Abstract
Introduction
Inhaled anesthetics are interfacially active, concentrating at interfaces such as the protein/water or bilayer/water interfaces. We tested the hypothesis that interfacial activity was a sufficient condition for anesthetic-like modulation of receptor function by applying surfactants to γ-aminobutyric acid type A (GABAA), glycine, and N-methyl-D-aspartate (NMDA) receptors. We defined anesthetic-like modulation as an increase in currents through native channels that isoflurane and ethanol increased currents through, and a decrease in currents through channels that isoflurane and ethanol decreased currents through. We also tested the null hypothesis that there would be no difference in modulation of channel currents by surfactants in receptors with point mutations that diminished their response to isoflurane and ethanol compared to the native version of these receptors.
Methods
The effect of seven surfactants with different head group charges (anionic, cationic, zwitterionic, and uncharged) and tail lengths (eight carbons and twelve carbons) on homomeric wild type α1 and mutant α1 (S267I) glycine receptors, wild type α1β2γ2s and mutant α1(S270I)β2γ2s GABAA receptors, and wild type NR1/NR2A and mutant NR1(F639A)/NR2A NMDA receptors was studied. Receptors were expressed in Xenopus laevis oocytes and studied using two-electrode voltage clamping.
Results
All seven surfactants, isoflurane, and ethanol potentiated GABAA receptor function. Six of seven surfactants, isoflurane, and ethanol potentiated glycine receptor function. Six of seven surfactants, isoflurane, and ethanol inhibited NMDA receptor function. For the mutant receptors, five of seven surfactants increased currents through GABAA receptors, while six of seven surfactants increased currents through glycine receptors. Six of seven surfactants decreased currents through the NMDA receptor. In contrast to isoflurane and ethanol, surfactants as a group did not diminish modulation of mutant compared to wild type receptors.
Conclusion
These findings identify another large class of compounds (surfactants) that modulate the function of GABAA, glycine, and NMDA receptors in a manner that is qualitatively similar to inhaled anesthetics. We cannot reject the hypothesis that interfacial activity is a sufficient condition for anesthetic-like modulation of these receptors. Mutations that diminish the modulatory effect of isoflurane and ethanol did not diminish the modulatory effect of the surfactants.
Keywords: Anesthesia, GABAA receptor, Glycine receptor, NMDA receptor, detergent, surface-active agents, interfacial activity
Introduction
Inhaled anesthetics have historically been discovered either serendipitously (1), or through an empirical process of trial and error. Once knowledge of the biological targets upon which inhaled anesthetics act is obtained, however, it should be possible to design new anesthetics; this has been reported for other drugs (2). Such knowledge may include that of putative binding sites on ion channels (3), or sites on soluble proteins like albumin (4) that are used as models for anesthetic interactions with proteins and for which there are x-ray diffraction crystal structures.
For 80 years the Meyer-Overton hypothesis (5) predicted best what compounds were inhaled anesthetics. Meyer and Overton hypothesized that solubility in a bulk lipid phase predicted the anesthetic potency of a molecule (6). However, in the past fifteen years exceptions have undermined the validity of the hypothesis. Some compounds (such as alcohols) are more potent than predicted by the hypothesis, and other compounds (transitional compounds) are less potent than predicted (7). Still others (nonimmobilizers) lack anesthetic effects altogether (8).
The interfacial theory of anesthesia remedied the deficiencies of the Meyer-Overton hypothesis. Interfacial concentration was found to predict anesthetic potency even for compounds that disobeyed the Meyer-Overton relationship, implying that interfacial activity was a necessary condition of inhaled anesthetic action (9). This theory envisages that inhaled anesthetics act at the boundary (interface) between a polar and nonpolar phase, such as the boundary between a bilayer and water, or possibly a protein and water. Nuclear magnetic resonance (NMR) spectroscopy has confirmed the predicted interfacial localization of volatile compounds for model membranes (10, 11).
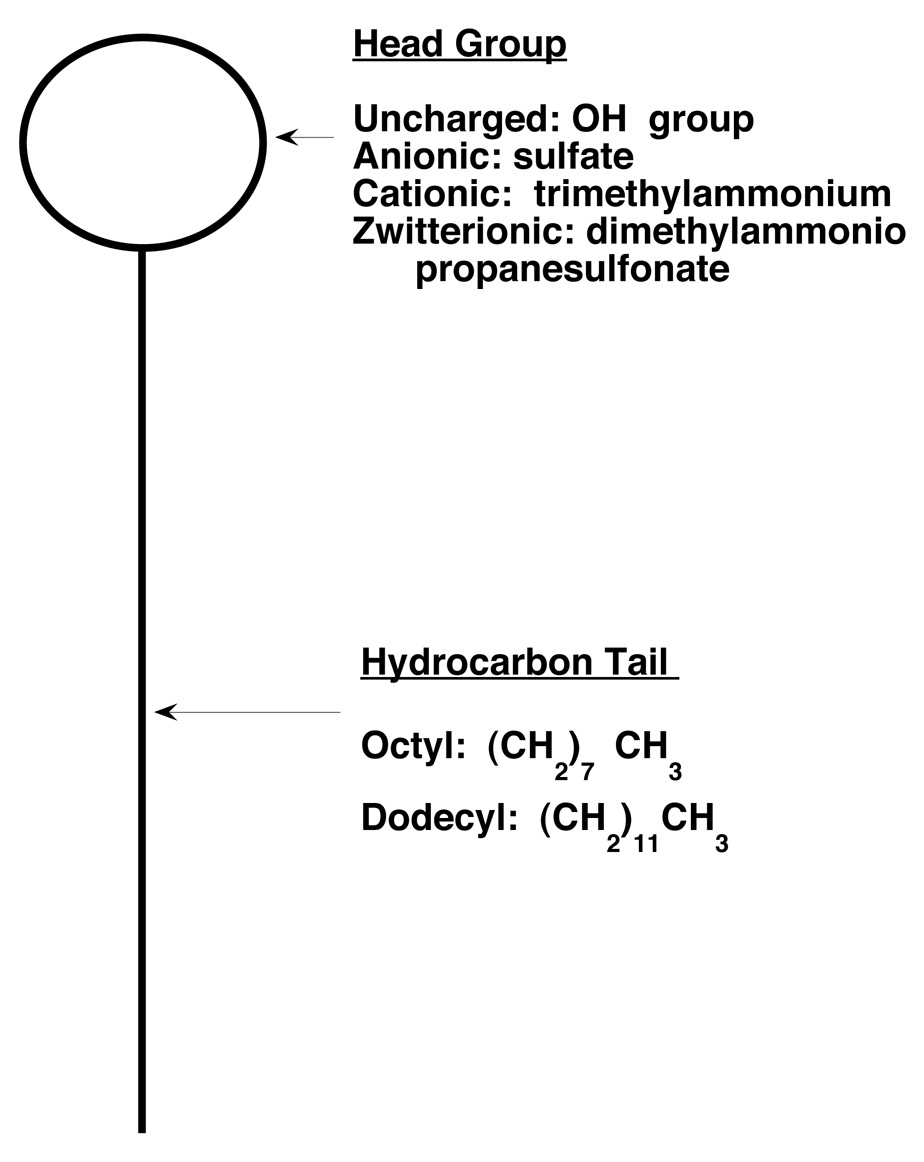
In the present study, we asked whether interfacial activity could predict compounds with anesthetic-like effects. A compound is interfacially active if it accumulates at an interface. Surfactants are interfacially active amphiphiles containing a hydrophobic “tail” and a hydrophilic “head” (Fig 1), which can interact favorably with the hydrophobic and hydrophilic phases present at an interface. We reasoned that if interfacial activity were a sufficient condition of anesthetic action, then surfactants should show anesthetic-like effects.
Fig 1.
Structure of surfactants. Surfactants are amphiphilic compounds, with a hydrophilic head group and a hydrophobic tail. In this study, uncharged, anionic, cationic, and zwitterionic surfactants with the designated head groups were used. Surfactants had either eight or twelve carbon tails.
Because many surfactants are large, charged molecules that cannot cross the blood brain barrier, we could not determine their potency in animals. We accordingly chose to test their potency on receptors which have been conjectured to mediate the effects of inhaled anesthetics. Our strategy for testing this hypothesis was as follows. We used surfactants with two tail lengths (eight and twelve carbons) and four head group charges (anionic, cationic, zwitterionic, and uncharged), for a total of seven surfactants (we could not obtain a zwitterionic eight carbon surfactant). We applied these compounds to three anesthetic-sensitive receptors: two inhibitory [γ-amino butyric acid type A (GABAA), and glycine] receptors and one excitatory [N-methyl-D-aspartate (NMDA)] receptor, predicting they would enhance the function of GABAA and glycine receptors and inhibit the function of NMDA receptors (12, 13) as the volatile anesthetics isoflurane and ethanol do.
We also tested these surfactants on GABAA, glycine, and NMDA receptors engineered with point mutations that diminish the modulatory effect of isoflurane and ethanol (12–17). The investigators who engineered these point mutations envisioned that the mutations altered a binding site for isoflurane and ethanol. In contrast, we considered it unlikely that the larger and sometimes charged surfactants we used would could all bind to a single site, and therefore hypothesized that the point mutations would not, in general, diminish the modulatory effect of the surfactants.
All surfactants were applied at the same aqueous concentration to each channel. We chose this to be 5 micromolar, the anesthetic EC50 for dodecanol in tadpoles (18), the uncharged 12 carbon alcohol and surfactant we studied.
Materials and Methods
Studies on animals were approved by the institutional animal care and use committee at University of California, San Francisco.
Materials
Wild-type and mutant α1(S270I)β2γ2s GABAA receptor and wild-type and mutant α1(S267I) glycine receptor clones were a gift of Professor R. Adron Harris (University of Texas, Austin). Wild-type and mutant NMDA receptor [NR1(F639A)/NR2A] clones were a gift of Professor John J. Woodward (Medical University of South Carolina). Xenopus laevis female frogs were purchased from Nasco (Modesto, CA). Absolute ethanol and the surfactants sodium dodecyl sulfate (SDS; 99% purity), dodecanol (97%), octanol (99%), sodium n-octyl sulfate (SOS; 99%), (1-octyl)trimethylammonium bromide (OTABR; 97%), (1dodecyl)trimethylammonium chloride (DTACL; 97%), 3-(dodecyldimethylammonio)propanesulfonate (DAPS) were purchased from Sigma-Aldrich (St. Louis, MO). Isoflurane was donated by Baxter Healthcare (Deerfield, IL).
Oocyte Expression
Ion channel expression in Xenopus laevis oocytes was described previously (19, 20).
We studied GABAA receptors comprised of human α1 (wild-type or S270I) and γ2s and rat β2 subunits (α1:β2:γ2s injected into oocytes in a molar ratio 1:1:6), homomeric human α1 (wild-type or S267I) glycine receptors, and NMDA receptors comprised of human NR1 (wild-type or F639A) and NR2A (injected into oocytes in a molar ratio 1:1). The cDNAs encoding GABAA receptor α1 wild-type and mutant subunits were cloned into a pBK-CMV vector, and cDNAs encoding GABAA receptor β2 and γ2s and glycine receptor α1 wild-type and mutant were cloned into a pCIS2 vector. The cDNAs encoding NMDA NR1 wild-type and NR2A were cloned into a pcDNA1 vector, whereas cDNAs encoding NMDA NR1 mutant were cloned into a pcDNA3 vector.
Two-electrode Voltage Clamp Recording
Two-electrode voltage clamping was performed as described previously (19, 20), with the following modifications.
Isoflurane was applied at a concentration of 0.3mM, and ethanol at 200mM. 5 µM of each surfactant was applied to oocytes expressing GABAA, glycine, or NMDA receptors. The GABA concentrations equivalent to 20% of the maximal effective concentration (EC20) and the glycine concentrations equivalent to EC5 were used in our studies. 10 µM glycine, which provides a maximal response for both wild-type and mutant receptors, was employed in all the studies of NMDA receptors. Glutamate concentrations equivalent to EC50 were applied.
For each oocyte, stable inward currents in response to application of agonist(s) were verified by application of agonist(s) for 20 seconds followed by a 5 to 6 minute washout (for GABAA and glycine receptors) or 3 to 4 minute washout (for NMDA receptors), which was repeated three times. Each surfactant was then applied to oocytes for 100 seconds, followed by coapplication of agonist(s) with the surfactant for 20 seconds. Return to baseline response to agonist(s) after washout of the test compound for 4 minutes was confirmed.
Data Analysis
Increase (potentiation) or decrease (inhibition) of currents was calculated as the percent change in current in oocytes clamped at −80mV during drug and agonist coadministration vs. agonist alone. All data are presented as mean ± SE, with 4 to 12 oocytes tested. Statistical significance was determined using Student’s t-Test. P<0.05 was considered significant.
We assessed whether the pattern of effects on receptor function by surfactants paralleled that of isoflurane and ethanol and thus might be termed an “anesthetic-like” effect. If receptor modulation by a surfactant differed significantly from zero and was in the same direction as that produced by isoflurane and ethanol on wild type receptors (potentiation of GABAA and glycine receptors, inhibition of NMDA receptors), then this was considered an “anesthetic-like” effect. Any other result was considered not an “anesthetic-like” effect. Seven surfactants were tested on three receptors, for a total of twenty one possible results. Statistical significance was determined by calculating the probability of obtaining the number of anesthetic-like effects observed (or a more extreme number) in twenty-one Bernoulli trials (21). We assumed that by chance alone, anesthetic-like and not anesthetic-like effects were equally likely.
Results
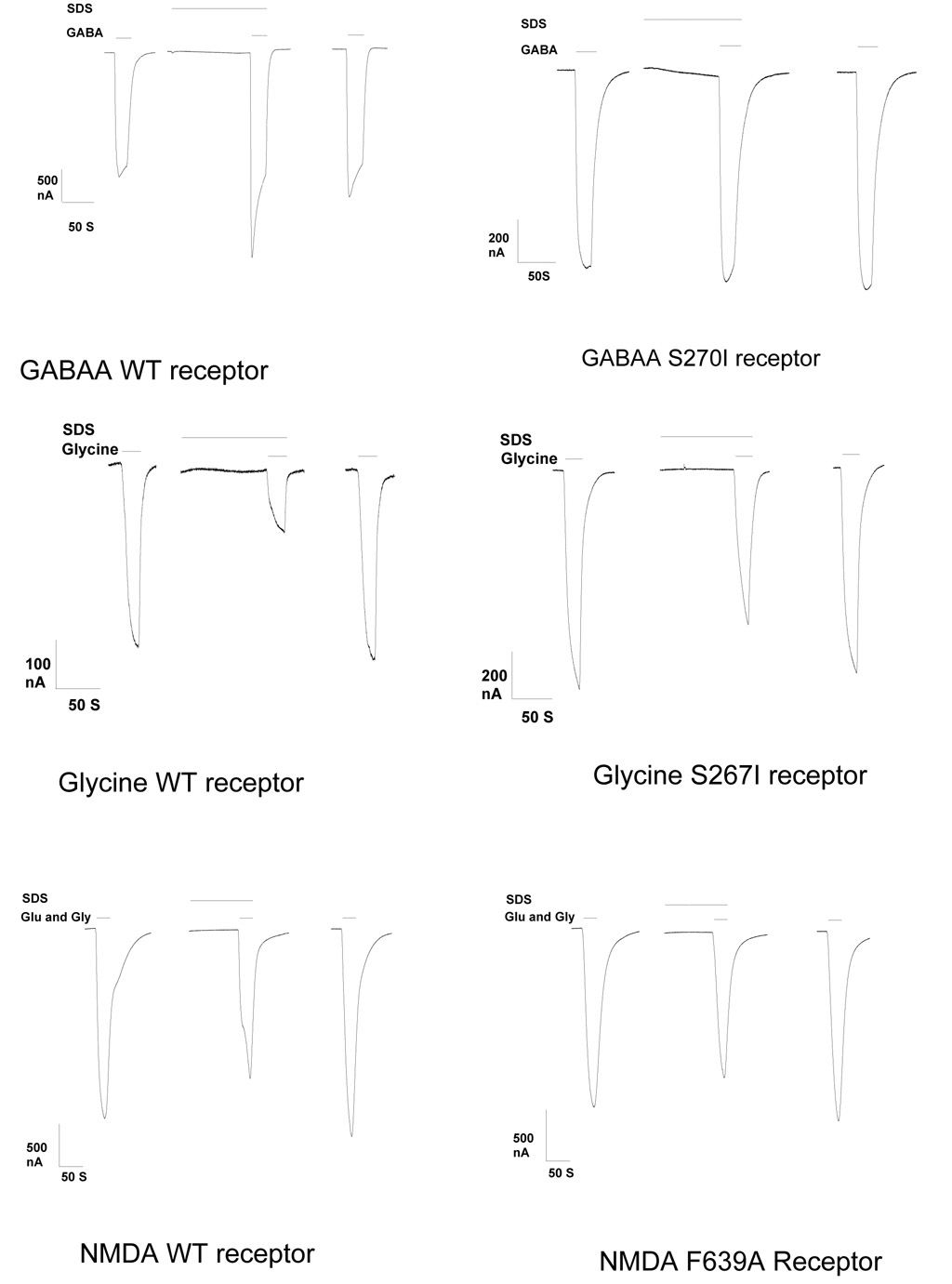
Current tracings showing the effect of a surfactant (SDS) on the wild type and mutant channels studied are in Fig 2.
Fig 2.
Current tracings show the responses to 5 micromolar sodium dodecyl sulfate (SDS) on wild type α1β2γ2s GABAA, α1 Glycine, or NMDA NR1 /NR2A receptors, or mutant receptors α1(S270I)β2γ2s GABAA, α1 (S267I) Glycine, or NMDA NR1 (F639A) / NR2A with point mutations that attenuated the response to isoflurane and ethanol, in Xenopus oocytes. Modulatory effects were reversible in all cases. Equi-effective concentrations of agonist were use on wild type and mutant receptors. The first tracing of each group shows the response to agonist prior to application of SDS. The second tracing shows the response to SDS and agonist. The third tracing shows the response to agonist after washout of SDS.
GABAA receptors
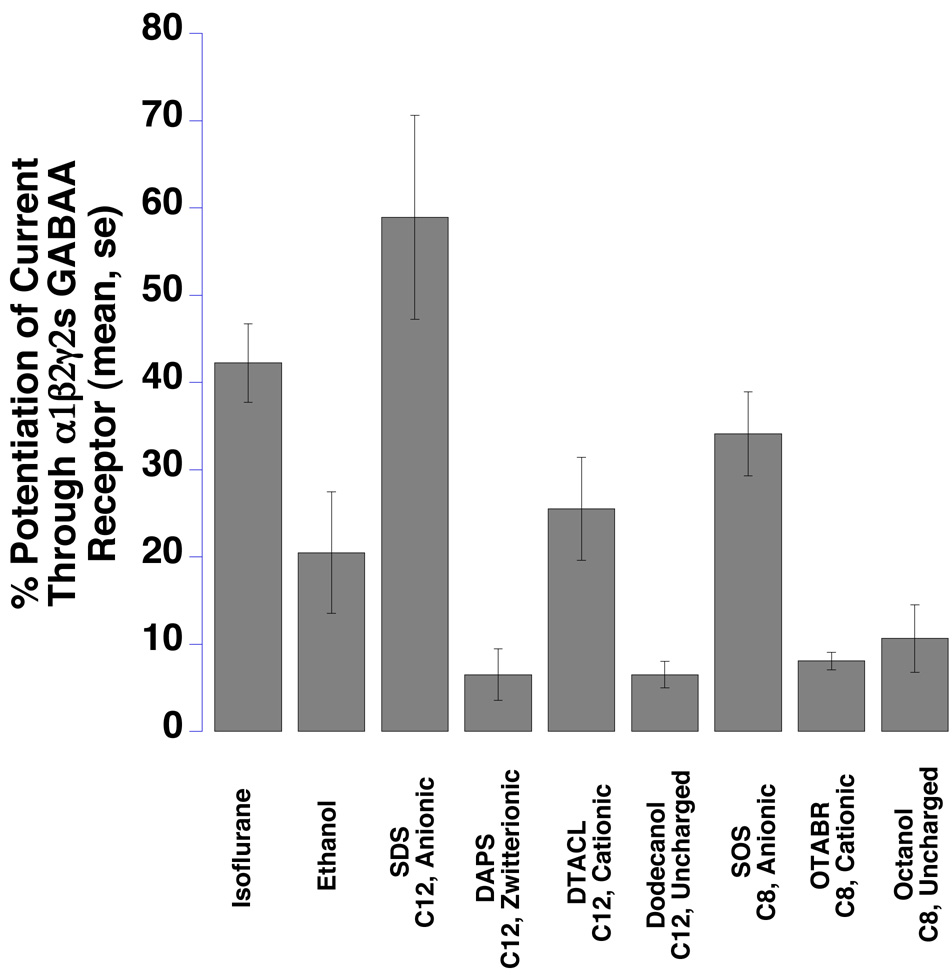
All surfactants, isoflurane, and ethanol enhanced GABAA receptor function (Fig 3 and Table 1).
Fig 3.
The function of α1β2γ2s GABAA receptors expressed in Xenopus oocytes was significantly potentiated by seven surfactants, isoflurane, and ethanol (P<0.05 for every compound). Bar graphs show the mean GABA-induced responses, at agonist EC20 concentrations, from multiple oocytes expressing GABAA receptors in the presence of test compounds. Means ± SE (N = 4–7) are displayed. SDS = sodium dodecyl sulfate, SOS = sodium n-octyl sulfate, OTABR = (1-octyl)trimethylammonium bromide, DTACL = (1dodecyl) trimethylammonium chloride, DAPS = 3-(dodecyldimethylammonio)propanesulfonate. C12 = 12 carbon tail, C8 = 8 carbon tail for the surfactants. Anionic, cationic, zwitterionic, and uncharged refer to head group charges for the surfactants.
Table 1.
Effect of mutations on the modulatory effect of surfactants
| Agent | GABAA Wild Type | GABAA Mutant | Glycine Wild Type | Glycine Mutant | NMDA Wild Type | NMDA Mutant |
|---|---|---|---|---|---|---|
| Isoflurane | 42 ± 5 | 9.5 ± 8* | 355 ± 38 | 240 ± 16* | −12 ± 4 | 1 ± 2* |
| Ethanol | 20 ± 7 | −9 ± 3* | 353 ± 50 | 180 ± 17* | −59 ± 3 | −41 ± 4* |
| SDS | 59 ± 12 | 2 ± 28* | −70 ± 4 | −33 ± 8* | −18 ± 6 | −15 ± 5 |
| DAPS | 7 ± 3 | 39 ± 5† | 284 ± 35 | 128 ± 23* | 17 ± 4 | −4 ± 4* |
| DTACL | 26 ± 6 | 16 ± 4 | 96 ± 30 | 70 ± 16 | −31 ± 3 | −8 ± 4* |
| Dodecanol | 6 ± 2 | 17 ± 2† | 164 ± 33 | 178 ± 38 | −13 ± 4 | −47 ± 11† |
| SOS | 34 ± 5 | 4 ± 3* | 243 ± 38 | 142 ± 6* | −27 ± 4 | −18 ± 5 |
| OTABR | 8 ± 1 | 7 ± 2 | 419 ± 59 | 170 ± 26* | −11 ± 2 | −42 ± 9† |
| Octanol | 11 ± 4 | 34 ± 5† | 174 ± 40 | 175 ± 67 | −20 ± 6 | −45 ± 7† |
Data are displayed as changes in current compared to agonist alone (percent ± SE)
indicates that the mutation significantly decreases the modulatory effect of the surfactant compared to the wild type receptor (p < 0.05). † indicates that the mutation significantly increases the modulatory effect of the surfactant compared to the wild type receptor (p < 0.05).
indicates that the mutation significantly increases the modulatory effect of the surfactant compared to the wild type receptor (p < 0.05).
The GABAA wild type receptor is α1β2γ2s. The mutant GABAa receptor contains an S267I mutation in the α1 subunit. The glycine wild type receptor is homomeric α1. The mutant glycine receptor contains an S267I mutation in the α1 subunit. The NMDA wild type receptor is NR1/NR2A. The mutant NMDA receptor contains an F639A mutation in the NR1 subunit.
Isoflurane was applied at 0.3mM. Ethanol was applied at 200 mM. All other agents were at 5 micromolar.
SDS = sodium dodecyl sulfate
DAPS = 3-(dodecyldimethylammonio)propanesulfonate
DTACL = (1dodecyl)trimethylammonium chloride
SOS = sodium n-octyl sulfate
OTABR = (1-octyl)trimethylammonium bromide
The 12-carbon anionic surfactant SDS showed the largest effect, while the 12-carbon zwitterionic surfactant DAPS and nonionic surfactants (12-carbon dodecanol and 8-carbon octanol) produced smaller effects. SOS, the 8-carbon anionic surfactant, potentiated the GABA response less than the 12-carbon SDS (P = 0.09). A similar pattern was seen with the two positively charged surfactants, the 12-carbon DTACL and the 8-carbon OTABR (P = 0.02).
Glycine Receptors
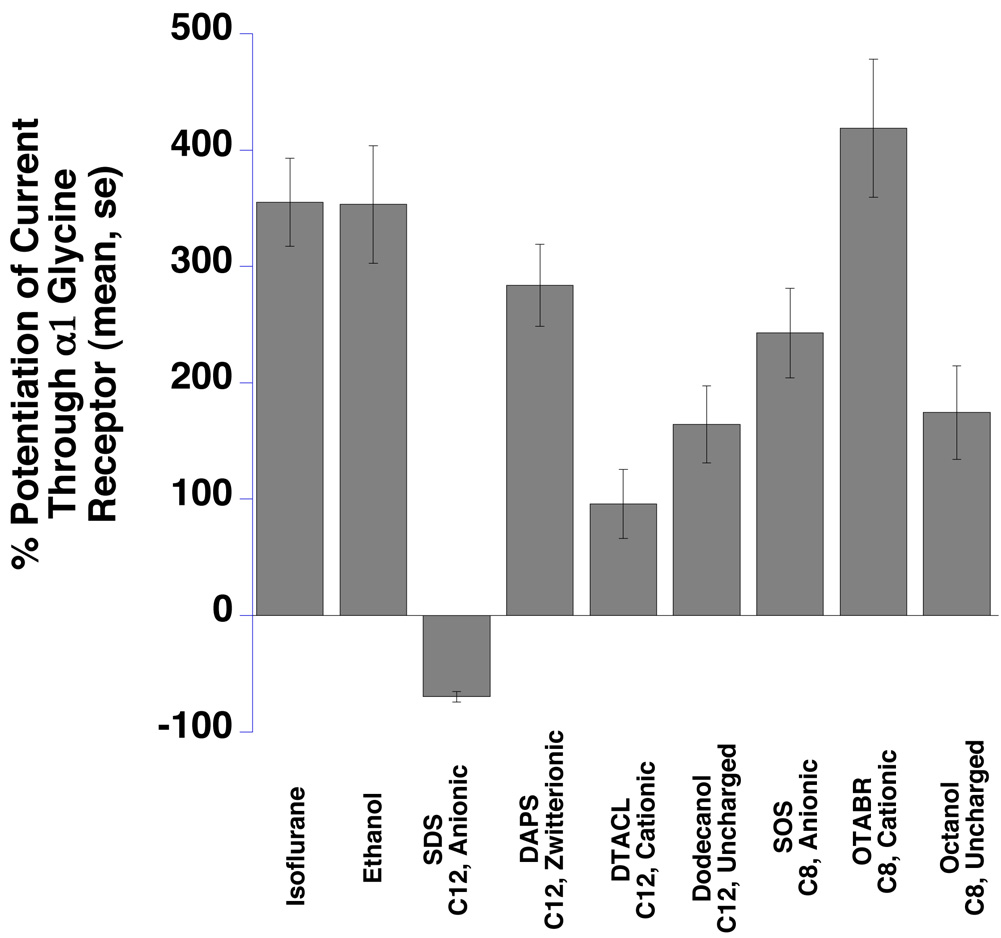
Isoflurane, ethanol, and six of seven surfactants enhanced glycine receptor function (Fig 4 and Table 1).
Fig 4.
α1 Glycine receptors expressed in Xenopus oocytes were significantly potentiated by isoflurane, ethanol, and six surfactants and inhibited by one (SDS) (all effects are significantly different from zero, P<0.05). Summary bar graphs show the mean glycine-induced responses, at EC5 agonist concentrations, from multiple oocytes expressing α1 glycine receptors in the presence of study compounds. Means ± SE (N = 4–7) are displayed. SDS = sodium dodecyl sulfate, SOS = sodium n-octyl sulfate, OTABR = (1-octyl)trimethylammonium bromide, DTACL = (1dodecyl) trimethylammonium chloride, DAPS = 3-(dodecyldimethylammonio)propanesulfonate. C12 = 12 carbon tail, C8 = 8 carbon tail for the surfactants. Anionic, cationic, zwitterionic, and uncharged refer to head group charges for the surfactants.
The exception was the 12-carbon anionic surfactant SDS, which inhibited receptor function. The other anionic surfactant, SOS, potentiated glycine receptor function. The 8-carbon cationic surfactant OTABR showed the greatest effect, while the corresponding 12-carbon DTACL produced the smallest effect (P = 0.006). The second greatest effect was produced by the 12-carbon zwitterionic surfactant DAPS. The two nonionic surfactants, dodecanol (12 carbon) and octanol (8 carbon) potentiated the EC5 glycine response similarly (P = 0.85).
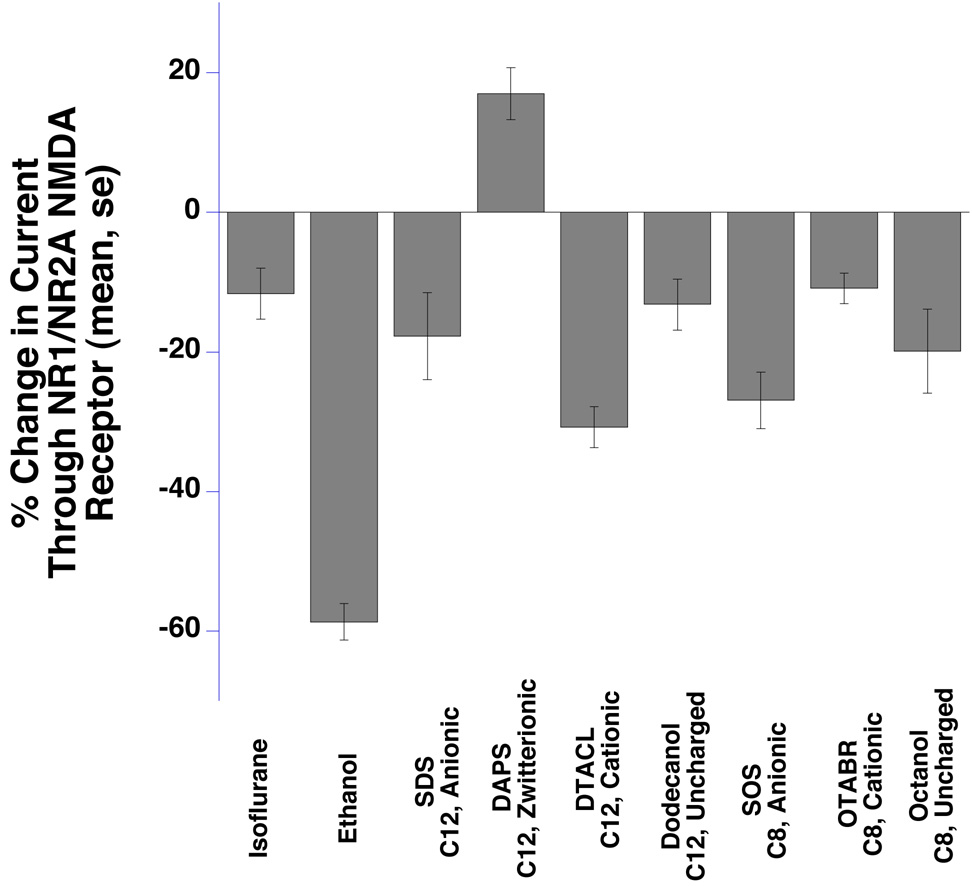
NMDA receptors
Isoflurane, ethanol, and six of seven surfactants inhibited NMDA receptor function (Fig 5 and Table 1).
Fig 5.
NR1 / NR2A N-methyl-D-Aspartate receptors expressed in Xenopus oocytes were inhibited by isoflurane, ethanol, and six surfactants and potentiated by DAPS (P<0.05). Bar graphs show the mean response induced by glutamate (EC50) and a saturating concentration of glycine. Means ± SE (N = 4–7) are displayed. SDS = sodium dodecyl sulfate, SOS = sodium n-octyl sulfate, OTABR = (1-octyl)trimethylammonium bromide, DTACL = (1dodecyl) trimethylammonium chloride, DAPS = 3-(dodecyldimethylammonio)propanesulfonate. C12 = 12 carbon tail, C8 = 8 carbon tail for the surfactants. Anionic, cationic, zwitterionic, and uncharged refer to head group charges for the surfactants.
The exception was the 12-carbon zwitterionic surfactant DAPS, which potentiated NMDA receptor function. The 12-carbon cationic surfactant DTACL showed the largest inhibitory effect, while the corresponding 8-carbon OTABR produced the smallest effect. Both the anionic surfactants SDS (12 carbon) and SOS (8 carbon) reduced NMDA receptor function, with former showing an insignificantly smaller effect (P = 0.23). A similar pattern was also seen between two nonionic alcohols, dodecanol (12 carbon) and octanol (8 carbon) (P = 0.37).
Surfactants, isoflurane, and ethanol have similar modulatory effects on receptor function
Seven surfactants were studied on three wild type receptors, for a total of 21 combinations of surfactants and receptors. In all cases, surfactants significantly modulated receptor function. In 19 of 21 cases, this modulation was in the same direction as isoflurane and ethanol (potentiation of currents in GABAA and glycine receptors, and inhibition of NMDA receptor currents). This is a significant result, having the same probability of occurring as obtaining 19 or more heads in 21 flips of a coin (P = 0.00011).
Effect of surfactants on mutant channels
Direction of modulation
Five of seven surfactants significantly increased currents through GABAA receptors, six of seven surfactants significantly increased currents through glycine receptors, and six of seven surfactants significantly decreased currents through the NMDA receptor (Table 1). Thus, in 17 of 21 cases mutant receptors were modulated in the direction observed in wild type receptors by isoflurane and ethanol (p < 0.01).
Change in modulation of mutant vs wild type receptors
The mutations diminished the modulatory effect of isoflurane and ethanol, as anticipated. By contrast, surfactants produced diverse effects on change in current through these receptors (Table 1). Through GABAA receptors, 3 of 7 surfactants diminished currents in mutant compared to wild type receptors, 3 of 7 increased currents, and one showed no significant change in current. For glycine receptors, 4 of 7 surfactants diminished currents in mutant compared to wild type receptors, and 3 of 7 surfactants had no effect. For NMDA receptors, 3 of 7 surfactants diminished currents in mutant compared to wild type receptors, 1 of 7 surfactants increased currents, and 3 of 7 had no significant change in current. Because in only 10 of 21 cases was there a diminished modulatory effect of the mutation, the null hypothesis that there is no diminished modulation of channel currents by these mutations cannot be rejected, (p > 0.05).
Discussion
This study identifies another large class of compounds (surfactants) that modulate the function of GABAA, glycine, and NMDA receptors in a manner that is qualitatively similar to isoflurane and ethanol (potentiation of GABAA and glycine receptors, inhibition of NMDA receptors) (Fig 3–Fig 5): in 19 of 21 cases, surfactants modulated these receptors in an anesthetic-like manner. We therefore cannot reject the hypothesis that interfacial activity is a sufficient condition for anesthetic-like modulation of these receptors. Results from other studies support the present results. For example, an anionic alkylbenzene sulfonate surfactant with a 12 carbon tail potentiates α2 glycine, and α1β2γ2s GABAA receptors, and inhibits DL--amino-3-hydroxy-5-methyl-4-isoxalonepropionic acid (AMPA) GluR3 receptor function (22).
Simulations (9) and NMR spectroscopy (10, 11) show that small uncharged inhaled anesthetics accumulate at interfaces. To the extent that surfactants and volatile anesthetics may act by a common mechanism to modulate channel function, surfactants may prove to be valuable probes of anesthetic action. At what interface might the actions described here of the surfactants be produced? As for volatile anesthetics, our results are consistent with either an action at a protein-water or a membrane-water interface. However, some observations seem to make the protein-water site of action less likely for surfactants. Given the range of surfactant charges, and the diversity of effects on the mutant channels we observed, if the surfactants act by binding to channel proteins, then different surfactants probably bind to different sites on the protein and yet produce similar modulatory effects. This would seem to be unlikely. Alternately, the hydrophilic region of the surfactants may interfere with charge interactions between the protein and head group regions of the membrane, or affect electrical potentials at the bilayer interface which may indirectly affect channel function. A theory has been proposed by which volatile anesthetics alter mechanical properties (stresses) of membranes as a result of their interfacial activity (23). This theory may also provide an explanation of our findings, particularly in light of the well-known effects of surfactants on surface tension (24).
Why do some compounds accumulate at interfaces? As noted previously, the favorable interactions of the hydrophobic tail and hydrophilic head groups of a surfactant with the hydrophobic and aqueous phases present at an interface promote the concentration of surfactant at the interface. These interactions should be smaller for inhaled anesthetics which in general have smaller hydrophobic tails.
The hexane/water interface was used as a model interface in initial molecular dynamic calculations. A consideration of the energetics of transfer of solute from water to hexane reveals another reason why these solutes accumulate at this interface. This transfer may be considered to consist of four steps (for a detailed discussion of the thermodynamics of solvation, see reference (25)). In the first step, a space is made for the solute in the phase to which it will be transferred. The second step requires breaking contacts between the solute and water. These two steps cost energy. The third step is insertion of the solute into its new chemical environment. In the fourth step, the cavity formed by removal of the solute from water is closed. These latter two steps are energetically favorable, because contacts are formed between molecules. It has been shown via simulations at hexane/water interfaces that for inhaled anesthetics, the balance between the cost of opening a cavity (which is least favorable in water) and the free energy of solvation (which is most favorable in water) is particularly important (9). These energies change in opposite directions in transferring the anesthetic from water across the interface. In particular, the minimal energetic cost of cavity formation at the interface, which results from the tendency of the immiscible phases to separate, favors the interfacial location of these small solutes at hexane/water interfaces.
Two polyhydroxyalkanes, each containing four −OH groups separated by six carbons from each other were recently synthesized and their anesthetic actions studied in tadpoles (26). These compounds were designed to be larger than the cavities anesthetics have been predicted to bind to in proteins. The alcohol groups did however render these compounds exceptionally interfacially active because each −OH tethers the molecule to the membrane interface, making the molecule more potent on a molar basis than alcohols with fewer −OH groups if interfacial activity determines anesthetic potency. That these compounds had the predicted anesthetic effects in tadpoles is consistent with a membrane mediated mechanism of anesthesia (26).
The length of the tail of the two anionic surfactants we studied affected the response of the glycine receptor. The 12 carbon anionic surfactant SDS inhibited and the 8 carbon anionic surfactant SOS potentiated glycine receptor function. Such a chain-length dependent crossover from potentiation to inhibition has been reported for a related cys-loop receptor, the neuronal nicotinic actylcholine receptor, in which short chain alcohols potentiate α4β2 receptor function, while longer chain alcohols inhibit currents through these receptors (27). Possibly a similar effect occurs with application of anionic surfactants to glycine receptors.
Homologous mutations, from serine to isoleucine at position 270 of the GABAA receptor α1 subunit, and position 267 of the α1 subunit of the glycine receptor, attenuated the modulatory effect of both anionic surfactants (SDS and SOS) on α1β2γ2s GABAA and α1 glycine receptors. This may indicate a shared mechanism between these compounds and isoflurane and ethanol, whose modulatory effects are also reduced by these mutations. However, for other surfactants with differing carbon chain lengths and head group charges, no clear pattern was observed on mutant receptors in comparison to wild type receptors, as conjectured.
Our results may have implications for the development of new anesthetics. Both surfactants and inhaled anesthetics are interfacially active, and our results suggest that they modulate receptor function similarly. This raises the possibility that interfacial activity may be used to predict function. To achieve sufficient interfacial concentration in the central nervous system to modulate receptor function, a compound must also cross the blood brain barrier. While uncharged inhaled anesthetics readily penetrate the blood brain barrier, large charged surfactants will not. Development or identification of interfacially active compounds that can cross the blood brain barrier may produce leads for new anesthetics. Possibly, some small metabolites which modulate channel function like anesthetics, and have anesthetic-like effects in animals fulfill these conditions (19, 28). We would predict that they are interfacially active and in particular that they will affect physical properties of lipids at interfaces.
Acknowledgments
This work was supported in part by NIGMS R01 GM069379
References
- 1.Fenster JM. Ether Day. New York: HarperCollins; 2001. [Google Scholar]
- 2.Powers RA, Morandi F, Shoichet BK. Structure-based discovery of a novel, noncovalent inhibitor of AmpC beta-lactamase. Structure. 2002;10(7):1013–1023. doi: 10.1016/s0969-2126(02)00799-2. [DOI] [PubMed] [Google Scholar]
- 3.Bertaccini EJ, Shapiro J, Brutlag DL, Trudell JR. Homology modeling of a human glycine alpha 1 receptor reveals a plausible anesthetic binding site. J Chem Inf Model. 2005;45(1):128–135. doi: 10.1021/ci0497399. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya AA, Curry S, Franks NP. Binding of the general anesthetics propofol and halothane to human serum albumin. High resolution crystal structures. J Biol Chem. 2000;275(49):38731–38738. doi: 10.1074/jbc.M005460200. [DOI] [PubMed] [Google Scholar]
- 5.Meyer HH. Theorie der Alkoholnarkose. Arch. Exp. Pathol. Pharmakol. 1899;42:109–118. [Google Scholar]
- 6.Taheri S, Halsey MJ, Liu J, Eger EI, 2nd, Koblin DD, Laster MJ. What solvent best represents the site of action of inhaled anesthetics in humans, rats, and dogs? Anesth Analg. 1991;72(5):627–634. doi: 10.1213/00000539-199105000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Sonner JM, Antognini JF, Dutton RC, Flood P, Gray AT, Harris RA, Homanics GE, Kendig J, Orser B, Raines DE, Rampil IJ, Trudell J, Vissel B, Eger EI., 2nd Inhaled anesthetics and immobility: mechanisms, mysteries, and minimum alveolar anesthetic concentration. Anesth Analg. 2003;97:718–740. doi: 10.1213/01.ANE.0000081063.76651.33. [DOI] [PubMed] [Google Scholar]
- 8.Koblin DD, Chortkoff BS, Laster MJ, Eger EI, 2nd, Halsey MJ, Ionescu P. Polyhalogenated and perfluorinated compounds that disobey the Meyer-Overton hypothesis. Anesth Analg. 1994;79(6):1043–1048. doi: 10.1213/00000539-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Chipot C, Wilson MA, Pohorille A. Interactions of anesthetics with the water-hexane interface. A molecular dynamics study. J Phys Chem B. 1997;101(5):782–791. doi: 10.1021/jp961513o. [DOI] [PubMed] [Google Scholar]
- 10.Tang P, Yan B, Xu Y. Different distribution of fluorinated anesthetics and nonanesthetics in model membrane: a 19F NMR study. Biophys J. 1997;72(4):1676–1682. doi: 10.1016/S0006-3495(97)78813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.North C, Cafiso DS. Contrasting membrane localization and behavior of halogenated cyclobutanes that follow or violate the Meyer-Overton hypothesis of general anesthetic potency. Biophys J. 1997;72(4):1754–1761. doi: 10.1016/S0006-3495(97)78821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogata J, Shiraishi M, Namba T, Smothers CT, Woodward JJ, Harris RA. Effects of anesthetics on mutant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2006;318(1):434–443. doi: 10.1124/jpet.106.101691. [DOI] [PubMed] [Google Scholar]
- 13.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 14.Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol. 1996;50(2):402–406. [PubMed] [Google Scholar]
- 15.Nishikawa K, Jenkins A, Paraskevakis I, Harrison NL. Volatile anesthetic actions on the GABAA receptors: contrasting effects of alpha 1(S270) and beta 2(N265) point mutations. Neuropharmacology. 2002;42(3):337–345. doi: 10.1016/s0028-3908(01)00189-7. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins A, Greenblatt EP, Faulkner HJ, Bertaccini E, Light A, Lin A, Andreasen A, Viner A, Trudell JR, Harrison NL. Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J Neurosci. 2001;21:RC136. doi: 10.1523/JNEUROSCI.21-06-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borghese CM, Werner DF, Topf N, Baron NV, Henderson LA, Boehm SL, 2nd, Blednov YA, Saad A, Dai S, Pearce RA, Harris RA, Homanics GE, Harrison NL. An isoflurane- and alcohol-insensitive mutant GABA(A) receptor alpha(1) subunit with near-normal apparent affinity for GABA: characterization in heterologous systems and production of knockin mice. J Pharmacol Exp Ther. 2006;319:208–218. doi: 10.1124/jpet.106.104406. [DOI] [PubMed] [Google Scholar]
- 18.Alifimoff JK, Firestone LL, Miller KW. Anaesthetic potencies of primary alkanols: implications for the molecular dimensions of the anaesthetic site. Br J Pharmacol. 1989;96(1):9–16. doi: 10.1111/j.1476-5381.1989.tb11777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brosnan RJ, Yang L, Milutinovic PS, Zhao J, Laster MJ, Eger EI, 2nd, Sonner JM. Ammonia has anesthetic properties. Anesth Analg. 2007;104:1430–1433. doi: 10.1213/01.ane.0000264072.97705.0f. [DOI] [PubMed] [Google Scholar]
- 20.Brosnan R, Gong D, Cotten J, Keshavaprasad B, Yost CS, Eger EI, 2nd, Sonner JM. Chirality in anesthesia II: stereoselective modulation of ion channel function by secondary alcohol enantiomers. Anesth Analg. 2006;103:86–91. doi: 10.1213/01.ane.0000221437.87338.af. [DOI] [PubMed] [Google Scholar]
- 21.Freund J. Mathematical Statistics. Fifth ed. Englewood Cliffs, New Jersey: Prentice Hall; 1992. [Google Scholar]
- 22.Machu TK, Mihic SJ, Dildy-Mayfield JE. Selective actions of a detergent on ligand-gated ion channels expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1998;284(1):32–36. [PubMed] [Google Scholar]
- 23.Cantor RS. The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Biochemistry. 1997;36(9):2339–2344. doi: 10.1021/bi9627323. [DOI] [PubMed] [Google Scholar]
- 24.Rosen MJ. Surfactants and Interfacial Phenomena. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 25.Dill K, Bromberg S. Molecular Driving Forces Statistical Thermodynamics in Chemistry and Biology. New York: Garland Science; 2003. [Google Scholar]
- 26.Mohr JT, Gribble GW, Lin SS, Eckenhoff RG, Cantor RS. Anesthetic potency of two novel synthetic polyhydric alkanols longer than the n-alkanol cutoff: evidence for a bilayer-mediated mechanism of anesthesia? J Med Chem. 2005;48(12):4172–4176. doi: 10.1021/jm049459k. [DOI] [PubMed] [Google Scholar]
- 27.Zuo Y, Aistrup GL, Marszalec W, Gillespie A, Chavez-Noriega LE, Yeh JZ, Narahashi T. Dual action of n-alcohols on neuronal nicotinic acetylcholine receptors. Mol Pharmacol. 2001;60:700–711. [PubMed] [Google Scholar]
- 28.Yang L, Zhao J, Milutinovic PS, Brosnan RJ, Eger EI, 2nd, Sonner JM. Anesthetic properties of the ketone bodies beta hydroxybutyric acid and acetone. Anesth Analg. 2007;105:673–679. doi: 10.1213/01.ane.0000278127.68312.dc. [DOI] [PubMed] [Google Scholar]