Abstract
PURPOSE
To determine if a positive response of macular cysts to treatment with dorzolamide eye drops in patients with juvenile X-linked retinoschisis (XLRS) can occur with mutations which result in different types of retinoschisin protein dysfunction.
DESIGN
Retrospective case series
METHODS
Thirteen eyes of 7 patients seen at the University of Illinois at Chicago with a known diagnosis of XLRS were included. Each patient had prior or was on current treatment with topical dorzolamide. One patient from each family was screened for a genetic mutation. Using the method of cell transfection and protein preparation, the mutation in each patient was further analyzed and categorized into three groups: 1. total absence of retinoschisin protein secretion, 2. decreased expression of the secreted protein or 3. secretion of a non-functional protein. The response to dorzolamide was observed using optical coherence tomography.
RESULTS
Significant improvement in the foveal zone thickness was observed with the use of dorzolamide in 3 of 4 patients with absence of protein secretion, in 2 patients with a lack of protein expression and in 1 patient with a non-functional protein secretion.
CONCLUSION
This study shows that the response of macular cysts to dorzolamide in patients with XLRS may be observed independent of the mechanism responsible for retinoschisin protein dysfunction. Hence, treatment with dorzolamide may be effective in patients with different mechanisms of dysfunction in retinoschisin.
Congenital X-linked retinoschisis (XLRS) is the most common form of juvenile onset macular degeneration that affects almost exclusively males.1,2 It presents with decreased visual acuity in the first decade of life and the presence of stellate-shaped foveal cystic changes on fundus examination. Electroretinography in this disease is characterized by a predominant reduction in b-wave amplitude. Evaluation using optical coherence tomography (OCT) reveals cystic spaces in the retina predominantly at the level of inner nuclear and outer plexiform layers in the macular region.3,4 Peripheral schisis may also be present in this disease.5–7
Mutations in the RS1 gene, which encodes for a 24-kDa protein retinoschisin, or RS1, are responsible for XLRS. 8 Retinoschisin consists of a 23 amino acid N-terminal leader sequence, 39 amino-acid RS1 domain, 157 amino acid discoidin domain and five amino acid C-terminal segment. 9 This protein has been proposed to function as a cell adhesion protein to maintain the retinal structure. 9,10 Three different mechanisms have been demonstrated to be responsible for X-linked retinoschisis in the various disease-linked missense mutations; most mutations in the discoidin domain result in protein misfolding and intracellular retention by the endoplasmic reticulum leading to absence of retinoschisin protein secretion; cysteine mutations in the RS1 domain and C-terminal segment cause defective octamer assembly leading to secretion of a non-functional protein; and mutations in the leader sequence prevent insertion of the polypeptide into the endoplasmic reticulum leading to decreased protein expression. 11,12 More recently, an additional mechanism has been inferred from in vitro analysis of the Arg141His mutant. 13,14 This mutant displays normal octamer assembly and secretion from cells, but is nonfunctional as indicated by its involvement in XLRS. This mutant may be defective in its capacity to interact with Na/K ATPase on the surface of photoreceptors and bipolar cells. 15 It should also be noted that these mechanisms are not necessarily mutually exclusive.
Topical and oral carbonic anhydrase inhibitors have been demonstrated to cause an improvement in the macular cystic cavities in XLRS, as documented by OCT, and by an improvement in visual acuity. 16,17 The purpose of this study was to determine if the topical carbonic anhydrase inhibitor, dorzolamide, can be effective for reducing the severity of the foveal cystic-appearing lesions and foveal thickness irrespective of the disease-causing mechanisms mentioned above that may lead to the loss of normal function in the retinoschisin protein.
METHODS
The clinical study was conducted in the Department of Ophthalmology at the University of Illinois at Chicago. A total of 7 patients, over the age of 18 years, from 6 families with a known diagnosis of XLRS were included. All patients were examined by one of the authors (GAF) and the diagnosis of XLRS was made based on the clinical findings, which included a decrease in visual acuity, stellate-shaped cavities in the macular region and a decrease in the b-wave amplitude on ERG examination.
All patients were started on 2% dorzolamide three times a day in one or both eyes. The dosage of dorzolamide was reduced to bid in two patients with sustained improvement; two other patients showed an increase in cystic lesions on OCT after reduction of the dosage and were reverted to tid dosage. The rest of the three patients were using the drops three times in a day. The patients were maintained on the above dosage for sustained response. The response to treatment was monitored with a time-domain OCT (StratusOCT, Carl Zeiss Meditec Inc., Dublin, CA; software version 4.0.1). Six radial scans of 6-mm each at 30° intervals passing through the center of the fovea were used for the time domain OCT testing. Each of these six scans was acquired individually and sequentially by the operator and consisted of 512 A-scans. The response to treatment was independently assessed in a qualitative manner by two observers (GAF and SW) and graded as 0-no response, 1-uncertain response, 2-mild, 3-moderate, and 4-marked response. The change in the foveal zone thickness (central 1000μm of the foveal region) from the beginning of the treatment to the final response was also calculated. A change in the value of FZT of more than 17.1% was considered significant. 16
Blood was drawn from one affected member in each family and analyzed for genetic mutations in the RS1 gene. At the University of Illinois at Chicago, genetic analysis was done by first isolating the DNA from human blood using the Genelute Blood Genomic DNA purification kit as described by the manufacturer (Sigma-Aldrich, St. Louis, MO). All six exons of the RS1 gene were then amplified using Sigma Genomic RedTaq (Sigma-Aldrich, St. Louis, MO) or KOD HotStart DNA Polymerase (EMD Biosciences, La Jolla, CA). For exon 1 to 3 and for exon 6, primers previously described were used. 8,18 For amplification of exons 4 and 5, the forward primer for exon 4 and the reverse primer for exon 5 were used. 8 PCR cycling conditions were: initially denaturation at 94°C for 3 minutes followed by 35 amplification cycles (30 sec at 94°C, 30 sec. at 57°C and 100 seconds at 72°C) and a final extension for 10 minutes at 72°C. PCR products were separated from primers using the Genelute PCR DNA purification kit according to manufacturer’s protocol (Sigma-Aldrich, St. Louis, MO). Sequencing of the total PCR reaction after purification was performed using fluorophore-labelled dye-terminator chemistry on an ABI automated sequencing instrument and the primers used for amplification.
At the University of Iowa, genetic analysis was done by isolating the DNA from human blood using the Autopure LS (Qiagen). One and a half μl of each patient’s stock DNA (~150ng) were used as template in a 30μl PCR containing: 3μl 10X buffer (100mM Tris-HCL pH 8.3, 500mM KCl, 15mM MgCl2), 9mM of each dCTP, dATP, dGTP and dTTP, 9μM of each primer and 0.25 units Biolase polymerase (Biolase). Samples were denatured for 5 minutes at 94 C and incubated for 35 cycles under the following conditions: exons 1, 3, 4 – 94° C for 30 sec, TD 60–55° C for 30 sec, 72° C for 30 sec, exons 5, 6 – 94° C for 30 sec, TD 65–60° C for 30 sec, 72° C for 30 sec and a final extension for 10 minutes at 72°C in a DNA thermocycler (ABI). For exon 2, one and a half microliters of each patient’s stock DNA (~150ng) were used as template in a 30μl PCR containing: 15μl MasterAmp 2x buffer E (100mM Tris-HCL pH 8.3, 100mM KCl, 5.0 mM MgCl2, 400 μM of each dCTP, dATP, dGTP and dTTP) (Epicentre), 0.45μM of each primer and 0.18 units MasterAmp polymerase (Epicentre). Samples were denatured for 5 minutes at 94 C and incubated for 35 cycles under the following conditions 94° C for 60 sec, 55° C for 60 sec, 72° C for 60 sec and a final extension for 10 minutes at 72°C. Samples were sequenced using fluorophore-labelled dye-terminator chemistry on an ABI automated sequencing instrument and the primers used for amplification. The sequences were analyzed using Sequencher software.
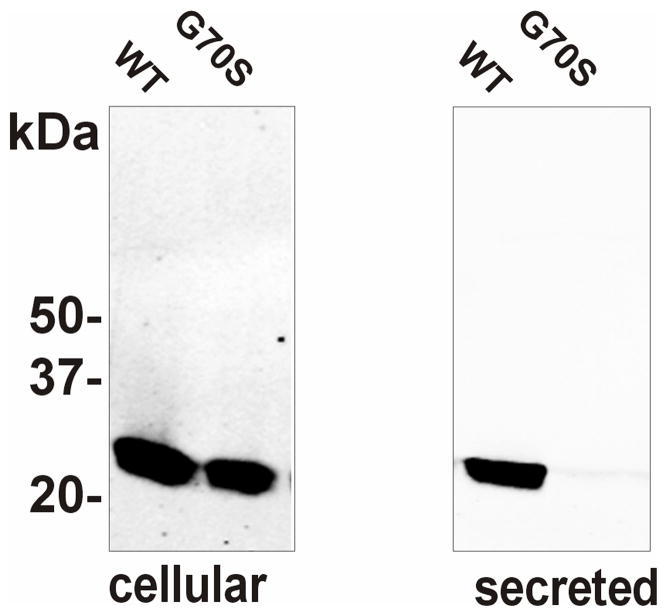
At the University of British Columbia, the effect of the Gly70Ser disease-linked mutation on octamer assembly and secretion of retinoschisin from EBNA 293 cells was examined as previously described. 11 Briefly, the Gly70Ser mutation was introduced using the quick change site-directed mutagenesis kit (Stratagene) into the human retinoschisin cDNA. EBNA 293 cells (American Type Culture Collection) were transfected with 20 μg of plasmid wild-type or mutant DNA, in 10 cm dishes using the calcium phosphate transfection procedure. The DNA containing medium was replaced with regular medium the next day and the cells were harvested two days later. Protein extraction, SDS gel electrophoresis (Figure 1) and Western blotting were performed as previously described.11 Similarly, other mutations that were determined in the present study were introduced into the retinoschisin cDNA and characterized.
Fig 1.
Expression and secretion of wild-type (WT) and G70S (Gly70Ser) mutant retinoschisin. Cells expressing WT or mutant retinoschisin were separated into the cellular (cells) and secreted fraction (medium). The expressed proteins were run on 10% SDS–polyacrylamide gels under reducing conditions. Western blots were labeled with the RS1 3R10 monoclonal antibody. In contrast to WT retinoschisin, the G70S mutant was not present in the secreted fraction
RESULTS
The average age of the patients included in the study was 29.7 years with a range of 19–38 years. Five patients were Caucasian and two were Hispanic. Table I displays the different mutations in our cohort and its effect on protein secretion.
TABLE I.
The distribution of age; RS1 mutation and its effect on protein secretion in our cohort.
| S.No. | Age | Race | Mutation | Protein function |
|---|---|---|---|---|
| 1 | 37 | Hispanic | Gly70Ser Exon 4 hemizygous | not secreted |
| 2 | 28 | Hispanic | Gly70Ser Exon 4 hemizygous | not secreted |
| 3 | 38 | Caucasian | Trp96Arg Exon 4 hemizygous | not secreted |
| 4 | 26 | Caucasian | 20 bp insertion Exon 4 codon 73 hemizygous | not expressed stably, premature stop codon |
| 5 | 37 | Caucasian | Gly70Ser Exon 4 hemizygous | not secreted |
| 6 | 23 | Caucasian | Arg141His Exon 5 hemizygous | nonfunctional, secreted |
| 7 | 19 | Caucasian | IVS4del ttCtcgg | not expressed, premature stop codon |
Age (years) has been recorded at the final visit for each patient
Moderate to marked response to the treatment with dorzolamide was observed in all patients except one. The patient who did not show a favorable response to dorzolamide was treated for just two months and was unwilling to continue further treatment. We noted that occasionally treatment may have to be continued for several months before any response is observed.
Both eyes were treated in six patients and one eye in one patient (the patient had nystagmus in the untreated eye more than the other eye, making it difficult to monitor the patient using OCT), for a total of 13 eyes. Eleven of the 13 eyes showed a moderate to marked decrease in the severity of their macular cysts from treatment with dorzolamide. The duration of treatment for each patient and the response to treatment has been tabulated in Table II.
TABLE II.
The duration of treatment with dorzolamide, foveal zone thickness (FZT) in each eye before the start of treatment and at the time of final response, the percentage change in FZT from baseline and the qualitative improvement on OCT as judged by the authors.
| S.No | Time (months) |
FZT OD-start |
FZT OS-start |
FZT OD- response |
FZT OS- response |
FZT OD- % change |
FZT OS- % change |
OD- Qualitative change |
OS- Qualitative change |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 323 | 218 | 230 | 133 | 28.79 | 38.99 | 3.5 | 4 |
| 2 | 2 | 255 | 231 | 392 | 356 | −53.72 | −54.11 | 0 | 0 |
| 3 | 26 | 315 | 262 | 162 | 171 | 48.57 | 34.73 | 4 | 4 |
| 4 | 26 | 664 | 606 | 164 | 167 | 75.3 | 72.44 | 4 | 4 |
| 5 | 18 | 416 | 395 | 178 | 176 | 57.21 | 55.44 | 4 | 4 |
| 6 | 23 | 525 | 551 | 431 | 153 | 17.9 | 72.23 | 3 | 4 |
| 7 | 6.5 | not treated | 272 | not treated | 170 | - | 37.5 | - | 4 |
Patients 1 &2 are siblings.
The cohort could be divided on the basis of retinoschisin protein secretion into three groups: four patients without retinoschisin protein secretion, two with a premature stop codon with absence of protein expression and one with a nonfunctional protein secretion (Table I). Significant improvement in the foveal zone thickness was observed from both eyes in 3 of 4 patients characterized by an absence of protein secretion, in both patients with a lack of protein expression (one of them was treated only in one eye) and in both eyes of the patient with non-functional protein secretion (Table II). In addition, the results on the qualitative response indicated that 3 of 4 patients in the first group showed a moderate to marked response in at least one eye; both patients showed a marked response in group 2, and the patient in group 3 showed a moderate to marked response.
The response to dorzolamide was independent of the initial foveal zone thickness, as can be seen in Table II. Variable responses were observed in two members of the same family (Table II).
As part of this study, the properties of the Gly70Ser retinoschisin mutant were studied. This mutation like most other disease-causing mutations in the discoidin domain prevented the secretion of retinoschisin from cells as shown in Figure 1.
DISCUSSION
Retinoschisin is secreted by the photoreceptors and localizes to the surface of photoreceptors and bipolar cells. 9 It has been shown that misfolding of the discoidin domain, defective-disulphide subunit assembly, and inability of retinoschisin to insert into the endoplasmic reticulum as part of the protein secretion process are three primary mechanisms responsible for loss in function of the RS1 protein and the pathogenesis of XLRS. We have shown here that the Gly70Ser mutation, like most other mutations in the discoidin domain, causes protein misfolding and retention of retinoschisin in cells. Based on the presence of discoidin domains in the RS1 protein and its association with the extracellular surface, it is believed to function in cell adhesion that maintains the structural and functional integrity of the retina.
The clinical effect of carbonic anhydrase inhibitors has been demonstrated through their action on the membrane bound CA IV receptors present in the retinal pigment epithelial layer. 19 Moreover, other carbonic anhydrases exist in the different cells of the neural retina that may also play a role in the clinical effect.20, 21 Prior studies have shown direct effects of these drugs both on retinal and RPE cell function by inducing an acidification in the subretinal space, a decrease in the standing potential, as well as increase in retinal adhesiveness. Retinal adhesiveness is probably enhanced by increasing the retinal pigment epithelium fluid transport. 22 These inhibitors have also been shown to decrease the volume of the subretinal space. 22 However, significant effect has not been demonstrated in macular blood flow with the use of carbonic anhydrase inhibitors. 23
We were able to demonstrate that a positive response of macular cysts in XLRS patients to dorzolamide may occur in any one of the mechanisms by which mutations in the retinoschisin gene can result in defective protein function. The observed effect of dorzolamide was independent of the initial foveal zone thickness (Table 2). It is possible that the observed decrease in cystic-like cavities on OCT with the use of dorzolamide may be related to its property of increasing retinal adhesiveness. 22 It has also been hypothesized earlier that the cystic spaces observed on OCT may represent the accumulation of soluble retinoschisin, which may undergo removal by the retinal pigment epithelium with the use of dorzolamide. 16 However, the present study demonstrates that a response to dorzolamide may be noted even in patients who lack the capacity for retinoschisin secretion and also in patients with a premature stop codon in the RS1 gene. The latter patients cannot synthesize any protein and are effectively a “knockout” for RS1. Thus, their positive response to dorzolamide, suggests that a mechanism independent of retinoschisin itself is responsible for the improvement in the degree of cystic spaces.
Studies in the past have shown that the cystic-like cavities in XLRS may coalesce and further form an atrophic lesion in the macula. 6 Hence, there is reason to believe that treatment of the cystic-like lesions may decrease the incidence of later onset atrophic lesions in XLRS patients. The present study demonstrates that dorzolamide may be effective in treating XLRS patients irrespective of their retinoschisin protein dysfunction.
Acknowledgments
Supported by funds from the Foundation Fighting Blindness, Owings Mills, Maryland; the Grant Healthcare Foundation, Lake Forest, Illinois; NIH core grant YO 1792 and EY 02422; an unrestricted departmental grant from Research to Prevent Blindness; National Eye Institute, Besthesda, Maryland; the Carver Endowment for Molecular Ophthalmology, Iowa City, Iowa; the Carver Family Center for Macular Degeneration, Iowa City, Iowa; the Howard Hughes Medical Institute, Chevy Chase, Maryland and postdoctoral fellowship funding from Foundation Fighting Blinding (FMD).
Biography
 Saloni Walia, MD is currently doing a fellowship in Retinal Electrophysiology and Medical Retina at the University of Illinois at Chicago. She is a graduate of Government Medical College at Chandigarh, India and did her Ophthalmology residency from Christian Medical College in India.
Saloni Walia, MD is currently doing a fellowship in Retinal Electrophysiology and Medical Retina at the University of Illinois at Chicago. She is a graduate of Government Medical College at Chandigarh, India and did her Ophthalmology residency from Christian Medical College in India.
Footnotes
The authors do not have any proprietary interest in this work.
Financial Disclosure: None
Author Contributions: Design of the study: (SW, GAF, RSM)
Conduct of the study: (SW, GAF, FMD, NMK, MAE, EMS)
Data collection: (SW, GAF, FMD, NMK, MAE, EMS)
Data analysis and interpretation: (SW, GAF, RSM, FMD, NMK, EMS)
Preparation of the manuscript: (SW, GAF, RSM, FMD, NMK, MAE, EMS)
Critical review of the manuscript: (SW, GAF, RSM, NMK, MAE, EM S)
Approval of the manuscript: (SW, GAF, RSM, FMD, NMK, MAE, EMS)
Statement about Conformity with Author Information: Approved by the institutional review board at the University of Illinois at Chicago. Informed consent was obtained from each participating patient. The study was conducted in accordance with Health Insurance Portability and Accountability Act.
Registered with http://www.clinicaltrials.gov, clinical trial number NCT00716586
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Forsius H, Krause U, Helve J, et al. Visual acuity in 183 cases of X-chromosomal retinoschisis. Can J Ophthalmol. 1973;8:385–393. [PubMed] [Google Scholar]
- 2.Sieving PA, Yashar BM, Ayyagari R. Juvenile retinoschisis: a model for molecular diagnostic testing of X-linked ophthalmic disease. Trans Am Ophthalmol Soc. 1999;97:451–464. [PMC free article] [PubMed] [Google Scholar]
- 3.Gao H, Kusumi R, Yung CW. Optical coherence tomographic findings in X-linked juvenile retinoschisis. Arch Ophthalmol. 2005;123:1006–1008. doi: 10.1001/archopht.123.7.1006. [DOI] [PubMed] [Google Scholar]
- 4.Apushkin MA, Fishman GA, Janowicz MJ. Correlation of optical coherence tomography findings with visual acuity and macular lesions in patients with X-linked retinoschisis. Ophthalmology. 2005;112:495–501. doi: 10.1016/j.ophtha.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Deutman AF. Sex-linked juvenile retinoschisis. In: Deutman AF, editor. The hereditary dystrophies of the posterior pole of the eye. Assen, the Netherlands: Van Gorcum; 1971. pp. 48–98. [Google Scholar]
- 6.George ND, Yates JR, Moore AT. X linked retinoschisis. Br J Ophthalmol. 1995;79:697–702. doi: 10.1136/bjo.79.7.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roesch MT, Ewing CC, Gibson AE, Weber BH. The natural history of X-linked retinoschisis. Can J Ophthalmol. 1998;33:149–58. [PubMed] [Google Scholar]
- 8.Sauer CG, Gehrig A, Warneke-Wittstock R, et al. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat Genet. 1997;17:164–170. doi: 10.1038/ng1097-164. [DOI] [PubMed] [Google Scholar]
- 9.Molday RS. Focus on molecules: retinoschisin (RS1) Exp Eye Res. 2007;84(2):227–228. doi: 10.1016/j.exer.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Weber BH, Schrewe H, Molday LL, et al. Inactivation of the murine X-linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure. Proc Natl Acad Sci U S A. 2002;99:6222–6227. doi: 10.1073/pnas.092528599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu WW, Molday RS. Defective discoidin domain structure, subunit assembly, and endoplasmic reticulum processing of retinoschisin are primary mechanisms responsible for X-linked retinoschisis. J Biol Chem. 2003;278:28139–28146. doi: 10.1074/jbc.M302464200. [DOI] [PubMed] [Google Scholar]
- 12.Wang T, Waters CT, Rothman AM, Jakins TJ, Römisch K, Trump D. Intracellular retention of mutant retinoschisin is the pathological mechanism underlying X-linked retinoschisis. Hum Mol Genet. 2002;11:3097–3105. doi: 10.1093/hmg/11.24.3097. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Zhou A, Waters CT, O’Connor E, Read RJ, Trump D. Molecular pathology of X linked retinoschisis: mutations interfere with retinoschisin secretion and oligomerisation. Br J Ophthalmol. 2006;90:81–86. doi: 10.1136/bjo.2005.078048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyka FM, Molday RS. Coexpression and interaction of wild-type and missense RS1 mutants associated with X-linked retinoschisis: its relevance to gene therapy. Invest Ophthalmol Vis Sci. 2007;48:2491–2497. doi: 10.1167/iovs.06-1465. [DOI] [PubMed] [Google Scholar]
- 15.Molday LL, Wu WW, Molday RS. Retinoschisin (RS1), the protein encoded by the X-linked retinoschisis gene, is anchored to the surface of retinal photoreceptor and bipolar cells through its interactions with a Na/K ATPase-SARM1 complex. J Biol Chem. 2007;282:32792–32801. doi: 10.1074/jbc.M706321200. [DOI] [PubMed] [Google Scholar]
- 16.Apushkin MA, Fishman GA. Use of dorzolamide for patients with X-linked retinoschisis. Retina. 2006;26:741–745. doi: 10.1097/01.iae.0000237081.80600.51. [DOI] [PubMed] [Google Scholar]
- 17.Ghajarnia M, Gorin MB. Acetazolamide in the treatment of X-linked retinoschisis maculopathy. Arch Ophthalmol. 2007;125:571–573. doi: 10.1001/archopht.125.4.571. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Omoto S, Takeuchi T, Kozaki K, Ueoka Y, Kitahara K. Four Japanese male patients with juvenile retinoschisis: only three have mutations in the RS1 gene. Am J Ophthalmol. 2004;138:788–798. doi: 10.1016/j.ajo.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 19.Wolfensberger TJ, Mahieu I, Jarvis-Evans J, et al. Membrane-bound carbonic anhydrase in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1994;35:3401–3407. [PubMed] [Google Scholar]
- 20.Wistrand PJ, Schenholm M, Lonnerholm G. Carbonic anhydrase isoenzymes CA I and CA II in the human eye. Invest Ophthalmol Vis Sci. 1986;27:419–428. [PubMed] [Google Scholar]
- 21.Ochrietor JD, Clamp MF, Moroz TP, et al. Carbonic anhydrase XIV identified as the membrane CA in mouse retina: strong expression in Müller cells and the RPE. Exp Eye Res. 2005;81:492–500. doi: 10.1016/j.exer.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Wolfensberger TJ. The role of carbonic anhydrase inhibitors in the management of macular edema. Doc Ophthalmol. 1999;97:387–397. doi: 10.1023/a:1002143802926. [DOI] [PubMed] [Google Scholar]
- 23.Grunwald JE, Mathur S, DuPont J. Effects of dorzolamide hydrochloride 2% on the retinal circulation. Acta Ophthalmol Scand. 1997;75:236–238. doi: 10.1111/j.1600-0420.1997.tb00763.x. [DOI] [PubMed] [Google Scholar]