Abstract
Common variants in CYP19A1 (the A alleles of rs749292 and rs727479) have been associated with a 10% to 20% increase in circulating estrogen levels in postmenopausal women. We hypothesized that the presence of one or both A alleles in these single nucleotide polymorphisms (SNP) is associated with increased endometrial cancer risk. We tested this hypothesis in a large pooled analysis of 4,998 endometrial cancer cases and 8,285 controls from 10 studies in the Epidemiology of Endometrial Cancer Consortium. The majority of women (>66%) were whites, with smaller proportions of other races and ethnic groups (blacks, Asians, and Latinas) also included in this pooled analysis. Unconditional logistic regression was used to model the association between SNPs/haplotypes and endometrial cancer risk. Carrying the A allele of either of these SNPs was associated with an increased risk of endometrial cancer, with pooled odds ratios per allele of 1.14, 95% confidence interval of 1.09-1.21, and P =7.1 × 10-7 for rs749292, and odds ratio per allele of 1.08, 95% confidence interval of 1.02-1.14, and P = 0.009 for rs727479. For rs749292, these associations were generally stronger among women age ≥55 years. For both SNPs, risk increased with increasing body mass index, and for rs727479, this pattern seemed stronger among women age ≥55 years (P interaction = 0.007). The combination of A alleles in the two SNPs, either by direct count or by haplotype analysis, did not increase risk above that observed for the individual SNPs. Our study provides evidence that CYP19A1 genetic variation influences susceptibility to endometrial cancer, particularly among older and obese women.
Introduction
Endometrial cancer is the most common gynecologic cancer and is the fourth most common cancer in women in the United States (1). Prolonged exposure to estrogens unopposed by progesterone plays an important role in the etiology of endometrial cancer (2, 3). Studies have shown that high endogenous levels of estrogens are related to increased risk of endometrial cancer (4-7).
Aromatase, encoded by CYP19A1, converts androstenedione to estrone and testosterone to estradiol. After menopause, the primary source of estrogens is via peripheral conversion of androgens in adipose tissue catalyzed by aromatase. Given its key role in estrogen biosynthesis, it is possible that polymorphisms in CYP19A1 that alter estrogen production could be involved in endometrial carcinogenesis. Previous studies have evaluated associations between various CYP19A1 polymorphisms and endometrial cancer risk (8-11), with mixed results; interactions with dietary factors have also been reported (12).
Recently, a comprehensive assessment of genetic variation at the CYP19A1 locus was conducted by resequencing of exons and determining linkage disequilibrium among 105 single nucleotide polymorphisms (SNP; ref. 13). This study revealed strong associations between seven tagging SNPs and endogenous estrogen levels in more than 3,000 healthy postmenopausal women who were not on hormone therapy. The strongest associations were observed for rs749292 (A allele) and rs727479 (A allele); these SNPs were independently associated with a 10% to 20% increase in estrogen levels, and the A-A haplotype was the strongest predictor of estrogen levels. None of the seven variants were associated with risk of breast cancer in that study.
These polymorphisms have not been studied in relation to endometrial cancer risk. Here we used data from the Epidemiology of Endometrial Cancer Consortium to evaluate the association between the two CYP19A1 SNPs most strongly related to circulating estrogen levels (rs749292 and rs727479) and endometrial cancer risk in a large pooled multiethnic study. We hypothesized that the A alleles of these SNPs are associated with increased risk. Because after menopause endogenous estrogens are predominantly produced in adipose tissue, we also hypothesized that the relative risk for the A alleles would be more pronounced in older women and particularly those with higher body mass index (BMI).
Materials and Methods
Study Population
Ten studies in the Epidemiology of Endometrial Cancer Consortium participated in this pooled analysis. The Epidemiology of Endometrial Cancer Consortium is an international consortium established to pool resources and data from many endometrial cancer studies in an effort to identify genetic and environmental risk factors for endometrial cancer. The participating studies in this pooled analysis were six population-based case-control studies (Estrogen, Diet, Genetics, and Endometrial Cancer; Fred Hutchinson Cancer Research Center case-control study; Women's Insights and Shared Experiences; Hawaii case-control study; Polish Endometrial Cancer Study;and Shanghai Endometrial Cancer Study), one hospitalbased case-control study (Toronto case-control), and three case-control studies nested within cohorts (California Teachers Study, Multiethnic Cohort, and Nurses' Health Study;Table 1). A detailed description of these studies is available in the Supplementary Materials. A total of 4,998 invasive endometrial cancer cases and 8,285 controls were available for the current analysis. Each study was approved by the institution's institutional review board and appropriate permission for the pooled analysis was obtained.
Table 1.
Characteristics of the 10 participating studies in the Epidemiology of Endometrial Cancer Consortium pooled analysis
| Study | Location | Study design | White (%) | No. cases | Age, y (mean) | No. controls | Age, y (mean) |
|---|---|---|---|---|---|---|---|
| CTS | California | Nested case-control | 95.3 | 356 | 65.4 | 686 | 66.1 |
| EDGE | New Jersey | Population-based case-control | 90.3 | 383 | 61.4 | 358 | 65.1 |
| FHCRC | Washington | Population-based case-control | 95.8 | 783 | 59.8 | 783 | 59.2 |
| Hawaii | Hawaii | Population-based case-control | 27.4 | 168 | 57.7 | 329 | 62.6 |
| MEC | Hawaii, California | Nested case-control | 22.8 | 299 | 64.6 | 1,534 | 62.9 |
| NHS | 11 US States | Nested case-control | 94.8 | 650 | 62.1 | 1,641 | 61.8 |
| PECS | Poland | Population-based case-control | 100.0 | 417 | 60.8 | 407 | 60.9 |
| SECS | China | Population-based case-control | - | 1,030 | 54.3 | 1011 | 54.3 |
| Toronto | Canada | Hospital-based case-control | 91.1 | 530 | 60.8 | 587 | 56.1 |
| WISE | Philadelphia | Population-based case-control | 80.5 | 382 | 62.6 | 949 | 61.1 |
| Pooled | 66.3 | 4,998 | 60.1 | 8,285 | 60.8 |
Abbreviations: CTS, California Teachers Study; EDGE, Estrogen, Diet, Genetics, and Endometrial Cancer; FHCRC, Fred Hutchinson Cancer Research Center; MEC, Multiethnic Cohort; NHS, Nurses' Health Study; PECS, Polish Endometrial Cancer Study; SECS, Shanghai Endometrial Cancer Study; WISE, Women's Insights and Shared Experiences.
Genotyping
Genomic DNA was extracted from buffy coat or buccal samples. The methods of specimen collection and handling differed somewhat among the studies (described in Supplementary Materials). Genotyping of rs749292 and rs727479 was done in individual laboratories using the same TaqMan assay protocols. The assay details including primers and probes are available online (http://www.uscnorris.com/MECgenetics). Depending on the study, 3% to 10% blinded quality control samples were included in each assay; the concordance rates ranged from 98% to 100%. The average genotyping call rate was 98% (range, 93-100%). With two exceptions, both SNPs were consistent with Hardy-Weinberg equilibrium in controls by race/ethnicity overall and in each study (P ≥ 0.12). The exceptions were the results for rs749292 in white controls in the California Teachers Study (P = 0.01) and those for rs727479 in controls in Poland (P = 0.03). Exclusion of these studies did not affect the results reported below.
Statistical Analysis
The A alleles of both SNPs were designated as “high-risk” alleles because of their association with higher estrogen levels (13). The GG genotype of rs749292 and the CC genotype of rs727479 were used as reference categories. We examined the joint association between these SNPs and endometrial cancer risk by classifying women as to the number of A alleles (0, 1, 2, 3, 4) and A-A haplotypes (0, 1, 2 copies). Haplotypes were estimated using the TagSNP program as previously described (13). In addition to genotype data, the following variables were available for this analysis: race/ethnicity (white, black, Asian, Hawaiian/Pacific Islander, mixed, other, and unknown), age (continuous), and BMI (continuous in kg/m2). Unconditional logistic regression was used to model the association of each SNP or haplotype with endometrial cancer risk [odds ratios (OR) and 95% confidence intervals (CI)] for each of the 10 studies. We combined the data from the 10 studies to calculate pooled OR, adjusting for age (continuous), race/ethnicity (categorical), and study (categorical). Heterogeneity of effects across studies and racial/ethnic groups was examined by the Q test. We also conducted analyses stratified by age (<55, 55-<65, and ≥65 y) and BMI (<25, 25-<30, and ≥30 kg/m2, representing underweight/normal, overweight, and obese categories, respectively). Because information on BMI was not available for the control subjects in the Toronto study, this study was excluded from the latter analysis. Interactions between genotypes and age and BMI were evaluated by including a multiplicative term in the logistic regression model. Trend tests were done by treating the number of A alleles as a continuous variable in the logistic regression model. All P values are two-sided. Analyses were done using SAS version 9.1 (SAS Institute Inc.) and STATA version 10 (StataCorp).
Results
A majority of women were aged ≥55 years and the average age was 60.1 and 60.8 years for cases and controls, respectively (Table 1). With the exception of the Multiethnic Cohort, Shanghai Endometrial Cancer Study, Hawaii, and Women's Insights and Shared Experiences studies, over 90% of women in each study were whites. The breakdown of race/ethnicity in each study is presented in Supplemental Table S1. The Asian category mainly comprised Chinese from the Shanghai Endometrial Cancer Study and Japanese from the Multiethnic Cohort and the Hawaii case-control study.
The race/ethnicity specific allele frequencies in control subjects were consistent across studies (Supplemental Table S2). The frequency of the rs749292 A allele ranged from 0.40 to 0.49 in whites, 0.43 to 0.45 in blacks, and 0.35 to 0.47 in Asians. The frequency of the rs727479 A allele ranged from 0.63 to 0.69 in whites, 0.77 to 0.82 in blacks, and 0.68 to 0.73 in Asians.
The A alleles of rs749292 and rs727479 were associated with higher endometrial cancer risk in a dose-dependent manner (rs749292 OR per allele, 1.14;95% CI, 1.09-1.21, and rs727479 OR per allele, 1.08;95% CI, 1.02-1.14). The OR for each SNP was ≥1.0 in all studies with no evidence of heterogeneity observed across studies (P ≥ 0.49; Fig. 1). Table 2 shows the association of rs749292 and rs727479 with endometrial cancer risk in all women combined and in whites, blacks, and Asians. In all women, the A allele of each SNP was associated with an increased risk of endometrial cancer: for rs749292, ORs of 1.19 (95% CI, 1.09-1.30) for AG versus GG and 1.30 (95% CI, 1.17-1.45) for AA versus GG, and for rs727479, ORs of 1.05 (95% CI, 0.93-1.20) for AC versus CC and 1.15 (95% CI, 1.01-1.31) for AA versus CC. The associations were consistent in whites, blacks, and Asians (P for heterogeneity across these three racial groups ≥0.87). When the analysis was repeated with BMI adjustment, results were similar (data not shown).
Figure 1.
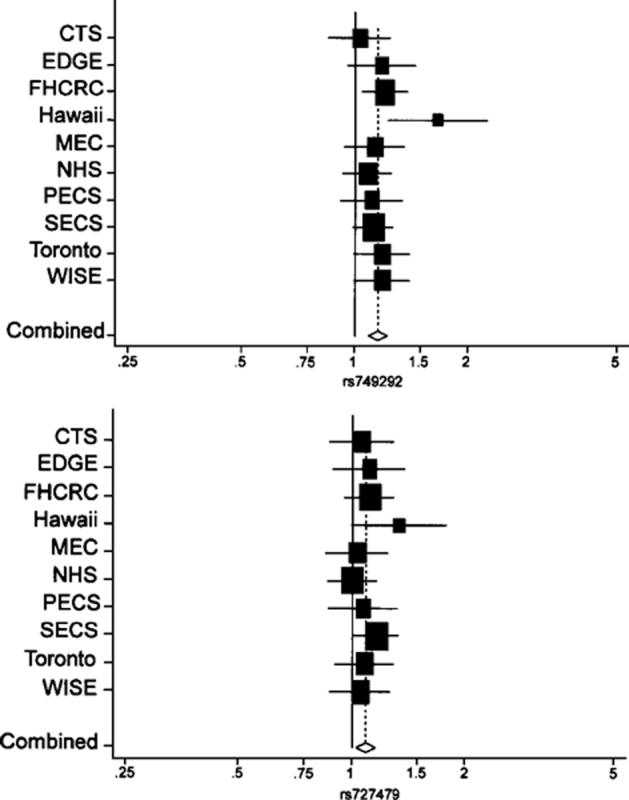
OR and 95% CI per allele of rs749292 (top) and rs727479 (bottom). ORs are shown on horizontal axis. Size of box is proportional to number of cases and controls in each study.
Table 2.
CYP19A1 SNPs and risk of endometrial cancer
| SNP | Race/ethnicity | Cases | Controls | OR* (95% CI) |
|---|---|---|---|---|
| rs749292 | All women | |||
| n | 4,814 | 7,742 | ||
| GG | 1,279 | 2,438 | 1.00 | |
| AG | 2,440 | 3,806 | 1.19 (1.09-1.30) | |
| AA | 1,095 | 1,495 | 1.30 (1.17-1.45) | |
| Per allele | 1.14 (1.09-1.21) | |||
| White women | ||||
| n | 3,241 | 4,934 | ||
| GG | 867 | 1,517 | 1.00 | |
| AG | 1,625 | 2,460 | 1.16 (1.04-1.29) | |
| AA | 749 | 957 | 1.32 (1.16-1.50) | |
| Per allele | 1.15 (1.08-1.22) | |||
| Black women | ||||
| n | 142 | 555 | ||
| GG | 39 | 168 | 1.00 | |
| AG | 70 | 277 | 0.99 (0.61-1.60) | |
| AA | 33 | 110 | 1.31 (0.74-2.31) | |
| Per allele | 1.14 (0.85-1.52) | |||
| Asian women | ||||
| n | 1,208 | 1,558 | ||
| GG | 300 | 507 | 1.00 | |
| AG | 639 | 746 | 1.31 (1.09-1.58) | |
| AA | 269 | 305 | 1.28 (1.02-1.61) | |
| Per allele | 1.14 (1.02-1.28) | |||
| rs727479 | All Women | |||
| n | 4,791 | 7,686 | ||
| CC | 470 | 861 | 1.00 | |
| AC | 2,020 | 3,348 | 1.05 (0.93-1.20) | |
| AA | 2,301 | 3,477 | 1.15 (1.01-1.31) | |
| Per allele | 1.08 (1.02-1.14) | |||
| White women | ||||
| n | 3,226 | 4,897 | ||
| CC | 355 | 589 | 1.00 | |
| AC | 1,413 | 2,244 | 1.01 (0.87-1.18) | |
| AA | 1,458 | 2,064 | 1.13 (0.97-1.31) | |
| Per allele | 1.08 (1.01-1.15) | |||
| Black women | ||||
| n | 140 | 540 | ||
| CC | 7 | 29 | 1.00 | |
| AC | 43 | 174 | 0.80 (0.31-2.07) | |
| AA | 90 | 337 | 0.97 (0.40-2.40) | |
| Per allele | 1.10 (0.77-1.56) | |||
| Asian women | ||||
| n | 1,201 | 1,547 | ||
| CC | 75 | 130 | 1.00 | |
| AC | 456 | 600 | 1.26 (0.91-1.74) | |
| AA | 670 | 817 | 1.36 (0.99-1.86) | |
| Per allele | 1.12 (0.99-1.27) |
The ORs and 95% CI were estimated using unconditional logistic regression adjusting for age, study and race/ethnicity when appropriate.
We examined the CYP19A1-endometrial cancer associations stratified by age and observed a stronger association between rs749292 and endometrial cancer risk in older age groups (age ≥55; Table 3). The OR per allele was 1.07 (95% CI, 0.97-1.18) for women age <55, 1.15 (95% CI, 1.05-1.25) for women 55≤ age <65 years, and 1.21 (95% CI, 1.10-1.32) for women >65 years (P interaction = 0.09). The association of rs727479 with endometrial cancer risk did not vary appreciably by age (P interaction = 0.47).
Table 3.
CYP19A1 SNPs and risk of endometrial cancer stratified by age
| SNP | Age <55 y |
55 ≤ Age < 65 y |
Age ≥ 65 y |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR* (95% CI) | Cases | Controls | OR* (95% CI) | Cases | Controls | OR* (95% CI) | |
| rs749292 | |||||||||
| n | 1,390 | 2,098 | 1,815 | 2,908 | 1,609 | 2,736 | |||
| GG | 385 | 625 | 1.00 | 468 | 903 | 1.00 | 426 | 910 | 1.00 |
| AG | 686 | 1,056 | 0.98 (0.83-1.16) | 918 | 1,409 | 1.25 (1.08-1.45) | 836 | 1,344 | 1.29 (1.11-1.50) |
| AA | 319 | 417 | 1.15 (0.94-1.41) | 429 | 596 | 1.31 (1.10-1.56) | 347 | 482 | 1.44 (1.19-1.73) |
| Per allele | 1.07 (0.97-1.18) | 1.15 (1.05-1.25) | 1.21 (1.10-1.32) | ||||||
| rs727479 | |||||||||
| n | 1,379 | 2,082 | 1,809 | 2,891 | 1,603 | 2,713 | |||
| CC | 137 | 222 | 1.00 | 167 | 328 | 1.00 | 166 | 311 | 1.00 |
| AC | 566 | 878 | 0.97 (0.76-1.24) | 766 | 1,264 | 1.14 (0.92-1.42) | 688 | 1,206 | 1.03 (0.82-1.28) |
| AA | 676 | 982 | 1.03 (0.81-1.32) | 876 | 1,299 | 1.25 (1.01-1.55) | 749 | 1,196 | 1.16 (0.93-1.44) |
| Per allele | 1.03 (0.93-1.15) | 1.11 (1.01-1.22) | 1.09 (0.99-1.21) | ||||||
The ORs and 95% CI were estimated using unconditional logistic regression adjusting for study and race/ethnicity.
We also examined the CYP19A1-endometrial cancer associations stratified by BMI (Table 4). The association with each of the SNPs increased with increasing BMI. For rs749292, the OR per allele was 1.08 (95% CI, 1.00-1.17) for normal weight women, 1.18 (95% CI, 1.06-1.31) for overweight women, and 1.22 (95% CI, 1.08-1.38) for obese women (P interaction = 0.07). For rs727479, the OR per allele was 1.00 (95% CI, 0.92-1.09) for normal weight women, 1.07 (95% CI, 0.96-1.20) for overweight women, and 1.23 (95% CI, 1.08-1.41) for obese women (P interaction = 0.009). For this SNP, the increased risk associated with the presence of one or more A alleles was highest in older women who were obese (OR per allele, 1.28; 95% CI, 1.10-1.49). Among women age ≥55 years, there was a strong interaction with BMI (P interaction = 0.007).
Table 4.
CYP19A1 SNPs and risk of endometrial cancer stratified by body mass index (kg/m2)
| SNP | BMI <25 kg/m2 |
25 ≤ BMI <30 kg/m2 |
BMI ≥30 kg/m2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR* (95% CI) | Cases | Controls | OR* (95% CI) | Cases | Controls | OR* (95% CI) | |
| rs749292 | |||||||||
| All women | |||||||||
| n | 1,697 | 3,954 | 1,309 | 2,076 | 1,278 | 1,125 | |||
| GG | 467 | 1,225 | 1.00 | 342 | 679 | 1.00 | 326 | 349 | 1.00 |
| AG | 866 | 1,974 | 1.10 (0.97-1.25) | 664 | 994 | 1.27 (1.07-1.51) | 648 | 559 | 1.23 (1.00-1.50) |
| AA | 364 | 755 | 1.17 (1.00-1.37) | 303 | 403 | 1.37 (1.11-1.69) | 304 | 217 | 1.49 (1.16-1.91) |
| Per allele | 1.08 (1.00-1.17) | 1.18 (1.06-1.31) | 1.22 (1.08-1.38) | ||||||
| Women age <55 y | |||||||||
| n | 549 | 1,115 | 344 | 439 | 327 | 268 | |||
| GG | 157 | 318 | 1.00 | 98 | 135 | 1.00 | 85 | 83 | 1.00 |
| AG | 282 | 574 | 0.93 (0.74-1.17) | 161 | 219 | 0.92 (0.64-1.33) | 161 | 136 | 1.08 (0.70-1.66) |
| AA | 110 | 223 | 0.97 (0.73-1.29) | 85 | 85 | 1.18 (0.76-1.82) | 81 | 49 | 1.55 (0.91-2.64) |
| Per allele | 0.98 (0.85-1.13) | 1.07 (0.86-1.34) | 1.23 (0.95-1.60) | ||||||
| Women age ≥55 y | |||||||||
| n | 1,148 | 2,839 | 965 | 1,637 | 951 | 857 | |||
| GG | 310 | 907 | 1.00 | 244 | 544 | 1.00 | 241 | 266 | 1.00 |
| AG | 584 | 1,400 | 1.18 (1.01-1.38) | 503 | 775 | 1.40 (1.15-1.71) | 487 | 423 | 1.26 (1.00-1.59) |
| AA | 254 | 532 | 1.26 (1.05-1.53) | 218 | 318 | 1.43 (1.13-1.82) | 223 | 168 | 1.47 (1.10-1.95) |
| Per allele | 1.13 (1.03-1.24) | 1.21 (1.08-1.36) | 1.21 (1.05-1.40) | ||||||
| rs727479 | |||||||||
| All women | |||||||||
| n | 1,688 | 3,905 | 1,296 | 2,067 | 1,277 | 1,127 | |||
| CC | 189 | 441 | 1.00 | 118 | 217 | 1.00 | 104 | 133 | 1.00 |
| AC | 720 | 1,688 | 0.91 (0.76-1.09) | 535 | 899 | 1.05 (0.81-1.37) | 541 | 491 | 1.41 (1.04, 1.91) |
| AA | 779 | 1,776 | 0.95 (0.80-1.14) | 643 | 951 | 1.14 (0.88-1.48) | 632 | 503 | 1.63 (1.21, 2.21) |
| Per allele | 1.00 (0.92-1.09) | 1.07 (0.96-1.20) | 1.23 (1.08, 1.41) | ||||||
| Women age <55y | |||||||||
| n | 541 | 1,102 | 344 | 436 | 324 | 268 | |||
| CC | 46 | 113 | 1.00 | 40 | 42 | 1.00 | 32 | 32 | 1.00 |
| AC | 235 | 463 | 1.03 (0.73-1.45) | 127 | 182 | 0.56 (0.33-0.97) | 134 | 109 | 1.21 (0.65-2.26) |
| AA | 260 | 526 | 1.02 (0.72-1.44) | 177 | 212 | 0.66 (0.38-1.13) | 158 | 127 | 1.31 (0.70-2.44) |
| Per allele | 1.00 (0.86-1.17) | 0.93 (0.73-1.17) | 1.12 (0.85-1.48) | ||||||
| Women age ≥55 y | |||||||||
| n | 1,147 | 2,803 | 952 | 1,631 | 953 | 859 | |||
| CC | 143 | 328 | 1.00 | 78 | 175 | 1.00 | 72 | 101 | 1.00 |
| AC | 485 | 1,225 | 0.86 (0.70-1.07) | 408 | 717 | 1.25 (0.92-1.70) | 407 | 382 | 1.47 (1.03-2.09) |
| AA | 519 | 1,250 | 0.93 (0.75-1.15) | 466 | 739 | 1.34 (0.99-1.82) | 474 | 376 | 1.76 (1.24-2.51) |
| Per allele | 1.00 (0.90-1.10) | 1.13 (0.99-1.28) | 1.28 (1.10-1.49) | ||||||
The ORs and 95% CI were estimated using unconditional logistic regression adjusting for age, study, and race/ethnicity when appropriate.
As shown in Table 5, the risk of endometrial cancer tended to increase with an increasing number of A alleles (0-4) in the two SNPs. The OR for those with 4 A alleles vs 0 A alleles was 1.21 (95% CI, 1.04-1.40). We also evaluated the association between the A-A haplotype (rs749292-rs727479) and endometrial cancer risk (Table 5). The OR for those with 2 copies of the A-A haplotype compared with those with 0 copies was 1.30 (95% CI, 1.17-1.45), similar to the OR among all women for the AA genotype in the rs749292 SNP alone. The correlation (r2) between the two SNPs was 0.4.
Table 5.
Association of combinations of CYP19A1 SNPs and haplotypes with endometrial cancer risk
| Cases | Controls | OR* (95% CI) | ||
|---|---|---|---|---|
| rs749292 | Rs727479 | |||
| GG | CC | 416 | 740 | 1.00 |
| AG | CC | 44 | 90 | 0.93 (0.62-1.37) |
| AA | CC | 3 | 9 | 0.69 (0.18-2.68) |
| GG | AC | 587 | 1,106 | 0.90 (0.77-1.06) |
| AG | AC | 1,358 | 2,109 | 1.09 (0.95-1.26) |
| AA | AC | 61 | 90 | 1.38 (0.96-1.98) |
| GG | AA | 255 | 525 | 0.90 (0.74-1.10) |
| AG | AA | 1,004 | 1,520 | 1.14 (0.98-1.32) |
| AA | AA | 1,019 | 1,361 | 1.21 (1.04-1.40) |
| Number of A alleles | ||||
| 0 | 416 | 740 | 1.00 | |
| 1 | 631 | 1,196 | 0.90 (0.77-1.06) | |
| 2 | 1,616 | 2,643 | 1.06 (0.92-1.22) | |
| 3 | 1,065 | 1,610 | 1.15 (0.99-1.34) | |
| 4 | 1,019 | 1,361 | 1.21 (1.04-1.40) | |
| P trend < 0.0001 | ||||
| Number of A-A haplotype† copies | ||||
| 0 | 1,343 | 2,546 | 1.00 | |
| 1 | 2,385 | 3,643 | 1.21 (1.10-1.32) | |
| 2 | 1,019 | 1,361 | 1.30 (1.17-1.45) | |
| Per copy | 1.15 (1.09-1.21) | |||
The ORs and 95% CI were estimated using unconditional logistic regression adjusting for age, study and race/ethnicity.
rs749292-rs727479 haplotype (numbers of cases and controls carrying A-A haplotypes were estimated).
Discussion
Estrogen plays a pivotal role in endometrial cancer etiology. After menopause, estrogen is produced primarily in adipose tissue, and production increases with advancing age as well as with increased body weight (14). It has been suggested that the increase in estrogen production associated with aging results from an increase in the specific activity of the aromatase enzyme in adipose cells, whereas the increase as a function of body weight is simply due to increased numbers of adipose cells (14, 15). In this large pooled analysis, we found that carrying the A allele of rs749292 or rs727479 in CYP19A1 was associated with increased risk of endometrial cancer. Furthermore, we found that the risk was more pronounced in older women (age ≥55 years) and among obese women (BMI ≥30 kg/m2), consistent with the biology of age and obesity on estrogen production.
Our findings were generally consistent with those of the earlier study that found that associations with circulating estrogen levels were similar for the two SNPs (13). However, that study also observed that the A-A haplotype was associated with the highest levels of circulating estrogens. In our study, we observed a similar association with risk among those with two copies of the A-A haplotype as among those with the AA genotype of rs749292.
These results differ from the null results reported earlier for breast cancer (13). Other candidate polymorphisms in CYP19A1 have been studied in breast cancer, with mixed results (16-21). Recent studies that have considered several variants throughout CYP19A1 have not shown associations with risk of breast cancer (22, 23).
One limitation of our study was that data on unopposed estrogen therapy were not available for analysis. We expect that the association between CYP19A1 and endometrial cancer would be obscured in users of unopposed estrogens. In addition, we were not able to classify cases according to histologic type of tumor. About 85% of endometrial cancers are “type I” (endometrioid) tumors (24) and are strongly related to excess estrogen, whereas the remaining “type II” tumors (e.g., serous, clear cell) are less estrogen-dependent (25). If the risk related to genotype were mainly found in the type I tumors, as we would expect, the inclusion of type II tumors in the case group would lead to attenuation of the ORs. Another limitation is the lack of information on stage of endometrial cancer for each study.
In conclusion, by combining data from 10 independent studies we provide supporting evidence that common CYP19A1 genetic variants are associated with increased endometrial cancer risk, particularly in older and obese women. We chose the two SNPs reported to be most strongly associated with circulating estrogens (13) for this study;future work should include evaluation of the other five SNPs also reported to be highly associated with estrogens. Because these SNPs were chosen as tagging SNPs, it is unknown whether other SNPs with which they are in linkage disequilibrium may be more strongly related to risk of endometrial cancer. Although these variants have been found to be related to circulating estrogen levels, to our knowledge, functional assessment of these variants in an experimental setting has not been conducted;this is another avenue for investigation in future studies. Interactions with other genes in the hormone biosynthesis and metabolism pathways are another area for future research.
Supplementary Material
Acknowledgments
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Grant support: CTS (NIH CA077398, CA91019), EDGE (NIH CA83918), Hawaii case-control (NIH CA33619, CA58598, CN67001, N01-PC-35137), FHCRC (NIH CA39779, CA75977, CA80636, N01 HD23166, CA92002, CA105212, CA112523 and funds from the Fred Hutchinson Cancer Research Center), MEC (NIH CA63464, CA54281), NHS (NIH CA82838, NICHD K12 HD051959-01, a grant from the American Cancer Society: RSG-00-061-04-CCE), PECS (Intramural Program of the NCI), SECS (NIH CA92585), Toronto (National Cancer Institute of Canada), and WISE (NIH CA77596). VW Setiawan is supported in part by the NCI Career Development Award grant NIH CA116543.
Footnotes
Note: Supplementary data for this article are available at Cancer Epidemiology Biomakers and Prevention Online (http://cebp.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.American Cancer Society . Cancer facts & figures 2008. American Cancer Society; Atlanta: 2008. [Google Scholar]
- 2.Henderson BE, Ross RK, Pike MC, Casagrande JT. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982;42:3232–9. [PubMed] [Google Scholar]
- 3.Key TJ, Pike MC. The dose-effect relationship between `unopposed' oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer. 1988;57:205–12. doi: 10.1038/bjc.1988.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen NE, Key TJ, Dossus L, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2008;15:485–97. doi: 10.1677/ERC-07-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukanova A, Lundin E, Micheli A, et al. Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. Int J Cancer. 2004;108:425–32. doi: 10.1002/ijc.11529. [DOI] [PubMed] [Google Scholar]
- 6.Potischman N, Hoover RN, Brinton LA, et al. Case-control study of endogenous steroid hormones and endometrial cancer. J Natl Cancer Inst. 1996;88:1127–35. doi: 10.1093/jnci/88.16.1127. [DOI] [PubMed] [Google Scholar]
- 7.Zeleniuch-Jacquotte A, Akhmedkhanov A, Kato I, et al. Postmenopausal endogenous oestrogens and risk of endometrial cancer: results of a prospective study. Br J Cancer. 2001;84:975–81. doi: 10.1054/bjoc.2001.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berstein LM, Imyanitov EN, Kovalevskij AJ, et al. CYP17 and CYP19 genetic polymorphisms in endometrial cancer: association with intratumoral aromatase activity. Cancer Lett. 2004;207:191–6. doi: 10.1016/j.canlet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Paynter RA, Hankinson SE, Colditz GA, et al. CYP19 (aromatase) haplotypes and endometrial cancer risk. Int J Cancer. 2005;116:267–74. doi: 10.1002/ijc.21041. [DOI] [PubMed] [Google Scholar]
- 10.Tao MH, Cai Q, Zhang ZF, et al. Polymorphisms in the CYP19A1 (aromatase) gene and endometrial cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2007;16:943–9. doi: 10.1158/1055-9965.EPI-06-1012. [DOI] [PubMed] [Google Scholar]
- 11.Olson SH, Orlow I, Bayuga S, et al. Variants in hormone biosynthesis genes and risk of endometrial cancer. Cancer Causes Control. 2008;19:955–63. doi: 10.1007/s10552-008-9160-7. Epub 2008 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu WH, Dai Q, Xiang YB, et al. Interaction of soy food and tea consumption with CYP19A1 genetic polymorphisms in the development of endometrial cancer. Am J Epidemiol. 2007;166:1420–30. doi: 10.1093/aje/kwm242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haiman CA, Dossus L, Setiawan VW, et al. Genetic variation at the CYP19A1 locus predicts circulating estrogen levels but not breast cancer risk in postmenopausal women. Cancer Res. 2007;67:1893–7. doi: 10.1158/0008-5472.CAN-06-4123. [DOI] [PubMed] [Google Scholar]
- 14.Cleland WH, Mendelson CR, Simpson ER. Effects of aging and obesity on aromatase activity of human adipose cells. J Clin Endocrinol Metab. 1985;60:174–7. doi: 10.1210/jcem-60-1-174. [DOI] [PubMed] [Google Scholar]
- 15.Simpson ER, Mendelson CR. Effect of aging and obesity on aromatase activity of human adipose cells. Am J Clin Nutr. 1987;45:290–5. doi: 10.1093/ajcn/45.1.290. [DOI] [PubMed] [Google Scholar]
- 16.Ahsan H, Whittemore AS, Chen Y, et al. Variants in estrogenbiosynthesis genes CYP17 and CYP19 and breast cancer risk: a family-based genetic association study. Breast Cancer Res. 2005;7:R71–81. doi: 10.1186/bcr951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunning AM, Dowsett M, Healey CS, et al. Polymorphisms associated with circulating sex hormone levels in postmenopausal women. J Natl Cancer Inst. 2004;96:936–45. doi: 10.1093/jnci/djh167. [DOI] [PubMed] [Google Scholar]
- 18.Haiman CA, Hankinson SE, Spiegelman D, Brown M, Hunter DJ. No association between a single nucleotide polymorphism in CYP19 and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:215–6. [PubMed] [Google Scholar]
- 19.Haiman CA, Stram DO, Pike MC, et al. A comprehensive haplotype analysis of CYP19 and breast cancer risk: the Multiethnic Cohort. Hum Mol Genet. 2003;12:2679–92. doi: 10.1093/hmg/ddg294. [DOI] [PubMed] [Google Scholar]
- 20.Kristensen VN, Harada N, Yoshimura N, et al. Genetic variants of CYP19 (aromatase) and breast cancer risk. Oncogene. 2000;19:1329–33. doi: 10.1038/sj.onc.1203425. [DOI] [PubMed] [Google Scholar]
- 21.Ralph DA, Zhao LP, Aston CE, et al. Age-specific association of steroid hormone pathway gene polymorphisms with breast cancer risk. Cancer. 2007;109:1940–8. doi: 10.1002/cncr.22634. [DOI] [PubMed] [Google Scholar]
- 22.Cai Q, Kataoka N, Li C, et al. Haplotype analyses of CYP19A1 gene variants and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2008;17:27–32. doi: 10.1158/1055-9965.EPI-07-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson JE, Ingle JN, Ma CX, et al. A comprehensive examination of CYP19 variation and risk of breast cancer using two haplotypetagging approaches. Breast Cancer Res Treat. 2007;102:237–47. doi: 10.1007/s10549-006-9324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristensen G, Trope C. Endometrial cancer: the management of highrisk disease. Curr Oncol Rep. 2004;6:471–5. doi: 10.1007/s11912-004-0078-2. [DOI] [PubMed] [Google Scholar]
- 25.Sherman ME, Sturgeon S, Brinton LA, et al. Risk factors and hormone levels in patients with serous and endometrioid uterine carcinomas. Mod Pathol. 1997;10:963–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


