Abstract
Purpose
The regimens of weekly irinotecan with platinum have been used for treatment of metastatic small-cell lung cancer (SCLC). We conducted a multi-institution phase II trial to evaluate a novel 21-day schedule of irinotecan and carboplatin in patients with relapsed or extensive SCLC.
Patients and Methods
Eighty patients were enrolled with the following characteristics: 39 male patients, 41 female patients; median age, 65 years; and Zubrod performance status, 0 to 1 in 85% and 2 in 15% of patients. Dosing schemas were based on the maximum-tolerated dose derived in a previous phase I study. Chemotherapy-naive patients with extensive SCLC were treated with irinotecan 200 mg/m2 and carboplatin area under the curve (AUC) of 5 (arm A). Patients, who had previously been treated with chemotherapy and had relapsed disease received irinotecan 150 mg/m2 and carboplatin AUC of 5 (arm B). In each study arm, the treatment was given every 21 days for up to six cycles.
Results
The most common grade 3 to 4 toxicities included neutropenia (54%), thrombocytopenia (22%), anemia (13%), diarrhea (22%), and nausea/emesis (11%) in both study arms. There were three treatment-related deaths owing to neutropenic sepsis. Among 72 assessable patients, response rates of 65% and 50% were observed, respectively, for arm A and arm B. The median survival for both study arms was identical at 10 months (95% CI, 6 to 14 months). A response rate of 65% was observed in the intracranial disease of 14 patients with known brain metastases.
Conclusion
This 21-day regimen of irinotecan and carboplatin seems promising for the treatment of relapsed SCLC.
INTRODUCTION
Chemotherapy has been the mainstay treatment for extensive small-cell lung cancer (SCLC). The current standard chemotherapeutic regimens for SCLC include cisplatin/etoposide and carboplatin/etoposide. Median survival time for patients with extensive SCLC treated with platinum-based chemotherapy has been approximately 10 months, with a 5-year survival rate of 1% to 2%.1,2 Prognosis for patients with relapsed SCLC is even poorer. In a randomized trial comparing single-agent topotecan with cyclophosphamide, doxorubicin, and vincristine (CAV) in patients with relapsed SCLC, the response rate was 24% for patients treated with topotecan and 18% for patients treated with CAV, with a median survival of 6 months in both groups.3
The combination of irinotecan and cisplatin has been studied in phase III trials as first-line therapy for patients with extensive SCLC. A phase III trial conducted by the Japan Clinical Oncology Group (JCOG) showed that the response rate and median survival were 84% and 12.8 months, respectively, for patients treated with irinotecan/cisplatin as compared with 67% and 9.4 months for patients treated with etoposide/cisplatin.4 However, in a phase III trial with identical design conducted by the Southwest Oncology Group, the response rates and median survival times between the two groups were not statistically different at 60% and 9.9 months versus 57% and 9.1 months, respectively.5
Carboplatin, which is generally better tolerated than cisplatin,6 has been combined with weekly irinotecan as a first-line treatment for extensive SCLC. Sohn et al7 treated 39 patients with newly diagnosed extensive SCLC with carboplatin (area under the curve [AUC] of 5) and irinotecan (50 mg/m2 on days 1, 8, and 15). The response rate was 69% and overall survival was 11 months. A phase II randomized trial reported by Schmittel et al8 combined carboplatin (AUC of 5) with either irinotecan (50 mg/m2 on days 1, 8, and 15) or etoposide (140 mg/m2 on days 1, 2, and 3) in 70 patients with newly diagnosed extensive SCLC. Although the response rates of 67% and 59%, respectively, were not significantly different, the progression-free survival was 9 months for patients treated with the irinotecan-containing regimen as compared with 6 months in the control arm (P = .03).
It is noteworthy that irinotecan has been administered on a weekly basis in most clinical trials, including those cited above. In practice, delivery of irinotecan on day 15 is often compromised by intracycle toxicity. In fact, in the JCOG trial, approximately 50% of patients were unable to receive the day 15 irinotecan dose. In a population-based phase I study, we investigated the regimen of irinotecan and carboplatin given every 21 days in patients with solid tumors at our institution.9 We reported that the maximum-tolerated dose (MTD) of irinotecan and carboplatin was 150 mg/m2 and AUC 5 for previously chemotherapy-treated patients and 200 mg/m2 and AUC 5 for chemotherapy-naive patients. As a follow-up trial, we conducted a phase II study of the 21-day regimen of carboplatin and irinotecan in previously chemotherapy-treated and -naive extensive SCLC.
PATIENTS AND METHODS
Eligibility Criteria
Patients were required to have a biopsy-proven diagnosis of SCLC; measurable disease; Zubrod performance status of 0 to 2; life expectancy of greater than 12 weeks; adequate hematologic (absolute neutrophil count > 1,500/μL and platelets > 100,000/μL) and hepatic (total bilirubin ≤ 1.5 mg/dL and aspartate transferase ≤ 2.5× upper limit of normal) functions. Patients with known brain metastases were eligible if they were asymptomatic or they had already received whole-brain irradiation. Patients were stratified into two arms: arm A consisted of patients who had extensive disease and had not been previously treated with chemotherapy, and arm B consisted of patients who had relapsed SCLC at least 90 days after previous chemotherapy. No radiation was allowed while the patients were receiving chemotherapy on the protocol. All patients signed informed consent in accordance with the guidelines of institutional review boards.
Treatment
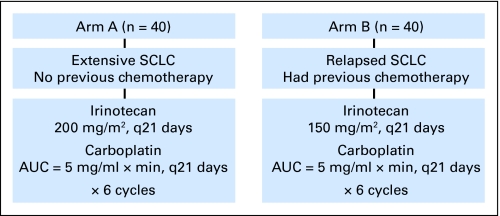
The treatment schema is shown in Figure 1. The schema was derived from a previous phase I study of population-based MTD of irinotecan and carboplatin.9 For arm A, irinotecan 200 mg/m2 was administered intravenously over 90 minutes followed by an intravenous bolus of carboplatin (AUC = 5 mg/mL × min) every 21 days for six cycles. The carboplatin dose was calculated according to the Calvert formula: carboplatin dose in milligrams = AUC × (25 + creatinine clearance [CrCl]). The method of Cockcroft and Gault was used to calculate CrCl.10 For arm B, the irinotecan dose was reduced to 150 mg/m2, and the dosage for carboplatin was identical to that of arm A, with an AUC of 5. Two precautionary measures were used to reduce the risk of overdosing of carboplatin. First, the ideal body weight was used to calculate CrCl if the actual body weight was more than 10% of the ideal. Second, the lowest limit for serum creatinine level was 1 mg/dL for calculating CrCl.
Fig 1.
Treatment schema of irinotecan and carboplatin for extensive or relapsed small-cell lung cancer. SCLC, small-cell lung cancer; AUC, area under the curve.
Dose Modifications
For arm A, the irinotecan dose was reduced to 150 mg/m2 if grade 4 neutropenia, grade 3 to 4 thrombocytopenia, or nonhematologic toxicity was encountered in the previous cycle. For arm B, the carboplatin dose was reduced to an AUC of 4 for grade 4 neutropenia and grade 3 to 4 thrombocytopenia, and the irinotecan dose was reduced to 100 mg/m2 in case of grade 3 to 4 diarrhea in the previous cycle.
Assessments
All adverse events were graded according to the National Cancer Institute Common Toxicity Criteria, version 2.0. Responses were evaluated after every two cycles of treatment according to the RECIST criteria.11
Statistics
The primary goals of this study were to evaluate the response rate and overall survival in each arm. A two-stage design was used for patient accrual. For the initial stage, 20 patients were accrued to each arm. If the overall response rate was ≤ 60% in arm A or less than 20% in arm B, further enrollment would cease in the respective arm. Otherwise, an additional 20 patients would be enrolled in each arm. The estimates were based on the response rates of 67% to 84% reported in the previous trial of JCOG with cisplatin/irinotecan and cisplatin/etoposide for untreated extensive SCLC4 and 18% to 24% reported by von Pawel et al for CAV or topotecan for previously chemotherapy-treated SCLC.3 Overall survival was determined according to the method of Kaplan-Meier using a Minitab statistical software program (State College, PA).
RESULTS
From July 2002 to October 2007, a total of 80 patients were enrolled in four medical centers. There were 40 patients in arm A and 40 in arm B. Patient characteristics are summarized in Table 1. There were 39 men and 41 women. The median age was 65 years, with a range of 43 to 81 years. Eighty-five percent of the patients had a Zubrod performance status of 0 or 1. Six patients in arm A and nine patients in arm B had brain metastases. All patients in arm B had previously received etoposide with either cisplatin or carboplatin at least 3 months before entering this trial.
Table 1.
Patient Characteristics
| Characteristic | Arm A | Arm B | Total |
|---|---|---|---|
| No. of patients | 40 | 40 | 80 |
| Sex | |||
| Male | 22 | 17 | 39 |
| Female | 18 | 23 | 41 |
| Age, years | |||
| Range | 50-81 | 43-80 | 43-81 |
| Median | 67 | 65 | 65 |
| Performance status | |||
| 0-1 | 34 | 34 | 68 |
| 2 | 6 | 6 | 12 |
| Brain metastases | 6 | 9 | 15 |
All patients received at least one cycle of treatment, with a median number of four cycles. Twenty-four patients, including 18 in arm A and six in arm B, required a dose reduction according to the dose modification rules as outlined above. All 80 patients were assessable for toxicity at least for the first cycle. As shown in Table 2, there was no apparent difference in the toxicity profile between the two arms. The most common grade 3 to 4 toxicity was bone marrow suppression, including neutropenia in 54%, thrombocytopenia in 21%, and anemia in 13% of the patients. Five patients (6%) developed neutropenic fever and three patients succumbed to sepsis in the setting of neutropenia after the first cycle of treatment. Common grade 3 to 4 nonhematologic toxicities included diarrhea in 21% and nausea/emesis in 11% of patients.
Table 2.
Grade 3 to 4 Toxicities (N = 80*)
| Toxicities | Arm A |
Arm B |
Total |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Neutropenia | 21 | 52 | 22 | 55 | 43 | 54 |
| Neutropenic fever | 4 | 10 | 1 | 3 | 5 | 6 |
| Thrombocytopenia | 8 | 20 | 9 | 23 | 17 | 21 |
| Anemia | 4 | 10 | 6 | 15 | 10 | 13 |
| Diarrhea | 9 | 23 | 8 | 20 | 17 | 21 |
| Nausea/emesis | 3 | 8 | 5 | 13 | 8 | 10 |
| Treatment-related death | 2 | 5 | 1 | 3 | 3 | 4 |
Includes 40 assessable patients in arm A and 40 in arm B.
Best responses, as assessed according to RECIST (Response Evaluation Criteria in Solid Tumors), are shown in Table 3. Of the 80 patients, 72 patients were assessable for response. For the remaining eight patients who were not assessable, six patients received only one cycle of chemotherapy and the other two patients were not evaluated for responses after two cycles of chemotherapy.
Table 3.
Best Response Among Assessable Patients*
| Response | Arm A |
Arm B |
Total |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Complete response | 4 | 12 | 5 | 13 | 9 | 13 |
| Partial response | 18 | 53 | 14 | 37 | 32 | 44 |
| Overall response | 22 | 65 | 19 | 50 | 41 | 57 |
| Stable disease | 5 | 15 | 14 | 37 | 19 | 26 |
| Progressive disease | 7 | 21 | 5 | 14 | 12 | 17 |
| Not assessable | 6 | 15 | 2 | 5 | 8 | 10 |
Includes 34 assessable patients in arm A and 38 in arm B.
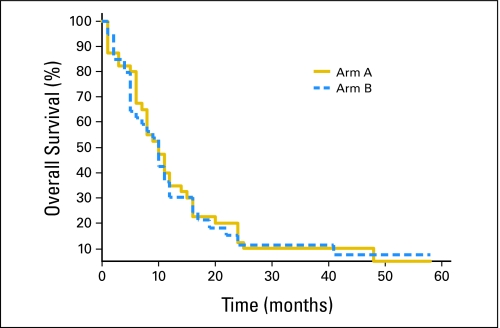
Among the 72 assessable patients, the overall response rates for arm A and arm B were 65% and 50%, respectively. Complete response rates were correspondingly 12% and 13%. Stable disease was observed in 15% of patients in arm A and 37% in arm B. Progressive disease was observed in 21% in arm A and 14% in arm B. By Kaplan-Meier analysis, the median survival for both arm A and arm B was identical at 10 months (95% CI, 6 to 14 months; Fig 2). There were 15 patients (19%) with brain metastasis in both arms; a response rate of 65% was observed in 14 assessable patients. The details of this latter observation have been published elsewhere.12
Fig 2.
Kaplan-Meier plot of overall survival of patients in arm A and arm B.
DISCUSSION
Most of the clinical trials conducted to date with irinotecan and platinum have used the weekly schedule of irinotecan for SCLC.4,5,7,8 In this study, we used the novel 3-week regimen of irinotecan and carboplatin in a phase II study consisting of two arms of patients with SCLC: One arm consisted of chemotherapy-naive extensive SCLC and the other consisted of relapsed SCLC after previous chemotherapy.
This 3-week approach was based on the results of a previous population-based phase I trial in which an MTD was derived for chemotherapy-naive or chemotherapy-treated patients.9 The tolerability of this regimen was similar in the two arms. The grade 3 to 4 toxicity profile of this study was comparable with studies using the weekly schedules of irinotecan with cisplatin as reported in randomized trials. In the randomized trial conducted by Noda et al, the most common grade 3 to 4 neutropenia and diarrhea were 65% and 16%, respectively, in the weekly irinotecan and carboplatin arm.4 In comparison, grade 3 to 4 neutropenia and diarrhea in our study were 54% and 21%, respectively. Likewise, treatment-related death in our study was 4%, which was identical to that reported in the study conducted by Noda et al.4 Most recently, a randomized Scandinavian study demonstrated that carboplatin (AUC 4 by Chatelut formula) and irinotecan (175 mg/m2) given every 3 weeks as front-line chemotherapy yielded a median survival of 8.5 months for patients with extensive SCLC.13 This median survival was statistically better than that of 7.1 months for patients who received the same dose of carboplatin and oral etoposide (120 mg/m2/d for 5 days) given every 3 weeks. Understandably, the delivery of the 3-week regimen is more convenient and presumably less costly.
The results of efficacy of this study were promising, especially for patients with relapsed disease. The response rate and overall survival of chemotherapy-naive patients were 65% and 10 months, respectively. These results are in line with clinical trials conducted to date on various combinations of anticancer drugs, including the irinotecan-containing regimens.1,2,5,6 In contrast, the response rate of 50% and a median overall survival of 10 months were remarkable for patients who had experienced treatment failure with previous platinum-based chemotherapy. In a randomized trial comparing CAV and topotecan in patients with relapsed SCLC, the response rate was 18% in the CAV arm and 24% in the topotecan arm, and the overall survival was only 6 months in each arm.3 Furthermore, in another randomized trial for relapsed SCLC, the median survival was 6.5 months for patients who received oral topotecan as compared with 4.5 months with best supportive care.14 In fact, although there has been growing optimism concerning the potential efficacy of amrubicin for relapsed SCLC, the response rate in patients with relapsed disease in the present trial exceeds that of United States–based phase II trials15,16 and is comparable to that seen in Japanese phase II studies.17
In our study, we enrolled patients with brain metastasis provided that they were without CNS symptoms or they had already received whole-brain irradiation. A total of 15 patients (19%) with brain metastasis were enrolled in both arms. We observed a surprisingly high rate of response of 65% of brain lesions to irinotecan and carboplatin, with an overall survival ranging from 1 to 24 months.12
In summary, the 3-week regimen of irinotecan and carboplatin was relatively well tolerated and efficacious in patients with SCLC, especially for relapsed disease and brain metastasis. The worth of this regimen for treatment of relapsed SCLC could be further evaluated by comparing it with topotecan in a phase III trial.
Footnotes
Supported by Grant No. K24CA10014 and Pfizer Inc.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: Derick Lau, Pfizer Honoraria: None Research Funding: Derick Lau, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Derick Lau
Provision of study materials or patients: Lou Fehrenbacher, Howard West, Primo N. Lara Jr, Leonid L. Yavorkovsky, Michael Russin, David Gandara, Derick Lau
Collection and assembly of data: Gigi Chen, Minh Huynh, Desiree Goldstein
Data analysis and interpretation: Gigi Chen, Minh Huynh, Derick Lau
Manuscript writing: Gigi Chen, Howard West, Primo N. Lara Jr, Leonid L. Yavorkovsky, Derick Lau
Final approval of manuscript: Gigi Chen, Minh Huynh, Lou Fehrenbacher, Howard West, Primo N. Lara Jr, Leonid L. Yavorkovsky, Michael Russin, Desiree Goldstein, David Gandara, Derick Lau
REFERENCES
- 1.Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: Perceptible progress. J Clin Oncol. 1999;17:1794–1801. doi: 10.1200/JCO.1999.17.6.1794. [DOI] [PubMed] [Google Scholar]
- 2.Sundstrøm S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: Results from a randomized phase III trial with 5 years' follow-up. J Clin Oncol. 2002;20:4665–4672. doi: 10.1200/JCO.2002.12.111. [DOI] [PubMed] [Google Scholar]
- 3.von Pawel J, Schiller JH, Sheperd FA, et al. Topotecan versus cyclophosphamide, doxorubicin and vincristine for the treatment of recurrent small cell lung cancer. J Clin Oncol. 1999;17:658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 4.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 5.Natale RB, Lara PN, Chansky K, et al. S0124: A randomized phase III trial comparing irinotecan/cisplatin (IP) with etoposide/cisplatin (EP) in patients (pts) with previously untreated extensive stage small cell lung cancer (E-SCLC). J Clin Oncol. 2008;26(suppl):400s. abstr 7512. [Google Scholar]
- 6.Skarlos DV, Samantas E, Kosmidis P, et al. Randomized comparison of etoposide-cisplatin vs etoposide carboplatin and irradiation in small cell lung cancer Hellenic Co-operation Oncology Group Study. Ann Oncol. 1994;5:601–607. doi: 10.1093/oxfordjournals.annonc.a058931. [DOI] [PubMed] [Google Scholar]
- 7.Sohn JH, Choi HJ, Chang J, et al. A phase II trial of fractionated irinotecan plus carboplatin for previously untreated extensive stage small cell lung cancer. Lung Cancer. 2006;54:365–370. doi: 10.1016/j.lungcan.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Schmittel A, Fischer von Weikersthal L, et al. A randomized phase II trial of irinotecan plus carboplatin versus etoposide plus carboplatin treatment in patients with extended disease small cell lung cancer. Ann Oncol. 2006;17:663–667. doi: 10.1093/annonc/mdj137. [DOI] [PubMed] [Google Scholar]
- 9.Wild C, Wang S, Gandara D, et al. Population-based maximum tolerated dose of irinotecan and carboplatin. Oncology. 2003;17:11–16. [PubMed] [Google Scholar]
- 10.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 11.James K, Eisenhauer E, Christian M, et al. Measuring response in solid tumors: Unidimensional versus bidimensional measurement. J Natl Cancer Inst. 1999;91:523–528. doi: 10.1093/jnci/91.6.523. [DOI] [PubMed] [Google Scholar]
- 12.Chen G, Huynh M, Chen A, et al. Chemotherapy for brain metastasis in small-cell lung cancer. Clin Lung Cancer. 2008;9:35–38. doi: 10.3816/CLC.2008.n.006. [DOI] [PubMed] [Google Scholar]
- 13.Hermes A, Bergman B, Bremnes R, et al. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: A randomized phase III trial. J Clin Oncol. 2008;26:4261–4267. doi: 10.1200/JCO.2007.15.7545. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien M, Ciuleanu T, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441–5447. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 15.Ettinger DS, Jotte RM, Gupta V, et al. A phase II trial of single-agent amrubicin (AMR) in patients with extensive small cell lung cancer (ED-SCLC) that is refractory or progressive within 90 days of completion of first-line platinum-based chemotherapy. J Clin Oncol. 2008;26(suppl):434s. abstr 8041. [Google Scholar]
- 16.Jotte RM, Conkling PR, Reynolds C, et al. A randomized phase II trial of amrubicin (AMR) vs topotecan as second-line treatment in extensive-disease small-cell lung cancer (SCLC) sensitive to platinum-based first-line chemotherapy. J Clin Oncol. 2008;26(suppl):433s. doi: 10.1200/JCO.2010.29.8851. abstr 8040. [DOI] [PubMed] [Google Scholar]
- 17.Onoda S, Masuda N, Seto T, et al. Phase II trial of amrubicin for treatment of refractory or relapsed small-cell lung cancer: Thoracic Oncology Research Group Study 0301. J Clin Oncol. 2006;24:5448–5453. doi: 10.1200/JCO.2006.08.4145. [DOI] [PubMed] [Google Scholar]