essex Pharma, and has received speaker’s honoraria from Gilead, Janssen-Cilag/Ortho-Biotech, MSD Sharp and Dohme/ Merck&Co., and Pfizer. H.P.L. has received honoraria from Gilead, MSD Sharp and Dohme, and Essex Pharma/Schering-Plough. A.J.U. has received research funding from Schering-Plough; has been a consultant for Astellas, Basilea, Gilead, MSD Sharp and Dohme/Merck&Co., Pfizer, and Schering-Plough; and has been a member of the speakers’ bureaus of Astellas, Gilead, MSD Sharp and Dohme/ Merck&Co., Pfizer, and Schering-Plough.
Footnotes
Presented in part at the Joint Annual Meeting of the German, Austrian and Swiss Society for Haematology and Oncology, Leipzig, Germany, November 4–8, 2006 (V377), and the International Society for Pharmacoeconomics and Outcomes Research 9th Annual European Congress, Copenhagen, Denmark, October 28–31, 2006 (A303).
Contributor Information
Peter Kaskel, Phone: +49-8945-611804, FAX: +49-8945-61281804, Email: peter_kaskel@msd.de.
Andrew J. Ullmann, Phone: +49-6131-176564, FAX: +49-6131-17476564, Email: ullmann@uni-mainz.de
References
- 1.Groll AH, Shah PM, Mentzel C et al (1996) Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect 33:23–32 [DOI] [PubMed]
- 2.Kullberg BJ, Oude Lashof AM (2002) Epidemiology of opportunistic invasive mycoses. Eur J Med Res 7:183–191 [PubMed]
- 3.Ruhnke M, Böhme A, Buchheidt D et al (2003) Diagnosis of invasive fungal infections in hematology and oncology. Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol 82:S141–S148 [DOI] [PubMed]
- 4.Hehlmann R, Berger U, Aul C et al (2004) The German competence network ‘Acute and chronic leukemias’. Leukemia 18:665–669 [DOI] [PubMed]
- 5.Maschmeyer G (2001) Pneumonia in febrile neutropenic patients: radiologic diagnosis. Curr Opin Oncol 13:229–235 [DOI] [PubMed]
- 6.Zaspel U, Denning DW, Lemke AJ et al (2004) Diagnosis of IPA in HIV: the role of the chest X-ray and radiologist. Eur Radiol 14:2030–2037 [DOI] [PubMed]
- 7.Walsh TJ, Teppler H, Donowitz GR et al (2004) Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med 351:1391–1402 [DOI] [PubMed]
- 8.Ullmann AJ, Sanz MA, Tramarin A et al (2006) Prospective study of amphotericin B formulations in immunocompromised patients in 4 European countries. Clin Infect Dis 43:e29–e38 [DOI] [PubMed]
- 9.Drummond M, O’Brien B, Stoddart G, Torrance G (eds) (1999) Methods for the economic evaluation of health care programmes, 2nd edn. Oxford University Press, Oxford New York Toronto
- 10.NN (2007) German DRG Calculation Scheme. www.g-drg.de/cms/index.php/inek_site.de/g_drg_system_2007/fallpauschalen_katalog
- 11.NN (2002) Standard Tariff of the German Hospital Association (DKG-NT), 28th edn. Deutsche Krankenhausgesellschaft, Berlin
- 12.NN (2007) Lump compensation scheme for the German DRG System 2007 www.g-drg.de/cms/index.php/inek_site.de/g_drg_system_2007
- 13.Laupacis A, Sackett D, Roberts R (1988) An assessment of clinically useful measures of the consequences of treatment. N Engl J Med 318:1728–1733 [DOI] [PubMed]
- 14.Bender R (2001) Calculating confidence intervals for the number needed to treat. Controlled Clinical Trials 22:102–110 [DOI] [PubMed]
- 15.Sekretariat der Roten Liste GmbH (2007) Rote Liste Online www.roteliste.de
- 16.NN (2007) Harmonized consumer price index. Federal Statistical Office Germany www.destatis.de/indicators/e/vpi120ae.htm
- 17.Spiegelhalter D, Abrams K, Myles J (2004) Cost-effectiveness, policy-making and regulation. Wiley, Hoboken, NJ, USA
- 18.Spiegelhalter D, Thomas A, Best N, Lunn D (2004) WinBUGS User Manual
- 19.Willan AR, Briggs AH (2006) Statistical analysis of cost-effectiveness data. Wiley, Hoboken, NJ, USA
- 20.Barnes GR, Cerrito PB (2001) The visualization of continuous data using PROC KDE and PROC capability. Proceedings of the 26th Annual SAS Users Group International Conference 26:176–186
- 21.Fan X, Felsovalyi A, Sivo S, Keenan S (2002) SAS(R) for Monte Carlo studies: a guide for quantitative researchers. SAS Institute, Cary, NC, USA
- 22.Wingard JR, Kubilis P, Lee L et al (1999) Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin Infect Dis 29:1402–1407 [DOI] [PubMed]
- 23.Walsh TJ, Finberg RW, Arndt C et al (1999) Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med 340:764–771 [DOI] [PubMed]
- 24.Wingard J, Leather H, Wood C, Gerth W, Lupinacci R, Berger M, Mansley E (2007) Pharmacoeconomic analysis of caspofungin versus liposomal amphotericin B as empirical antifungal therapy for febrile neutropenia. Am J Health Syst Pharm 64:637–643 [DOI] [PubMed]
- 25.Harbarth S, Burke JP, Lloyd JF et al (2002) Clinical and economic outcomes of conventional amphotericin B-associated nephrotoxicity. Clin Infect Dis 35:e120–e127 [DOI] [PubMed]
- 26.Bruynesteyn K, Gant V, McKenzie C et al (2007) A cost-effectiveness analysis of caspofungin vs. liposomal amphotericin B for treatment of suspected fungal infections in the UK. Eur J Haematol 78:532–539 [DOI] [PMC free article] [PubMed]
- 27.Billing A, Thalhammer M, Eissner HJ et al (2004) Economic aspects of intensive care medicine–cost and reimbursement according to diagnosis related grouping. Zentralbl Chir 129:440–446 [DOI] [PubMed]
- 28.Mishra V, Andresen S, Brinch L et al (2005) Cost of autologous peripheral blood stem cell transplantation: the Norwegian experience from a multicenter cost study. Bone Marrow Transplant 35:1149–1153 [DOI] [PubMed]
- 29.Mishra V, Vaaler S, Brinch L (2001) A prospective cost evaluation related to allogeneic haemopoietic stem cell transplantation including pretransplant procedures, transplantation and 1 year follow-up procedures. Bone Marrow Transplant 28:1111–1116 [DOI] [PubMed]
- 30.Müller M, Irps S, Röder N, DRG Research Group (2007) GDRG Grouping result for MDC17/R60E/PCCL4 [C92.00/D70.3/D69.59/D.64.8/Z51.1!, 34 days]. University of Münster DRG Research Group http://drg.uni-muenster.de/de/webgroup/m.webgroup.php