Abstract
Accumulation of triacylglycerol (TAG) and lipid intermediates in skeletal muscle plays an important role in the etiology of insulin resistance and type 2 diabetes mellitus. Disturbances in skeletal muscle lipid turnover and lipolysis may contribute significantly to this. So far, knowledge on the regulation of muscle lipolysis is limited. Recently the identification of a new lipase was reported: adipose triglyceride lipase (ATGL). ATGL deficient animals show significant lipid accumulation in skeletal muscle, which may indicate that ATGL plays a pivotal role in skeletal muscle lipolysis. However, until now, it is still unknown whether ATGL protein is expressed in human skeletal muscle. Therefore, the aim of the present study was to investigate whether ATGL is expressed at the protein level in human skeletal muscle, and to examine whether its expression is fiber-type specific. To accomplish this, we established an imunohistochemical and immunofluorescent staining procedure to study ATGL protein expression in relation to fiber type in human vastus lateralis muscle of eight male subjects (BMI range: 21.0–34.5 kg/m2 and age: 38–59 years). In the present paper we report for the first time that ATGL protein is indeed expressed in human skeletal muscle. Moreover, ATGL is exclusively expressed in type I (oxidative) muscle fibers, suggesting a pivotal role for ATGL in intramuscular fatty acid handling, lipid storage and breakdown.
Keywords: ATGL, Skeletal muscle, Fiber type, Obesity, Protein
Introduction
The prevalence of obesity and type 2 diabetes mellitus has reached epidemic proportions both in developing as well as developed countries. More information is required on the etiology of obesity and insulin resistance to improve nutritional recommendations and/or to identify new targets for pharmacological interventions. Recent evidence indicates that increased storage of triacylglycerol (TAG) and lipid intermediates in non-adipose tissues (i.e. ectopic fat deposition), such as skeletal muscle plays an important role in the etiology of insulin resistance and type 2 diabetes mellitus (Petersen and Shulman 2006). Disturbances in the breakdown of stored fat (endogenous lipolysis) may be a factor contributing to an increased fat storage in the form of intramuscular triacylglycerol (IMTG) and lipid intermediates (e.g. diacylglycerol (DAG), long-chain fatty-acyl CoA and ceramides) (Blaak et al. 2004). The enzymatic regulation of lipolysis in skeletal muscle is poorly understood, even in healthy volunteers. The general view is that fat stores can be mobilized by hormone-sensitive lipase (HSL), which is controlled by the action of catecholamines (Langfort et al. 1999) and muscle contraction (Langfort et al. 2000). Recently the identification of a new lipase that is primarily responsible for the hydrolysis of TAG was reported, namely adipose triglyceride lipase (ATGL) (Zimmermann et al. 2004). ATGL mRNA expression has been demonstrated in skeletal muscle of rodents (Zimmermann et al. 2004). Interestingly, this lipase may play a pivotal role in skeletal muscle lipolysis, since ATGL deficient animals show significant TAG accumulation in non-adipose tissues, including skeletal muscle (Haemmerle et al. 2006). However, until now, it is still unknown whether ATGL protein is expressed in human skeletal muscle. Therefore, the aim of the present study was to investigate whether ATGL is expressed at the protein level in human skeletal muscle. In addition, we examined whether ATGL protein expression is fiber type-specific to obtain better insight into its physiological role in human skeletal muscle. To accomplish this we established a combined immunohistochemical an immunofluorescent staining procedure to study ATGL protein expression in relation to fiber type in human vastus lateralis muscle.
Materials and methods
Subjects
Skeletal muscle biopsies were taken under local anesthesia (Xylocaine®, AstraZeneca BV, Zoetermeer, The Netherlands) by needle biopsy from the vastus lateralis muscle of eight male subjects after an overnight fast. Three lean (BMI < 25 kg/m2) and five obese (BMI > 30 kg/m2) male subjects participated in this study. Anthropometric and clinical data of the subjects are summarized in Table 1. Muscle tissue was immediately frozen in isopentane, followed by liquid nitrogen, and stored at −80°C until further analysis. All subjects were asked to refrain from drinking alcohol and to perform no strenuous exercise for a period of 24 h prior to the biopsy. After subjects voided their bladder body weight was determined on a calibrated electronic scale, accurate to 0.1 kg. Waist and hip circumference measurements to the nearest 1 cm were made midway between the lower rib and iliac crest with participants standing upright. Body weight and body density (by hydrostatic weighing) used for calculations of percentage body fat (%BF), fat mass (FM) and fat-free mass (FFM), were determined as previously described (Goossens et al. 2007). Insulin sensitivity was assessed by the homeostasis model assessment index for insulin resistance (HOMAIR), calculated from fasting glucose and insulin concentrations (Matthews et al. 1985). The Medical Ethical Review Committee of Maastricht University approved the study protocol and the clinical investigations were performed according to the Declaration of Helsinki. All subjects gave their written informed consent before participating in the study.
Table 1.
Athropometric and clinical data for the subjects
| n | Eight men (three lean and five obese) |
|---|---|
| Age (year) | 50 ± 3 (38–59) |
| BMI (kg/m2) | 28.9 ± 1.9 (21.0–34.5) |
| %BF | 28.9 ± 1.7 (22.2–34.2) |
| FM (kg) | 26.6 ± 3.1 (16.5–40.3) |
| FFM (kg) | 65.2 ± 2.8 (53.3–77.6) |
| WHR | 0.96 ± 0.02 (0.85–1.06) |
| HOMAir | 3.05 ± 0.4 (1.6–4.1) |
Data are presented as mean ± standard error (SE) and (range)
n Number of subjects, BMI body mass index, %BF percentage body fat, FM fat mass, FFM fat-free Mass, WHR waist-to-hip ratio, HOMAir homeostasis model assessment for insulin resistance
Biochemical analysis
A fasting venous blood sample was collected in tubes containing EDTA as anti-coagulant and centrifuged for 10 min at 1,000g, 4°C. The plasma was used for the enzymatic colorimetric quantification of glucose concentration (ABX Diagnostics, Montepellier, France) on a COBAS MIRA automated spectrophotometer (Roche Diagnostica, Basel, Switzerland). Plasma insulin was measured with a double antibody radioimmunoassay (Linco Research Inc., St. Charles, Missouri, USA).
Immunostaining and immunofluorescence protocol
Transverse serial sections (10 μm) were cut from each biopsy and each section was placed on a glass slide (Menzel GmbH & CoKG, Braunschweig, Germany) and air dried at room temperature. Skeletal muscle ATGL protein expression and fiber type were investigated by means of a combined immunostaining and immunofluorescence protocol. The sections were fixed using 0.3% H2O2 in methanol. After washing with phosphate buffered saline (PBS) the sections were incubated overnight with a polyclonal antibody raised against human ATGL (Cayman Chemical, Michigan, USA) in PBS at room temperature. Thereafter, slides were incubated with a biotin labeled swine-anti-rabbit secondary antibody (DAKO, Glostrup, Denmark). Subsequently, slides were washed with 0.05% Tween in PBS and incubated with the ABC peroxidase kit (Vectastain Elite PK610, Vector, Burlingame, California). The ATGL immunostaining was visualized with diaminobenzidin (DAB) solution (Fluka Chemie, GmbH, Buchs, Germany) diluted in 0.05M Tris, pH 7.6 and 0.03% H2O2. Coloring was followed by microscope and stopped with water. For determining muscle fiber type the same slides were briefly washed in PBS and then incubated with a monoclonal antibody raised against adult human slow myosin heavy chain and a monoclonal antibody reactive with adult human fast IIa myosin heavy chain (Cho et al. 1993). The antibodies were diluted in PBS and incubated at room temperature. The following secondary antibodies were used: goat anti-mouse IgM conjugated with Alexa Fluor 555 (red) (GAMIgM-Alexa555) and goat anti-mouse IgG1 conjugated with Alexa Fluor 488 (green) (GAMIgG1-Alexa488; Molecular Probes Europe, Leiden, The Netherlands), diluted in PBS and incubated at room temperature. Finally, nuclei were colored using Haematoxilin and slides were included in Mowiol (Merck Chemicals Ltd., Nottingham, UK). Sections were viewed and photographed using a Nikon Eclipse E800 microscope mounted with an Axiocam color CCD camera (Nikon, Melville, NY USA). About 200 to 300 muscle fibers were analyzed per person.
Results
Antropometric and clinical data for the subjects are summarized in Table 1.
ATGL protein expression and fiber typing in human skeletal muscle
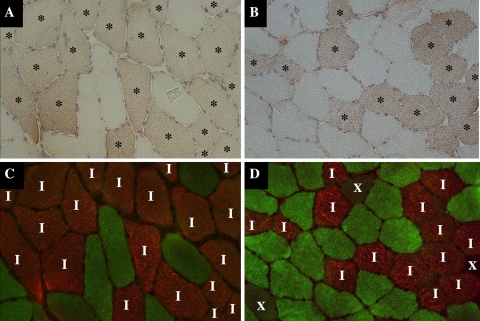
We found that ATGL protein is expressed in skeletal muscle of both lean and obese subjects, respectively (visualized as brown DAB staining in Fig. 1a, b). This observation was representative for all lean (BMI < 25 kg/m2, n = 3) and obese (BMI > 30 kg/m2, n = 5) subjects. Pre-incubation of the ATGL antibody with the ATGL peptide or incubation of the slides without ATGL antibody resulted in the complete disappearance of this staining, indicating that this staining is highly ATGL specific (data not shown). The dual-immunofluorescent staining of skeletal muscle fibers is shown in Fig. 1c (lean subjects) and d (obese subjects). Type I fibers stained red and type IIa fibers stained green; type IIx fibers were unstained. When these images were combined, it appeared that ATGL is expressed exclusively in type I fibers of both lean and obese subjects. All type I fibers stained positive for ATGL. None of the type II fibers showed a positive staining for ATGL.
Fig. 1.
Immunohistochemical ATGL staining combined by immunofluorescent fiber typing in skeletal muscle of a lean (BMI: 21.0 kg/m2, a, c) and an obese (BMI: 32.4 kg/m2, b, d) subject. a, b Muscle fibers that contain ATGL stained brown using DAB (fibers positively stained for ATGL are indicated by an asterisk). c, d Dual-immunofluorescent staining of muscle fibers; type I muscle fibers stained red (indicated by I), type IIa muscle fibers stained green and type IIx muscle fibers were unstained (indicated by X). Images are of the same area of the same section from the same lean (a, c) or obese (b, d) individual and demonstrate that ATGL is expressed exclusively in type I muscle fibers
Discussion
The present study examined ATGL protein expression in human skeletal muscle for the first time. The major finding is that ATGL protein is exclusively expressed in type I oxidative fibers of human skeletal muscle.
It is known that type I (slow-twitch, oxidative) muscle fibers have an increased TAG content compared to type IIa and IIx (fast-twitch, oxidative-glycolytic, predominantly glycolytic) fibers (Malenfant et al. 2001). The present finding that ATGL protein is exclusively expressed in type I fibers of human skeletal muscle, characterized by high TAG content compared with type II fibers, may suggest that ATGL plays an important role in intramuscular fatty acid handling and TAG turnover in humans. It has previously been shown that HSL is also expressed in human skeletal muscle (Roepstorff et al. 2004). Although HSL expression is not fiber type specific, higher levels are also found in type I fibers (Langfort et al. 1999). Taken together, the physiological role of ATGL in skeletal muscle lipolysis certainly warrants further investigation, both in healthy volunteers and in obese insulin resistant subjects. It is tempting to speculate that an imbalance between ATGL and HSL expression might increase the storage of TAG or lipid intermediates in skeletal muscle of obese insulin resistant subjects. In addition, it should be examined whether a possible impairment can be reversed by interventions, such as weight loss or physical activity that improve insulin sensitivity.
In summary, the present data show for the first time that ATGL protein is present in human skeletal muscle, and is exclusively expressed in type I muscle fibers. ATGL may play a pivotal role in skeletal muscle fatty acid handling, lipid storage and lipolysis. These data indicate that there is an urgent need to revisit lipolysis in human skeletal muscle.
Acknowledgments
The authors greatly appreciated the willingness of the volunteers to participate in this study. This research was supported by a grant from The Netherlands Organisation for Health Research and Development (NWO-ZonMw contract no. 015.01.095) to Prof. Dr. E.E. Blaak.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Blaak EE, Schiffelers SL, Saris WH, Mensink M, Kooi ME (2004) Impaired beta-adrenergically mediated lipolysis in skeletal muscle of obese subjects. Diabetologia 47:1462–1468 [DOI] [PubMed]
- Cho M, Webster SG, Blau HM (1993) Evidence for myoblast-extrinsic regulation of slow myosin heavy chain expression during muscle fiber formation in embryonic development. J Cell Biol 121:795–810 [DOI] [PMC free article] [PubMed]
- Goossens GH, Jocken JW, Blaak EE, Schiffers PM, Saris WH, van Baak MA (2007) Endocrine role of the renin-angiotensin system in human adipose tissue and muscle: effect of beta-adrenergic stimulation. Hypertension 49:542–547 [DOI] [PubMed]
- Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R (2006) Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312:734–737 [DOI] [PubMed]
- Langfort J, Ploug T, Ihlemann J, Saldo M, Holm C, Galbo H (1999) Expression of hormone-sensitive lipase and its regulation by adrenaline in skeletal muscle. Biochem J 340(Pt 2):459–465 [DOI] [PMC free article] [PubMed]
- Langfort J, Ploug T, Ihlemann J, Holm C, Galbo H (2000) Stimulation of hormone-sensitive lipase activity by contractions in rat skeletal muscle. Biochem J 351:207–214 [DOI] [PMC free article] [PubMed]
- Malenfant P, Joanisse DR, Theriault R, Goodpaster BH, Kelley DE, Simoneau JA (2001) Fat content in individual muscle fibers of lean and obese subjects. Int J Obes Relat Metab Disord 25:1316–1321 [DOI] [PubMed]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed]
- Petersen KF, Shulman GI (2006) Etiology of insulin resistance. Am J Med 119:S10–S16 [DOI] [PMC free article] [PubMed]
- Roepstorff C, Vistisen B, Donsmark M, Nielsen JN, Galbo H, Green KA, Hardie DG, Wojtaszewski JF, Richter EA, Kiens B (2004) Regulation of hormone-sensitive lipase activity and Ser563 and Ser565 phosphorylation in human skeletal muscle during exercise. J Physiol 560:551–562 [DOI] [PMC free article] [PubMed]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383–1386 [DOI] [PubMed]