Abstract
Purpose
153Sm-ethylenediaminetetramethylenephosphonic acid (EDTMP; Quadramet®) is indicated for the treatment of painful bone metastases, whereas zoledronic acid (Zometa®) is indicated for the prevention of skeletal complications. Because of the different therapeutic effects, combining the treatments may be beneficial. Both, however, accumulate in areas with increased osteoblastic activity. Possible drug interactions were investigated.
Methods
Patients with hormone-refractory prostate cancer were treated with 18.5 MBq/kg 153Sm-EDTMP in weeks 1 and 3 and with 37 MBq/kg in week 15. Treatment with 4 mg zoledronic acid began in week 3 and continued every 4 weeks through week 23. In weeks 3 and 15, zoledronic acid was administered 2 days before 153Sm-EDTMP treatment. Urine was collected 48 h after injection of 153Sm-EDTMP, and whole-body images were obtained 6, 24 and 48 h post-injection. The effect of zoledronic acid on total bone uptake of 153Sm-EDTMP was measured indirectly by the cumulative activity excreted in the urine in weeks 1, 3 and 15. Biodistribution, safety, tolerability and effect on prostate-specific antigen level were also studied.
Results
The urinary excretion in week 3 divided by the urinary excretion in week 1 (baseline) times 100% was mean 98.4 ± 11.6% (median 96.2%). From week 1 to 15, after four zoledronic acid treatments, the mean ratio was 101.9 ± 10.7% (median 101.8%). Bioequivalence could be concluded by using a two-sample t test for both per-protocol (n = 13) and full-analysis sets (n = 18). Toxicity was comparable to of monotherapy with 153Sm-EDTMP.
Conclusion
Zoledronic acid treatment does not influence 153Sm-EDTMP skeletal uptake. Combined treatment is feasible and safe.
Keywords: Bone metastases, Pain, Samarium, Zoledronic acid, Nuclear therapy
Introduction
Prostate cancer is one of the most common malignancies worldwide. Approximately 50–70% of patients present at a locally advanced stage, and ~15–30% have bone metastases at the time of diagnosis [1]. Metastatic disease may be found upon presentation or may develop after treatment for localised disease.
In advanced prostate cancer, spread of the disease to the skeleton occurs in the majority of patients, with skeletal metastases being predominantly osteoblastic in nature. The clinical course of metastatic bone disease in prostate cancer is relatively long, with patients experiencing complications over a period of several years. These complications include bone pain, fractures, hypercalcemia and spinal cord compression, all of which may profoundly impair a patient’s quality of life. Careful attention to pain management by care providers is crucial.
External radiotherapy is the best treatment for localised metastatic bone pain [2]. Nevertheless, external beam radiotherapy is less favourable when the disease has metastasised globally because the effective radiation dose is limited by toxicity in adjacent or overlapping critical structures and organs. Radionuclide therapy has been proposed as an alternative modality for the management of bone pain. These radiopharmaceuticals localise preferentially in active bone and mainly at metastatic lesions, allowing site-directed radiotherapy [3].
Samarium-153-ethylenediaminetetramethylphosphonic acid (153Sm-EDTMP or 153Sm-lexidronam; Quadramet®) is a radiopharmaceutical compound that has an affinity for skeletal tissue and concentrates in areas of increased bone turnover [4]. 153Sm-EDTMP is indicated for the relief of pain in patients with osteoblastic metastatic bone lesions at a dose of 37 MBq/kg (1.0 mCi/kg). With a half-life of 46.3 h, the radioisotope emits a 103-keV gamma ray (29%) for external imaging and beta particles (average energy 233 keV) for localised radiotherapy. The average range of emission of 153Sm-electrons is only 1.7 mm in bone, limiting the exposure of bone marrow and other adjacent tissues to radiation. The combination of radiopharmaceuticals with other treatment options (systemic or local) needs to be investigated to further improve efficacy [5, 6].
To minimise the incidence of skeletal-related events, bisphosphonates may be indicated as a systemic treatment for patients with osseous metastases. Bisphosphonates are characterised by a central phosphorus–carbon–phosphorus structure. They bind tightly to the calcified bone matrix and are powerful inhibitors of osteoclast-mediated bone resorption. Zoledronic acid, a new-generation bisphosphonate, exhibits a more potent inhibitory activity of osteoclasts compared with other bisphosphonates [7]. In July 2002, the European Agency for the Evaluation of Medicinal Products granted marketing authorisation in the European Union for Zometa® (zoledronic acid) for the prevention of skeletal-related events in patients with advanced malignancies involving bone. These malignancies include multiple myeloma, prostate cancer, breast cancer, lung cancer, renal cancer and other solid tumors.
To manage potential complications related to bone metastasis in prostate cancer (pain, pathological fractures, etc.), several approaches are being developed, including radiopharmaceuticals and bisphosphonates. With regard to pain relief, radiopharmaceuticals are indicated in prostate cancer. In contrast, the efficacy of bisphosphonates for pain management has not been clearly demonstrated [8]. Combined treatment with both pharmaceuticals may improve palliative care and enhance overall efficacy [9].
However, there are conflicting data as to whether bisphosphonates inhibit the uptake of radiolabelled phosphonates in bone metastases. Some studies reported that bone uptake of 99mTc-labelled bone scanning agents was decreased in patients receiving etidronate intravenously or orally [10–12]. On the other hand, other studies reported the feasibility of the combined use of bisphosphonates with radiolabelled phosphonates [13–15]. With regard to bone-seeking radiopharmaceuticals, proper studies evaluating the influence of bisphosphonates on bone uptake are non-existent.
Patients with an indication for 153Sm-EDTMP therapy often receive monthly infusions of bisphosphonates such as zoledronic acid, and these related compounds are both taken up by the bone. Thus, the purpose of the present clinical trial was to investigate the effects of zoledronic acid on the bone uptake of 153Sm-EDTMP and on bone metabolism and to assess the safety of the combined use of both products.
Materials and methods
Study population
Patients with histologically documented adenocarcinoma of the prostate, progressive hormone-refractory disease and more than one bone metastasis were included in this open-label prospective study. Other inclusion criteria were a Karnofsky performance status of at least 70%, life expectancy of at least 8 months, age of at least 18 years and the ability to understand and willingness to sign an informed consent document. Patients receiving bisphosphonate therapy had to discontinue their treatment for at least 3 months before study entry, and patients under luteinising hormone–releasing hormone agonists had to continue their treatment. Patients with pathologic long-bone fractures or metastatic involvement of >75% of the ribs, vertebrae and pelvic bones and patients with known malignancies other than prostate cancer (not including basal cell carcinoma of the skin) were excluded. Other exclusion criteria were chemotherapy (including Estracyt®) within the past 5 years; prior treatment with systemic radiotherapeutic bone agents; receipt of any other investigational drug within 4 weeks of study entry; previous hemi-body external radiation therapy (for >25% of the bone marrow within 90 days); concomitant treatment with aminoglycosides; clinically significant bleeding disorders; hypersensitivity to phosphonate compounds, mannitol or zoledronic acid; concurrent illnesses or treatments that might preclude study completion; active central nervous system or epidural brain metastasis; absolute neutrophil count <2 × 109/l; platelet count <150 × 109/l; hemoglobin <6.2 mmol/l; serum creatinine >177 μmol/l; or total prostate-specific antigen (PSA) <5 ng/ml. The study was approved by the local ethics committee, and written informed consent was obtained from all patients.
Treatment
Included patients were treated with 18.5 MBq/kg (0.5 mCi/kg) 153Sm-ethylenediaminetetramethylphosphonic acid (EDTMP; Quadramet®; CIS bio International, Saclay, France) in week 1 (mean ± 1 SD, 1,606 ± 252 MBq) and week 3 (mean ± 1 SD, 1,672 ± 293 MBq) and with 37 MBq/kg (1.0 mCi/kg) 153Sm-EDTMP in week 15 (mean ± 1 SD, 3,319 ± 609 MBq). The three intravenous injections contained the same absolute amount of EDTMP.
To avoid an effect of progressive disease with significant changes in skeletal metastatic load, a short interval of 2 weeks was chosen between the first two treatments. Half the usual dose of 153Sm-EDTMP was administered to avoid unacceptable toxicity. Although this regimen is unusual, it seems acceptable from a therapeutic standpoint. A dose response relation for 153Sm-EDTMP was not clearly found in all studies [16]. Furthermore, repeated treatments at short intervals have shown favourable responses [17]. The interval between the second and third treatment with 153Sm-EDTMP (12 weeks) was chosen to allow (a) bone marrow recovery from the first two treatments and (b) four consecutive administrations of zoledronic acid before the third 153Sm-EDTMP treatment. Another 12-week interval between the third treatment and the final visit in week 27 was chosen to allow bone marrow recovery.
Treatment with 4 mg zoledronic acid (Zometa®; Novartis, Stein, Switzerland) every 4 weeks started in week 3 and continued through week 23 (six treatments total). In weeks 3 and 15, zoledronic acid was administered 2 days (48 h) before 153Sm-EDTMP treatment. Zoledronic acid treatments were injected as single 15-min intravenous infusions in a daycare setting. After infusion, vital signs were recorded every 30 min for the first 2 h, after which, patients were discharged. In weeks 1, 3 and 15, patients were hospitalised for 48 h after 153Sm-EDTMP administration. Urine was collected during the 48 h after injection of 153Sm-EDTMP in five portions (0–4, 4–8, 8–12, 12–24 and 24–48 h). Whole-body images were captured with a dual-head gamma camera at 6, 24 and 48 h post-injection (anterior and posterior). Patients with lower urinary tract obstruction or incontinence had to consent to catheterisation of the bladder for up to 48 h.
Analysis
After intravenous injection of 153Sm-EDTMP, most of the activity is rapidly cleared from the blood and excreted in urine. No appreciable extraskeletal uptake was observed on the scintigraphic images because the remaining activity in the body was almost completely localised to the skeletal mass. Previous animal studies confirm the minimal uptake of 153Sm-EDTMP by extraskeletal tissues [4, 18]. Therefore, the uptake of 153Sm-EDTMP by the whole skeleton was studied indirectly by measuring the urinary excretion of activity [19, 20].
After the initial injection of 153Sm-EDTMP, urine was collected over the next 48 h. The amount of activity in these samples was determined by measurement of 15-ml non-diluted samples with a dose calibrator. For comparison with the administered activity, the exact injected dose was determined by measurement of the syringe before and after administration. This procedure enabled determination of the amount of activity excreted and, as a corollary, the relative amount of activity retained within the body. The primary endpoint of the study was the effect of zoledronic acid on the urinary excretion of 153Sm-EDTMP, which was measured in weeks 1 and 3, i.e. before and after the first zoledronic acid infusion. The effect of repeated zoledronic acid treatment on the urinary excretion of 153Sm-EDTMP in weeks 1 and 15 was a secondary endpoint.
Secondary endpoints also included the effects of the combined use of zoledronic acid and 153Sm-EDTMP on the uptake of 153Sm-EDTMP in bone metastases, liver and kidneys. The scintigraphic images were evaluated by an independent certified nuclear physician with expertise in skeletal scintigraphy, blinded for patient data and the week the images were acquired. Images of each week (6, 24 and 48 h after injection) were shown together for each patient, as the reader could anyhow identify the different points in time by the decrease in activity. The reader started with the ‘6-h’ image and drew regions of interest (ROIs; at least 25 pixels) for three metastatic bone lesions, normal bone (femur), background (soft tissue of the thigh), the liver (ROIs without overlap of ribs. Anterior image: paravertebral space at height Th11–Th12; posterior image: interribonspace 9–10 or 10–11) and kidneys (arithmetic mean of both kidneys was used). ROIs were copied to the other images of the same patient. From all ROIs, 30% isocontour ROIs were automatically drawn. Geometric means of anterior and posterior images were calculated for these ROIs and corrected for background. Evaluation of biodistribution was based on a quantitative analysis of the pixel-normalised lesion-to-normal bone ratios and pixel-normalised organ-to-normal bone ratios. Blood sampling was not performed.
Other secondary endpoints were the following: safety and tolerability of the combined use of 153Sm-EDTMP and zoledronic acid (vital signs, laboratory values and adverse events according to the NCI Common Terminology Criteria for Adverse Events, version 3.0), the effect on markers of bone metabolism as measured in blood and urine samples [serum bone-specific alkaline phosphatase (BAP), serum procollagen type 1 N propeptide (PINP) and the urinary N-terminal type 1 collagen peptide (NTX)] and the effect on PSA levels.
The present study was not designed to investigate the efficacy of the combined treatment.
Statistical methods
Descriptive statistics [n, mean, standard deviation (SD), minimum, quartile 1 (Q1), median, quartile 3 (Q3), and maximum] were calculated for quantitative variables; frequency counts by category were determined for qualitative variables. A bioequivalence test was performed using the classical 90% confidence interval method for the ratio of the cumulated activities excreted in urine within 48 h after administration in weeks 3 and 1 [(excreted urine week 3)/(excreted urine week 1) × 100%]. For a broad range of drugs, the Food and Drug Administration (FDA) has chosen a limit of 80–125% for the confidence interval of a bioequivalence test. Generally, the limit of 80–125% is based on a clinical judgment that a test product with measures outside this range should be denied market access. If the confidence interval lay completely within the equivalence interval of 80–125%, equivalence was concluded.
The primary target variable was analysed with a two-sample t test. It was assumed that this parameter was log-normally distributed; therefore, the logarithms (base e) were analysed.
Results
A total of 20 patients were enrolled in this study. Two patients were not treated because of low platelet counts. Major protocol deviations leading to exclusion from analysis of the primary objective were found in five patients. One patient had not stopped anti-androgen medication; one patient received a dosage in week 3 higher than the required dose due to miscalculation of his weight, and this patient also started anti-androgen treatment; one patient went off-study before treatment in week 3 due to progressive disease (lymphangitis carcinomatosa); and two patients had no or incomplete urine collection. Thus, 18 patients were included in the full-analysis set (FAS) and 13 in the per-protocol set (PPS). All patients in the FAS were white with a mean age of 67.3 years (range 61–74).
Among the 18 patients who entered the treatment period, seven withdrew before the end of the treatment period (before week 23), and 11 completed both the treatment period and the study course (27 weeks). Two patients were withdrawn because of adverse events (increase in creatinine level, disseminated intravascular coagulation), four patients were recommended to start with radiotherapy or chemotherapy because of progressive disease, and one patient was withdrawn due to decreasing PSA level after the start of anti-androgen treatment.
Of the 18 included patients, 13 were evaluable for the primary endpoint (PPS). Urinary excretion data expressed as percentage of administered activity are shown in Table 1. The mean ratio of urinary excretion of 153Sm in week 3 to that at baseline (week 1) expressed as a percentage was 98.4 ± 11.6% (median 96.2%, range 81.1–118.9%; Table 2). The point estimate and the 90% confidence interval for the assessment of bioequivalence were 97.83% (92.39–103.60%) for weeks 1 to 3, which was completely within the presumed equivalence range used by the FDA (80–125%). Hence, bioequivalence could be concluded.
Table 1.
Urinary excretion of 153Sm-EDTMP (percentage of administered activity)
| Patient | Week 1 | Week 3 | Week 15 |
|---|---|---|---|
| 1 | 36.3 | 9.2a | 39.3 |
| 2 | 48.5 | 57 | 55.9 |
| 3 | Screening failureb | ||
| 4 | 37.6 | 44.7 | 42.8 |
| 5 | 43.7 | 46 | 44.3 |
| 6 | 55.6 | 48.8 | 50.2 |
| 7 | 43.8 | 46.7 | 50.1 |
| 8 | –a | 37.8 | Off study |
| 9 | 43.5 | 49.2 | 48.5 |
| 10 | 47.5 | 43.1 | 48.6 |
| 11 | Screening failureb | ||
| 12 | 46.6 | 49.2 | 47 |
| 13 | 32 | 30 | Off study |
| 14 | 38.1 | 37 | 31.7 |
| 15 | 30.8 | 27.1 | Off study |
| 16 | 33.7 | 38.1 | Off study |
| 17 | 31.8 | 25.8 | Off study |
| 18 | 39.6 | 38.1 | 37.1 |
| 19 | 39.8 | 36.3 | 41.1 |
| 20 | 51.2 | Off study | Off study |
aCollection of urine was not complete/not performed
bExclusion due to low trombocyte count
Table 2.
Descriptive statistics of ratios (%) of urinary excretion of 153Sm-EDTMP
| N | Mean (%) | SD | Min | Q1 | Median (%) | Q3 | Max | |
|---|---|---|---|---|---|---|---|---|
| Week 3/Week 1 | ||||||||
| PPS | 13 | 98.44 | 11.58 | 81.13 | 90.74 | 96.21 | 105.58 | 118.88 |
| FAS | 16 | 95.70 | 22.00 | 25.34 | 89.36 | 96.66 | 109.84 | 118.88 |
| Week 15/Week 1 | ||||||||
| PPS | 10 | 101.85 | 10.71 | 83.20 | 93.69 | 101.84 | 113.83 | 115.26 |
| FAS | 12 | 103.18 | 10.20 | 83.20 | 97.27 | 102.79 | 112.66 | 115.26 |
| Week 15/Week 3 | ||||||||
| PPS | 10 | 100.48 | 8.59 | 85.68 | 95.75 | 97.72 | 107.28 | 113.22 |
| FAS | 12 | 127.55 | 94.68 | 85.68 | 96.03 | 98.32 | 110.02 | 427.17 |
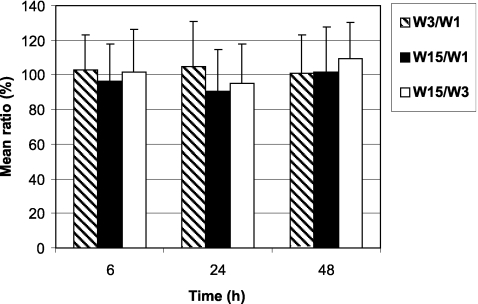
As shown in Fig. 1, there was no change in the fraction of 153Sm-EDTMP excreted in urine after one administration of zoledronic acid (week 3) or after repeated administrations of zoledronic acid (week 15) compared with the urinary excretion at baseline (week 1). Descriptive statistics were similar in the PPS and the FAS except for the mean ratio of 153Sm-EDTMP excretion between weeks 15 and 3, which was higher for FAS (127.55%) due to the low value of urinary excretion in one patient at week 3. This patient was excluded from the PPS because the collection of his urine at week 3 was not complete (Tables 1 and 2).
Fig. 1.
Urinary excretion of 153Sm-EDTMP in week 3 (combined therapy with zoledronic acid) divided by urinary excretion of 153Sm-EDTMP in week 1 (without zoledronic acid) times 100%. Also, for week 15 (after repeated zoledronic acid administrations) vs week 1 and week 15 vs week 3. All data for the per-protocol set (PPS). Zoledronic acid does not change the excretion nor the uptake of 153Sm-EDTMP because no changes between different weeks were observed (all ratios are close to 100%)
The uptake of 153Sm-EDTMP in bone metastases or organs vs uptake in normal bone was determined by calculation of the lesion- or organ-to-normal bone ratios at each time point (skeletal scintigrams were obtained 6, 24 and 48 h post-injection). Figure 2 shows the uptake of 153Sm-EDTMP in normal bone 6, 24 and 48 h after each administration of 153Sm-EDTMP. The uptake of activity in normal skeleton was dosage- and time-dependent because it decreased with increasing time after injection (from 6 to 48 h post-injection) and increased proportionally with increased injected dose (18.5 MBq/kg at weeks 1 and 3 vs 37 MBq/kg at week 15). 153Sm-EDTMP was bound to the bone in a stable manner because the decrease in uptake between 6 and 48 h was due solely to the physical decay of 153Sm. Importantly, the FAS results showed no effect of one dose (week 3) or repeated doses (week 15) of zoledronic acid on the uptake of 153Sm-EDTMP by normal bone. Similar results were obtained for the PPS.
Fig. 2.
Mean (±1 SD) uptake (counts per pixel) of 153Sm-EDTMP in normal bone in post-treatment skeletal scintigrams obtained 6, 24 and 48 h after administration for the full-analysis set (FAS). Weeks 1, 3, and 15 are compared. q = 18.5 MBq/kg 153Sm-EDTMP, Q = 37 MBq/kg 153Sm-EDTMP; Z Zoledronic acid
The 153Sm-EDTMP uptake ratios of metastases/normal bone, kidneys/normal bone and liver/normal bone were calculated at baseline (week 1), week 3 and week 15. Figure 3 shows the differences between these time points given as ratios, e.g. ratio of metastases/normal bone at week 3 to metastases/normal bone at baseline × 100% (given numbers are thus ratios of ratios). The uptake of 153Sm-EDTMP in bone metastases was relatively insensitive to variation in the injected dose of 153Sm-EDTMP and to repeated administrations of zoledronic acid and 153Sm-EDTMP. In addition, the uptake of 153Sm-EDTMP in kidneys and liver was not affected by the activity of the injected dose of 153Sm-EDTMP or by repeated administrations of zoledronic acid and 153Sm-EDTMP. Similar results were obtained for the FAS and the PPS. No significant changes were found. None of the images showed liver uptake or any other extraskeletal uptake besides kidneys and bladder. Activity in the kidneys and bladder was only visualised on the early images.
Fig. 3.
Per patient (FAS), a total of nine scintigrams were performed at three different weeks (weeks 1, 3 and 15) and three different time points post injection (6, 24 and 48 h p.i.). Lesion-to-normal bone ratios were calculated on every scintigram. Comparison between different weeks were made for every time point post-injection as follows: [lesion-to-normal bone ratio] week 3, 6 h p.i. divided by [lesion-to-normal bone ratio] week 1, 6 h p.i., times 100%. Zoledronic acid does not change the uptake of 153Sm-EDTMP in lesions compared to normal bone (all ratios are close to 100%)
Blood sampling was not performed. Blood plasma activity clearance was therefore not measured. Because uptake in normal bone, metastatic bone and organs, as well as urinary excretion of activity were stable, it is reasonable to assume that the retention of activity in the blood pool compartment did not change either. Any change in the retention of activity in this compartment would lead to a secondary change in either urinary excretion of activity or uptake in the skeleton. This hypothesis is supported by stable and low background activity as measured in the thigh. Besides minimal visualisation of activity in soft tissue, the measured soft tissue activity was approximately 30–40 times less than normal bone activity [0.6 ± 0.1 counts per pixel compared to 22.1 ± 5.7 counts per pixel 6 h p.i. in week 1; 0.7 ± 0.1 counts per pixel compared to 22.3 ± 4.3 counts per pixel 6 h p.i. in week 3 (PPS)].
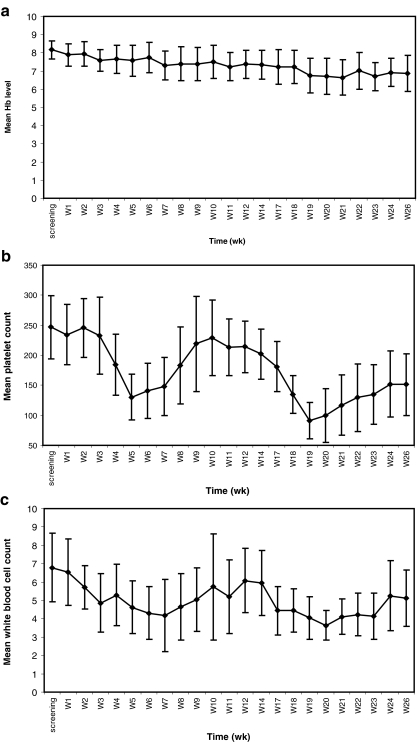
After onset of the study treatment, hematological parameters were measured on a weekly basis (Fig. 4) and serum chemistry (including creatinine level) on a monthly basis. All patients experienced the expected temporary decline in platelet count in week 5 (mean ± 1 SD, 42.2 ± 18.8% decline, 4 weeks after the first 153Sm-EDTMP administration) and in week 19 (64.3 ± 9.9% decline, 4 weeks after the third 153Sm-EDTMP administration), with subsequent recovery. In addition, as expected, there was a temporary decline in white blood cell count in week 7 (36.5 ± 23.8% decline, 6 weeks after the first 153Sm-EDTMP administration) and in week 20 (40.5 ± 15.2% decline, 5 weeks after the third 153Sm-EDTMP administration), with recovery thereafter. Hemoglobin levels steadily declined throughout the study course with a mean decrease of 12.5 ± 8.0% at the end of the study compared with baseline. This is consistent with the disease progression (PSA increase) observed for most patients during the study course.
Fig. 4.
Mean hematological parameters (FAS) during the whole study period: hemoglobin (a), platelet count (b) and white blood cell count (c)
Grades 2, 3, and 4 hematological toxicity occurred in 12, 1 and 2 patients, respectively. Creatinine levels were stable throughout the study period for all, except one patient who experienced a sudden increase in creatinine level (grade 2) in week 10 (after two doses of 18.5 MBq/kg 153Sm-EDTMP and two infusions of 4 mg zoledronic acid) due to bilateral ureter obstruction caused by pathologically enlarged lymph nodes. This patient went off-study, and creatinine levels returned to normal after relief of the obstruction.
Other adverse events included pain and fatigue due to disease progression and ‘influenza-like symptoms,’ nausea, vomiting and hypocalcemia related to zoledronic acid administration. One patient experienced a pathological hip fracture, and another patient had monoparesis as a result of epidural metastatic disease. A third patient had disseminated intravascular coagulation, causing him to develop grade 3 hematoma and grade 4 thrombopenia. These patients went off-study. No further unexpected toxicity occurred (Table 3).
Table 3.
Number of patients experiencing adverse events
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Disseminated intravascular coagulation | – | – | 1 | – |
| Abdominal pain | 4 | – | – | – |
| Constipation | 4 | – | – | – |
| Diarrhea | 2 | – | – | – |
| Nausea | 6 | 1 | 1 | – |
| Vomiting | 1 | – | 2 | – |
| Asthenia | 2 | 1 | – | – |
| Crepitations | – | 1 | – | – |
| Fatigue | 1 | 1 | 1 | – |
| Influenza-like illness | 13 | – | – | – |
| Malaise | 1 | 1 | – | – |
| Pain | 4 | 4 | 4 | 1 |
| Pyrexia | 3 | – | – | – |
| Hip fracture | – | – | 1 | – |
| Anaemia | 4 | 7 | – | 1 |
| Leucopenia | 4 | 8 | 0 | – |
| Neutropenia | 1 | 7 | 1 | – |
| Thrombopenia | 12 | 2 | – | 2 |
| Hypocalcaemia | 11 | 1 | 2 | – |
| Creatinine increase | 1 | 1 | – | – |
| Urea increase | 3 | 1 | ||
| Weight decreased | 7 | – | – | – |
| Dizziness | 2 | 1 | – | – |
| Headache | 5 | 1 | – | – |
| Monoparesis | – | – | – | 1 |
| Paraesthesia | 2 | – | – | – |
| Euphoric mood | – | 1 | – | – |
| Obstructive uropathy | – | 1 | – | – |
| Urinary retention | 2 | – | – | – |
| Cough | 2 | 1 | – | – |
| Dysnpoea | – | 3 | – | – |
| Epistaxis | 2 | – | – | – |
| Haemoptysis | 2 | – | – | – |
| Hypoxia | – | – | 1 | – |
| Hyperhidrosis | 3 | – | – | – |
| Night sweats | 2 | – | – | – |
| Haematoma | – | – | 1 | – |
| Hypertension | 4 | 2 | – | – |
| Lymphangitis | – | 1 | – | – |
This table includes adverse events which are related or unrelated to study drugs, with either an intensity >grade 1 (according to NCI Common Terminology Criteria for Averse Events) or frequency >1 patient
Secondary endpoints for efficacy were bone metabolism markers and PSA response. Figure 5 shows the change from baseline of bone metabolism markers (FAS). Urinary NTX corrected to creatinine excretion, which reflects bone resorption, was reduced markedly, falling rapidly (median decrease ~70%) within 1 month after the first administration of zoledronic acid (week 3) and remaining suppressed during the entire study course. Serum BAP, reflecting bone formation by osteoblasts, decreased, with a mean reduction of ~25% at week 15. An additional 25% decrease in BAP was noticed after the administration of a full dose of 153Sm-EDTMP at week 15, leading to a 50% total decrease from baseline. The increase in BAP at week 15 may reflect some disease progression before the 153Sm-EDTMP treatment at week 15. Serum PINP decreased progressively over 15 weeks to a mean reduction of 50% after four to five repeated administrations of zoledronic acid.
Fig. 5.
Median change (%) from baseline of bone markers during the study period of 27 weeks (FAS). BAP Serum bone-specific alkaline phosphatase, PINP serum procollagen type 1 N propeptide, corr NTX urinary creatinine-corrected N-terminal type 1 collagen peptide
Baseline levels of total PSA measured before administration of the study drugs ranged from 15 to 2,400 ng/ml. Of the 18 treated patients, 14 had baseline levels <400 ng/ml and four had baseline levels >1,000 ng/ml. After the start of treatment, PSA levels of the 15 evaluable patients (two patients did not stop or started anti-androgen treatment, one patient went off-study before week 3) were either increasing (nine patients), stabilised (three patients) or decreasing (three patients). Eight weeks after the final administration of 153Sm-EDTMP in week 23, PSA levels in most patients had increased by >50%, reflecting prostate cancer progression. Two patients had remarkably decreased PSA levels [from 24 to 6.9 μg/l (−71%) and from 30.7 to 17.6 μg/l (−43%)] at week 23.
Discussion
Various therapies for the treatment of painful skeletal metastases are currently available. These can be in the form of local or systemic therapy and include analgesics, chemotherapy, hormonal therapy, surgery, bisphosphonates, external beam radiation and systemically administered radiopharmaceuticals. A multidisciplinary approach to the treatment of cancer pain has been advocated [20]. Besides optimising radiopharmaceutical dosing schemes and treatment regimens [21, 22], synergy between different pharmaceuticals could ultimately enhance efficacy to move beyond mere palliation [6].
The combined use of bisphosphonates and bone-seeking radiopharmaceuticals could have clinical benefit [9]. The prevention of skeletal-related events could have an additive effect on the palliative treatment of bone-seeking radiopharmaceuticals. Nevertheless, the European label of 153Sm-EDTMP includes the contraindication that “it should not be used concurrently with other bisphosponates if an interference is shown on the 99mTc-labelled bisphosphonate bone scan.” This contraindication is based on the hypothesis that as both drugs interact at the hydroxyapatite crystal surface of the skeleton, competition might exist for uptake by bone. However, the present study shows that the combined use of zoledronic acid has no effect on the uptake of 153Sm-EDTMP in skeletal metastases of hormone-refractory prostate carcinoma. These results are consistent with the American label of 153Sm-EDTMP, which does not include the contraindication of the combined use of 153Sm-EDTMP with other bisphosphonates. The present results show that combining 153Sm-EDTMP and zoledronic acid is both feasible and safe. No competition for uptake by bone was observed, and only the known adverse drug reactions associated with 153Sm-EDTMP and zoledronic acid were encountered. Regarding the conflicting data on the effect of bisphosphonates on radiolabelled phosphonates, it should be noted that reduced uptake observed on 99mTc-labelled bone scans after initiating therapy with bisphosphonates does not necessarily indicate an interaction between these agents. The reduced uptake of radiolabelled phosphonates could also reflect a true decrease in metabolic bone activity due to the therapeutic effect of the bisphosphonates, especially when repeated bone scintigraphy takes place after a long interval of bisphosphonate treatment [23]. This was not the case in the present study.
Secondly, in studies that reported an adverse interaction between bisphosphonates and radiolabelled phosphates, bisphosphonates were administered because of malignancy-induced hypercalcemia [10, 12]. High serum calcium levels may lead to complex formation between calcium ions and radiolabelled phosphates, resulting in impaired imaging [24]. Furthermore, in patients with malignant hypercalcemia in association with renal failure, progressive soft tissue uptake of radiolabelled phosphates may occur due to metastatic microcalcification. This soft tissue uptake may occur at in vivo calcium phosphate ion product concentrations of >5 mmol2/l2 [25, 26]. In patients with normal calcium levels, interactions between bisphosphonates and radiolabelled phosphates could not be confirmed. In patients with prostate cancer treated with alendronate (oral 40 mg daily) and in patients with breast cancer treated with clodronate (i.v. 300 mg daily), no effects of these bisphosphonates on repeated bone scintigraphy were observed [13, 15]. All patients in our study had either normal or below-normal serum calcium levels throughout the study course.
The recent identification of specific and sensitive biochemical markers reflecting the overall rate of bone formation and bone resorption has improved the noninvasive assessment of bone turnover abnormalities in patients with prostate cancer. Several studies have shown a rapid decrease in bone resorption markers in patients with prostate cancer and bone metastases after treatment with bisphosphonates. It has been reported that the magnitude of the decrease correlated with the efficacy of the treatment [27, 28]. Our results are consistent with data reported by Saad et al. [7] for metastatic prostate cancer patients treated with zoledronic acid (4 mg every 3 weeks). In our study, urinary NTX corrected to creatinine excretion showed a 70% decrease and remained suppressed during the entire study course. Serum BAP decreased, with a mean reduction of ~25% at week 15. Similarly, Saad et al. reported a 25% decrease in serum BAP, which was stable for ~1 year, in hormone-refractory prostate cancer patients treated with zoledronic acid. However, in our study, an additional 25% decrease in BAP was observed after administration of a full dose of 153Sm-EDTMP at week 15, leading to a 50% total decrease from baseline. The additional decrease may be attributed to a BAP-reducing effect of 153Sm-EDTMP. In support of this idea, the bone markers had already started to decrease after 153Sm-EDTMP treatment alone. Bone marker responses may suggest an additive effect of the two treatments. Besides that, it is interesting to note that while bone markers declined, most patients showed a PSA increase. Presumably, the decrease in bone metabolism was not enough to lead to an objective anti-tumour effect in this study population. However, the present study was not designed to investigate the efficacy of combined treatment because the number of treated patients is small. Although overall results on PSA decline do not seem to parallel the data on bone marker decline, two patients did respond very well. In addition to a remarkable and durable PSA decline (−71 and −43% at week 23), all bone markers decreased (−65 and −90% at week 23), and clinical responses were complete.
To enhance the efficacy of bone-seeking radiopharmaceuticals, the search for effective combination therapies is crucial. Individual patients in our study responded very well to the combined treatment of bisphosphonates and bone-seeking radiopharmaceuticals. In these patients, significant bone marker decline paralleled a significant clinical and PSA response. Because the present study was not designed for evaluating treatment efficacy, the value of this particular combination therapy merits further investigation. Furthermore, it is of growing importance for the optimisation of palliative treatment and enhancement of efficacy to characterise the bone marker responses of those patients that respond well to the proposed treatment regimen. This characterisation may prove important for individualised therapy monitoring.
Conclusion
Combined treatment with the bisphosphonate zoledronic acid has no effect on the skeletal uptake of 153Sm-EDTMP in patients with hormone-refractory prostate carcinoma and normal calcemia. Combined treatment is feasible and safe. The potential additive effect on efficacy of the two treatments should be studied in future trials.
Acknowledgements
We thank Alice van Dongen for her input. This study was financially supported by CIS bio International, Saclay, France. Zoledronic acid was kindly provided by Novartis, Stein, Switzerland.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Dijkman GA, Debruyne FM. Epidemiology of prostate cancer. Eur Urol 1996;30(3):281–95. [DOI] [PubMed]
- 2.Auclerc G, Antoine EC, Cajfinger F, Brunet-Pommeyrol A, Agazia C, Khayat D. Management of advanced prostate cancer. Oncologist 2000;5(1):36–44. [DOI] [PubMed]
- 3.Bayouth JE, Macey DJ, Kasi LP, Fossella FV. Dosimetry and toxicity of samarium-153-EDTMP administered for bone pain due to skeletal metastases. J Nucl Med 1994;35(1):63–9. [PubMed]
- 4.Goeckeler WF, Edwards B, Volkert WA, Holmes RA, Simon J, Wilson D. Skeletal localization of samarium-153 chelates: potential therapeutic bone agents. J Nucl Med 1987;28(4):495–504. [PubMed]
- 5.Rago R. Management of hormone-sensitive and hormone-refractory metastatic prostate cancer. Cancer Control 1998;5(6):513–21. [DOI] [PubMed]
- 6.Ricci S, Boni G, Pastina I, Genovesi D, Cianci C, Chiacchio S, et al. Clinical benefit of bone-targeted radiometabolic therapy with 153Sm-EDTMP combined with chemotherapy in patients with metastatic hormone-refractory prostate cancer. Eur J Nucl Med Mol Imaging 2007;34(7):1023–30. [DOI] [PubMed]
- 7.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002;94(19):1458–68. [DOI] [PubMed]
- 8.Berry S, Waldron T, Winquist E, Lukka H. The use of bisphosphonates in men with hormone-refractory prostate cancer: a systematic review of randomized trials. Can J Urol 2006;13(4):3180–8. [PubMed]
- 9.Storto G, Klain M, Paone G, Liuzzi R, Molino L, Marinelli A, et al. Combined therapy of Sr-89 and zoledronic acid in patients with painful bone metastases. Bone 2006;39(1):35–41. [DOI] [PubMed]
- 10.Hommeyer SH, Varney DM, Eary JF. Skeletal nonvisualization in a bone scan secondary to intravenous etidronate therapy. J Nucl Med 1992;33(5):748–50. [PubMed]
- 11.Krasnow AZ, Collier BD, Isitman AT, Hellman RS, Ewey D. False-negative bone imaging due to etidronate disodium therapy. Clin Nucl Med 1988;13(4):264–7. [DOI] [PubMed]
- 12.Sandler ED, Parisi MT, Hattner RS. Duration of etidronate effect demonstrated by serial bone scintigraphy. J Nucl Med 1991;32(9):1782–4. [PubMed]
- 13.Carrasquillo JA, Whatley M, Dyer V, Figg WD, Dahut W. Alendronate does not interfere with 99mTc-methylene diphosphonate bone scanning. J Nucl Med 2001;42(9):1359–63. [PubMed]
- 14.Marcus CS, Saeed S, Mlikotic A, Mishkin F, Pham HL, Javellana T, et al. Lack of effect of a bisphosphonate (pamidronate disodium) infusion on subsequent skeletal uptake of Sm-153 EDTMP. Clin Nucl Med 2002;27(6):427–30. [DOI] [PubMed]
- 15.Pecherstorfer M, Schilling T, Janisch S, Woloszczuk W, Baumgartner G, Ziegler R, et al. Effect of clodronate treatment on bone scintigraphy in metastatic breast cancer. J Nucl Med 1993;34(7):1039–44. [PubMed]
- 16.Tian JH, Zhang JM, Hou QT, Oyang QH, Wang JM, Luan ZS, et al. Multicentre trial on the efficacy and toxicity of single-dose samarium-153-ethylene diamine tetramethylene phosphonate as a palliative treatment for painful skeletal metastases in China. Eur J Nucl Med 1999;26(1):2–7. [DOI] [PubMed]
- 17.Palmedo H, Manka-Waluch A, Albers P, Schmidt-Wolf IG, Reinhardt M, Ezziddin S, et al. Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: randomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonate. J Clin Oncol 2003;21(15):2869–75. [DOI] [PubMed]
- 18.Singh A, Holmes RA, Farhangi M, Volkert WA, Williams A, Stringham LM, et al. Human pharmacokinetics of samarium-153 EDTMP in metastatic cancer. J Nucl Med 1989;30(11):1814–8. [PubMed]
- 19.Farhanghi M, Holmes RA, Volkert WA, Logan KW, Singh A. Samarium-153-EDTMP: pharmacokinetic, toxicity and pain response using an escalating dose schedule in treatment of metastatic bone cancer. J Nucl Med 1992;33(8):1451–8. [PubMed]
- 20.Serafini AN. Therapy of metastatic bone pain. J Nucl Med 2001;42(6):895–906. [PubMed]
- 21.Strigari L, Sciuto R, D'Andrea M, Pasqualoni R, Benassi M, Maini CL. Radiopharmaceutical therapy of bone metastases with 89SrCl2, 186Re-HEDP and 153Sm-EDTMP: a dosimetric study using Monte Carlo simulation. Eur J Nucl Med Mol Imaging 2007;34(7):1031–8. [DOI] [PubMed]
- 22.Sartor O, Reid RH, Bushnell DL, Quick DP, Ell PJ. Safety and efficacy of repeat administration of samarium Sm-153 lexidronam to patients with metastatic bone pain. Cancer 2007;109(3):637–43. [DOI] [PubMed]
- 23.Smith ML, Fogelman I, Ralston S, Boyce BF, Boyle IT. Correlation of skeletal uptake of 99mTc-diphosphonate and alkaline phosphatase before and after oral diphosphonate therapy in Paget’s disease. Metab Bone Dis Relat Res 1984;5(4):167–70. [DOI] [PubMed]
- 24.Koyano H, Schimizu T, Shishiba Y. The bisphosphonate dilemma. J Nucl Med 1995;36(4):705–6. [PubMed]
- 25.Low RD, Hicks RJ, Arkles LB, Gill G, Adam W. Progressive soft tissue uptake of Tc-99m MDP reflecting metastatic microcalcification. Clin Nucl Med 1992;17(8):658–2. [DOI] [PubMed]
- 26.Eagel BA, Stier SA, Wakem C. Non-osseous bone scan abnormalities in multiple myeloma associated with hypercalcemia. Clin Nucl Med 1988;13(12):869–73. [DOI] [PubMed]
- 27.Garnero P, Buchs N, Zekri J, Rizzoli R, Coleman RE, Delmas PD. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br J Cancer 2000;82(4):858–64. [DOI] [PMC free article] [PubMed]
- 28.Garnero P. Markers of bone turnover in prostate cancer. Cancer Treat Rev 2001;27(3):187–92. [DOI] [PubMed]