Abstract
Purpose
Hepatocellular carcinoma (HCC) is the third leading cause of cancer mortality worldwide. Incidence rates are increasing in the United States. Monitoring incidence, survival, and mortality rates within at-risk populations can facilitate control efforts.
Methods
Age-adjusted incidence trends for HCC were examined in the Surveillance, Epidemiology, and End Results (SEER) registries from 1975 to 2005. Age-specific rates were examined for birth cohorts born between 1900 and 1959. Age-adjusted incidence and cause-specific survival rates from 1992 to 2005 were examined in the SEER 13 registries by race/ethnicity, stage, and treatment. United States liver cancer mortality rates were also examined.
Results
Age-adjusted HCC incidence rates tripled between 1975 and 2005. Incidence rates increased in each 10-year birth cohort from 1900 through the 1950s. Asians/Pacific Islanders had higher incidence and mortality rates than other racial/ethnic groups, but experienced a significant decrease in mortality rates over time. From 2000 to 2005, marked increases in incidence rates occurred among Hispanic, black, and white middle-aged men. Between 1992 and 2004, 2- to 4-year HCC survival rates doubled, as more patients were diagnosed with localized and regional HCC and prognosis improved, particularly for patients with reported treatment. Recent 1-year survival rates remained, however, less than 50%.
Conclusion
HCC incidence and mortality rates continue to increase, particularly among middle-aged black, Hispanic, and white men. Screening of at-risk groups and treatment of localized-stage tumors may contribute to increasing HCC survival rates in the United States. More progress is needed.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer mortality worldwide.1 This malignancy occurs more often among men than women, with the highest incidence rates reported in East Asia.2 The incidence rates of HCC in the United States have historically been lower than in many countries. However, in recent decades, HCC age-adjusted incidence rates have doubled3 and primary liver cancer mortality rates have increased faster than mortality rates for any other leading cause of cancer.4,5 Approximately 90% of primary liver cancers in the United States are HCCs, while most of the remaining 10% are intrahepatic cholangiocarcinomas.6 The pathway leading to HCC generally begins with an acute hepatic insult which progresses over decades. Fibrosis and cirrhosis are typically precursors of HCC.7 Among patients with localized stage HCC, treatment options may include resection or transplantation. Chemoembolization, a combination of chemotherapy and occlusion of the tumor blood supply, is reported to improve survival in well-selected patients with unresectable HCC.8 Many patients who are diagnosed with HCC, however, have advanced disease and are only candidates for palliative care, contributing to a relatively low reported 5-year survival rate of approximately 10%.6
Most HCC is thought to be associated with either chronic hepatitis C virus (HCV) or hepatitis B virus (HBV) infection.9 In the United States, more than 3 million people are chronically infected with HCV.10 Many of these individuals were exposed as young adults beginning in the 1960s.10,11 HCV risk factors include contaminated blood product transfusion and injected drug use.10,11
Chronic infection with HBV, a major global risk factor for HCC, is less common overall in the United States than HCV infection is. Among some United States' ethnic groups, however, HBV is a more common risk factor than HCV. For example, a report from Los Angeles found that 74% of HCC among Asians was linked to HBV, while 90% of HCC in whites was linked to HCV.9
The etiology of HCC is likely to involve interactions between multiple risk factors. In a study which utilized the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked databases,12 the most commonly reported risk-factor was nonspecific cirrhosis (21%), followed by alcohol-induced liver disease (16%), HCV infection (10%), and HBV infection (5%). Obesity and type II diabetes6 are also suspected to increase risk.
To monitor changes in the burden of HCC in the United States, this report provides analyses of incidence and survival of HCC and mortality of liver cancer. To define at-risk populations, data were examined by sex, race/ethnicity, and age.
METHODS
HCC Incidence Data
HCC patients in the SEER registries were defined by the International Classification of Diseases (ICD)-O-2/ICD-O-3 topography C22.0 (liver) and morphology 8170 (hepatocellular carcinoma, not otherwise specified). With release of ICD-O-3 in 2001, morphology codes for HCC were expanded to include specific histologies: 8171, 8172, 8173, 8174, and 8175,13 which were also included in the case definition for this report. Data were examined for 1975 through 2005 from the SEER 9 registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah), and for 1992 through 2005 from the SEER 13 registries (SEER 9 plus Los Angeles, San Jose-Monterey, Rural Georgia, Alaska Native Tumor Registry). The SEER 9 registries cover approximately 10% and the SEER 13 registries cover approximately 14% of the United States' population.
Before 1990, the Census Bureau provided population denominators for white, black and other races. As of 1990, population denominators were available for Asians/Pacific Islanders, American Indians/Alaska Natives, and Hispanic ethnicity. Alaska Native registry data on Hispanic ethnicity included missing values and were not used in the present analyses. Place of birth was ascertained for patients diagnosed between 2000 and 2005 in the SEER 13 registries. Patients were classified as foreign-born versus United States natives including United States' territories. Point estimates and ranges of percent foreign birth were determined for racial/ethnic groups based on completeness of data.14
Annual age-adjusted HCC incidence rates were examined from 1975 to 2005. Age-adjusted HCC incidence rates were also examined by race and ethnicity for 1992 to 1993 and 3-year time periods from 1994 to 1996 to 2003 to 2005. Standard United States' population estimates were used to assign weights for 19 age groups: younger than 1, 5-year age groups up to 80 to 84, and 85+ years of age.15 Louisiana incidence data for the second half of 2005 were excluded due to hurricane-related disruptions. No adjustments were made for delays in case reporting to registries.16 In SEER 9 registries, approximately 5% of liver and intrahepatic bile duct cancer cases remain unreported in the first data submission for a surveillance year, with a 1% delay in case reporting 5 years after initial submission.17
A cohort analysis of incidence rates was performed with data from 1975 to 2005 with age-specific rates calculated by birth decade. The model included 22 age-specific rates, 18 with complete data for the age-range and four with data for 88% of person-years. The last three birth years in these four cohorts had yet to span the full age range.
Liver Cancer Mortality Data
Cancer deaths in the United States are reported by primary cancer site. Deaths from liver cancer other than intrahepatic bile duct cancer were examined. This site corresponds closely with HCC for SEER incidence data. Mortality rates were age-adjusted according to the United States 2000 standard population.15 The 2005 population estimate adjusted for hurricane-related shifts in the Gulf Coast area.
Age-adjusted mortality rates were estimated by race/ethnicity for the same time periods examined for incidence rates. Nine states were excluded from mortality rate analyses for Hispanic ethnicity because of missing data: Connecticut, Maine, Maryland, Minnesota, New Hampshire, New York, North Dakota, Oklahoma, and Vermont.
Cause-Specific Survival
Cause-specific HCC survival estimates survival in the absence of other causes of death using standard life-tables, with other causes of death censored. These rates were calculated for time intervals from 1992 to 1993 through 2003 to 2004. Survival rates were also examined by stage at diagnosis and initial treatment after diagnosis.
Statistical Methods
Joinpoint models were used to fit age-adjusted HCC incidence trends from 1975 to 2005, as well as liver cancer mortality rates from 1992 to 2005 (Joinpoint 3.3; IMS, Silver Spring, MD).18 Cause-specific 12-month survival rates were fit from 1992 to 2004, with follow-up of vital status through 2005. Up to four joinpoints were allowed for trends from 1975 to 2005 and up to three joinpoints were allowed for trends from 1992 to 2004/2005. A minimum of four observations were required between joinpoints, with at least three observations in initial and final joinpoint segments.
RESULTS
Age-Adjusted HCC Incidence Data
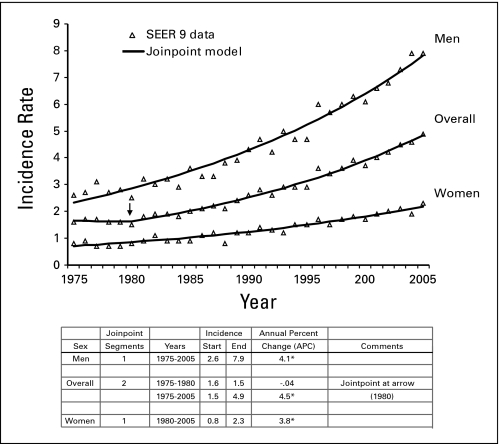
Overall age-adjusted incidence rates of HCC tripled between 1975 and 2005, rising from 1.6 per 100,000 to 4.9 per 100,000 (Fig 1). Incidence rates were approximately three times higher among men than among women throughout this time period. The best fitting model for overall HCC incidence trends had two segments. The first segment, from 1975 to 1980, showed no change in HCC incidence. The second segment, fitting HCC incidence trends from 1980 to 2005, estimated an annual percent change of 4.5% (P ≤ .05). When rates among males and females were modeled separately, the best-fitting joinpoint models had one segment. The annual percent changes in HCC incidence from 1975 to 2005 were statistically significant in both models (P ≤ .05).
Fig 1.
Annual age-adjusted incidence rates per 100,000 and trends, all hepatocellular carcinoma cases and by sex, 1975 to 2005 (Surveillance, Epidemiology, and End Results 9 [SEER9]). (*) The overall joinpoint model had two segments, with a change point at 1980 (arrow). Asterisks indicate annual percent change (APC) differed from zero, P ≤ .05.
Birth Cohort Analyses
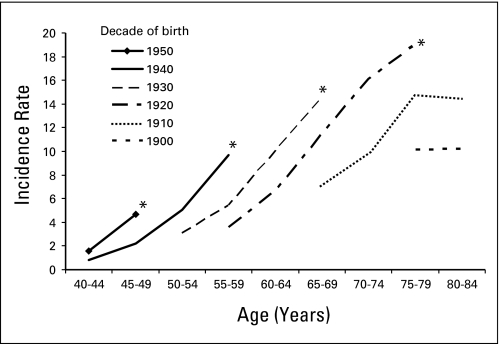
Examining age-specific incidence by birth cohort revealed that rates increased in each successive birth cohort born between 1900 and 1959 (Fig 2). Incidence rates for the most recent surveillance years (marked with asterisks) were not adjusted for delayed reporting or final cases in the last birth years of each cohort.
Fig 2.
Age-specific hepatocellular carcinoma incidence rates per 100,000 by decade of birth (cohort), 1975 to 2005 (Surveillance, Epidemiology, and End Results 9). (*) Indicates rates for the most recent surveillance years.
Age-Adjusted Rates by Race and Ethnicity
Between 1992 and 2005, overall incidence rates of HCC increased, with an annual percent change of 4.3% (P ≤ .05; Table 1). Asians/Pacific Islanders had the highest age-adjusted HCC incidence rates throughout the period, followed by Hispanics, blacks, American Indians/Alaska Natives and whites (Table 1). Although Asians/Pacific Islanders had the highest incidence, they experienced a smaller annual percent change in HCC incidence rates (annual percent change [APC] = 1.0%) than other racial/ethnic groups. American Indians/Alaska Natives experienced the greatest significant increase (APC = 5.0%), followed by blacks (APC = 4.9%), whites (APC = 4.6%), and Hispanics (APC = 4.0%). For each trend, the best-fitting joinpoint model had one segment.
Table 1.
Age-Adjusted HCC Incidence Rates, Liver Cancer Death Rates, and 1-Year Cause-Specific Survival Rates for HCC, From 1992 to 2005 by Time Period and Race/Ethnicity, With APC
| Histology by Race/Ethnicity | Diagnosis Years* |
1992-2004/2005 |
|||||
|---|---|---|---|---|---|---|---|
| 1992-1993 | 1994-1996 | 1997-1999 | 2000-2002 | 2003-2004/2005 | Count | APC | |
| Incidence | |||||||
| HCC | |||||||
| Overall | 3.1 | 3.6 | 4.2 | 4.6 | 5.1 | 20,444 | 4.3† |
| Asian/Pacific Islander | 10.0 | 11.1 | 11.2 | 11.4 | 11.7 | 5501 | 1.0† |
| Hispanic† | 4.8 | 6.0 | 6.9 | 7.7 | 8.0 | 3320 | 4.0† |
| Black | 4.2 | 4.4 | 5.3 | 6.0 | 7.0 | 2401 | 4.9† |
| American Indian/Alaska Native | 3.5 | 4.8 | 3.9 | 5.1 | 6.6 | 229 | 5.0† |
| White | 2.6 | 2.6 | 3.1 | 3.5 | 3.9 | 12,246 | 4.6† |
| Mortality | |||||||
| Liver cancer excluding intrahepatic bile duct | |||||||
| Overall | 3.3 | 3.5 | 3.6 | 3.7 | 4.0 | 139,738 | 1.6† |
| Asian/Pacific Islander | 9.5 | 9.6 | 9.2 | 8.9 | 8.9 | 9934 | −0.9† |
| Hispanic† | 5.5 | 5.7 | 6.4 | 6.4 | 6.7 | 11,833 | 1.7† |
| Black | 4.9 | 5.2 | 5.3 | 5.3 | 5.8 | 19,161 | 1.3† |
| American Indian/Alaska Native | 3.5 | 3.7 | 4.5 | 4.8 | 4.9 | 915 | 2.8 |
| White | 2.9 | 3.1 | 3.3 | 3.3 | 3.5 | 109,728 | 1.7† |
| One-year survival, % | |||||||
| HCC | |||||||
| Overall | 25 | 29 | 34 | 40 | 47 | 15,288 | 6.0† |
| Asian/Pacific Islander | 28 | 34 | 40 | 48 | 49 | 4,305 | 5.0† |
| Hispanic† | 23 | 27 | 31 | 41 | 47 | 2,378 | 6.6† |
| Black | 20 | 23 | 26 | 32 | 40 | 1,775 | 7.1† |
| American Indian/Alaska Native | 36 | 36 | 45 | 37 | 41 | 174 | −0.6 |
| White | 24 | 28 | 32 | 38 | 47 | 8,987 | 6.3† |
NOTE. Incidence per 100,000 and 1-year (12-month) survival in Surveillance, Epidemiology, and End Results 13 registries; deaths per 100,000, United States.
Abbreviations: HCC, hepatocellular carcinoma; APC, annual percent change.
Final time period for incidence and death rates: 2003-2005, for survival rates: 2003-2004 with follow-up through 2005.
Slope of APC regression line not equal to zero, P ≤ .05.
‡Surveillance, Epidemiology, and End Results data for Hispanic ethnicity exclude data from the Alaska Native Registry. Mortality data for Hispanic ethnicity excludes data from CT, ME, MD, MN, NH, NY, ND, OK, and VT. Hispanic ethnicity is not exclusive of race.
United States Versus Foreign Place of Birth
Data on place of birth were available for 81% of persons with HCC diagnosed in the SEER 13 registries between 2000 and 2005, including more than 80% of patients in all racial groups and 77% of Hispanic patients (data not shown). Among patients with data, 31% were born outside the United States; however, 80% of Asians/Pacific Islanders with data were born outside the United States, more than any other racial or ethnic group. Among Hispanics with HCC, 40% were born outside the United States. Compared with Asian/Pacific Islander and Hispanic HCC cases, foreign birth occurred less frequently among white (17%), black (6%), and American Indian/Alaska Native HCC patients (3%) with data.
Recent Incidence Trends
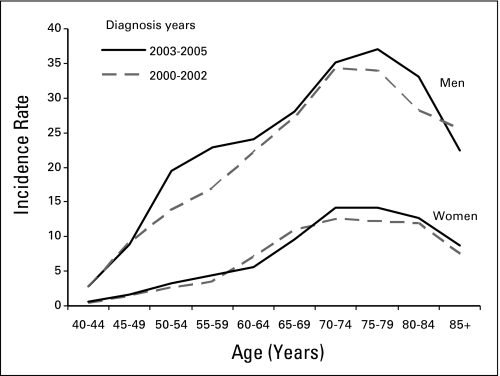
An examination of age-specific HCC incidence rates in SEER 13 registries in 2000 to 2002 and 2003 to 2005 revealed marked increases in incidence rates among men 50 to 59 years of age and 75 to 84 years of age (Fig 3) with slight increases in incidence rates among women in these age groups.
Fig 3.
Age-specific incidence rates for hepatocellular cancer by gender for two time periods of diagnosis, 2000 to 2002 and 2003 to 2005, Surveillance, Epidemiology, and End Results 13 registries.
Within the 50- to 59-year age group, the annual percent change in HCC incidence rates significantly increased from 2000 to 2005 for Hispanic men, black men, and white men, and for black and white women (P ≤ .05), with HCC incidence rates among blacks exceeding those of Asians/Pacific Islanders during 2003 to 2005 (Table 2). Among persons 75 to 84 years of age, increases in HCC incidence were seen among all men and white women (P ≤ .05). No other significant annual percent changes were seen from 2000 to 2005 by age, sex, or race/ethnicity (data not shown).
Table 2.
Age-Specific Hepatocellular Carcinoma Incidence Rates by Sex and Race/Ethnicity From 2000 to 2002 and 2003 to 2005 in the Two Age Groups With Increases in Incidence Rates
| Parameter | Diagnosis Years |
APC*: 2000-2005 | |
|---|---|---|---|
| 2000-2002 | 2003-2005 | ||
| Age 50-59 years | |||
| Male | |||
| Overall | 15.3 | 21.0 | 10.1† |
| Asian/Pacific Islander | 31.2 | 35.5 | 4.6 |
| Hispanic | 23.0 | 32.8 | 11.6† |
| Black | 28.7 | 40.8 | 10.2† |
| American Indian/Alaska Native | 18.5 | 29.1 | 12.7 |
| White | 11.5 | 16.5 | 11.6† |
| Female | |||
| Overall | 3.1 | 3.8 | 10.3† |
| Asian/Pacific Islander | 6.8 | 5.9 | −0.2 |
| Hispanic | 5.4 | 5.7 | 7.7 |
| Black | 5.2 | 6.9 | 11.4† |
| American Indian/Alaska Native | 2.4 | 8.3 | ‡ |
| White | 2.2 | 3.0 | 13.8† |
| Age 75-84 years | |||
| Male | |||
| Overall | 31.7 | 35.5 | 3.7† |
| Asian/Pacific Islander | 73.9 | 83.1 | 2.0 |
| Hispanic | 64.5 | 53.2 | −3.2 |
| Black | 41.8 | 37.6 | 2.6 |
| American Indian/Alaska Native | 22.9 | 46.8 | ‡ |
| White | 25.6 | 28.8 | 3.6 |
| Female | |||
| Overall | 12.1 | 13.6 | 5.5 |
| Asian/Pacific Islander | 36.6 | 37.9 | 5.6 |
| Hispanic | 25.5 | 25.1 | 5.3 |
| Black | 10.6 | 13.6 | 1.4 |
| American Indian/Alaska Native | 24.0 | 26.6 | −2.8 |
| White | 9.5 | 10.2 | 4.1† |
NOTE. Rates per 100,000, Surveillance, Epidemiology, and End Results 13 registries. Rates among people of Hispanic ethnicity exclude data from the Alaska Native Registry.
Abbreviation: APC, annual percentage change.
APC indicates incidence rates from 2000 to 2005. No other statistically significant APC was seen by age, sex, or race/ethnicity.
Slope of the APC regression line differs from zero, P ≤ .05.
APC could not be calculated.
Age-Adjusted Liver Cancer Mortality Data
Between 1992 and 2005, patterns in liver cancer mortality were similar to patterns in HCC incidence. Overall age-adjusted mortality rates increased, with an annual percent change of 1.6% (P ≤ .05). Mortality rates were highest for Asians/Pacific Islanders, followed by Hispanics, blacks, American Indians/Alaska Natives, and finally whites (Table 1). Rates increased significantly among Hispanics and whites (APC = 1.7%) as well as blacks (APC = 1.3%). Mortality rates among Asians/Pacific Islanders, however, significantly declined (APC = −0.9%), with stable mortality rates among American Indian/Alaska Native populations.
Survival Rates
One-year cause-specific survival rates increased over time for all racial/ethnic groups except American Indians/Alaska Natives (Table 1). During the recent years, 2003 to 2004, the overall 1-year survival rate was 47% (range, 40% for blacks to 49% for Asian/Pacific Islanders).
Overall, survival rates increased for short, intermediate, and longer-term follow-up intervals over the time period of study, with a doubling of 2- to 4-year cause-specific survival rates (Table 3). These increases in survival rates occurred as more patients were diagnosed with localized stage HCC (28% in 1992 to 1993, 44% in 2003 to 2004) and 1-year survival rates for patients with localized HCC increased from 41% to 67%. Among patients with localized HCC that reported therapy, 1-year survival rates increased from 65% in 1992 to 1993 to 83% in 2003 to 2004. One-year survival rates for patients with localized HCC who received surgery increased from 81% to 91% between 1992 to 1993 and 2003 to 2004 (data not shown). Survival rates also increased among patients with regional HCC who reported therapy. Although changes in survival rates for cases with distant HCC were less pronounced, patients reporting treatment had higher survival rates.
Table 3.
Cause-Specific Hepatocellular Carcinoma Survival Rates From 1992 to 2004, by Time Period, Stage of Diagnosis, and Reported Initial Treatment
| Parameter | Diagnosis Years (%) |
||||
|---|---|---|---|---|---|
| 1992-1993 | 1994-1996 | 1997-1999 | 2000-2002 | 2003-2004 | |
| No. of patients | 1,635 | 2,868 | 3,498 | 4,118 | 3,169 |
| Overall survival by year | |||||
| 1 | 25 | 29 | 34 | 40 | 47 |
| 2 | 16 | 18 | 23 | 29 | 35 |
| 3 | 11 | 14 | 18 | 24 | * |
| 4 | 10 | 12 | 15 | 20 | * |
| 5 | 8 | 10 | 13 | * | * |
| Patients by stage at diagnosis | |||||
| Localized | 28 | 30 | 33 | 40 | 44 |
| Regional | 22 | 26 | 28 | 30 | 29 |
| Distant | 22 | 21 | 19 | 18 | 17 |
| 1-year survival by stage | |||||
| Localized | 41 | 48 | 57 | 59 | 67 |
| Regional | 25 | 25 | 28 | 34 | 39 |
| Distant | 12 | 13 | 11 | 14 | 15 |
| Patients by stage and initial treatment (%) | |||||
| Localized | |||||
| Treated | 44 | 48 | 55 | 57 | 62 |
| Not treated | 53 | 50 | 44 | 41 | 37 |
| Regional | |||||
| Treated | 41 | 35 | 41 | 43 | 45 |
| Not treated | 57 | 62 | 57 | 54 | 54 |
| Distant | |||||
| Treated | 34 | 37 | 32 | 36 | 34 |
| Not treated | 63 | 61 | 66 | 62 | 63 |
| 1-year survival by stage and initial treatment | |||||
| Localized | |||||
| Treated | 65 | 67 | 73 | 77 | 83 |
| Not treated | 20 | 28 | 34 | 33 | 38 |
| Regional | |||||
| Treated | 39 | 43 | 45 | 56 | 60 |
| Not treated | 15 | 15 | 15 | 17 | 21 |
| Distant | |||||
| Treated | 25 | 20 | 20 | 23 | 30 |
| Not treated | 5 | 8 | 7 | 9 | 6 |
NOTE. Proportions of patients by stage and treatment status do not sum to 100% due to exclusion of cases with unstaged disease and unknown treatment status.
Not enough intervals to produce a rate.
DISCUSSION
The principal findings in this report were that incidence rates of HCC tripled in the United States from 1975 through 2005, with marked recent increases among middle-aged black, Hispanic, and white males; there was a birth-cohort effect on risk; and overall 1-year cause-specific survival rates for new HCC patients nearly doubled from 1992 to 2004 as more patients were diagnosed with low-stage HCC and their prognosis improved. Increases in cause-specific survival rates were experienced by all racial and ethnic groups, except for American Indians/Alaska Natives. However, despite increasing HCC survival rates, improvement is needed, with the 1-year cause-specific survival rate remaining lower than 50%.
Much of the increase in incidence between 2000 and 2005 occurred among men age 50 to 59 years. Not all racial/ethnic groups were equally affected, with black, Hispanic, and white men experiencing the greatest increases. Between 2003 and 2005, for both sexes, blacks in this age group had higher incidence rates than Asian/Pacific Islanders. The changing racial pattern of HCC in this age group may be partially attributable to an epidemic of HCV infection that occurred approximately four decades earlier, in the 1960s, when they were young adults.10 Several findings in this report suggest, however, that risk of HCC is likely to be driven by additional factors. There has been a steady increase in age-adjusted HCC incidence rates among men and women since 1975. Age-specific rates have increased in each successive birth cohort between 1900 and 1959. Etiologic studies of recent HCC patients are recommended to elucidate factors contributing to the ongoing increase in HCC incidence.
In contrast with other racial groups, most Asians/Pacific Islanders with HCC were born outside the United States. This predominance of foreign births is consistent with HBV infection being a major risk factor among most groups of Asians/Pacific Islanders9,20 as chronic HBV infection is notably more common in eastern Asian countries than in the United States, with higher HCC incidence rates among Southeast Asians compared with Filipinos, Japanese, and Asian Indians/Pakistanis.19 An exception is Japan, where more HCC patients are linked to HCV infection than HBV infection.21 The modest increase in HCC incidence rates and decrease in liver cancer mortality rates from 1992 to 2005 suggests that Asians/Pacific Islander populations born in the United States have, thus far, not been as affected by the factors that are driving the HCC increases in other populations. Worldwide, the incidence of HCC among Asians/Pacific Islanders is likely to decline as HBV vaccination becomes more widespread in Asian countries.22
In this study, HCC incidence rates among Hispanics were lower than among Asians/Pacific Islanders, but higher than among whites. Approximately one half of Hispanic HCC patients were born in the United States. In contrast to Asians/Pacific Islanders, HCC incidence rates are reported to be higher among Hispanics born in the United States than among foreign-born Hispanics.23 This suggests that factors associated with life in the United States, perhaps including HCV infection,10 alcohol abuse,24 or obesity,25 may be adversely affecting Hispanic populations.
Despite a paucity of new therapies for HCC during the surveillance years in this report, 1-year cause-specific survival rates for HCC nearly doubled between 1992 to 1993 and 2003 to 2004. This supports earlier evidence that survival rates for HCC are increasing.26 Initially, the increase in survival was restricted to short-term follow-up27; however, in this report 4-year survival rates doubled and 5-year survival rates increased by more than 60%. Furthermore, more patients were diagnosed with localized and regional stage HCC and survival rates increased for these patients. With greater awareness of HCC, more patients are being diagnosed with asymptomatic disease via active screening employing serum alpha-fetoprotein testing, abdominal ultrasound, and diagnostic imaging.28 Furthermore, aggressive treatments including transplantation and resection of localized-stage tumors appear to be improving long-term survival.29 The advent of targeted HCC therapies holds promise for further improvement in prognosis among patients with regional and distant-stage HCC.30
Racial variation was seen in 1-year cause-specific survival rates. In 2003 to 2004, survival was greatest among Asians/Pacific Islanders (49%) and lowest among blacks (40%) and American Indians/Alaska Natives (41%). In another study, racial and ethnic variations in survival were partially explained by stage at diagnosis and the therapy received by blacks and Hispanics compared with whites.26 While improvements in survival are needed across all racial and ethnic groups, blacks and American Indians/Alaska Natives may particularly benefit from targeted HCC control efforts.
Coordination of existing HCC prevention efforts is needed. Primary HCC prevention measures include hepatitis B vaccination programs,22 screening of the blood supply for hepatitis viruses,31 and campaigns to discourage intravenous drug abuse. Secondary prevention measures focus on detecting asymptomatic HCC among at-risk individuals28 through periodic screening of high-risk patients by ultrasound, with follow-up tests when suspicious lesions are detected.32 High-risk individuals include cirrhotic patients, adult HBV carriers, and persons infected with HCV. Gaps in HCC screening in the United States include limited HCV testing of current and former injected drug users,33 pre-1990 transfusion recipients,34 and incomplete HBV testing of foreign-born Asians/Pacific Islanders.35 In addition, cultural and economic barriers to HCC screening of chronic viral carriers exist.36,37 Tertiary measures for HCC vary by stage of disease and comorbidity but include surgical resection, transplantation, radiofrequency ablation, and chemoembolization.28 A SEER-Medicare linked study of HCC patients diagnosed in the 1990s indicated that only one third of cases with favorable tumor features received potentially curative therapy.38
A limitation of this study is the absence of information on risk factors. There is a need for studies to estimate the current proportion of HCCs that are attributed to HCV and to HBV including differences across demographic groups.9,39 Studies are needed of the oncogenic potential of viral genotypes.40,41 Associations with suspected cofactors for HCC should also be examined, including alcoholism24 and steatohepatitis,25 obesity,42 diabetes mellitus,43 and iron storage diseases.44
This report may underestimate recent increases in HCC incidence rates because of delays in reporting16 and an estimated rate of under-reporting of 2.5% because of absence of data on Veterans' Affairs hospital patients,5 a population which may be at elevated risk for HCC compared with the general population.45 Despite these limitations, the findings strongly suggest that HCC incidence and mortality continue to increase in the United States. In addition, this report provides reason for optimism that, with more HCC screening of high-risk groups and treatment of low-stage disease, the burden of HCC can be lessened.
Acknowledgment
We thank Brenda Edwards, Lynn Ries, Barry Miller, Kathy Cronin, and Carol Kosary for assistance with analysis and review.
Footnotes
Supported by the Division of Cancer Control and Population Sciences, Surveillance Research Program, National Cancer Institute, National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Sean F. Altekruse, Marsha E. Reichman
Collection and assembly of data: Sean F. Altekruse, Marsha E. Reichman
Data analysis and interpretation: Sean F. Altekruse, Katherine A. McGlynn, Marsha E. Reichman
Manuscript writing: Sean F. Altekruse, Katherine A. McGlynn, Marsha E. Reichman
Final approval of manuscript: Sean F. Altekruse, Katherine A. McGlynn, Marsha E. Reichman
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden: GLOBOCAN 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Curado MP, Edwards B, Shin HR, et al., editors. Vol. IX. Lyon, France: IARC; 2007. Cancer Incidence in Five Continents. IARC Scientific Publications, No. 160. [Google Scholar]
- 3.El-Serag HB, Davila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: An update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 4.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–2152. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 5.Ries LAG, Melbert D, Krapcho M, et al., editors. Bethesda, MD: National Cancer Institute; 2008. SEER Cancer Statistics Review, 1975-2005. http://seer.cancer.gov/csr/1975_2005. [Google Scholar]
- 6.London WT, McGlynn KA. Liver cancer, In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. ed 3. New York, NY: Oxford University Press; 2006. pp. 763–786. [Google Scholar]
- 7.Seeff LB. Introduction: The burden of hepatocellular carcinoma. Gastroenterology. 2004;127:S1–S4. doi: 10.1053/j.gastro.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 9.Barazani Y, Hiatt JR, Tong MJ, et al. Chronic viral hepatitis and hepatocellular carcinoma. World J Surg. 2007;31:1243–1248. doi: 10.1007/s00268-007-9041-3. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 11.Wasley A, Grytdal S, Gallagher K. MMWR Surveillance Summaries. Vol. 57. Atlanta, GA: 2008. Surveillance for acute viral hepatitis — United States, 2006; pp. SS–2. 1–24. [PubMed] [Google Scholar]
- 12.Davila JA, Morgan RO, Shaib Y, et al. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: A population-based study. Gastroenterology. 2004;127:1372–1380. doi: 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Fritz A, Percy C, Jack A, et al. ed 3. Geneva, Switzerland: World Health Organization; 2000. International Classification of Diseases for Oncology. [Google Scholar]
- 14.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States) Cancer Causes Control. 2006;17:771–781. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 15.United States Department of Health and Human Services. Bethesda, MD: National Cancer Institute; Population estimates used in NCI's SEER*Stat Software. http://seer.cancer.gov/popdata/methods.html. [Google Scholar]
- 16.Clegg LX, Feuer EJ, Midthune DN, et al. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94:1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 17.United States Department of Health and Human Services. Bethesda, MD: National Cancer Institute; Cancer Query Systems: Delay-Adjusted SEER Incidence Rates. http://srab.cancer.gov/delay/canques.html. [Google Scholar]
- 18.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed F, Perz JF, Kwong S, et al. National trends and disparities in the incidence of hepatocellular carcinoma, 1998-2003. Prev Chronic Dis. 2008;5:A74. [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenblatt KA, Weiss NS, Schwartz SM. Liver cancer in Asian migrants to the United States and their descendants. Cancer Causes Control. 1996;7:345–350. doi: 10.1007/BF00052940. [DOI] [PubMed] [Google Scholar]
- 21.Kiyosawa K, Umemura T, Ichijo T, et al. Hepatocellular carcinoma: Recent trends in Japan. Gastroenterology. 2004;127:S17–S26. doi: 10.1053/j.gastro.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Geneva, Switzerland: World Health Organization; 2006. WHO vaccine-preventable diseases: Monitoring system, 2006 global summary. [Google Scholar]
- 23.El-Serag HB, Lau M, Eschbach K, et al. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167:1983–1989. doi: 10.1001/archinte.167.18.1983. [DOI] [PubMed] [Google Scholar]
- 24.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87–S96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Caldwell SH, Crespo DM, Kang HS, et al. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–S103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Davila JA, El-Serag HB. Racial differences in survival of hepatocellular carcinoma in the United States: A population-based study. Clin Gastroenterol Hepatol. 2006;4:104–110. [PubMed] [Google Scholar]
- 27.Capocaccia R, Sant M, Berrino F, et al. EUROCARE Working Group: Hepatocellular carcinoma: Trends of incidence and survival in Europe and the United States at the end of the 20th century. Am J Gastroenterol. 2007;102:1661–1670. doi: 10.1111/j.1572-0241.2007.01337.x. [DOI] [PubMed] [Google Scholar]
- 28.El-Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz RE, Smith DD. Trends in local therapy for hepatocellular carcinoma and survival outcomes in the US population. Am J Surg. 2008;195:829–836. doi: 10.1016/j.amjsurg.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Atlanta, GA, MMWR Recomm Rep. 1998;47:1–39. [PubMed] [Google Scholar]
- 32.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma: Conclusions of the Barcelona-2000 EASL Conference. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 33.Edlin BR, Carden MR. Injection drug users: The overlooked core of the hepatitis C epidemic. Clin Infect Dis. 2006;42:673–676. doi: 10.1086/499960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rustgi VK. The epidemiology of hepatitis C infection in the United States. J Gastroenterol. 2007;42:513–521. doi: 10.1007/s00535-007-2064-6. [DOI] [PubMed] [Google Scholar]
- 35.Hu KQ. Hepatitis B virus (HBV) infection in Asian and Pacific Islander Americans (APAIs): How can we do better for this special population? Am J Gastroenterol. 2008;103:1–10. doi: 10.1111/j.1572-0241.2008.01878.x. [DOI] [PubMed] [Google Scholar]
- 36.Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: Tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98:1934–1939. [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen TT, Taylor V, Chen MS, Jr, et al. Hepatitis B awareness, knowledge, and screening among Asian Americans. J Cancer Educ. 2007;22:266–272. doi: 10.1007/BF03174128. [DOI] [PubMed] [Google Scholar]
- 38.El-Serag HB, Siegel AB, Davila JA, et al. Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: A population-based study. J Hepatol. 2006;44:158–166. doi: 10.1016/j.jhep.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Yu L, Sloane DA, Guo C, et al. Risk factors for primary hepatocellular carcinoma in black and white Americans in 2000. Clin Gastroenterol Hepatol. 2006;4:355–360. doi: 10.1016/j.cgh.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Bruno S, Crosignani A, Maisonneuve P, et al. Hepatitis C virus genotype 1b as a major risk factor associated with hepatocellular carcinoma in patients with cirrhosis: A seventeen-year prospective cohort study. Hepatology. 2007;46:1350–1356. doi: 10.1002/hep.21826. [DOI] [PubMed] [Google Scholar]
- 41.Chan HL, Tse CH, Mo F, et al. High viral load and hepatitis B virus subgenotype ce are associated with increased risk of hepatocellular carcinoma. J Clin Oncol. 2008;26:177–182. doi: 10.1200/JCO.2007.13.2043. [DOI] [PubMed] [Google Scholar]
- 42.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 43.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: A systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Ko C, Siddaiah N, Berger J, et al. Prevalence of hepatic iron overload and association with hepatocellular cancer in end-stage liver disease: Results from the National Hemochromatosis Transplant Registry. Liver Int. 2007;27:1394–1401. doi: 10.1111/j.1478-3231.2007.01596.x. [DOI] [PubMed] [Google Scholar]
- 45.Harris RE, Hebert JR, Wynder EL. Cancer risk in male veterans utilizing the Veterans Administration medical system. Cancer. 1989;64:1160–1168. doi: 10.1002/1097-0142(19890901)64:5<1160::aid-cncr2820640533>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]