Abstract
Background
The expression of HLA-G during allogeneic recognition is associated with better graft acceptance. The inhibitory receptor ILT2 is expressed on activated T cells and serves to shut down T cell activation, culminating in T cell death or induction of anergy. One of the potential mechanisms in the immunosuppressive accomplishment of HLA-G-ILT2 interactions involves the expansion of myeloid-derived suppressor cells (MDSCs). The potential of MDSCs in transplantation has not yet been exploited.
Methods
(1) Detailed phenotypic characteristics, immunosuppressive potential of MDSCs expanded via inhibitory receptor ILT2 and its ligands, and allogeneic transplant-activated MDSCs were obtained in mice. (2) Oligo- and Real-time pathway-specific PCR Arrays were performed to characterize ILT2-specific MDSCs. (3) Skin allograft survival after adoptive transfer of MDSCs was studied.
Results
Engagement of ILT2 receptors, especially by HLA-G, expanded the population of MDSCs with enhanced suppressive activity. Adoptive transfer of MDSCs generated via ILT2 receptor and its ligands prolonged graft survival in recipients of allogeneic skin transplant. We have proposed pathways for enhancement of immunosuppressive activities and expansion of MDSCs via ILT2 and HLA-G.
Conclusions
Our results suggest that induction of MDSCs using ILT2 inhibitory receptor/HLA-G ligand may be an attractive strategy for preventing rejection of highly immunogenic organs/tissues in clinical transplantation.
Keywords: Immunosuppression, Myeloid-derived suppressor cells, HLA-G, Inhibitory receptor, Allograft survival, Mice
Introduction
The induction of immune tolerance continues to be an important goal of clinical organ and tissue transplantation. The immunological acceptance of a fetal semiallograft during pregnancy is a natural model of immune tolerance, and its underlying mechanisms can be exploited to prevent allograft rejection in clinical transplantation. One of the potential mechanisms involves HLA-G, a human immunosuppressive non-classical MHC molecule (1–4). Evidence suggests that HLA-G protects the fetus from attack by natural killer cells, macrophages, dendritic cells (DCs), monocytes, and T cells by interacting with immune inhibitory receptors, such as immunoglobulin-like transcript 2 (ILT2) and ILT4, and modifying cell functions (5–12). The unique characteristics of both cell-surface and soluble isoforms of HLA-G, the formation of disulfide-bonded dimers with the potential to augment inhibitory receptor signaling, the function of HLA-G as a preferential ligand for the ILTs, and its presence in patients with kidney, kidney/liver, and heart allografts, make HLA-G very important in fundamental approaches for modulation of immune responses to improve allogeneic graft survival in clinical transplantation.
ILT2, also known as LILRB1, LIR-1, and CD85j, is an inhibitory receptor broadly expressed on human T and B lymphocytes and on antigen presenting cells, modulating their functions. Mechanisms of the modulation of immune responses by HLA-G and ILT2 include generation and/or expansion of cell populations that negatively regulate T cell functions (11, 13, 14). One example of these powerful natural regulatory cells, the myeloid-derived suppressor cells (MDSCs), have not yet exploited for the induction and maintenance of transplantation tolerance. MDSCs express CD11b, a specific marker for myeloid cells of the macrophage lineage, and Gr-1, a marker for granulocytes, and display features of undifferentiated myeloid cells, containing precursors of different myeloid cell subsets. MDSCs are an intrinsic part of the normal process of myelopoiesis, and they are present in relatively small numbers in naive hosts. Their number significantly increases in tumor-bearing subjects, as well as during bacterial and parasitic infections, immunization with potent antigens and superantigens, and in other conditions associated with impaired immune reactivity, such as cyclophosphamide treatment, irradiation, and traumatic stress. MDSCs are a potent suppressor of both CD4+ and CD8+ T cells and are responsible for down-regulation of immunity through complex molecular pathways. MDSCs can induce loss or significant decrease of the expression of the T cell receptor ζ chain (CD3ζ), which is an essential part of the TCR complex, and they can inhibit CD3/CD28-induced T cell activation/proliferation, inhibit interferon-γ production by CD8+ T cells in response to specific peptide, and prevent development of cytotoxic T lymphocytes in vitro (15–19). Although the involvement of CD11b+/Gr-1+cells in tumor-mediated suppression has been established, the signals that induce the proliferation of these MDSCs and the mechanisms by which they operate are not completely understood.
Our previous data demonstrate that the expression of ILT2 in transgenic mice significantly down-modulates T cell function in vivo, resulting in long-term skin graft survival or acceptance of allografts (11). Here, we show that ILT2 binds with its natural ligand HLA-G and induces expansion of the CD11b+/Gr-1+ population. These cells are directly involved in the prolongation of allograft survival in mice and are characterized by unique potential with enhanced immunosuppressive activities. Our data demonstrate a novel mechanism used by the host to exploit inhibitory receptor/ligand interactions to promote allograft acceptance. This provides new strategies for the design of better therapies for preventing the rejection of highly immunogenic organs/tissues in clinical transplantation.
Materials and Methods
Mice
C57BL/6 (H-2b) and B6.C-H-2bm12 (bm12) mice were obtained from Jackson Laboratory (Bar Harbor, ME). ILT2 transgenic mice have been established and maintained on a C57BL/6 background as described previously (11). MHC class I-deficient (β2m−/−) and ILT2+/β2m−/− mice were also maintained on a C57BL/6 background. Experiments were conducted on mice at 8–12 weeks and in accordance with institutional guidelines for animal care and use.
Preparations of cell suspensions
Spleens from 6- to 8-wk-old mice were removed and disrupted between glass slides and washed twice with IMDM medium (Life Technologies, Gaithersburg, MD). CD11b+/Gr-1+ cells were isolated from spleen using MACS magnetic microbeads (Miltenyi Biotec, Auburn, CA). The purity of cell separation ranged between 89 and 96%.
Skin grafting
Specific pathogen-free C57BL/6 (H-2b) mice and ILT2 transgenic mice (H-2b) (8 to 10 weeks of age) were used as skin graft recipients throughout the study. Donor skin was from MHC class II-disparate B6.C-H-2bm12 (bm12, H-2b) mice. Allogeneic skin grafts were performed by standard methods as described previously (20).
Cytotoxic T lymphocyte (CTL) assay
Effector cells were restimulated in vitro and tested in a standard 51Cr-release assay. Briefly, spleen cells (5 × 106/ml) from wild-type (WT) or ILT2 transgenic mice that had been transplanted with allogeneic skin grafts were co-cultured with irradiated (20Gy) allogeneic splenocytes (5 × 106/ml) for 5 days. Cytotoxicity tests were performed in triplicate at effector:target (E:T) ratios of 30:1, 10:1, 3:1, and 1:1. Target cells were incubated for 90 min at 37°C with 100 µCi 51Cr (GE Healthcare Bio-Sciences, Piscataway, NJ) before being mixed with effector cells. After 4 h at 37°C, 25 µl of supernatant was collected and counted (Betaplate 1205, Wallac, Turku, Finland). T cell blasts were generated by cultured splenocytes with 2 µg/ml of Con A (Sigma-Aldrich, St. Louis, MO) and used as targets. Allospecific CTL activity was determined as % of specific lysis by a standard formula.
Antibodies and flow cytometry
Cells were stained with FITC-, PE-, CyChrome- or allophycocyanin-conjugated mAbs specific for CD3 (145-2C11), CD4 (RM4–5), CD8α (53–6.7), CD11c (N418), CD11b (M1/70), CD80 (16-10A1), CD86 (GL1), CD124 (mIL4R-M1), B220 (RA3-6B2), Gr-1 (RB6-8C5), MHC class I (CTKb), or MHC class II (M5/114.15.2) and with isotype controls (rat IgG2a, rat IgG2b, mouse IgG2a, armenian hamster IgG) from eBioscience (San Diego, CA), or Biolegend (San Diego, CA), or Caltag (Carlsbad, CA). Cells were incubated with primary antibodies in PBS containing 2% BSA for 30 min at 4°C and washed twice. A BD Biosciences FACSCanto (Mountain View, CA) was used for data acquisition, and CellQuest software was used for analysis.
T cell proliferation assay
2 × 105 naïve T cells were stimulated with 2.5 µg/ml anti-CD3 mAb plus 2.5 µg/ml anti-CD28 mAb (eBioscience). A total of 1 × 103 to 1 × 105 CD11b+/Gr-1+ cells isolated from control or skin-grafted mice were co-cultured with stimulated T cells using DMEM medium containing 150 µM L-arginine at 37°C for 48 h. Nontreated T cells served as controls. Each well was pulsed with 1 µCi [3H] methyl-thymidine (GE Healthcare Bio-Sciences) for the final 18 h of incubation. Cells were harvested onto a filter (Wallac), and isotope incorporation was measured by 1450 MicroBeta TRILUX liquid scintillation counter.
ELISA assay
To evaluate the effect of CD11b+/Gr-1+ cells on IL-2 production by T cells, the level of IL-2, IFN-γ, in cell-free supernatants was measured by ELISA using the Mouse IL-2 and IFN-γ ELISA kits (eBioscience). T cells (2 × 105 T cells per well) were placed in the 96-well plates and stimulated with 2.5 µg/ml plate-bound anti-CD3 mAb and 2.5 µg/ml anti-CD28 mAb (eBioscience). Control or skin graft-induced CD11b+/Gr-1+ cells (1 × 105) were mixed with T cells. Cell-free supernatants were collected at 48 h and analyzed by ELISA according to the manufacturer’s instruction.
RNA isolation, Oligo GEArray, and Real-time PCR Array analysis
Total RNA from CD11b+/Gr-1+ cells from ILT2 and control C57BL/6 mice was isolated with RNA STAT-60TM Kit (Tel-Test, Friendswood, TX). For Oligo GEArray analysis, three micrograms of total RNA was used and cRNA probes were generated according to the manufacturer’s instructions (SuperArray Bioscience, Frederick, MD). Probes were hybridized to the Mouse Angiogenesis Array at 60°C for 16 h. Data were analyzed by the Image Quant 1.2 Software (Amersham Biosciences, Piscataway, NJ) with STORM™ 840 Gel and Blot Imaging System (Amersham Biosciences). The signal for each transcript was normalized by comparison to the housekeeping gene GAPDH.
For Real-time PCR Array analysis, one microgram of total RNA was used to generate cDNAs with the PCR Array RT2 First Strand kit (SuperArray). cDNA was added to RT2 SYBR qPCR Master Mix, and then added to the Mouse Inflammatory Cytokines and Receptors RT2 Profiler PCR Array. PCR cycles were performed according to the manufacturer’s instructions. Data were analyzed by Web-Based PCR Array Data Analysis software (SuperArray). The cycle threshold (Ct) value for each transcript was normalized by comparison to housekeeping genes.
Statistical analysis
Mann-Whitney (Stat 7.0 Software) analysis was performed to evaluate the significance of differences between experimental groups. For a single comparison of two groups, Student’s t test was used after evaluation of normality. For all analyses p< 0.05 was considered significant. All experiments were performed at least three times.
Results
Engagement of ILT2 receptors and allogeneic skin transplantation enhances the recruitment and expansion of CD11b+/Gr-1+ MDSCs
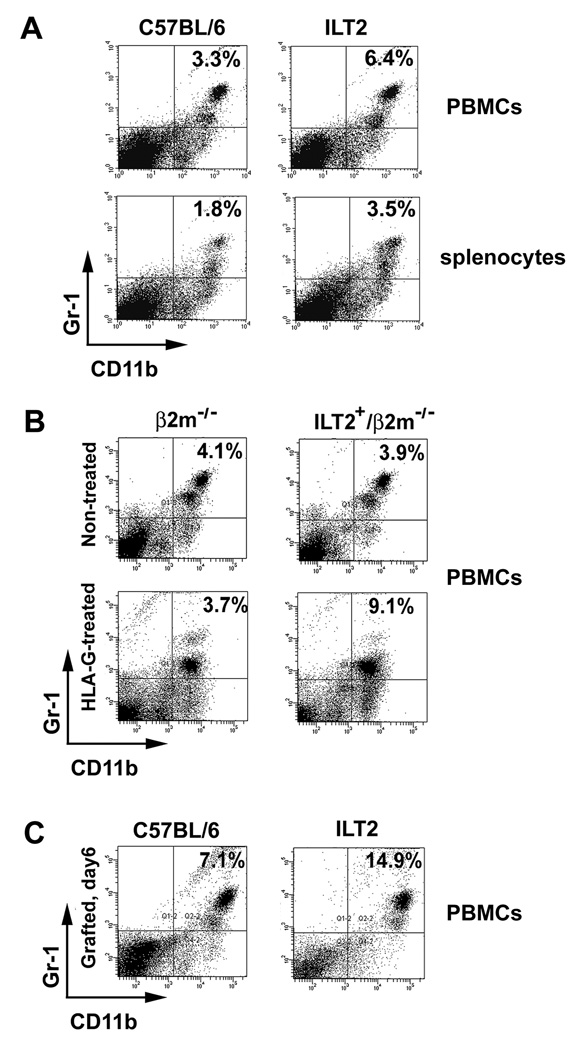
CD11b+/Gr-1+ cells represent a heterogeneous population of myeloid-derived cells containing immature dendritic cells (DCs), macrophages, granulocytes, and other myeloid cells at early stages of differentiation. The cells can be defined in mice as MDSCs by expression of CD11b and Gr-1 markers and by their potential to suppress immune responses. In healthy mice, MDSCs can be detected in the bone marrow, peripheral blood, and spleen. We observed that in non-manipulated mice expressing inhibitory receptor ILT2 (ILT2 mice), the number of CD11b+/Gr-1+ cells in peripheral blood mononuclear cells (PBMCs) have been elevated (6.6% ± 1.3%, mean ± SD, n=8, p < 0.05 in ILT2 mice compared with 3.4% ± 0.4%, n=10 in wild-type mice, Fig. 1A). In addition, an increased number of CD11b+/Gr-1+ cells have been determined in spleen of ILT2 mice (3.6% ± 0.3%, n=8, p < 0.05 in ILT2 mice compared with 1.7% ± 0.2%, n=10, in wild-type mice, Fig. 1A). Since ILT2 mice already are constitutively phosphorylated by murine ligand H-2Db (11), it was necessary to investigate the role of the ligand/receptor interaction in the expansion of MDSCs. The endogenous ligand-free ILT2-positive mice, ILT2+/β2m−/− (ILT2 transgenic on β2-microglobulin knockout background) described previously (11) have a similar number of MDSCs compared with control β2m−/− mice (Fig. 1B). However, treatment of mice with HLA-G ligand gives rise to MDSCs (9.4% ± 0.1%, n=6, p < 0.05 in ILT2+/β2m−/− mice compared with 3.6% ± 0.2% in β2m−/− mice, n=8, Fig. 1B). Since ILT2 receptors are phosphorylated in ILT2+/β2m−/− mice by engagement with natural ligand HLA-G (11), these data indicate the important role of the HLA-G and ILT2 interaction in the control of expansion or induction of MDSCs in vivo. Beyond the progressive accumulation of MDSCs during tumor development, infection, and inflammation, trauma induces activation and accumulation of MDSCs under some circumstances (21–23). We determined that allogeneic skin transplantation increased the numbers of MDSCs in control mice from 1.7% ± 0.2% to 7.3% ± 0.9 (n=10) and from 3.5% ± 0.62% to 14.7% ± 2.1% (n=12) in ILT2-recipient mice (Fig. 1C). Previous studies by our laboratory showed that allogeneic skin transplants were accepted in ILT2 mice or there was robust prolongation of allografts (11). The accumulation of MDSCs in ILT2 mice with long-term survival of skin allograft or acceptance of allograft suggest the unique properties of this special subset of regulatory cells and their potential in prolongation of allograft survival. Thus, the analysis of the phenotype, suppressive function, and unique molecular characteristics of these cells is of interest.
Figure 1. Increased number of MDSCs in naïve ILT2 mice, following treatment with HLA-G, and after allogeneic skin transplantation.
(A) Increased MDSCs in peripheral blood mononuclear cells (PBMCs) (upper panels) and splenocytes (lower panels) in ILT2 mice compared to controls. PBMCs and splenocytes were harvested and stained with anti-CD11b (PE) and anti-Gr-1 (FITC) mAbs and examined by flow cytometry. (B) Treatment of mice with HLA-G increased the numbers of MDSCs. HLA-G-coated beads were injected i.v. into ILT2+/β2m−/− mice and β2m−/− mice (lower panels). PBMCs were harvested and stained with anti-CD11b (PE) and anti-Gr-1 (FITC) mAbs and examined by flow cytometry. (C) Increased number of MDSCs in PBMCs from ILT2 mice after allogeneic skin transplantation. ILT2 and control C57BL/6 recipient mice were given tail skin grafts from MHC class II-disparate mutant mice (B6.C-H-2bm12, bm12) that carry the I-Abm12 alloantigen. On day 6, PBMCs were harvested, stained with anti-CD11b (PE) and anti-Gr-1 (FITC) mAbs and examined by flow cytometry. Numbers indicate percentage of positive cells. Data are representative of four independent experiments.
Allogeneic transplantation induces a specific phenotype of MDSCs in ILT2 mice
CD11b+/Gr-1+ cells isolated from ILT2 transgenic and control wild-type mice undergoing skin graft were compared at different time points. Neither skin graft-induced CD11b+/Gr-1+ cells from C57BL/6 mice nor skin graft-induced CD11b+/Gr-1+ cells from ILT2 mice significantly expressed markers of mature macrophages, DCs, lymphocytes, or NK cells such as CD3, B220, CD8, CD4, CD11c, F4/80, and NK 1.1 (Fig. 2 and data not shown). However, allogeneic skin graft-induced CD11b+/Gr-1+ cells from ILT2 mice expressed lower levels of MHC class I (22.7%) than skin graft-induced CD11b+/Gr-1+ cells from wild-type mice (48.5%). Considerably less expression of pan DC marker CD11c was determined in MDSCs from ILT2 mice (16.3%) compared with wild-type mice (43.4%). The majority of these cells also were MHC class II-positive (71.8%). Decreased levels of expression of B220, CD4, and CD8 were observed in MDSCs from ILT2 mice compared to MDSCs from wild-type mice. Other markers studied were inconsistently expressed and included costimulatory molecules CD80 and CD86 (Fig. 2).
Figure 2. Unique phenotype of MDSCs from ILT2 mice.
Splenocytes from the indicated mice were isolated and stained with PE-conjugated anti-CD11b, FITC-conjugated anti-Gr-1, and APC-conjugated mAbs and examined by FACS. Histograms shown were gated on CD11b+/Gr-1+ population. Filled histograms represent isotype controls. Numbers indicate percentage of positive cells of total gated cells. The results are from one representative experiment of four performed.
The presence of IL-4Rα on CD11b+/Gr-1+ cells appeared to be critical for their suppressive activity. The analyses of the cell surface expression of IL-4Rα (CD124) determined increased expression of IL-4Rα on MDSCs from ILT2 mice (46.6% ± 5.1%, n=10) compared with MDSCs from C57BL/6 mice, where 29.3% ± 6.2%, n=8 were positive for CD124 (Fig. 2).
Thus, these data demonstrate that allogeneic skin graft-induced CD11b+/Gr-1+ cells from ILT2 mice represent a more undifferentiated phenotype of cells with potential suppressive activity.
Augmented suppressive functions of MDSCs from ILT2 mice induced by allogeneic skin transplant
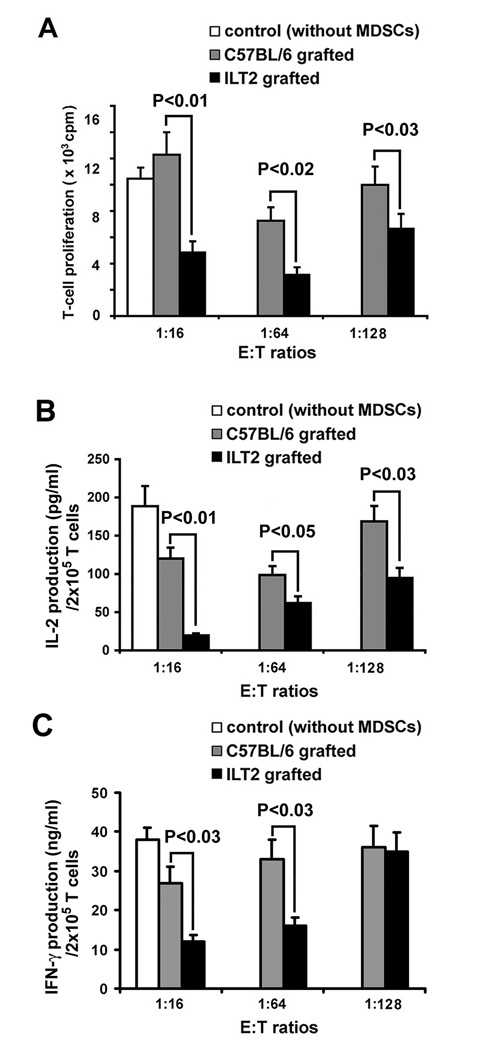
To analyze the suppressive potential of MDSCs, naive T cells were co-cultured with skin graft-induced CD11b+/Gr-1+ cells isolated from wild-type or ILT2 mice. T cells from control syngeneic mice stimulated with anti-CD3 and anti-CD28 antibodies and co-cultured in the presence of CD11b+/Gr-1+ cells from ILT2 mice exhibited a significant decrease in proliferation as measured by [3H] thymidine incorporation, compared with cells co-cultured with CD11b+/Gr-1+ cells from wild-type mice or cultured alone (Fig. 3A). The most significant suppressive effect of CD11b+/Gr-1+ on T cell proliferation was observed at 1:16 effector:target (E:T) ratio with a two-fold inhibition of T cell proliferation (12280 ± 1412 for controls vs. 4900 ± 586 cpm for ILT2 mice; p < 0.01) (Fig. 3A).
Figure 3. Augmented suppressive capacity of MDSCs from ILT2 mice.
(A) MDSCs suppress T cell proliferation. MDSCs were isolated by cell sorting from spleens of ILT2 and control mice on day 6 after allogeneic skin transplant. 2 × 105 naïve T cells were stimulated with 2.5 µg/ml of anti-CD3 and 2.5 µg/ml of anti-CD28 mAbs and co-cultured at different ratios with MDSCs from ILT2 mice (black bars) or MDSCs from control C57BL/6 mice (grey bars) for 48 h. Naïve T cells without MDSCs served as control (open bar). T cell proliferation was measured in triplicate by [3H] thymidine incorporation after 16 h pulse and is expressed in cpm. Results are given as mean ± SD from three representative independent experiments. (B) Decrease IL-2 and IFN-γ production by T cells cultured for 48 h with MDSCs. Concentration of IL-2 and IFN-γ in cell-free supernatant of cultures as described above was determined by ELISA. Results are given as mean ± SD from three representative independent experiments.
One of the potential immunosuppressive mechanisms used by MDSCs involves down-regulation of IL-2 production. To determine whether CD11b+/Gr-1+ cells isolated from ILT2 mice subjected to allogeneic skin graft affected IL-2 production, we measured IL-2 accumulation in the culture medium of T cell: CD11b+/Gr-1+ cell co-cultures. Our results demonstrate up to a 76% inhibition in IL-2 accumulation for T cells co-cultured with CD11b+/Gr-1+ cells from ILT2 mice (37.48 ± 4.9 pg/ml (n=8), compared with IL-2 production by T cells co-cultured with CD11b+/Gr-1+ cells from C57BL/6 control mice (120.2 ± 11.8 pg/ml, n=9) at an E:T ratio of 1:16 (p < 0.01, Fig. 3B). In addition, we determined that CD11b+/Gr-1+ cells from ILT2 mice have more capacity to inhibit production of IFN-γ by activated T cells, compared with CD11b+/Gr-1+ cells from C57BL/6 control mice (Fig. 3C). Importantly, these data are in agreement with our results demonstrating suppression of T cell proliferation by CD11b+/Gr-1+ cells after skin graft (Fig. 3A).
CD11b+/Gr-1+ MDSCs from ILT2 mice exhibit a unique transcriptional profile
To understand the mechanisms of expansion and enhancement of suppressive function of MDSCs from ILT2 mice, we analyzed their gene expression profile using a pathway-focused RT2 Profiler PCR Array System (SuperArray). This PCR Array is designed to profile the expression of 84 inflammatory pathway-specific genes potentially associated with the function of MDSCs. We compared the gene expression profiles of nonactivated MDSCs isolated from spleen of ILT2 mice and control C57BL/6 mice. This comparison was performed to identify differentially expressed genes affected by ILT2. In addition, the comparison between allogeneic transplant-activated MDSCs from both groups of mice was analyzed to determine the influence of allogeneic transplantation on the most affected genes. Expression levels of most transcripts were similar. However, 29 genes from the nonactivated MDSCs from ILT2 mice were specifically affected, the majority of which were down-regulated (Table 1). The most affected genes were in the group of interleukin receptors and chemokine receptors. Highly overexpressed genes in MDSCs from ILT2 mice were CCL8/MCP-2, CXCL5/ENA-78, CXCL13/BLC, and CCL19/ELC. Since CCL8 functions through chemokine receptors CCR1, CCR2, CCR3, and CCR5, which are highly expressed on activated T cells, it is most likely that CCL8 is one of the preferential chemokines for recruitment of activated T cells to MDSCs from ILT2 mice. Significant down-regulation of transcription of CCR2 and CCR5 on MDSCs from ILT2 mice suggest that at least CCR2 and CCR5 signaling is prevented on MDSCs and is probably not important for enhancing their suppressive function. However, the tenfold overexpression of the CXCL5 gene on MDSCs from ILT2 mice suggested that this chemokine could act as a suppressor molecule, since CXCL5 has shown strong myelosuppressive activity (24). Some proteins produced by MDSCs may be involved in the expansion of these cells. IL-1β was increased by three fold in MDSCs from ILT2 mice compared to normal mice. It has been previously shown that IL-1β induces the expansion of MDSCs indirectly (25). In addition, the 5.6 fold increase of IL-4 and 4.6 fold increase of IL-13 (Table 1), and the increased expression of the marker for immunosuppressive activity of MDSCs, CD124 (IL-4Rα), on MDSCs from ILT2 mice support our hypothesis that these cytokine/receptor pathways play an important role in enhancing the immunosuppressive activity of MDSCs from ILT2 mice.
Table 1.
Transcripts changed in MDSCs from ILT2 mice compared with MDSCs from C57BL/6 control micea
| Transcripts downregulated | Transcripts upregulated | ||||||
|---|---|---|---|---|---|---|---|
| Cytokines, cytokine receptors, growth factors, growth factor receptors | |||||||
| Gene name | Gene Symbol | Fold change | Gene name | Gene symbol | Fold change | ||
| Interleukin 6 signal transducer | IL6st | −10.88 | (−2.64)b | Interleukin-4 | IL4 | 5.68 | (1.19) |
| Tumor necrosis factor receptor 1β | TNFRII | −5.22 | (−3.53) | Interleukin-13 | IL13 | 4.58 | (2.63) |
| Interleukin 5 receptor α | IL5Rα | −4.39 | (−2.25) | Interleukin-1β | IL1β | 3.02 | (1.71) |
| Transforming growth factor, beta 1 | TGFβ1 | −4.12 | (−2.58) | Interferon gamma | IFNγ | 1.86 | (−1.11)c |
| Lymphotoxin beta | LTβ | −3.52 | (−3.2) | ||||
| Interleukin 2 receptor β chain | IL2Rβ | −3.37 | (−5.39) | ||||
| Interleukin 10 receptor α | IL10Rα | −3.24 | (−3.97) | ||||
| Interleukin 6 receptor α | IL6Rα | −2.92 | (−1.95) | ||||
| Interleukin 2 receptor γ chain | IL2Rγ | −2.65 | (−1.36) | ||||
| Interleukin 16 | IL16 | −2.47 | (−4.38) | ||||
| Chemokines, chemokine receptors, other genes | |||||||
| Chemokine (C-C motif) receptor 2 | CCR2 | −10.96 | (−2.36) | Chemokine (C-C motif) ligand 8 | CCL8 | 18.97 | (10.63) |
| Chemokine (C-C motif) receptor 5 | CCR5 | −8.36 | (−1.04) | Chemokine (C-X-C motif) ligand 5 | CXCL5 | 10.03 | (2.87) |
| Chemokine (C-C motif) receptor 7 | CCR7 | −6.79 | (1.34)c | Chemokine (C-X-C motif) ligand 13 | CXCL13 | 6.85 | (1.58) |
| Complement component 3 | C3 | −4.07 | (−4.17) | Chemokine (C-C motif) ligand 19 | CCL19 | 5.16 | (1.33) |
| Burkitt lymphoma receptor 1 | BLR1 | −3.52 | (−2.35) | Chemokine (C-X-C motif) receptor 3 | CXCR3 | 3.15 | (1.17) |
| B-cell leukemia/lymphoma 6 | Bcl6 | −3.26 | (−1.99) | Chemokine (C-X-C motif) ligand 4 | CXCL4 | 2.82 | (1.69) |
| Chemokine (C-C motif) ligand 2 | CCL2 | −1.87 | (3.18)c | Chemokine (C-C motif) ligand 7 | CCL7 | 2.82 | (2.04) |
| Chemokine (C-C motif) ligand 5 | CCL5 | 2.4 | (−1.13)c | ||||
The average fold changes were obtained by comparison of nonactivated MDSCs isolated from splenocytes of ILT2 mice with MDSCs isolated from splenocytes of control C57BL/6 mice.
Fold changes in allogeneic transplant-activated MDSCs from ILT2 mice compared with allogeneic transplant-activated MDSCs from C57BL/6 mice indicated in bracket.
Opposite changes.
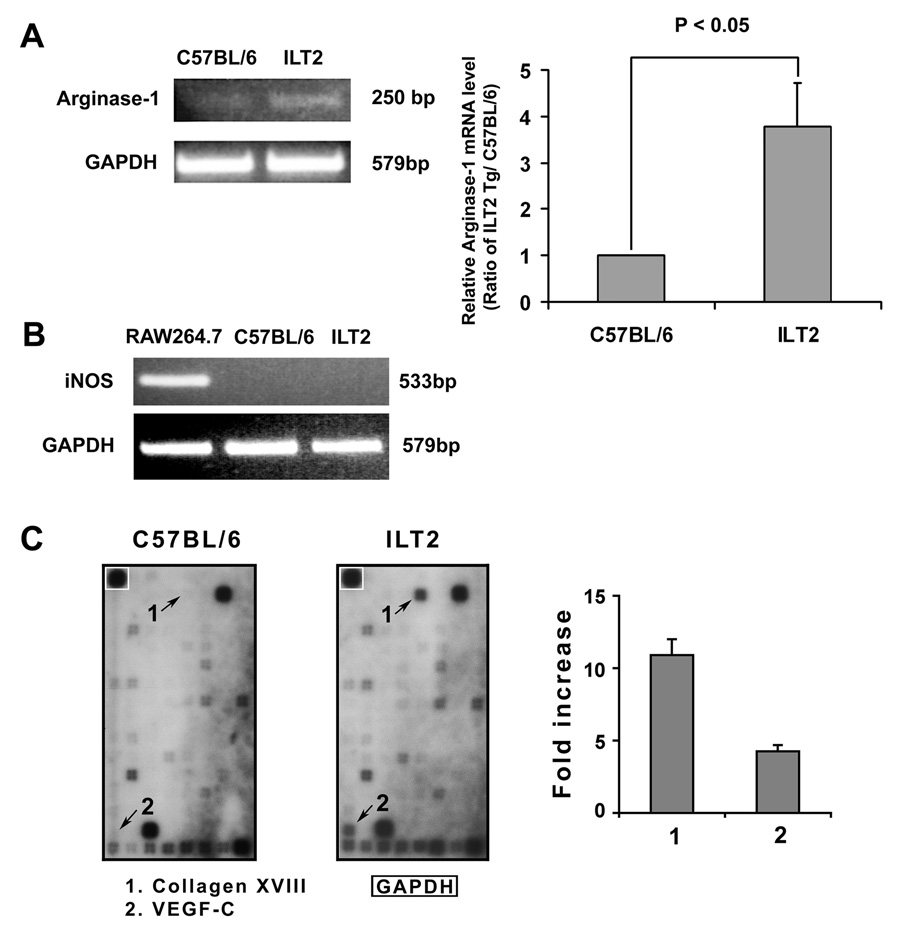
Arginase 1 (Arg1) and iNOS2 are key enzymes induced in myeloid cells and play a significant role in the mechanisms of immunosuppressive activity of MDSCs by the regulation of T cell function through arginine depletion. We determined that MDSCs from ILT2 mice have a 3.8-fold increase in the level of transcription of Arg 1 compared to MDSCs from control mice (Fig. 4A). However, the transcription level of iNOS2 was not detectable on either MDSCs from ILT2 or C57BL/6 control mice (Fig. 4B).
Figure 4. Distinct transcriptional profile of MDSCs from ILT2 mice.
Elevated level of Arginase 1 transcript in MDSCs from ILT2 mice. Total RNA was prepared from MDSCs from the indicated mice for RT-PCR analysis of transcription of Arginase 1 (A) and iNOS (B). iNOS-specific sequence (533bp) and Arginase-1-specific sequence (250bp) were detected by agarose gel electrophoresis. Murine RAW264.7 macrophage cell line treated with lipopolysaccharide (4 µg/ml) for 4 h were used as positive control for the analysis of iNOS transcription. PCR for GAPDH was performed to verify the similar initial cDNA content of the samples. The experiment was repeated three times and similar results were obtained. Density ratio over GAPDH was measured by densitometry. (C) Hybridization intensity of the genes of MDSCs on Mouse Dendritic and Antigen-presenting Cell Gene Array. Total RNA was extracted from MDSCs of ILT2 and C57BL/6 control mice. Arrows indicate the most affected differentially expressed genes. Densitometry scans of bands were obtained to determine the differences in the expression of the genes. Data represent three independent experiments.
To further identify unique characteristics of MDSCs from ILT2 mice, we performed gene expression profiles on MDSCs from both ILT2 transgenic and control B6 mice, focusing on genes involved in MDSC activation. We used an Oligo GEArray that includes 128 genes encoding proteins important for mouse angiogenesis, including genes encoding signal transduction molecules, growth factors and receptors, and adhesion molecules (Fig 4C). The expression levels of most of the transcripts were similar. However, these experiments revealed differences in expression of more than 10 genes (IFN-γ, chemokine ligand 2, angiopoietin 1, leptin, thrombospondin 2, angiopoietin-like 3, VEGF-C, procollagen type XVIII, ephrin B2, and prostaglandin-endoperoxide synthase 2). The most affected overexpressed genes collagen XVIII (11.2-fold increase) and VEGF-C (4.6-fold increase). This overexpression was confirmed by RT-PCR for VEGF-C and collagen XVIII (data not shown). Since most of the differentially expressed genes play a crucial role in migration into inflamed tissue and in cell adhesion, it is most likely that MDSCs from ILT2 transgenic mice have a high capacity to migrate to the site of the allograft and play an important role in the prolongation of allograft survival. Microarray analysis has been performed by the Bronte lab on MDSCs from tumor-bearing mice (15). The results demonstrate that MDSCs freshly isolated from tumor-bearing mice differed from normal counterparts in regard to the presence of 2 main signatures. One signature identifies multiple genes coding either for enzymes characterizing polymorphonuclear cells or for molecules associated with acute inflammatory responses. The other signature comprises cytokines, membrane molecules, and markers associated with alternative activation of macrophages. This cluster includes chitinase 3-like 3, complement component 4, IL-6, IL-10, IL-1α, IL-1 receptor II, TGF-β-induced transcript 4, and the C-type mannose receptor. The most upregulated genes were Arg1 and Nos2, which are well-recognized markers of alternative macrophage activation and they are coding critical enzymes for inhibition of activated T cells. Other genes were found to be upregulated that belong to the pathway activated by Th2-type cytokines such as IL-4 and IL-13, suggesting the presence of MDSC-released autocrine factors driving activation/maturation of MDSCs. To our knowledge, this is the first report demonstrating a microarray analysis on transplant-activated or inhibitory receptor-activated MDSCs. Our data confirmed that transplant-activated and inhibitory receptor-activated MDSCs have similar signatures with tumor-bearing MDSCs regarding characteristics associated with acute inflammatory responses. However, transplant-activated MDSCs and inhibitory receptors-activated MDSCs have more markers (chemokine receptors, chemokine ligands) associated with increased migration capacity and enhanced recruitment of activated T cells to MDSCs. In addition, inhibitory receptor-activated MDSCs have more myelopoietic factors such as VEGF, involved in expansion, recruitment, and activation of MDSCs. Another unique signature of the inhibitory receptor-activated MDSCs is that they produced the Arg1 enzyme only involved in L-arginine metabolism, whereas MDSCs from tumor-bearing mice MDSCs utilized both Arg1 and iNOS.
MDSCs from ILT2 mice directly promote long-term survival of skin allografts
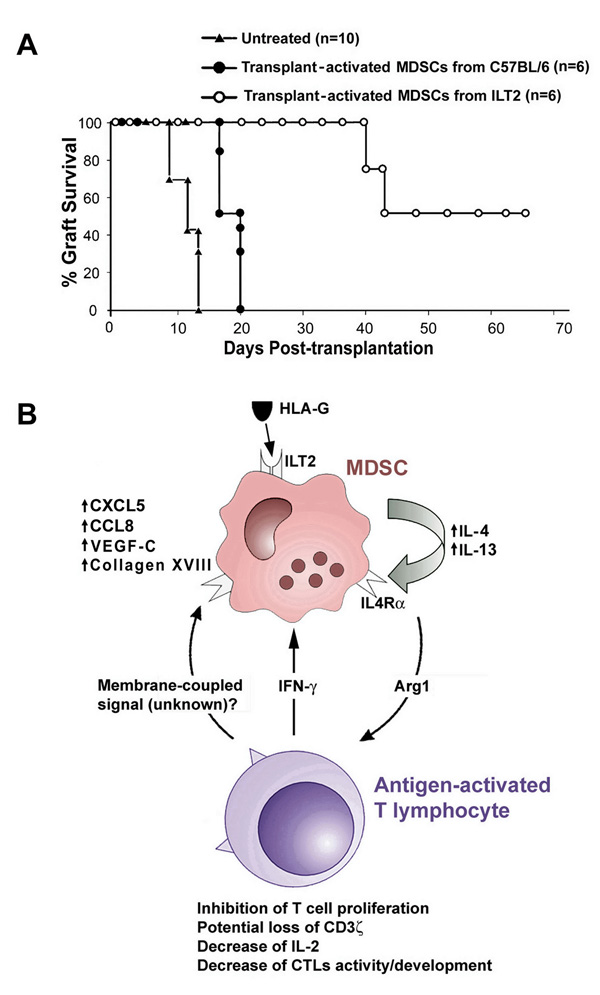
To investigate the biological significance of unique MDSCs from ILT2 mice, we performed adoptive transfer of CD11b+/Gr-1+ cells into wild-type mice, the recipient of allogeneic skin. 2×105 CD11b+/Gr-1+ cells from ILT2 transgenic mice that had prolonged bm12 mouse skin grafts were administered to C57BL/6 mice two times. All mice significantly delayed rejection of bm12 skin allografts. In contrast, all control C57BL/6 mice, that received the same number of CD11b+/Gr-1+ cells from C57BL/6 mice that had bm12 skin grafts, rapidly rejected the skin allografts (Fig. 5A). In addition, we observed that adoptively transferred MDSCs from ILT2 mice significantly inhibited the development of alloantigen-specific cytotoxic T lymphocytes (Supplementary Fig.1).
Figure 5. Prolongation of skin allograft survival by MDSCs from ILT2 mice and potential mechanisms of enhancing the immunosuppressive activities of MDSCs via ILT2 and HLA-G.
(A) Prolongation of allograft survival on C57BL/6 mice treated with MDSCs from ILT2 mice. C57BL/6 mice very strongly recognize the MHC class II-disparate mutant bm12 mouse that carries the I-Abm12 alloantigen (▲) (untreated group, mean of survival time (MST)=12.7, n=10). 2.0 × 105 CD11b+/Gr-1+ cells isolated from bm12 allograft-activated ILT2 or control C57BL/6 mice were injected into C57BL/6 recipient mice 24 h before and 72 h after bm12 skin transplantation. Allogeneic skin grafts were rejected at 16.8 days (n=6) in recipient mice that received allograft-activated MDSCs from C57BL/6 mice (●). A significant prolongation of skin allograft occurred in C57BL/6 mice that received allograft-activated MDSCs from ILT2 mice (○) (MST=54.8, n=6, p=0.0026). (B) Proposed mechanism of enhancing the immunosuppressive activities of MDSCs via ILT2 and HLA-G. ILT2 and ligand (HLA-G) interaction on MDSCs enhances the release of IL-4 and IL-13 and increases the expression of IL-4Rα. This results in increased production and activation of Arginase 1, which inhibits T cell proliferation and IL-2 production, causes potential loss of CD3 ζ chain, and inhibits CTL development and activity. In addition, release of IFN-γ from antigen-activated T cells further promotes the activation of MDSCs with enhanced expression of chemokine ligands CXCL5 and CCL8 to augment their suppressive effect on antigen-activated T cells.
Discussion
The establishment and maintenance of peripheral allograft tolerance is an active, highly regulated, multi-step process (26). The accumulation of evidence from both experimental and clinical studies indicates that, in the case of tolerance, a balance between regulation and deletion of responder T cells is an effective strategy to control immune responsiveness after organ or cell transplantation (27–29). The majority of regulatory cells comprise a subset of T lymphocytes that can suppress immune responses, control immune responsiveness to donor alloantigens, and have the potential to play a role in both inducing and maintaining transplant tolerance in vivo (27, 30–32). In addition to regulatory T cells, MDSCs represent natural regulatory cells with a strong potential to control the adaptive immune response but they have not yet been exploited in the induction and maintenance of transplantation tolerance. The induction and expansion of regulatory and immunosuppressive cells is a major goal to achieve transplantation tolerance. Several mechanisms likely contribute to the expansion of MDSCs. Our data demonstrate that expansion of MDSCs occurs after engagement of ILT2 receptors by murine ligand in ILT2 mice or by treatment of ligand-free ILT2 mice with HLA-G. Upon ligand binding, the immunoreceptor tyrosine-based inhibitory motif (ITIM) domains of ILT2 receptor became phosphorylated, which is known to be mediated by Lyn kinase (33). Following tyrosine phosphorylation, ILT2 recruits the tyrosine phosphatase SHP-1 and the C-terminal Src (Csk) kinase (33, 34). Lyn is a hematopoietic Src family kinase that is expressed in all blood cell lineages (with the exception of T cells) and is known to support survival of hematopoietic cells (35, 36). It is most likely that Lyn kinase, tyrosine phosphatase, and Csk kinase play major roles in the expansion of MDSCs. We do not exclude the possibility that other factors, such as cytokines or growth factors, play a role in the expansion of MDSCs as a result of the HLA-G-ILT2 interaction. The major potential candidates for this are VEGF and GM-CSF. We have demonstrated that MDSCs from ILT2 mice overexpress the VEGF-C gene. Future experiments will be necessary to determine the molecular mechanisms of expansion of MDSCs via HLA-G and ILT2.
MDSCs express two enzymes involved in L-arginine metabolism, Arg1 and iNOS, and the activity of both enzymes can result in T cell apoptosis through the production of reactive nitrogen oxide species (16, 18, 21, 37, 38). Our data demonstrate that MDSCs from ILT2 mice up-regulate the expression of Arg1 only and not NOS2. We determined that engagement of ILT2 receptor by its ligand up-regulated the expression of IL-4 and IL-13 on MDSCs, which are directly involved in the up-regulation of Arg1. This indicates that the production of IL-4 and IL-13 by MDSCs is the major factor contributing to the unique properties of ILT2-ligand-mediated MDSCs with enhanced suppressive activity. Based on evaluation of the molecular and cellular characteristics of MDSCs expanded via ILT2 and its interaction with ligand, we propose potential mechanisms of the unique suppressive functions of MDSCs, as summarized in Fig. 5B. The presence of the α-chain of the IL-4 receptor (IL-4Rα, CD124) on MDSCs is also crucial for their suppressive activity (15, 18). It will be necessary to determine which cytokine produced by MDSCs, IL-4 or IL-13, plays the major role for suppression via IL-4Rα and inhibition of the generation of alloreactive cytotoxic T lymphocytes.
Our results indicate that the interaction of human ILT2 receptor with its ligand in vivo creates a microenvironment where immature myeloid cells develop. These cells suppress alloantigen-activated T cells and inhibit cytotoxic T cell-mediated rejection. At the same time, immature myeloid cells are important for wound repair and participate in the formation of transient lymphatic vessels. In addition, histological evaluation of skin allografts shows that adoptively transferred MDSCs from ILT2 mice have a high capacity to migrate to the site of the graft to prolong allograft survival (Supplementary Fig. 2). Thus, the exogenously activated immature myeloid cells may hold promise for human therapies.
The results obtained in a mouse model have important potential clinical applications. Similar to MDSCs in mice, human MDSCs may be important participants in down-regulation of the immune response in tissue/organ transplant patients. Treatment regimens using the induction of specific MDSCs in vivo via ILT2 receptors and its strong natural ligand, such as HLA-G, will be a potential tool to increase the efficiency of organ and tissue transplants and can be used as maintenance therapy, replacing the current drugs that are associated with chronic toxicities. Alternatively, MDSCs could be generated by manipulating myelomonocytic cells isolated from the recipient in vitro via HLA-G-ILTs. After testing, these MDSCs could be re-infused into the transplant recipient. We currently are investigating the involvement of MDSCs in the induction and/or maintenance of peripheral allograft tolerance in kidney transplant patients.
Supplementary Material
Acknowledgements
The authors are very grateful to Drs. Rhea-Beth Markowitz and Dimitrios Moskofidis for helpful discussions and critical reading of the manuscript.
This study was supported by grants from the National Institutes of Health, RO1 AI055923, and the Roche Organ Transplantation Research Foundation.
Abbreviations
- Arg1
arginase 1
- DCs
dendritic cells
- ILT
immunoglobulin-like transcript
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- MDSCs
myeloid-derived-suppressor cells
- SHP
src homology (SH2)-containing phosphotyrosine phosphatase
Footnotes
Conflict of interest disclosure: The authors declare no potential conflict of interest.
References
- 1.Carosella ED, Moreau P, Le Maoult J, Le Discorde M, Dausset J, Rouas-Freiss N. HLA-G molecules: from maternal-fetal tolerance to tissue acceptance. Adv Immunol. 2003;81:199. doi: 10.1016/s0065-2776(03)81006-4. [DOI] [PubMed] [Google Scholar]
- 2.Rouas-Freiss N, LeMaoult J, Moreau P, Dausset J, Carosella ED. HLA-G in transplantation: a relevant molecule for inhibition of graft rejection? Am J Transplant. 2003;3:11. doi: 10.1034/j.1600-6143.2003.30103.x. [DOI] [PubMed] [Google Scholar]
- 3.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. Faseb J. 2005;19:681. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 4.Carosella ED, Moreau P, Lemaoult J, Rouas-Freiss N. HLA-G: from biology to clinical benefits. Trends Immunol. 2008;29:125. doi: 10.1016/j.it.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Hunt JS, Jadhav L, Chu W, Geraghty DE, Ober C. Soluble HLA-G circulates in maternal blood during pregnancy. Am J Obstet Gynecol. 2000;183:682. doi: 10.1067/mob.2000.106762. [DOI] [PubMed] [Google Scholar]
- 6.Le Bouteiller P, Legrand-Abravanel F, Solier C. Soluble HLA-G1 at the maternofoetal interface. A review. Placenta. 2003;24:S10. doi: 10.1053/plac.2002.0931. [DOI] [PubMed] [Google Scholar]
- 7.LeMaoult J, Krawice-Radanne I, Dausset J, Carosella ED. HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci U S A. 2004;101:7064. doi: 10.1073/pnas.0401922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajagopalan S, Long EO. Viral evasion of NK-cell activation. Trends Immunol. 2005;26:403. doi: 10.1016/j.it.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA-G. Eur J Immunol. 2005;35:1133. doi: 10.1002/eji.200425741. [DOI] [PubMed] [Google Scholar]
- 10.LeMaoult J, Zafaranloo K, Le Danff C, Carosella ED. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. Faseb J. 2005;19:662. doi: 10.1096/fj.04-1617fje. [DOI] [PubMed] [Google Scholar]
- 11.Liang S, Zhang W, Horuzsko A. Human ILT2 receptor associates with murine MHC class I molecules in vivo and impairs T cell function. Eur J Immunol. 2006;36:2457. doi: 10.1002/eji.200636031. [DOI] [PubMed] [Google Scholar]
- 12.Liang S, Ristich V, Arase H, Dausset J, Carosella ED, Horuzsko A. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6-STAT3 signaling pathway. Proc Natl Acad Sci U S A. 2008;105:8357. doi: 10.1073/pnas.0803341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feger U, Tolosa E, Huang YH, et al. HLA-G expression defines a novel regulatory T-cell subset present in human peripheral blood and sites of inflammation. Blood. 2007;110:568. doi: 10.1182/blood-2006-11-057125. [DOI] [PubMed] [Google Scholar]
- 14.Rouas-Freiss N, Naji A, Durrbach A, Carosella ED. Tolerogenic functions of human leukocyte antigen G: from pregnancy to organ and cell transplantation. Transplantation. 2007;84(1 Suppl):S21. doi: 10.1097/01.tp.0000269117.32179.1c. [DOI] [PubMed] [Google Scholar]
- 15.Gallina G, Dolcetti L, Serafini P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol. 2005;175:4583. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang S, Baibakov B, Horuzsko A. HLA-G inhibits the functions of murine dendritic cells via the PIR-B immune inhibitory receptor. Eur J Immunol. 2002;32:2418. doi: 10.1002/1521-4141(200209)32:9<2418::AID-IMMU2418>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Ochoa JB, Bernard AC, O'Brien WE, et al. Arginase I expression and activity in human mononuclear cells after injury. Ann Surg. 2001;233:393. doi: 10.1097/00000658-200103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuei BJ, Bernard AC, Shane MD, et al. Surgery induces human mononuclear cell arginase I expression. J Trauma. 2001;51:497. doi: 10.1097/00005373-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 24.Broxmeyer HE, Cooper S, Hangoc G, Chang CH. Class II transactivator-mediated regulation of major histocompatibility complex class II antigen expression is important for hematopoietic progenitor cell suppression by chemokines and iron-binding proteins. Exp Hematol. 2006;34:1078. doi: 10.1016/j.exphem.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 26.Zheng XX, Sanchez-Fueyo A, Domenig C, Strom TB. The balance of deletion and regulation in allograft tolerance. Immunol Rev. 2003;196:75. doi: 10.1046/j.1600-065x.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 27.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 28.Wells AD, Li XC, Strom TB, Turka LA. The role of peripheral T-cell deletion in transplantation tolerance. Philos Trans R Soc Lond B Biol Sci. 2001;356:617. doi: 10.1098/rstb.2001.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood KJ, Jones ND, Bushell AR, Morris PJ. Alloantigen-induced specific immunological unresponsiveness. Philos Trans R Soc Lond B Biol Sci. 2001;356:665. doi: 10.1098/rstb.2001.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waldmann H, Cobbold S. Regulating the immune response to transplants. a role for CD4+ regulatory cells? Immunity. 2001;14:399. doi: 10.1016/s1074-7613(01)00120-0. [DOI] [PubMed] [Google Scholar]
- 31.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195:1641. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bach JF. Regulatory T cells under scrutiny. Nat Rev Immunol. 2003;3:189. doi: 10.1038/nri1026. [DOI] [PubMed] [Google Scholar]
- 33.Dietrich J, Cella M, Colonna M. Ig-like transcript 2 (ILT2)/leukocyte Ig-like receptor 1 (LIR1) inhibits TCR signaling and actin cytoskeleton reorganization. J Immunol. 2001;166:2514. doi: 10.4049/jimmunol.166.4.2514. [DOI] [PubMed] [Google Scholar]
- 34.Sayos J, Martinez-Barriocanal A, Kitzig F, Bellon T, Lopez-Botet M. Recruitment of C-terminal Src kinase by the leukocyte inhibitory receptor CD85j. Biochem Biophys Res Commun. 2004;324:640. doi: 10.1016/j.bbrc.2004.09.097. [DOI] [PubMed] [Google Scholar]
- 35.Lowell CA. Src-family kinases: rheostats of immune cell signaling. Mol Immunol. 2004;41:631. doi: 10.1016/j.molimm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Karur VG, Lowell CA, Besmer P, Agosti V, Wojchowski DM. Lyn kinase promotes erythroblast expansion and late-stage development. Blood. 2006;108:1524. doi: 10.1182/blood-2005-09-008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 38.Frey AB. Myeloid suppressor cells regulate the adaptive immune response to cancer. J Clin Invest. 2006;116:2587. doi: 10.1172/JCI29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.