Abstract
In a randomized, placebo-controlled trial in Ghana, 67 onchocerciasis patients received 200-mg/day doxycycline for 4–6 weeks, followed by ivermectin (IVM) after 6 months. After 6–27 months, efficacy was evaluated by onchocercoma histology, PCR and microfilariae determination. Administration of doxycycline resulted in endobacteria depletion and female worm sterilization. The 6-week treatment was macrofilaricidal, with >60% of the female worms found dead, despite the presence of new, Wolbachia-containing worms acquired after the administration of doxycycline. Doxycycline may be developed as second-line drug for onchocerciasis, to be administered in areas without transmission, in foci with IVM resistance and in areas with Loa co-infections.
Keywords: Doxycycline, Ghana, ivermectin, macrofilaricidal, Onchocerca volvulus, Wolbachia
Introduction
Onchocerciasis (river blindness) is endemic in many sub-Saharan countries in Africa, with minor foci in Latin America and Yemen [1]. The infection is transmitted through the bites of blackflies (Simulium spp.) carrying infective third-stage larvae, which have developed in the vector from microfilariae (mf) acquired from other humans during a preceding blood meal. The female adult worms measure up to 60 cm in length and have an average life expectancy of 10 years [2], producing millions of mf. The mf migrate to the skin, causing dermatitis and atrophy, or to the eye, where they cause inflammation leading to reduced vision and blindness. Apart from blindness, dermatitis has been identified as a separate factor that significantly reduces the lifespan of infected people [3]. For many years, onchocerciasis was thought to have affected 17 million people [1]. Since more attention has recently been paid to remote areas in Central African countries, the estimated number of infections has risen to more than 37 million [4].
There has been a longstanding effort to reduce the burden imposed by onchocerciasis using vector control and mass treatment. Efforts were made to improve the dosage scheme of ivermectin (IVM), either to include albendazole, or to develop new drugs such as amocarzine. Current elimination programmes are based on mass administration of the antifilarial drug IVM. The African Programme for Onchocerciasis Control (APOC) relies on a strategy of annual mass treatment [5]. However, IVM is mainly a microfilaricidal and temporarily sterilising drug [6], which has to be given for many years until all adult worms are sterile or dead. Some studies indicate that after several rounds of IVM treatment, female worms are no longer inseminated [7]. But insemination and fertility of worms did resume after interruption of IVM administration before all worms had died [8]. Macrofilaricidal activity of IVM has been reported after frequent doses [9, 10]. The recent Conference on the Eradicability of Onchocerciasis [11] concluded that eradication would not be feasible at coverage rates of 65% for 35 or more number of years. In addition, there is concern that in some geographic areas worm populations exist that may exhibit some degree of resistance to IVM [12–14].
Equally important are the costs to individuals, who, in the absence of a long-acting drug, are faced with the prospect of remaining infected for a decade or more with the necessity of repeated IVM treatments to prevent ocular or dermal damage due to the mf. Therefore, it has been a long-standing aim to identify a non-toxic macrofilaricidal principle against onchocerciasis. Recently, Wolbachia endosymbiotic bacteria have been exploited as targets for antifilarial drugs with high efficacy [15]. An earlier, open study showed that a 6-week administration of 100-mg/day doxycycline to onchocerciasis patients led to a depletion of Wolbachia from adult worms [16–18]. This was associated with a long-term cessation of embryogenesis that was still observed 18 months after doxycycline treatment. In lymphatic filariasis due to Wuchereria bancrofti, administration of 200-mg/day doxycycline for 6–8 weeks led to more than 80% of macrofilaricidal activity [19, 20].
We therefore undertook a placebo-controlled, randomized study with 200 mg/day of doxycycline, administered for 4-6 weeks, to onchocerciasis patients. The results confirmed a depletion of Wolbachia as well as interruption of embryogenesis that led to a cessation of production of new mf at 20 and 27 months after the onset of doxycycline treatment. We also observed a macrofilaricidal activity of more than 60% in the 6-week treatment arm at these points of time.
Methods
Participants
Study site
In the Central Region of Ghana, the area south of the Offin and west of the Pra rivers is endemic to onchocerciasis but not for other human filarial infections. Both rivers are breeding sites of the vector blackflies Simulium sanctipauli that have flight ranges of up to 12 km [21]. The study participants were recruited in three neighbouring villages in Assin district situated where the Offin flows into the Pra. The distance between the villages and Offin is less than 1.5 km. In 1999, the focus was found to be hyperendemic during a rapid assessment of 30 men aged more than 19 years in each village: 75, 86 and 93% nodule carriers and 80, 89 and 90% mf carriers were found (R. Horstmann, Bernhard Nocht Institute, personal communication). Mass treatment with IVM started in 1999 in the district. This focus lies south of the area of the Onchocerciasis Control Programme in West Africa (OCP) and transmission is ongoing. Due to the remoteness of the villages the coverage of IVM mass treatment was rather low until the end of this study [21]. A reduction in the infection rates of the flies was not observed by Garms and colleagues in 2002 (for a detailed map and vector epidemiology see [21]). During another assessment of the transmission in 2006 the Simulium infectivity parameters closely resembled those from other hyperendemic areas without intervention. The entomologists concluded that a person might easily receive one or several infective bites per week (R. Garms, Bernhard Nocht Institute, unpublished report). The nodule loads found during recruitment for this study in September 2003 also indicated a high endemicity.
Ethical aspects and study design
The design of this randomized, placebo-controlled, double-blind study was approved by the Committee on Human Research and Ethics of the School of Medical Sciences of the Kumasi University of Science and Technology (KNUST), Kumasi, Ghana, as well as by the Ethics Committee of the University of Liverpool. The latter acted as a control body for several studies on anti-Wolbachia treatment in onchocerciasis and lymphatic filariasis, which were carried out by a network of groups funded by the European Union (ICA4-2002-10051). The study conformed to the principles of the Helsinki Declaration of 1964 (last amended 2002). A Trial Steering Committee (TSC) as well as a Data Monitoring and Ethics Committee (DMEC) were established. The trial is registered at Current Controlled Trials and has the registration number ISRCTN 71141922.
Informed consent
During a meeting with the village elders and interested villagers the study was explained in English and then in the local language Twi. The participants were requested to ask questions and these were answered. During a survey persons identified as eligible to the study were informed individually first in English by the study physician and then in Twi by an experienced teacher and biologist, who was a team member since 1999. Informed, signed/thumbprinted, or witnessed consent was obtained from all participants. Before nodulectomies, the surgeon again explained the procedure in Twi, in the ward, and answered questions.
Recruitment and examination of patients
Individuals eligible for participation were: nodule carriers of both sexes, aged 18–62 years, with a body weight of more than 40 kg, in good health, and without any clinical condition requiring chronic medication. Physical examinations by SM and MB included inspection and palpation of onchocercomas. Onchocercal skin disease was recorded as papular and lichenified dermatitis and depigmentation (“leopard skin”) [1]. The mf density was assessed by two skin snips as described in [18]. Before doxycycline resp. placebo treatment and again 3 weeks after treatment onset, hepatic and renal functions as well as pregnancy were assessed by dipstick chemistry using venous blood. Exclusion criteria were: palpation of less than two onchocercomas, abnormal hepatic and renal enzymes (AST [0–40 IU/l], ALT [0–45 IU/l], and creatinine [3–126 μmol/l]), pregnancy, breast-feeding, intolerance to doxycycline, and alcohol or drug abuse.
Interventions
Patients were treated in September2003 and October 2003 (Fig. 1). There were three treatment arms: (1) 6 weeks 200 mg/day doxycycline; (2) 4 weeks 200 mg/day doxycycline, followed by 2 weeks matching placebo; (3) 6 weeks matching placebo. Participants received two 100 mg capsules of Vibramycin® or matching placebo supplied by Pfizer. The treatment was monitored by a trial physician (MB) as daily-observed treatment (DOT). This included daily monitoring in the villages of adverse side effects on a hardcopy standardized patient form.
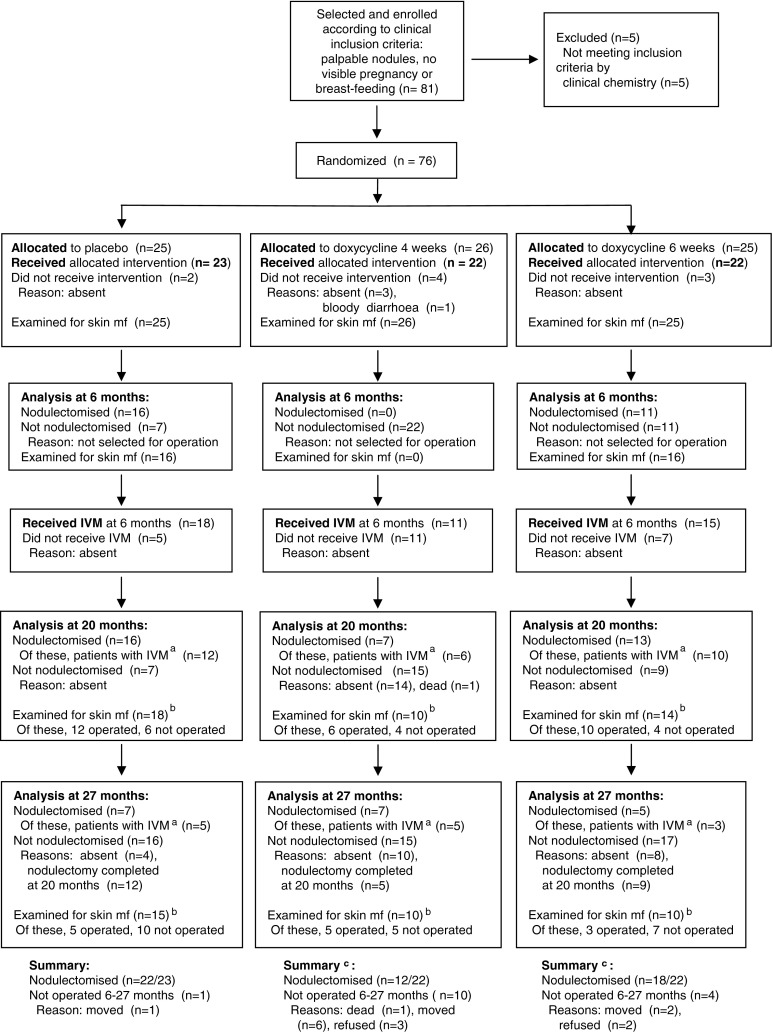
Fig. 1.
Flow chart of patients who took part in the study. a Patients who had received ivermectin (IVM) at the 6 months point of time. b Only patients who had received ivermectin at 6 months were analysed. The total numbers comprise the patients who underwent nodulectomy and had received ivermectin (see footnote a) plus patients who were only available for skin snipping (but not for nodulectomy) at the respective point of time. c Four additional patients (two from each of the doxycycline groups), three of whom were not available for nodulectomy between 6 and 27 months, were nodulectomised at 39 months, increasing the total number of nodulectomised patients to 14/22 and 19/22 in the 4 and 6-week groups, respectively
After 6 months, a limited number of nodules were excised from some patients of the 6-week doxycycline and the placebo groups harbouring many onchocercomas, to confirm the depletion of Wolbachia. One nodulectomy on patients with several sites with onchocercomas does not decrease the mf densities (Büttner, unpublished data). Six months after the beginning of the study and after this first round of nodulectomy, the patients received 0.15 mg/kg IVM to more quickly deplete skin mf.
Based on the results of previous studies ([18], and unpublished data), patients were nodulectomised 20 and/or 27 months after the start of the study in May2005 and November 2005. In addition, three patients who had been absent before, and one patient who had not been completely nodulectomised, were operated after 39 months in November 2006. For the nodulectomies patients were admitted at the district hospital in Dunkwa for two or more days. The nodules were excised aseptically under local anaesthesia in a separate room by an experienced surgeon (PK) as previously described [18, 22]. Treatment of the wounds was started in the hospital and continued in the villages by the trial physician. During drug and wound treatment the patients and a few other villagers were also treated for other medical conditions such as respiratory and intestinal infections, fungal infections of the skin, fever assumed to be caused by malaria, fresh wounds and old ulcers. Additionally, four study patients underwent hernia operations.
The study patients had been asked not to participate in IVM mass treatment between September 2003 and November 2005. They were treated by us with IVM in November 2005–2006, when a final examination for nodules was made after 39 months in November 2006.
Objectives
The study had two objectives: (1) To study in a placebo-controlled manner the efficacy of 4 and 6-week treatment with doxycycline followed by IVM, in order to define the minimum regimen needed to achieve Wolbachia depletion and complete sterilization of adult female worms, leading to a reduction of skin mf over a long period. Two and 3 weeks of doxycycline were not sufficient in the previous studies (unpublished data). (2) To assess a potential macrofilaricidal activity of doxycycline on Onchocerca volvulus.
Outcomes
The primary outcome was the assessment of sustained effects of doxycycline treatment on worm fertility and worm survival. Worm fertility was measured (1) by observation of the presence or absence of normal or degenerated embryos in female worms and of mf in the human nodule tissue using histology, and (2) by analysis of the presence and quantity of skin mf. Worm survival was measured as the proportion of living and dead worms detected by histology.
Sample size
The sample size was calculated for the presence of mf in the patients’ skin. Applying Fisher’s exact test, the power calculation resulted in a sample size of 20 patients per treatment arm for a power of 90% at a significance level of 0.001, in order to observe a significantly higher proportion of doxycycline-treated patients without skin mf compared to placebo. Allowing a dropout rate of 20% the calculation suggested starting treatment with 25 patients for each arm. This sample size was also suggested on the basis of histological analysis of female worms in extirpated onchocercomas: Twenty patients with two or more nodules containing two or more female worms would result in the examination of about 100 female worms, a number found to be sufficient in earlier studies [16–18, 23–25].
Randomisation
The random allocation sequence was computer-generated by AH (StatView® version 4.5. for Macintosh). Simple randomisation without restrictions was used. The random allocation sequence was implemented by packing tablet containers according to the consecutive running numbers to which the mode of treatment had been allocated and which had been assigned to the patients.
Blinding
Blinding was assured by the exclusion of persons involved in randomisation or tablet packaging in any clinical or laboratory assessments as described [20]. At the beginning of the study and during drug treatment all members of the team and the patients were blinded. Patients were blinded to group allocation. A few nodules were excised from patients with many onchocercomas at 6 months after the beginning of the study to confirm the depletion of Wolbachia. These patients had been selected by AH without giving information on treatment to other members of the team (only a list with patient identification numbers was provided). All persons involved with the assessment of nodules and skin biopsies in the laboratory were kept blinded until the end of the analysis.
Laboratory methods
Nodule preservation
For histological analysis, we used either complete small nodules or more than half of medium or large nodules to allow assessment of worm numbers and status. Definitions of “medium” and “large” were diameters of 5–10 mm and more than 10 mm, respectively. For histology the nodules or portions of them were fixed in 80% ethanol or 4% phosphate-buffered formaldehyde solution. For PCR analysis the minor portions or total small nodules were placed immediately into ice-cold RNAlater solution (Ambion, Cambridgeshire, UK), kept at 4°C overnight and then frozen until further processing. The presence of worms was verified by worm actin PCR. If possible, cuts were made such that the piece that was cut away for PCR constituted the minor portion of worm-containing tissue (“worm nest”) of which the larger one corresponded to the piece used for histology. Since this could not always be guaranteed macroscopically we analysed the pieces of the nodules for PCR and histology independently.
Histology
Samples were embedded in paraffin and several sections were stained by hematoxylin and eosin. For selected sections, Giemsa stains and Gomori’s method for iron were used. For immunostaining, the alkaline phosphatase anti-alkaline phosphatase technique (APAAP) was applied according to the manufacturer’s instructions (DakoCytomation, Hamburg, Germany). To demonstrate the presence of Wolbachia, a rabbit anti-serum against Dirofilaria immitis Wolbachia surface protein (DiWsp) was used at a dilution of 1:1,000 [26]. Worm vitality was assessed with rabbit anti-serum against a cathepsin D-like lysosomal aspartic protease of O. volvulus (APR) at a dilution of 1:1,000 [27]. As secondary antibody, an anti-rabbit mouse monoclonal antibody was used (clone MR12/53, DakoCytomation). Fast Red TR salt (Sigma, Deisenhofen, Germany) was applied as the chromogen, and hematoxylin functioned as a counter stain (Merck, Darmstadt, Germany).
Assessment criteria were as previously described [18, 23]. Criteria for dead filariae were worm portions calcified without cuticle or nearly completely absorbed. Further characteristics for death of a worm included loss of body wall integrity, loss of nuclei [23], and absence of APR-staining [27] (Fig. 2). Very degenerated worms, still APR-positive, were classified as moribund according to Duke et al. [28, 29] and grouped in the category “dead”. But this criterion was used rarely, mainly for worms with neoplasms. “Living” means alive at the time of nodulectomy. All developmental stages from the stage of two cells to the stretched mf in the uterus were classified as “embryos” [18, 23]. When only the stretched mf were degenerated and the other embryos were not, embryogenesis was recorded as “normal” for the purpose of this doxycycline-oriented study. It was assumed that degenerated stretched mf were due to IVM treatment [30, 31]. The sections were assessed by two authors (DWB and SS) independently. When the examiners did not agree on the findings, the slides were re-examined, which always led to a consensus. Examination and re-examination were performed without information on the treatment.
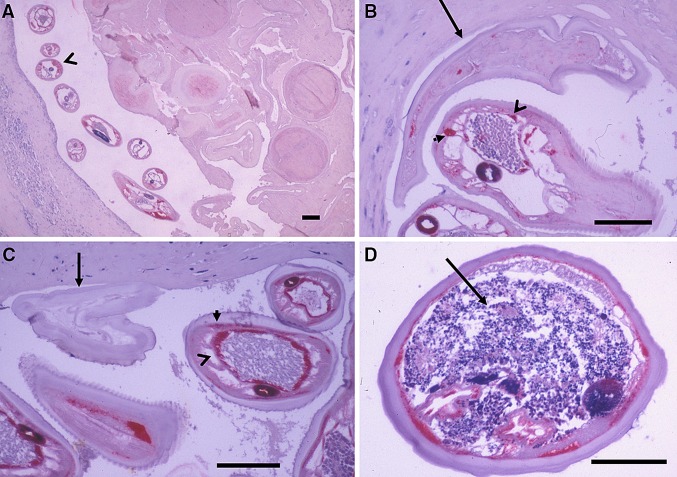
Fig. 2.
Differentiation between living and dead O. volvulus worms by immunohistology. “Living” is defined as alive before fixation of nodules. a At left, a living male worm labelled red (arrowhead) and at right, a dead female with strongly degenerated organs after doxycycline and ivermectin. b Detail of the same slide as (a) but from another region showing the red labelling of the epithelium of the testis by antiserum against APR (open arrowhead) and of the median chord (closed arrowhead) of the living male in contrast to the dead female (arrow). The testis is filled with sperms. c A living male with well-labelled epithelium of the testis (open arrowhead) and hypodermis (closed arrowhead) and an unlabelled dead female (black arrow) from a placebo patient not treated with ivermectin. d A moribund female with a pleomorphic neoplasm in the pseudocoeloma cavity (black arrow) from a placebo patient 14 months after ivermectin. The intense blue staining of the gut indicates the older age of these worms and it hides the red labelling of APR on the photos. All patients were operated 27 months after the onset of the study. Sections were stained with antiserum against a lysosomal aspartic protease (APR) of O. volvulus only labelling the organs of “living” but not of dead filariae. Scale bar = 100 μm
PCR
For the analysis of the extent of Wolbachia depletion by quantitative PCR, only nodules positive for worm actin were included in the analysis. DNA was extracted using Trizol reagent following the manufacturer’s protocol (Invitrogen, Karlsruhe, Germany) by homogenising 4 × 30 s at 6,800 rpm in a Precellys 24 (PeqLab, Erlangen, Germany) using 2.8-mm steel beads. DNA was dissolved in 0.8 mM NaOH, pH adjusted to 7.4 with 1 M HEPES (Invitrogen), and further purified using the QIAamp Mini Kit (Qiagen, Hilden, Germany) following the protocol for crude cell lysates.
The Wolbachia ftsZ gene was quantified from the purified DNA by real-time PCR (qPCR) using the following conditions: 1× HotStar Taq Polymerase buffer (Qiagen), 200 μM dNTP, 200 nM each of forward (5′-AGGAATGGGTGGTGGTACTG-3′) and reverse (5′-CTTTAACCGCAGCTCTTGCT-3′) primers, 0.2 μl of Sybr Green (1:1,000 diluted in DMSO, Roche, Mannheim, Germany), 2.5 U HotStar Taq, and 2 μl DNA in a 20 μl reaction. The gene was amplified in a Rotorgene 3000 (Corbett Research, Sydney, Australia) using the following conditions: 1 × 15 min at 95°C, 40 cycles of 94°C for 15 s, 55°C for 30 s, 72°C for 20 s. Fluorescence was acquired on the FAM channel. Nematode actin was measured as described [32]. Copy numbers for each gene were calculated using a modification [20] of the comparative quantification formula as described in [33].
Microfilariae
Microfilariae were assessed at recruitment and at 6, 20 and 27 months after the onset of doxycycline/placebo treatment. For mf analysis, two skin biopsies of 1–3 mg were taken from the buttocks using a Holth punch. Each biopsy was immersed in 100 μl of 0.9% NaCl solution in a well of a microtiter plate (Nunc, Roskilde, Denmark). The skin biopsies were incubated at room temperature for 6–20 h. The solution was then transferred onto a slide for microscopic examination. The biopsies were weighed using a Sartorius electronic balance (Göttingen, Germany). Microfilarial data are given as median with range, and as geometric mean. For the latter, the calculation according to Williams was performed as used by WHO for standardization of mf results [34]. Since the geometric mean cannot use zero, the calculation uses mf +1 values, and from the calculated mean, 1 is subtracted afterwards [34].
Deviations from the study protocol
The methods described above were those actually used. New information based on the results of the studies that we had performed in 2000–2003 led to some deviations from the original study protocol. The time for nodulectomy was postponed from 4, 18 and 24 months to 6, 20 and 27 months for a better assessment of worm fertility and survival. Patients of the 4 weeks group were not nodulectomised at 6 months in order to save their nodules for later follow-up times since data analysis from other studies [35] suggested that 4 weeks of doxycycline might be the optimal regimen, and it was clear that the anti-parasitic effect of doxycycline would be best observed at later points of time. We also decided to include patients who had taken one or two doses of IVM and those with less than 10 mf/mg, since we knew that we could differentiate between the effects of doxycycline and of IVM taken before recruitment, as long as IVM had not been taken more than one to two times. We also accepted patients older than 50 years with many nodules.
Since IVM was planned to be given after the first round of nodulectomies, it had to be administered at 6 months instead of 4 months. Since skin snipping without the prospect of nodulectomy would have led to reduced compliance at later follow-up times, we omitted mf analysis at 6 months in the 4-week doxycycline group. Sonography was used to detect onchocercomas and these results will be published elsewhere.
A third objective mentioned in the protocol was the analysis of whether treatment with IVM several months after the end of the doxycycline treatment led to a reduction of adverse reactions to IVM. Severe side effects as described by Awadzi [36] and as we had seen in Uganda [37], were not observed. Since no difference between placebo and doxycycline was seen, we do not report this topic here.
Statistical methods
The presence of mf in the skin of the patients, was compared between the three treatment groups by Fisher’s exact test, comparing all treatment groups first, followed by all possible pair-wise comparisons in case of a significant result for the first test.
In addition, regression analyses were performed using alternating logistic regression [38, 39], as implemented in the SAS-Procedure Genmod®, to analyse and estimate the rate of nodules with “normal” embryogenesis, “living” female worms, or mf in the human tissues of the nodule and compare them between placebo and the two doxycycline treatment arms. This procedure allowed taking into consideration a potential dependency between the nodules in one patient.
Further analyses were performed on the per nodule level using the Kruskal–Wallis test, the Mann–Whitney-U test and the Wilcoxon Signed Rank test for quantitative data. The Chi-square test was used for qualitative data. These calculations were done using Stat View® software version 4.5.
Results
Participant flow and recruitment
Figure 1 illustrates the trial profile in the way the study was performed. Deviations of the original protocol are described in the section, “Methods” In September 2003 during surveys in the three villages, 76 patients who met the clinical inclusion criteria were recruited from a potential 81 candidates. Five of the 81 had at least one exclusion criterion in clinical chemistry when tests were performed subsequent to clinical examination. The 76 patients were randomly allocated to the 3 treatment arms, and 67 patients completed the allocated treatment. Adherence to treatment was generally high. Two to four individuals in each treatment group failed to complete the course of doxycycline or placebo. The failure was usually due to absence from the village. In one case the treatment was stopped after bloody diarrhoea on day 3. A number of patients who had received placebo or doxycycline for 6 weeks underwent a nodulectomy on one site of the body 6 months after recruitment. The excised nodules contained 29 and 32 living females (Table 2), enough filariae to assess the early activity against the Wolbachia. The main points of time for the assessment were 20 and 27 months after the beginning. Four of the patients absent at that time were offered another chance for nodulectomy and were nodulectomised 1 year later in November 2006. Including these, a total of 22 of 23 placebo patients, 19 of 22 patients treated 6 weeks with doxycycline, and 14 of the 22 patients of the 4-week group were nodulectomised. Most of those who did not undergo nodulectomy had moved since study onset. The nodules excised in 2005 permitted an assessment of 149 females and 48 males filariae in the 6-week group and 171 females and 53 males in the placebo group, providing more worms than needed for a judgement of worm vitality.
Table 2.
Effect of doxycycline treatment on presence of Wolbachia in worms: results of immunohistology
| Treatment group | Time (months) | Number of patients/nodules | Number of living female worms | Number of living male worms | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | With Wolbachia | Alla | With Wolbachia | |||||||
| Many | Few | None | Many | Few | None | |||||
| Placebo | 6 | 13/14 | 29 | 26 | 0 | 3 | 8 | 7 | 1 | 0 |
| 20 | 16/51 | 70 | 58 | 7 | 5 | 25 | 18 | 2 | 3 | |
| 27 | 7/38 | 61 | 50 | 9 | 2 | 25 | 14 | 3 | 5 | |
| Doxycycline (4 weeks) | 20 | 7/36 | 27 | 3 | 4 | 20b | 21 | 3 | 2 | 16b |
| 27 | 7/23 | 20 | 4 | 2 | 14b | 9 | 6 | 2 | 1 | |
| Doxycycline (6 weeks) | 6 | 11/19 | 32 | 1 | 8 | 23b | 18 | 1 | 2 | 14b |
| 20 | 13/62 | 42 | 9 | 033 | 33b,d | 20 | 3 | 2 | 15b | |
| 27 | 5/27 | 15 | 5 | 0 | 10b | 14 | 2 | 0 | 11c,e | |
aWhile the number of all living worms is given to make the numbers consistent to the other tables, in some instances it was not possible to distinguish if a male worm had many, few or no Wolbachia, due to too little worm material in the respective histological sections. Therefore, “All” is not always a summary of the three categories for males
b,cSignificant difference between the respective doxycycline and the placebo group regarding the distribution between many, few and no Wolbachia at the respective time points (bP < 0.0001; cP < 0.002, Chi-square test)
d,eSignificant difference between the two doxycycline groups regarding the distribution between many, few and no Wolbachia at the respective time points (dP = 0.03; eP = 0.003, Chi-square test)
Baseline data
Table 1 shows the characteristics of the three groups of patients that completed the allocated treatment.
Table 1.
Characteristics of patient groups that completed treatment (baseline data)
| Characteristics of patient groups | Placebo | Doxycycline (4 weeks) | Doxycycline (6 weeks) |
|---|---|---|---|
| Baseline data | |||
| No. of individuals | 23 | 22 | 22 |
| Sex ratio, male/female | 15/8 | 18/4 | 18/4 |
| Mean age, years (range) |
39 (20–61) |
39 (23–58) |
41 (19–59) |
| Mean weight, kg (range) |
54 (40–67) |
54 (49–65) |
56 (40–73) |
|
No. of palpable nodules Median (range) |
5 (2–13) |
6 (2–13) |
5 (2–13) |
| No. of mf carriers | 20 | 20 | 22 |
|
No. of mf/mg in the skin Median (range) |
9 (0–98) |
6 (0–78) |
21 (2–175) |
| No. with onchodermatitis | 1 | 5 | 7 |
| No. with depigmentation | 2 | 8 | 3 |
|
No. of individuals who took previously ivermectina Months before recruitment Mean (range) |
16 18 (5–41) |
8 15 (1–41) |
14 19 (5–40) |
|
No. with complete treatment Less days of treatment |
22 1 × 28 |
20 2 × 41b |
19 1 × 40, 2 × 41 |
aThree patients had taken ivermectin two times before study onset; the rest had taken ivermectin a single time
bThe 1 day that the two patients missed was during the doxycycline part of the treatment
Before study onset, the numbers of onchocercomas did not differ between the three groups. The mf loads of the 6-week doxycycline group were higher than those in the other two groups.
Outcomes
Depletion of Wolbachia by doxycycline
The presence of Wolbachia endobacteria in worm tissues was examined by immunohistology using anti-serum against Wolbachia surface protein (Table 2). Worms were classified as having many, few, or no bacteria as described previously [18]. Depletion of Wolbachia after doxycycline was already clearly visible at 6 months after the onset of treatment with 72% of 32 female and 78% of 18 male worms being Wolbachia-negative in the 6-week doxycycline group in contrast to 10% of 29 female and 0% of 8 male worms in the placebo group. At 20 months most placebo-treated living worms contained many bacteria, and only 7% of 70 female and 12% of 25 male worms stained as Wolbachia-negative. Since Wolbachia are not evenly distributed in the filarial tissues, this was expected and it had previously been observed [18]. In contrast to the placebo group, after 6 weeks of doxycycline treatment, 79% of 42 female and 75% of 20 male worms did not contain bacteria anymore. After 4 weeks of doxycycline 74% of 27 female and 76% of 21 male worms were found Wolbachia-negative. Equivalent differences were still seen at 27 months after treatment (Table 2). At 39 months after doxycycline 13 of 21 female worms in 20 nodules from 4 patients showed no bacteria. The presence of Wolbachia in the placebo group was retained after IVM treatment.
The data obtained by histology were corroborated by real-time PCR, where the Wolbachia load can be analysed quantitatively (Table 3). Since there is variation between nodules with regard to the amount of human tissue in relation to worms we normalized the Wolbachia loads to Onchocerca actin, as described [32]. Nodules without an actin signal were not considered for further analysis. Depletion of Wolbachia after doxycycline, demonstrated by quantitative PCR, was observed at all follow-up points of time. At 6, 20 and 27 months nodules from both doxycycline groups showed Wolbachia levels that were almost tenfold lower than the nodules from the placebo-treated group (Table 3). This was sustained over the observation period, with no statistical indication of an increase.
Table 3.
Effect of doxycycline treatment on Wolbachia loads in worm tissue: results of quantitative PCR
| Treatment group | Time (months) | Number of patients/nodules | Index ftsZ/actina
(10–90th percentiles) |
P valueb |
|---|---|---|---|---|
| Placebo | 6 | 12/31 |
3.24 (0.3–19.5) |
– |
| 20 | 16/70 |
3.04 (0.2–27.0) |
– | |
| 27 | 7/19 |
1.85 (0.1–29.1) |
– | |
| Doxycycline (4 weeks) | 20 | 7/13 |
0.47 (0.01–3.2) |
0.0016 |
| 27 | 5/15 |
0.20 (0.04–3.31) |
0.0011 | |
| Doxycycline (6 weeks) | 6 | 10/33 |
0.19 (0.02–4.4) |
0.0005 |
| 20 | 12/22 |
0.30 (0.04–6.9) |
<0.0001 | |
| 27 | 5/11 |
0.13 (0.04–1.4) |
0.0004 |
DNA was prepared from portions of onchocercomas extirpated at the indicated time points, and the copy numbers of the Wolbachia single copy gene ftsZ and the nematode gene actin were determined by quantitative PCR
aMedian
bSignificant differences in the respective doxycycline compared to the placebo group (Mann–Whitney U test). No significant differences between the two doxycycline groups were observed at any time point
Interruption of embryogenesis by doxycycline
For this analysis one has to consider the different effects on embryogenesis by doxycycline and by one or two doses of IVM, as described in the “Methods” (histology). Since the focus of this study is the doxycycline-induced effects, the term “degeneration” (Table 4) refers to early embryogenesis and is affected only by doxycycline; while degeneration only of the stretched filariae, an effect of IVM, was counted as “normal” (Table 4). In the placebo group, we observed that 27 of 70 female worms (39%) had normally developed embryos at 20 months, and mf were observed in 24% of the nodules analysed (Table 4). In contrast, only 3 of 27 (11%) and 3 of 42 (7%) of the female worms were found with normal embryogenesis in the 4 and 6 weeks doxycycline treatment arms, respectively. No mf were found in the 98 onchocercomas excised at 20 months from the patients of the doxycycline arms. Equivalent differences were seen at 27 months and were already observed at 6 months in the 6 weeks doxycycline group. At 39 months after doxycycline, 2 of 21 female worms showed embryogenesis and 2 of 20 nodules from four patients contained mf, respectively (data not shown). These data were in accordance with those of our previous study [18].
Table 4.
Effect of doxycycline treatment on microfilariae production: results of immunohistology
| Treatment group | Time (months) | Number of patients | Number of living female worms | Number of nodules | ||||
|---|---|---|---|---|---|---|---|---|
| All | Embryos | All | With intact mf | |||||
| None | Degenerated | Normala | ||||||
| Placebo | 6 | 13 | 29 | 16 | 5 | 8 | 14 | 6 (43%) |
| 20 | 16 | 70 | 26 | 17 | 27 | 51 | 12 (24%) | |
| 27 | 7 | 61 | 30 | 10 | 21 | 38 | 10 (26%) | |
| Doxycycline (4 weeks) | 20 | 7 | 27 | 13 | 11 | 3b | 36 | 0 (0)e |
| 27 | 7 | 20 | 10 | 8 | 2b | 23 | 2 (9%) | |
| Doxycycline (6 weeks) | 6 | 11 | 32 | 10 | 21 | 1c | 19 | 1 (5%)f |
| 20 | 13 | 42 | 24 | 15 | 3d | 62 | 0 (0)g | |
| 27 | 5 | 15 | 9 | 5 | 1 | 27 | 0 (0)h | |
No significant differences between the two doxycycline groups were observed at any time point
aNormal embryogenesis only refers to the younger embryonic stages, excluding degenerated stretched mf
b, c, dSignificant differences between doxycycline and placebo groups regarding the presence of degenerated and normal embryogenesis at the respective time point. bP = 0.03; c P = 0.0003, dP = 0.001 (Chi-square test)
e, f, g, hSignificant differences between doxycycline and placebo groups regarding the proportions of nodules with intact mf at the respective time point. eP = 0.001; f P = 0.03; g P < 0.0001, h P = 0.004 (Fisher’s exact test)
Since the nodules of one patient and the nodules stemming from the same site of the body of a patient could a priori not be regarded as independent, we used alternating logistic regression (see “Methods”), as implemented in the SAS-Procedure Genmod® [39], to analyse the rate of normal embryogenesis and the presence of nodular mf, and mathematically taking into account the potential interdependency of worms in nodules and nodules in patients. This analysis confirmed that both the rate of normal embryogenesis per nodule and the rate of the modelled likelihood for the presence of mf in the nodule tissues, based on the data given in Table 4, are much lower in nodules from 4 to 6 weeks doxycycline than from placebo patients at month 20. The estimated rate of nodules with normal embryos was 8.3% [CI 4.3–15.3%] for 4 weeks doxycycline, 4.9% [CI 2.2–10.4%] for 6 weeks doxycycline, and 44.2% for placebo, showing clear differences between both treatment groups and placebo (P < 0·0001 for both comparisons) but no evidence for a difference between 4 and 6 weeks doxycycline (P = 0.3). The estimated likelihood for the presence of nodular mf was 0 [CI 0.0–10.3%] for the 4 weeks doxycycline group and 1.7% [CI 0.4–6.5%] for the 6 weeks doxycycline group as compared to placebo with 31.5% [CI 20.4–45.3%], P < 0·0001.
Together, these results confirm our earlier findings [17, 18] that one of the effects of doxycycline on female worms is a sustained cessation of embryogenesis and mf production in all or most of the worms (see “Discussion”).
Sustained reduction of skin mf loads by doxycycline
Table 5 presents the skin mf load before treatment and at follow-up points of time. All patients who were available for follow-up underwent skin snipping regardless of their IVM uptake. However, IVM itself affects skin mf. Therefore, for the analysis of skin mf in Table 5 only patients who had taken IVM at 6 months were included. The data from patients without IVM were measured but were not considered, although inclusion of these patients did not alter the significance of the data (not shown).
Table 5.
Effect of doxycycline treatment on microfilariae production: mf loads in skin snips
| Treatment group | Time (months) | Number of patients assessed | Mf levelsa | Number of patients with skin mf (% total) |
|||
|---|---|---|---|---|---|---|---|
| GMI | Range | Median | P valueb | ||||
| Placebo | 0 | 25 | 8.5 | 0/98 | 10.3 | – |
23 (92%) |
| 6 | 16 | 14.3 | 0/64 | 27.6 | 0.379 |
15 (94%) |
|
| 20 | 18 | 4.4 | 0/58 | 4.3 | 0.011 |
17 (94%) |
|
| 27 | 15 | 5.5 | 0/75 | 3.0 | 0.394 |
12 (80%) |
|
| Doxycycline (4 weeks) | 0 | 26 | 6.5 | 0/78 | 6.2 | – |
25 (94%) |
| 20 | 10 | 0.7 | 0/6 | 0.3 | 0.012 |
6c (60 %) |
|
| 27 | 10 | 1.3 | 0/23 | 0.4 | 0.839 |
5 (50%) |
|
| Doxycycline (6 weeks) | 0 | 25 | 19.1 | 0.3/175 | 13.2 | – |
25 (100%) |
| 6 | 16 | 14.7 | 0.4/60 | 25.2 | 0.049 |
16 (100%) |
|
| 20 | 14 | 0.1 | 0/1 | 0·0 | 0.001 |
3d (15%) |
|
| 27 | 10 | 0.8 | 0/6 | 0·6 | 0.007 |
7 (70%) |
|
aGeometric mean intensity (GMI), median values, and ranges of mf/mg skin are shown. The GMI was calculated using the log (x + 1) method according to Williams
bChanges in median levels from baseline (at 0 month) in skin mf levels at 6, 20, and 27 months in doxycycline or placebo groups were assessed (Wilcoxon signed rank test)
c, dSignificantly fewer patients were mf-positive in the respective doxycycline groups compared to the placebo group at 20 months after study onset. cP = 0·041; dP < 0·0001 (Fisher`s exact test). No significant differences between the two doxycycline groups were observed at any time point
At month 20, in the placebo group that had received only IVM, mf loads were reduced but 17 of 18 patients were still positive for skin mf. In contrast, in both groups that had received doxycycline plus IVM, the median mf level was much more reduced, being zero in the doxycycline 6 weeks group. In both doxycycline groups, the proportion of patients, who were still positive for skin mf, was significantly lower than in the placebo group (Fisher’s exact test, Table 5). At month 27, the significant and sustained reduction of skin mf as compared to placebo was still maintained in the 6 weeks doxycycline group. There was less reduction of skin mf in the 4 weeks group, while in the placebo group, there was a clear resumption of mf production, confirming earlier reports that show that production of mf starts a few months after the administration of IVM [24, 40].
Macrofilaricidal activity of doxycycline
After a long observation period of 20 and 27 months, a distinct macrofilaricidal effect on female worms was observed in onchocercomas from the doxycycline-treated groups (Table 6). Examples of dead compared to living worms in onchocercoma sections are presented in Fig. 2. At 6 months after study onset, the proportions of dead female and male worms in the 6-week doxycycline group were similar to the placebo group. In the latter, proportions of dead worms remained stable through all follow-ups and they were in accordance to other published results [18, 23, 25, 28]. In the placebo group a few polymorphic neoplasms were observed (Fig. 2d), a killing mechanism of IVM described by Duke et al. [29]. In contrast, in both doxycycline groups a significant and biologically relevant increase of dead females was seen at 20 and 27 months, compared to placebo. The proportion of dead females was highest in the 6-week group at 20 and 27 months (>60%). A lower but significant increase in dead males was seen in both doxycycline treated groups. The ratio of all living female to living male worms, summarised for both doxycycline groups and for 20 and 27 months, was 1.6/1 compared to 2.6/1 in the placebo group. The difference is significant (P = 0.037, Chi-square test) and shows the lower lethality of doxycycline on male worms.
Table 6.
Macrofilaricidal activity of doxycycline treatment: percentage of dead and living filariae. Results of immunohistology
| Treatment group | Time (months) | Number of patients/nodules | Number of female worms | Number of male worms | Number of living worms per nodule | |||
|---|---|---|---|---|---|---|---|---|
| All | Dead females (%) |
All | Dead males (%) |
Female | Male | |||
| Mean (SEe) |
Mean (SEe) |
|||||||
| Placebo | 6 | 13/14 | 35 |
6 (17%) |
8 |
0 (0) |
2.1 (0.5) |
0.6 (0.2) |
| 20 | 16/51 | 93 |
23 (25%) |
27 |
2 (7%) |
1.4 (0.2) |
0.5 (0.1) |
|
| 27 | 7/38 | 78 |
17 (22%) |
26 |
1 (4%) |
1.6 (0.2) |
0.7(0.1) | |
| Doxycycline (4 weeks) | 20 | 7/36 | 55 |
28 (51%)a |
28 |
7 (25%) |
0.8f (0.2) |
0.6 (0.1) |
| 27 | 7/23 | 39 |
19 (49%)b |
16 |
7 (44%)c |
0.6g (0.2) |
0.4 (0.1) |
|
| Doxycycline (6 weeks) | 6 | 11/19 | 41 |
9 (22%) |
18 |
0 (0) |
1.7 (0.3) |
0.9 (0.3) |
| 20 | 13/62 | 111 |
69 (62%)a |
31 |
11 (35%)d |
0.7h (0.2) |
0.3 (0.1) |
|
| 27 | 5/27 | 38 |
23 (61%)a |
17 |
3 (18%) |
0.6i (0.2) |
0.5 (0.1) |
|
a,bSignificant difference between the respective doxycycline and the placebo groups regarding the proportion of dead female worms at the respective time point compared to the placebo group: aP < 0.0001; b P = 0.005 (Fisher’s exact test)
c,dSignificant difference between the respective doxycycline and the placebo groups regarding the proportion of dead male worms at the respective time point: c P = 0.003; d P = 0.013 (Fisher’s exact test). No significant differences between the two doxycycline groups were observed at any time point
eArithmetic mean and standard error (SE)
f,g,h,iSignificant differences in the respective doxycycline compared to the placebo groups at the respective point of time: f P = 0.009; g P = 0.0004; h P < 0.0001; i P = 0.0002 (Mann–Whitney-U test). No significant differences between the two doxycycline groups were observed at any time point
Table 6, right set of columns, illustrates the changes in the number of living female worms per nodule. This calculation is not compromised by the potential bias that some dead worms may have been already absorbed. The data confirm the results shown by histology. At 6 months no macrofilaricidal effect by doxycycline was seen. At 20 and 27 months the numbers of living females per nodule were significantly lower in both doxycycline groups when compared to the placebo group.
Alternating logistic regression analysis was used to estimate the rates of nodules with living female worms at 20 months. In the 4-week doxycycline group, 52.2% of nodules were calculated to contain a living female [CI 32.1–71.6%] and in the 6-week doxycycline group, 38.2% of nodules were calculated to contain a living female [CI 22.5–56.0%], in contrast to 79.2% [CI 58.3–90.3%] in the placebo group. The proportions of dead and living female worms are in this analysis are almost identical to the results in Table 6, where the dead and living worms were analysed without considering the potential interdependency of worms in nodules.
Ancillary analyses
Palpation of nodules in November 2006
At 39 months after study onset, 46 of the 67 treated and four new untreated patients were examined for nodules and the findings compared with the previous survey and the records made at the times of nodulectomy. Three of the 46 treated patients had not yet been nodulectomised at all, and one patient had not been completely nodulectomised (see Fig. 1, footnote c). Of the 42 nodulectomised patients, 27 had no palpable nodules. Only two patients had one nodule each, which had been observed already before the nodulectomies. Thirteen patients had 32 new nodules. One patient presented 11 new nodules. His profession suggested an extremely high exposure to Simulium flies (“I catch crabs in the river Offin everyday”). We conclude that since all palpable nodules except for two had been excised for analysis, there had been no selection of nodules. The eight patients, who had not been nodulectomised, presented 33 nodules (range 3–6). The mean number of nodules was 4.1 compared to 0.8 in the nodulectomised patients (P < 0.0001). The eight nodule carriers were nodulectomised after examination (see results on month 39 in the sections before).
Doxycycline without IVM
A sub-analysis was done based on eight patients from the doxycycline groups who had never received IVM before or during the study. Nodulectomy of two 6-week patients resulted in 12 nodules with 17 living female worms (82% dead). From the six 4-week patients, 44 nodules were obtained with 78 female worms (40% dead). Although the numbers are small and comprise all late points of time (20, 27 and 39 months), the data suggest that doxycycline is macrofilaricidal on its own.
Twelve nodules from 2 patients from the 6-week doxycycline group, and with 44 nodules from 6 patients from the 4-week doxycycline group,
Adverse events
One patient in the 4-week group presented with bloody diarrhoea on day 3 of treatment. The treatment was stopped and the patient recovered quickly after treatment with metronidazole. Day 3 may have been too early for antibiotic-associated colitis. Other side effects were mild, did not last longer than 3 days and did not occur more frequently after administration of doxycycline. No serious adverse events were observed. One patient from the 4-week doxycycline group died 8 months after doxycycline treatment without confirming any diagnosis. Dying adult filariae in superficial tissues may perforate the tissue, as it has been observed with suramin treatment of onchocerciasis or diethylcyarbamazine (DEC) treatment of Brugia infections. If this happens, the patients always become aware of it. None of our doxycycline-treated patients reported a perforation and all scars observed in November 2006 were identical with the sites of nodulectomies. Since doxycycline does not show a fast microfilaricidal activity, no side effects known to occur with DEC or IVM were observed during the treatment or reported later by the patients.
Discussion
Selection of villages and treatment with placebo
The three neighbouring villages selected for the study were located in an area with ongoing transmission [21]. This probably led to the acquisition of new worms during the study, presenting a problem for the diagnosis of worm vitality (see below). However, performing the study in an area with strongly reduced or interrupted transmission would have biased the results, as older worm populations present more degenerated and dead worms [23]. This would also present a bias for the diagnosis of any macrofilaricidal activity. Differing from our previous study [16–18] the present study was placebo-controlled and randomised. The recruitment of placebo patients in a randomized way from the same villages is nowadays necessary, since comparison to historical data sets may be biased due to IVM mass treatment or vector control, resulting in a different age pattern of the worms.
Remaining living worms and acquisition of new worms after doxycycline
At 20 and 27 months after doxycycline treatment, the tables show a few living worms with many Wolbachia (Table 2), female worms with normal embryogenesis (Table 3), and approximately 40 and 50% surviving females in the 6 and 4-week groups, respectively (Table 4). To explain these findings we consider three hypotheses, which probably are all true for some of the filariae:
(1) Some worms survive for longer periods without the endobacteria but they do not resume mf production for several years or for the rest of their lives. Such survival of worms after treatment is well known for O. volvulus after suramin [25]. Cure rates at this extent are also known from other tissue-dwelling helminths and are accepted provided a reduction of the worm load is achieved. For example, Schistosoma cure rates of 60–95% are accepted by WHO [41]. Also for loiasis and lymphatic filariasis for some patients several courses of DEC are needed for a cure. (2) Not all endobacteria have been eliminated from the filariae and these may multiply over a period of time, as it is known from Rickettsia. We suppose that survival of a few bacteria in degenerated oocytes or embryos might happen when suboptimal doses of the antibiotic are used. (3) Based on our observations we are convinced that the worms with many bacteria (Fig. 3) and with either undisturbed oogenesis or embryogenesis were newly acquired after the doxycycline administration. Given that a female worm’s average life span is 10 years [2] and the worm load in adult humans in areas with ongoing transmission such as the study area, is rather stable, one has to consider a yearly turnover of approximately 10% of the total adult worm load. Therefore, during an observation period of up to 27 months, a rate of 20% of newly acquired young worms would have to be expected. These young female worms can be differentiated from older worms by their morphology [18, 23, 42–44]. The young female worms are characterized by: a smaller diameter, no thickening of the cuticle, prominent lateral cords and somatic muscles, lack of inclusion bodies or other signs of degeneration in hypodermis, muscles, and epithelia. Most are still nulliparous. Little or no intense brown or blue staining is seen in the gut and none in other organs because of less iron deposition [45]. In accordance with these results, the APR-positive lysosomes are not found in immature worms, and they are rare in the gut and more so in the other tissues of young worms compared to the older ones [27].
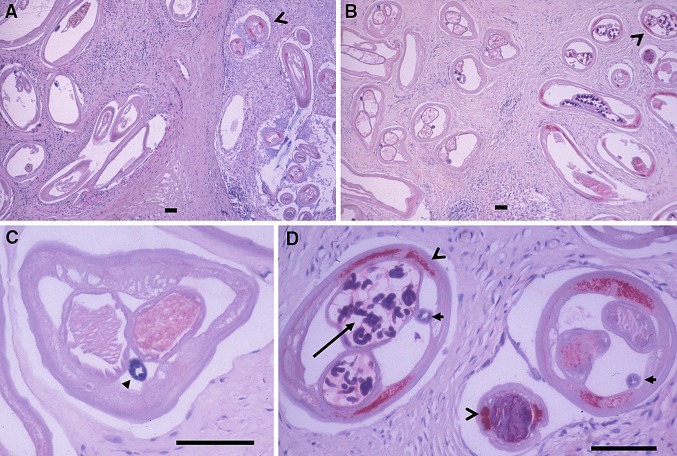
Fig. 3.
Living O. volvulus worms acquired before and after doxycycline treatment without and with Wolbachia endobacteria. a At left an old female worm without endosymbionts and at right a young newly acquired female and a male worm with red labelled Wolbachia (open arrowhead). Onchocercoma excised at 27 months. b As in (a), at left an old female and at right new young worms at 20 months (open arrowhead). c Another section of the old female shown in (b)—but from another place of the same slide—without Wolbachia and embryos and with a dark, pigmented gut (closed arrowhead). d Detail of (b) showing both the young female and male worms with endobacteria labelled in red (open arrowheads). The gut is less pigmented (closed arrowhead) and the uterus contains coiled microfilariae (arrow). Sections stained with antiserum against Wolbachia surface protein (DiWsp). Scale bar = 100 μm
In our histological analyses we observed the presence of such worms, especially when we examined the slides stained by anti-APR-serum (Fig. 3) or by Gomori’s method for iron. Applying these criteria and subtracting these newly acquired living worms from the total, the proportion of dead female worms would be higher, around 70% in the 6-week doxycycline group at both months 20 and 27, compared to only 30% in the placebo group. In particular, the alternating logistic regression analysis for the rates of living female worms per nodule at 20 months is disproportionately affected by the newly acquired worms, as most of the nodules in the doxycycline group with living worms are comprised only of new worms. In addition, some dead worms may have been absorbed. Assuming that 70% or more worms have died, up to 30% of the female and a higher percentage of male worms would have survived without endobacteria, albeit sterile and degenerated. This represents a considerable reduction of the adult worm load and together with the complete sterilisation (except for new worms) apparently meets the criteria of cure applied for other helminths [41].
Interdependency issues
The primary outcome of this study was the assessment of sustained effects of doxycycline treatment on worm fertility and worm survival. This outcome was evaluated also by taking into consideration possible interference, since worms aggregate in nodules, arguably in order to enhance their chances to mate and produce offspring, and to survive. Therefore, we used alternating logistic regression to compare, between the treatment arms, the rate of nodules with normal embryogenesis, the rate of nodules with mf, and the rate of nodules with living female worms. Interestingly, the results for alternating logistic regression showed almost identical proportions of living and dead female worms compared to the analysis of individual worms, as shown in Table 4. However, analysed by alternating logistic regression, the data on this are now comparable to the mode of analysis by Duke and colleagues [28].
Macrofilaricidal activity of doxycycline
It may be asked whether the macrofilaricidal effect seen in this study in the patients who received doxycycline has only been achieved in conjunction with the administration of IVM. A preliminary answer to this may be given from the analysis of the small number of nodules from patients who had received only doxycycline, because they had missed the IVM administration in our study. The findings from these nodules allowed the assumption that doxycycline is macrofilaricidal on its own. This was confirmed by the data of another study where the patients did not receive IVM (Hoerauf et al., in preparation).
Safety of doxycycline treatment
Doxycycline for bacterial infections has been used by millions of people against bacterial infections, including probably tens of thousands with filarial co-infections. It is a safe drug as long as the contraindications are observed. We have used it previously to treat bacterial infections of onchocerciasis patients. Considering the large number of treated people, it was certainly not an objective of this study to assess the safety of doxycycline in general. However, we did consider which side effects might be expected regarding anti-filarial activity of doxycycline. Since doxycycline has no or at least no fast microfilaricidal activity, adverse side effects known from DEC or IVM were not to be expected. Therefore we assumed that an ophthalmologic examination was not needed for this study. Regarding the macrofilaricidal activity, subcutaneous abscesses and rarely perforation tissue b moribund or dead filariae may occur, as with suramin treatment of onchocerciasis or DEC with Brugia infections. This did not occur during our study.
Conclusions
The present study showed that doxycycline not only interrupts embryogenesis in the long-term, but also has a macrofilaricidal activity in human onchocerciasis. The treatment has a low toxicity except for pregnant and lactating women and children below the age of 9 years. The sustained absence of embryogenesis, of skin mf and the macrofilaricidal activity seemed to be better after 6 weeks as compared to 4 weeks of doxycycline administration (P = 0.07 for the combined 20 and 27 months groups, Chi-square test); simulating the same proportions of dead and living worms with higher numbers would result in a statistically significant difference. Therefore, doxycycline should rather be used for 6 weeks, if possible. Larger studies may show that the 4-week regimen is equivalent for certain indications, such as long-term sterilisation.
The new chemotherapeutic principle is beneficial to the treatment of individual patients, who have left a transmission area and thus can achieve a strong reduction of the adult worm load and a clearance of mf. The new regimen may also be considered in areas where there has been evidence for a sub-optimal performance of IVM as in the Asubende focus in Ghana [12–14]. Worm populations that might have acquired some degree of resistance may thus be eliminated with an existing drug to prevent resistance from further spreading. Doxycycline without IVM may also be considered for treatment in areas with co-endemicity of onchocerciasis and loiasis. In addition, the elimination of onchocerciasis in the Americas has reached “end-game” scenarios where transmission has apparently been interrupted and there are only a limited number of people infected with onchocerciasis in some foci. Administration of doxycycline to these people has been suggested ([46], and F. Richards, Atlanta, personal communication) on the basis of its long-term embryotoxicity. The new data on the macrofilaricidal effect further underscores this argumentation. In these foci children are usually no longer infected and pregnant and lactating women can be treated later. The anti-wolbachial chemotherapeutic principle may also be exploited for the search of new macrofilaricidal drugs, which can be focussed on Wolbachia-depleting antibiotics. Indications have been dealt with in more detail in [47].
In summary, anti-wolbachial chemotherapy of human onchocerciasis with doxycycline can be orally applied for special indications to sterilise the female filariae and to reduce the adult worm load. It cannot replace IVM for mass treatment in areas with ongoing transmission.
Acknowledgments
This study received major funding by the European Commission (ICA4-2002-10051). AYD received a PhD scholarship from the German Academic Exchange Service (DAAD). Pfizer Inc., Karlsruhe, donated Vibramycin® capsules and matching placebos. Technical assistance by Daniel Tagoe, Kumasi, Ingeborg Albrecht, Frank Geisinger, Hamburg, and Karin Lemke, Bonn, is gratefully acknowledged. We are indebted to the inhabitants of the villages Nyarduam, Asamang and Akwanhyiam, Central Region, Ghana, for taking part in the study.
Author contributions
AH designed the study, coordinated the compiling of the data including basic statistical analysis and wrote the first draft and the final version of the manuscript; SS preserved the nodules, performed immunohistology, was the second analyser of the histological sections and helped with the basic statistical analysis and with manuscript finalisation; MB and SM recruited the patients, MB distributed the tablets, examined the patients before and during treatment and at follow up, treated the nodulectomy wounds; KP designed the PCR technique and supervised PCR analysis; YMD, AYD and JAL performed the information campaigns in the villages, recorded clinical and treatment data and organized the nodulectomies; PK performed the nodulectomies; CB and NB produced the antisera used for immunohistology; NB helped with manuscript finalisation; AA and AYD performed the PCR studies; JAL and LB did skin snipping and determined the skin mf; OA co-designed the study, interacted with the local authorities (ethics committees, health districts etc.) and supervised the local data collection; DWB was the first analyser of the histological sections, compiled the histological data and wrote major parts of the manuscript. RF performed the regression analyses and advised in all the other statistical procedures.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- APR
O. volvulus aspartic protease
- GMI
Geometric mean index
- IVM
Ivermectin
- Mf
Microfilaria(e)
- DiWsp
Dirofilaria immitis Wolbachia surface protein
Footnotes
Achim Hoerauf and Sabine Specht contributed equally to the study.
The trial registration number is ISRCTN 71141922 (Current Controlled Trials).
An erratum to this article is available at http://dx.doi.org/10.1007/s00430-007-0072-z.
References
- 1.WHO Onchocerciasis and its control. WHO Tech Rep Ser. 1995;852:1–103. [PubMed] [Google Scholar]
- 2.Habbema JDF, Plaisier AP, van Oortmarssen GJ, Remme J. Prospective evaluation of onchocerciasis control strategies. Acta Leiden. 1990;59:387–398. [PubMed] [Google Scholar]
- 3.Little MP, Breitling LP, Basanez MG, Alley ES, Boatin BA. Association between microfilarial load and excess mortality in onchocerciasis: an epidemiological study. Lancet. 2004;363:1514–1521. doi: 10.1016/S0140-6736(04)16151-5. [DOI] [PubMed] [Google Scholar]
- 4.Basanez MG, Pion SD, Churcher TS, Breitling LP, Little MP, Boussinesq M. River blindness: a success story under threat? PLoS Med. 2006;3:e371. doi: 10.1371/journal.pmed.0030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boatin BA, Richards FO., Jr Control of onchocerciasis. Adv Parasitol. 2006;61:349–394. doi: 10.1016/S0065-308X(05)61009-3. [DOI] [PubMed] [Google Scholar]
- 6.Awadzi K, Attah SK, Addy ET, Opoku NO, Quartey BT. The effects of high-dose ivermectin regimens on Onchocerca volvulus in onchocerciasis patients. Trans R Soc Trop Med Hyg. 1999;93:189–194. doi: 10.1016/S0035-9203(99)90305-X. [DOI] [PubMed] [Google Scholar]
- 7.Kläger SL, Whitworth JA, Downham MD. Viability and fertility of adult Onchocerca volvulus after 6 years of treatment with ivermectin. Trop Med Int Health. 1996;1:581–589. doi: 10.1111/j.1365-3156.1996.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 8.Kläger S, Whitworth JA, Post RJ, Chavasse DC, Downham MD. How long do the effects of ivermectin on adult Onchocerca volvulus persist? Trop Med Parasitol. 1993;44:305–310. [PubMed] [Google Scholar]
- 9.Duke BO. Evidence for macrofilaricidal activity of ivermectin against female Onchocerca volvulus: further analysis of a clinical trial in the Republic of Cameroon indicating two distinct killing mechanisms. Parasitology. 2005;130:447–453. doi: 10.1017/S0031182004006766. [DOI] [PubMed] [Google Scholar]
- 10.Cupp EW, Cupp MS. Short report: impact of ivermectin community-level treatments on elimination of adult Onchocerca volvulus when individuals receive multiple treatments per year. Am J Trop Med Hyg. 2005;73:1159–1161. [PubMed] [Google Scholar]
- 11.Dadzie Y, Neira M, Hopkins D. Final report of the Conference on the Eradicability of Onchocerciasis. Filaria J. 2003;2:2. doi: 10.1186/1475-2883-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awadzi K, Boakye DA, Edwards G, Opoku NO, Attah SK, Osei-Atweneboana MY, Lazdins-Helds JK, Ardrey AE, Addy ET, Quartey BT, Ahmed K, Boatin BA, Soumbey-Alley EW. An investigation of persistent microfilaridermias despite multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann Trop Med Parasitol. 2004;98:231–249. doi: 10.1179/000349804225003253. [DOI] [PubMed] [Google Scholar]
- 13.Awadzi K, Attah SK, Addy ET, Opoku NO, Quartey BT, Lazdins-Helds JK, Ahmed K, Boatin BA, Boakye DA, Edwards G. Thirty-month follow-up of sub-optimal responders to multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann Trop Med Parasitol. 2004;98:359–370. doi: 10.1179/000349804225003442. [DOI] [PubMed] [Google Scholar]
- 14.Osei-Atweneboana MY, Eng JK, Boakye DA, Gyapong JO, Prichard RK. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: a two-phase epidemiological study. Lancet. 2007;369:2021–2029. doi: 10.1016/S0140-6736(07)60942-8. [DOI] [PubMed] [Google Scholar]
- 15.Hoerauf A, Nissen-Pähle K, Schmetz C, Henkle-Dührsen K, Blaxter ML, Büttner DW, Gallin MY, Al-Qaoud KM, Lucius R, Fleischer B. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J Clin Invest. 1999;103:11–18. doi: 10.1172/JCI4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoerauf A, Volkmann L, Hamelmann C, Adjei O, Autenrieth IB, Fleischer B, Büttner DW. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet. 2000;355:1242–1243. doi: 10.1016/S0140-6736(00)02095-X. [DOI] [PubMed] [Google Scholar]
- 17.Hoerauf A, Mand S, Adjei O, Fleischer B, Büttner DW. Depletion of Wolbachia endobacteria in Onchocerca volvulus by doxycycline and microfilaridermia after ivermectin treatment. Lancet. 2001;357:1415–1416. doi: 10.1016/S0140-6736(00)04581-5. [DOI] [PubMed] [Google Scholar]
- 18.Hoerauf A, Mand S, Volkmann L, Büttner M, Marfo-Debrekyei Y, Taylor M, Adjei O, Büttner DW. Doxycycline in the treatment of human onchocerciasis: kinetics of Wolbachia endobacteria reduction and of inhibition of embryogenesis in female Onchocerca worms. Microbes Infect. 2003;5:261–273. doi: 10.1016/S1286-4579(03)00026-1. [DOI] [PubMed] [Google Scholar]
- 19.Taylor MJ, Makunde WH, McGarry HF, Turner JD, Mand S, Hoerauf A. Macrofilaricidal activity following doxycycline treatment of Wuchereria bancrofti: a double-blind randomised controlled trial. Lancet. 2005;365:2116–2121. doi: 10.1016/S0140-6736(05)66591-9. [DOI] [PubMed] [Google Scholar]
- 20.Debrah AY, Mand S, Specht S, Marfo-Debrekyei Y, Batsa L, Pfarr K, Larbi J, Lawson B, Taylor M, Adjei O, Hoerauf A. Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis. PLOS Pathogens. 2006;2(9):e92. doi: 10.1371/journal.ppat.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutin K, Kruppa TF, Brenya R, Garms R. Efficiency of Simulium sanctipauli as a vector of Onchocerca volvulus in the forest zone of Ghana. Med Vet Entomol. 2004;18:167–173. doi: 10.1111/j.0269-283X.2004.00496.x. [DOI] [PubMed] [Google Scholar]
- 22.Albiez EJ, Büttner DW, Duke BO. Diagnosis and extirpation of nodules in human onchocerciasis. Trop Med Parasitol. 1988;39:331–346. [PubMed] [Google Scholar]
- 23.Büttner DW, Albiez EJ, von Essen J, Erichsen J. Histological examination of adult Onchocerca volvulus and comparison with the collagenase technique. Trop Med Parasitol. 1988;39:390–417. [PubMed] [Google Scholar]
- 24.Awadzi K, Addy ET, Opoku NO, Plenge-Bönig A, Büttner DW. The chemotherapy of onchocerciasis XX: ivermectin in combination with albendazole. Trop Med Parasitol. 1995;46:213–220. [PubMed] [Google Scholar]
- 25.Awadzi K, Hero M, Opoku NO, Addy ET, Büttner DW, Ginger CD. The chemotherapy of onchocerciasis XVIII. Aspects of treatment with suramin. Trop Med Parasitol. 1995;46:19–26. [PubMed] [Google Scholar]
- 26.Bazzocchi C, Jamnongluk W, O’Neill SL, Anderson TJ, Genchi C, Bandi C. wsp gene sequences from the Wolbachia of filarial nematodes. Curr Microbiol. 2000;41:96–100. doi: 10.1007/s002840010100. [DOI] [PubMed] [Google Scholar]
- 27.Jolodar A, Fischer P, Büttner DW, Miller DJ, Schmetz C, Brattig NW. Onchocerca volvulus: expression and immunolocalization of a nematode cathepsin D-like lysosomal aspartic protease. Exp Parasitol. 2004;107:145–156. doi: 10.1016/j.exppara.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Gardon J, Boussinesq M, Kamgno J, Gardon-Wendel N, Demanga N, Duke BO. Effects of standard and high doses of ivermectin on adult worms of Onchocerca volvulus: a randomised controlled trial. Lancet. 2002;360:203–210. doi: 10.1016/S0140-6736(02)09456-4. [DOI] [PubMed] [Google Scholar]
- 29.Duke BO, Marty AM, Peett DL, Gardo J, Pion SD, Kamgno J, Boussinesq M. Neoplastic change in Onchocerca volvulus and its relation to ivermectin treatment. Parasitology. 2002;125:431–444. doi: 10.1017/S0031182002002305. [DOI] [PubMed] [Google Scholar]
- 30.Albiez EJ, Walter G, Kaiser A, Ranque P, Newland HS, White AT, Greene BM, Taylor HR, Büttner DW. Histological examination of onchocercomata after therapy with ivermectin. Trop Med Parasitol. 1988;39:93–99. [PubMed] [Google Scholar]
- 31.Duke BO, Zea-Flores G, Munoz B. The embryogenesis of Onchocerca volvulus over the first year after a single dose of ivermectin. Trop Med Parasitol. 1991;42:175–180. [PubMed] [Google Scholar]
- 32.Gilbert J, Nfon CK, Makepeace BL, Njongmeta LM, Hastings IM, Pfarr KM, Renz A, Tanya VN, Trees AJ. Antibiotic chemotherapy of onchocerciasis: in a bovine model, killing of adult parasites requires a sustained depletion of endosymbiotic bacteria (Wolbachia species) J Infect Dis. 2005;192:1483–1493. doi: 10.1086/462426. [DOI] [PubMed] [Google Scholar]
- 33.Meijerink J, Mandigers C, van de Locht L, Tonnissen E, Goodsaid F, Raemaekers J. A novel method to compensate for different amplification efficiencies between patient DNA samples in quantitative real-time PCR. J Mol Diagn. 2001;3:55–61. doi: 10.1016/S1525-1578(10)60652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remme J, Ba O, Dadzie KY, Karam M. A force-of-infection model for onchocerciasis and its applications in the epidemiological evaluation of the Onchocerciasis Control Programme in the Volta River basin area. Bull World Health Organ. 1986;64:667–681. [PMC free article] [PubMed] [Google Scholar]
- 35.Debrah AY, Mand S, Marfo-Debrekyei Y, Larbi J, Adjei O, Hoerauf A. Assessment of microfilarial loads in the skin of onchocerciasis patients after treatment with different regimens of doxycycline plus ivermectin. Filaria J. 2006;5:1. doi: 10.1186/1475-2883-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Awadzi K. Clinical picture and outcome of serious adverse events in the treatment of onchocerciasis. Filaria J. 2003;2(Suppl 1):S6. doi: 10.1186/1475-2883-2-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kipp W, Bamhuhiiga J, Rubaale T, Büttner DW. Adverse reactions to ivermectin treatment in Simulium neavei-transmitted onchocerciasis. Am J Trop Med Hyg. 2003;69:621–623. [PubMed] [Google Scholar]
- 38.Carey V, Zeger SL, Diggle P. Modelling multivariate binary data with alternating logistic regressions. Biometrika. 1993;80:517–526. doi: 10.1093/biomet/80.3.517. [DOI] [Google Scholar]
- 39.SAS Institute Inc. SAS OnlineDoc® 9.1.2. Cary: SAS Institute Inc.; 2004. [Google Scholar]
- 40.Awadzi K, Dadzie KY, Schulz-Key H, Haddock DR, Gilles HM, Aziz MA. The chemotherapy of onchocerciasis X. An assessment of four single dose treatment regimes of MK-933 (ivermectin) in human onchocerciasis. Ann Trop Med Parasitol. 1985;79:63–78. [PubMed] [Google Scholar]
- 41.WHO . Drugs used in parasitic diseases. 2nd edn. Geneva: WHO; 1995. [Google Scholar]
- 42.Franz M. The morphology of adult Onchocerca volvulus based on electron microscopy. Trop Med Parasitol. 1988;39:359–366. [PubMed] [Google Scholar]
- 43.Schulz-Key H. The collagenase technique: how to isolate and examine adult Onchocerca volvulus for the evaluation of drug effects. Trop Med Parasitol. 1988;39:423–440. [PubMed] [Google Scholar]
- 44.Duke BO. Observations and reflections on the immature stages of Onchocerca volvulus in the human host. Ann Trop Med Parasitol. 1991;85:103–110. doi: 10.1080/00034983.1991.11812536. [DOI] [PubMed] [Google Scholar]
- 45.Wildenburg G, Henkle-Dührsen K. Onchocerca volvulus: immunolocalization of the extracellular CuZn superoxide dismutase using antibodies raised against a 15-mer epitope of this enzyme. Exp Parasitol. 1999;91:1–6. doi: 10.1006/expr.1999.4352. [DOI] [PubMed] [Google Scholar]
- 46.WHO Meeting of the international task force for disease eradication. Wkly Epidem Rec. 2007;82:197–208. [PubMed] [Google Scholar]
- 47.Hoerauf A, Pfarr K. Wolbachia Endosymbionts: an achilles’ heel of filarial nematodes. In: Hoerauf A, Rao R, editors. Wolbachia: a bug’s life in another bug. Basel: Karger; 2007. pp. 31–51. [Google Scholar]