Abstract
Growth of the green algae Chlamydomonas reinhardtii and Chlorella sp. in batch cultures was investigated in a novel gas-tight photobioreactor, in which CO2, H2, and N2 were titrated into the gas phase to control medium pH, dissolved oxygen partial pressure, and headspace pressure, respectively. The exit gas from the reactor was circulated through a loop of tubing and re-introduced into the culture. CO2 uptake was estimated from the addition of CO2 as acidic titrant and O2 evolution was estimated from titration by H2, which was used to reduce O2 over a Pd catalyst. The photosynthetic quotient, PQ, was estimated as the ratio between O2 evolution and CO2 up-take rates. NH4+, NO2−, or NO3− was the final cell density limiting nutrient. Cultures of both algae were, in general, characterised by a nitrogen sufficient growth phase followed by a nitrogen depleted phase in which starch was the major product. The estimated PQ values were dependent on the level of oxidation of the nitrogen source. The PQ was 1 with NH4+ as the nitrogen source and 1.3 when NO3− was the nitrogen source. In cultures grown on all nitrogen sources, the PQ value approached 1 when the nitrogen source was depleted and starch synthesis became dominant, to further increase towards 1.3 over a period of 3–4 days. This latter increase in PQ, which was indicative of production of reduced compounds like lipids, correlated with a simultaneous increase in the degree of reduction of the biomass. When using the titrations of CO2 and H2 into the reactor headspace to estimate the up-take of CO2, the production of O2, and the PQ, the rate of biomass production could be followed, the stoichiometrical composition of the produced algal biomass could be estimated, and different growth phases could be identified.
Key words: Chlamydomonas reinhardtii, Chlorella sp., photosynthetic quotient, lumostat, nitrogen limitation
Introduction
When microorganisms are grown in batch cultures, growth progresses through several phases, conventionally described as a lag phase, an exponential growth phase, and a stationary phase. However, this model is often too simplistic to give a reasonable description of batch growth in cultures of phototrophic algae and cyanobacteria. Growth is seldom exponential since increased biomass concentrations result in self-shading and decreased specific growth rates (Eriksen et al. 1996), and increased competition for light will often cause phototrophic microorganisms to increase their pigment contents (Richardson et al. 1983) and thereby change their composition.
Nutrient depletion, which is not necessarily followed by an immediate entry into a stationary phase, may also result in changes in biomass composition. During nutrient depleted growth phases, starch and other carbon and energy storage compounds may accumulate and constitute a major part of the biomass production in green algae (Rigano et al. 2000; Ball 2002; Zhila et al. 2005). In nitrogen depleted and carbon sufficient bacteria, accumulation of carbon and energy storage compounds may account for all the produced biomass (Stenholm et al. 1998). Depletion of nutrients, in particular the nitrogen source, also results in break-down of the photosynthetic apparatus (Coleman et al. 1988), including the photosynthetic pigments (Eriksen and Iversen 1995).
As the composition of the produced biomass changes, so will the specific rates of substrate uptake and product formation. In phototrophs, CO2 and O2 are quantitatively the most important substrate and product, respectively. The ratio between O2 evolution rate and CO2 uptake rate (the photosynthetic quotient, PQ) depends on the composition of the produced biomass and the substrates that are utilised. Especially oxidised nitrogen sources, which must be reduced before they are incorporated into the biomass, affect the PQ. When biomass composition equals the Redfield ratio, CH2O(NH3)0.15 (Redfield et al. 1963), and NO3− is the nitrogen source
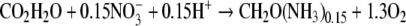 |
1 |
a PQ of 1.3 will be expected. With the lesser reduced NO2− as nitrogen source, the expected PQ is 1.2. Growth on NH4+,
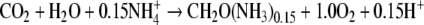 |
2 |
which is of similar degree of reduction as nitrogen in the biomass, should result in a PQ of 1. Changes in PQ are therefore expected if especially NO3− or NO2− are depleted and reduction of nitrogen no longer takes place.
If the composition of the produced biomass is altered, the PQ is likely to change since different molecules, which are incorporated into the biomass, are not equally reduced. The PQ can be predicted from the degree of reduction of the biomass, γx (see Roels 1980), which describes the number of electrons per carbon atom available for oxidation reactions. When no organic products in addition to biomass are produced, and NH4+ is the nitrogen source (Eq. 2), the molar ratio between oxygen production and carbon fixation, 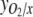 can be found from a degree of reduction balance
can be found from a degree of reduction balance
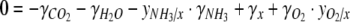 |
3 |
where 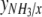 is the molar ratio between ammonium uptake and carbon fixation. The degrees of reduction of CO2, H2O and NH3,
is the molar ratio between ammonium uptake and carbon fixation. The degrees of reduction of CO2, H2O and NH3, 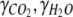 , and
, and 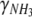 , respectively, all equal zero, and the degree of reduction of O2,
, respectively, all equal zero, and the degree of reduction of O2, 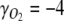 (Roels 1980). For the biomass, x=4 if carbohydrates are the only compounds formed, while synthesis of proteins and lipids results in higher x values. When no organic products are secreted from the cells, all CO2 taken up is incorporated into the biomass, and the PQ is equal to
(Roels 1980). For the biomass, x=4 if carbohydrates are the only compounds formed, while synthesis of proteins and lipids results in higher x values. When no organic products are secreted from the cells, all CO2 taken up is incorporated into the biomass, and the PQ is equal to 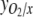 . Equation 3 can then be rewritten
. Equation 3 can then be rewritten
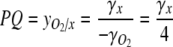 |
4 |
Equation 4 predicts that synthesis of carbohydrates results in a PQ=1, while synthesis of more reduced cell components results in higher PQ values. The PQ in cultures using NO2− or NO3− as nitrogen source can also be predicted by Eq. 4, although x in cultures using these nitrogen sources will be assigned slightly higher values (see Roels 1980).
In this paper, we have investigated the stoichiometry of gas exchange and biomass production in batch cultures of the green algae Chlamydomonas reinhardtii and Chlorella sp. grown on reduced and oxidised nitrogen sources, NH4+, NO2−, and NO3−, respectively. All growth experiments were carried out in a novel gas-tight photobioreactor, in which CO2 uptake was estimated from the addition of CO2 as acidic titrant to maintain constant pH, and O2 evolution was estimated from titration with H2, which was used to reduce O2 over a Pd catalyst and maintain constant dissolved oxygen partial pressure.
Materials and methods
Strain and growth medium
Chlamydomonas reinhardtii UTEX2337 was obtained from the Culture Collection of Algae at The University of Texas at Austin, USA. Chlorella sp. was a gift from Dr. Šetlíc, Department of Autotrophic Microorganisms, Třeboň, Czech Republic. Both strains were maintained by sequential transfer into photoautotrophic batch cultures grown at room temperature in the growth medium for Chlamydomonas described by Starr (1978). For growth experiments, NH4+, NO2−, or NO3− was included in the growth medium as the final cell density limiting substrate.
Bioreactor
Batch cultures were grown in a 3.0 l Applikon BTS05 bioreactor (Applikon, The Netherlands) containing 2.1 L of culture. The bioreactor consisted of a cylindrical glass jar with a diameter of 13 cm fitted with a top plate of stainless steel. The bioreactor was placed in a cabinet of mirrors equipped with 12 Osram 18W/20 cool white fluorescent tubes. The temperature was maintained at 25°C and the culture was stirred at 500 rpm by a Rushton turbine and aerated with 1.4 L min−1 of an aeration gas which was circulated through a closed loop of gas tight polypropylene tubing (Figure 1). The exit gas from the reactor was passed through a condenser at 4°C, a 0.25 L foam trap, a 50-ml glass column (inner diameter=2.1 cm) containing 25 g of a palladium catalyst (Pd coated porous allumina pellets of approximately 1×3 mm supplied by Haldor Topsoe, Denmark) with a void fraction of 0.8 L, and a membrane compressor with a gas tight viton membrane, before it was re-introduced into the culture through a sparger placed below the impeller. An open-ended tubing submerged in 15 cm of a saturated NaCl solution served as a safety device against build-up of excess pressure in the headspace.
Figure 1.
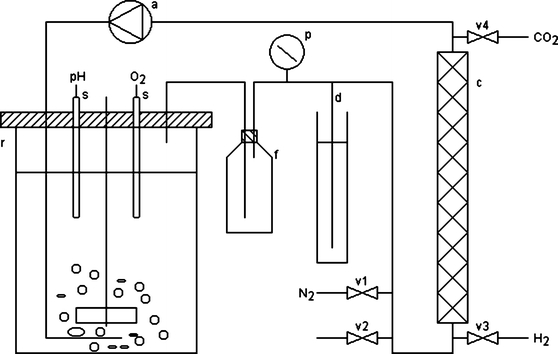
Gas-tight photobioreactor, r reactor vessel, s sensors for pH and dissolved oxygen tension, f foam trap, p pressure gauge, d open ended tubing submerged in 15 cm of water, c catalytic column containing Pd catalyst, a air compressor, v1–v4 solenoid valves controlling addition of N2, release of excess pressure, addition of H2, and addition of CO2, respectively.
Headspace pressure
The pressure in the gas circulation loop was measured by a differential pressure transducer (Honeywell 26PC) and maintained at 1.0–1.6 kPa above ambient pressure. Fixed amounts of N2 (129 ± 5 μmol) could be added to increase the pressure in the gas loop from a pressurised N2 supply by opening a solenoid valve (Sirai z0300A, Italy) for 0.1 s. The pressure of the N2 supply was 5.4 kPa above ambient pressure. Pressure could be released from the gas loop by a second solenoid valve. When opened for 0.1 s, the headspace pressure decreased by 0.1 kPa, and the amount of gas in the gas loop was lowered by 62 ± 3 μmol.
Dissolved oxygen tension and O2 production
Dissolved oxygen tension in the growth medium was measured by an autoclavable oxygen electrode (Applisens), and maintained at 80% air saturation by reduction of O2 in the gas loop on a Pd surface (see e.g., Nowakowski et al. 2002). Fixed amounts of H2 (223 ± 13 μmol) were delivered through a third solenoid valve (opened for 0.1 s) located at a position just before the gas entered the Pd catalyst column (Figure 1). At the surface of the Pd catalyst, O2 was reduced to H2O by the consumption of 2 moles of H2 per mol O2. The pressure of the H2 supply was 3.9 kPa above ambient pressure, and the H2 additions were regulated by a dose-pause controller. The minimal pause between two H2 additions was 10 s to allow time for the oxygen depleted aeration gas to dissipate oxygen from the liquid phase before a new dose of H2 was added.
The number of additions of H2 to the gas circulation loop was recorded by a computer and the molar amount of photosynthetically produced O2, 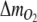 in time interval Δt was estimated as
in time interval Δt was estimated as
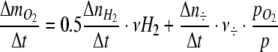 |
5 |
where 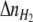 and
and 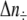 are the number of H2 additions and pressure releases, respectively, in time interval Δt,
are the number of H2 additions and pressure releases, respectively, in time interval Δt, 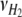 and
and 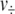 are moles of H2 added or gas molecules released from the headspace per H2 addition or pressure release, respectively, and
are moles of H2 added or gas molecules released from the headspace per H2 addition or pressure release, respectively, and 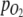 and p are oxygen partial pressure and total pressure in headspace, respectively. It was assumed that O2 in the headspace and dissolved O2 in the culture were at equilibrium, and that
and p are oxygen partial pressure and total pressure in headspace, respectively. It was assumed that O2 in the headspace and dissolved O2 in the culture were at equilibrium, and that 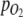 measured in the medium therefore also represented
measured in the medium therefore also represented 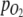 in the gas loop. The total amount of O2 produced was found by integration of Eq. 5.
in the gas loop. The total amount of O2 produced was found by integration of Eq. 5.
pH control and CO2 uptake
Culture pH was measured by an autoclavable pH electrode (Mettler Toledo). When pH increased above pH 7.5, a pulse of 101 ± 3 μmol of CO2 was added as acidic titrant to the gas loop by opening a fourth solenoid valve for 0.1 s. In the growth medium, CO2 dissolved partly as carbonic acid, resulting in a decrease in pH. Because CO2 adsorbs reversibly onto Pd surfaces (see, e.g., Liu et al. 2001), the CO2 addition valve was placed after the Pd catalytic column (Figure 1). The minimal pause between successive CO2 additions was 30 s.
Uptake of CO2 was the major process in the culture that affected medium pH, and the CO2 uptake was estimated from the CO2 addition rate. However, uptake of the nitrogen source also affected medium pH (see Eqs. 1 and 2). With NO3− or NO2− as nitrogen sources, more CO2 than that removed by the cells had to be added to maintain constant pH, since reduction of 1 mol of NO3− or NO2− consumes 1 mol of protons (Eq. 1). These protons had to be replaced by dissociation of carbonic acid. The concentration of HCO3− in the growth medium therefore increased in proportion to the decrease in the concentration of the nitrogen source, and this increase also increased other pools of inorganic carbon in the photobioreactor.
With NH4+ as nitrogen source, 1 mol of protons was excreted into the growth medium for each mol of NH4+ taken up by the cells (Eq. 2), and less CO2 than that removed by the cells were needed to maintain constant pH. The concentration of HCO3− therefore decreased in proportion to the decrease in concentration of NH4+, and therefore other pools of inorganic carbon in the photobioreactor also decreased when NH4+ was the nitrogen source.
The molar uptake of CO2, 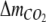 per time was also corrected for losses of CO2 via ventilation of excess pressure, and calculated as
per time was also corrected for losses of CO2 via ventilation of excess pressure, and calculated as
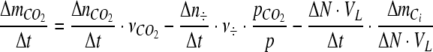 |
6 |
where 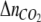 is the number of CO2 additions,
is the number of CO2 additions, 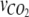 is the moles of CO2 added per addition,
is the moles of CO2 added per addition, 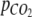 is CO2 partial pressure, ΔN is the change in concentration of the nitrogen source, VL is the volume of the growth medium, and
is CO2 partial pressure, ΔN is the change in concentration of the nitrogen source, VL is the volume of the growth medium, and 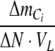 is the relationship between the overall change in total inorganic carbon content and nitrogen content in the photobioreactor. Calculations of
is the relationship between the overall change in total inorganic carbon content and nitrogen content in the photobioreactor. Calculations of 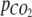 and
and 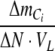 are described in Appendix A, and ΔN was estimated from Eq. 13 (see below). The photosynthetic quotient, PQ was estimated by dividing Eq. 5 with Eq. 6
are described in Appendix A, and ΔN was estimated from Eq. 13 (see below). The photosynthetic quotient, PQ was estimated by dividing Eq. 5 with Eq. 6
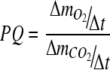 |
7 |
Since additions of CO2 and H2 as well as ventilation of excess pressure was not continuous functions but discrete events, a relatively large Δt = 4 h was used for estimation of the PQ values.
Regulation of light intensity
The light intensity on the reactor, measured as the average light intensity at 6 positions on the reactor wall and at the bottom by a Licor LI-250 Light Meter fitted with a quantum sensor, could be controlled between 0 and 220 μmol photons m−2 s−1 by a dimming transformer. The dimming transformer was regulated by a 0–10 V analog signal controlled by the computer. The light intensity was automatically changed during batch cultivations in order to maximise the CO2 addition rate and allow the culture to grow exponentially using a modified version of the approach described by Eriksen et al. (1996). The time taken for 15 CO2 pulses to be added to the gas loop after a change in light intensity was compared to the time taken for the preceding 15 CO2 additions. If the last 15 pulses were added during a shorter time interval than the preceding 15 pulses, the light intensity was changed by 1–8.5 μmol photons m−2 s−1 in the same direction as the previous change (the largest changes occurred at the lowest light intensities because of non-linearity between dimming transformer output and light intensity). If the time taken for the 15 CO2 pulses to be added had increased, the light intensity was changed by 1–8.5 μmol photons m−2 s−1 in the direction opposite to the previous change. By this approach, the light intensity was automatically maintained at the optimal intensity resulting in the fastest CO2 consumption rate, and was increased along with the increase in cell density.
Measurements of biomass, starch, chlorophyll a, and biomass composition
The concentration of biomass was measured spectrophotometrically at 750 nm (OD750) at which wavelength there was no absorbance from pigments. If necessary, the samples were diluted to OD750 values below 0.3. Biomass dry weight (DW) of samples was measured after filtration onto pre-dried, pre-weighed 0.22-μm Millipore filters and dryed at 85°C overnight. The relationship between OD750 and biomass dry weight was rectilinear at all cell densities.
Starch was extracted from C. reinhardtii in 0.05 M acetate buffer, pH 5, after disruption of the cells in a B.Braunn Mikro-Dismembrator S rotating at 2,500 rpm for 5 min. The cell homogenate was diluted in the same buffer in order to obtain a concentration of glucose equivalents not higher than 320 mg L−1. The starch was hydrolysed to glucose by mixing 500 μL of the cell homogenate with 60 μL of a mixture containing 3 amylolytic enzymes (8 KNU of α-amylase, BAN240L, 5 AGU of glucoamylase, AMG 300L, and 9 PUN of pullunase, Promozyme 400L) from Novozymes, Denmark (KNU, AGU, and PUN are enzyme activity units used by Novozymes). The mixture was incubated for 1 h at 65°C followed by 5 min of centrifugation at 8,050 g. The starch content was measured as glucose equivalents, after subtraction of the glucose content in cell extracts not treated with amylolytic enzymes.
Chlorophyll a was measured spectrophotometrically after 24 h of dark extraction in 80% ethanol, followed by 5 min of centrifugation at 8,050 g using an extinction coefficient of 83 L g−1 cm−1 (Arvola 1981).
The composition of the major elements, carbon, hydrogen, nitrogen, and oxygen in samples of C. reinhardtii harvested from the photobioreactor was analysed on a Carlo Erba elemental analyser.
Measurements of NH4+, NO2−, and NO3−
Concentrations of NH4+, NO2−, and NO3− in supernatants of samples removed from the cultures were measured using a Technicon Traacs 800 auto-analyser. NH4+ was reacted with basic hypochlorite and salicylate to form a green indophenol complex, which could be measured at 660 nm. NO2− was reacted with sulphanilamide and N-(1-naphtyl)ethylenediamine dihydrochloride and the product was recorded at 520 nm. NO3− was reduced to NO2− over a Cd column, and otherwise detected as NO2−.
Modelling growth in nitrogen limited batch culture
In order to describe observed changes in biomass composition during batch cultivations and the consumption of the nitrogen sources, a kinetic model describing growth of Chlamydomonas reinhardtii and Chlorella sp. in nitrogen limited, light sufficient batch cultures, was developed. The specific uptake rate of the nitrogen source, qN (N-mol C-mol−1 h−1) per unit of non-starch biomass was modelled using saturation kinetics
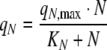 |
8 |
where qN,max is the maximal specific uptake rate of the nitrogen source, N is the concentration of the nitrogen source in the growth medium (M), and KN is the affinity constant for the nitrogen source (M). The specific rate of fixation of CO2 into the biomass, qC (C-mol C-mol−1 h−1) per unit of non-starch biomass in light sufficient cells, was negatively affected by the starch content per unit of biomass, S (C-mol C-mol−1) and was modelled using a logistic relationship
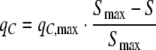 |
9 |
where qC,max is the maximal specific carbon uptake rate per unit of non-starch biomass and Smax is the maximum specific starch concentration per unit of biomass, respectively. The specific growth rate of the non-starch biomass, μ (h−1) is controlled by qN
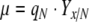 |
10 |
where Yx/N is the molar ratio between carbon and nitrogen in the starch-free biomass (C-mol N-mol−1) and accumulation of non-starch biomass, x (C-mol L−1) was described by
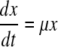 |
11 |
Accumulation of starch in the culture, s (C-mol L−1) is described as the difference between the total carbon uptake rate and the production of non-starch biomass
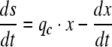 |
12 |
and the decrease in concentration of the nitrogen source depends on the production of non-starch biomass
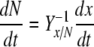 |
13 |
In order to describe x, s, and N during batch cultures, Eqs. 11–13 were solved numerically. From Eq. 13, the amount of consumed nitrogen, ΔN was estimated and used in the calculation of the CO2 uptake by Eq. 6.
Results
Chlamydomonas reinhardtii, NH4+ as nitrogen source
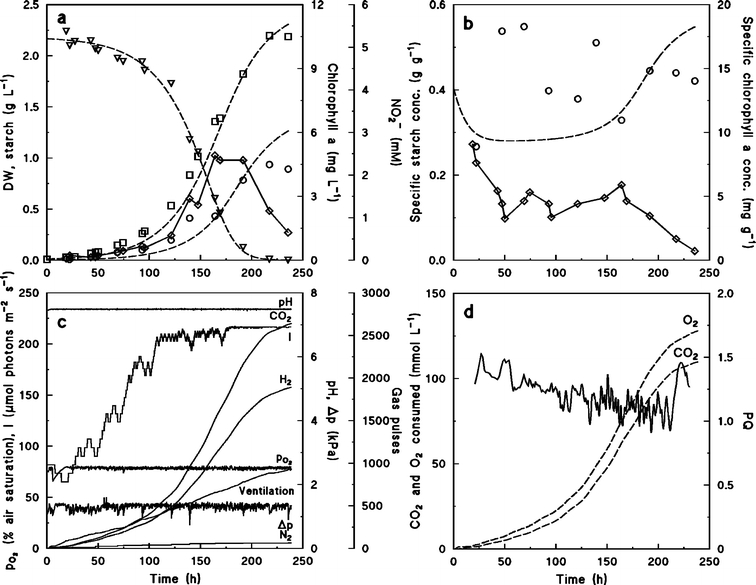
Figure 2 shows a batch culture of Chlamydomonas reinhardtii grown on NH4+ as the nitrogen source. Growth proceeded exponentially for approximately 100 h until NH4+ was depleted (Figure 2a) and the culture became nitrogen limited. Nitrogen limitation was clearly indicated also by the decrease of total and specific chlorophyll a concentration in the culture (Figure 2b). However, the concentration of biomass dry weight was still increasing for the following 100 h despite nitrogen limitation.
Figure 2.
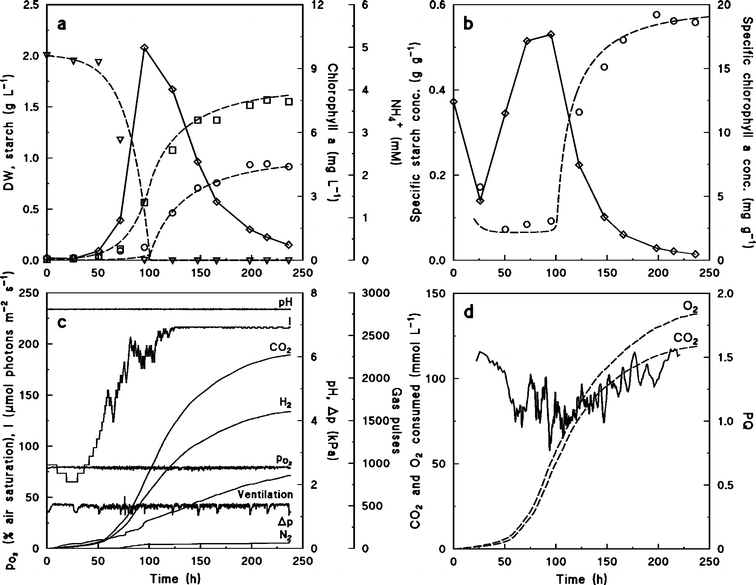
Batch culture of Chlamydomonas reinhardtii grown on NH4+ as nitrogen source. (a) Concentration of biomass dry weight (□), starch (○), NH4+ (▿), and chlorophyll a (⋄). Symbols are experimental values, broken lines are calculated from Eqs. 11–13. Parameters used in Eqs. 11–13, qN,max=0.01 mol C-mol−1 h−1, KN=0.05 mM, qC,max=0.06 C-mol C-mol−1 h−1, Smax=0.6 C-mol C-mol−1, Yx/N=5 C-mol mol–1. (b) Specific concentrations of chlorophyll a (⋄) and starch (○). Symbols are experimental values, broken line is calculated from Eqs. 11 and 12. (c) On-line measurements of pH, dissolved oxygen tension, headspace pressure, and number of pressure releases and additions of CO2, H2, and N2. (d) Estimated amounts of produced O2 and assimilated CO2 from Eqs. 5 and 6, respectively (broken lines), and PQ values estimated from Eq. 7 (solid line).
Accumulation of starch in the cells accounted for the entire increase in biomass concentration during the NH4+ depleted growth phase. Before NH4+ was depleted, the specific starch concentration in the cells was 0.1 g g−1 but increased and reached 0.6 g g−1 (Figure 2b). In the same period, the specific nitrogen concentration decreased, partly due to the dilution effect caused by accumulation of the starch, but nitrogen was also lost from the biomass, indicating that lysis of some of the cells occurred during the nitrogen limited phase (Table 1). Additionally, foam development on the surface of the culture indicated accumulation of macromolecular lysis products in the growth medium.
Table 1.
Biomass composition and PQ values during batch cultures of Chlamydomonas reinhardtii grown on NH4+ as nitrogen source
| t (h) | x (g L−1) | Biomass composition | x | Nx (mM) | Δt (h) | Biomass production Δx |
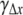 |
Calculated PQ | Measured PQa |
|---|---|---|---|---|---|---|---|---|---|
| 45 | 0.14 | CH1.71O0.53N0.13 | 4.26 | 0.7 | |||||
| 77 | 1.08 | CH1.76O0.55N0.09 | 4.38 | 3.6 | 45–77 | CH1.77O0.55N0.09 | 4.40 | 1.10 | 1.13 |
| 97 | 1.65 | CH1.82O0.64N0.05 | 4.39 | 2.9 | 77–97 | CH1.93O0.82 | 4.30 | 1.07 | 1.01 |
| 144 | 1.86 | CH1.83O0.60N0.03 | 4.54 | 2.2 | 97–144 | CH1.88O0.42 | 5.04 | 1.26 | 1.16 |
| 161 | 2.20 | CH1.85O0.59N0.02 | 4.59 | 1.7 | 144–161 | CH1.96O0.57 | 4.82 | 1.21 | 1.36 |
Biomass dry weight, x and biomass composition were measured on samples withdrawn from the culture at time t, x is the degree of reduction of the biomass, and Nx is the total concentration of nitrogen in the biomass. Biomass production is the calculated composition of biomass produced in a time interval Δt, and  γΔx is the degree of reduction of the produced biomass. Calculated PQ values were obtained from Eq. 4. Measured PQ values were obtained using Eq. 7.
γΔx is the degree of reduction of the produced biomass. Calculated PQ values were obtained from Eq. 4. Measured PQ values were obtained using Eq. 7.
aThe measured PQ is from the experiment shown in Figure 2 while other data are from a replicate batch culture
In order to maintain constant pH and dissolved oxygen partial pressure during progress of the culture, CO2 and H2 were added to the closed gas loop during the nitrogen sufficient as well as during the nitrogen limited phase (Figure 2c). Gas also had to be released from the gas loop at a regular rate to avoid build up of pressure due to evaporation of water vapour from the culture medium and the catalytic column, and therefore parts of the O2 produced or CO2 added were lost via this route. Figure 2c also shows how the light intensity was automatically increased during the cultivation.
Figure 2d shows the integrated amounts of CO2 and O2 consumed or produced by the cells, respectively calculated from Eqs. 5 and 6, and the PQ value calculated from Eq. 7 using a Δt = 4 h. During the nitrogen sufficient phase, PQ = 1.08 ± 0.13. The PQ was hardly affected by nitrogen depletion, but increased gradually during nitrogen limitation. Since the C. reinhardtii biomass during the nitrogen sufficient phase was more reduced (x= 4.26–4.38; Table 1) than a biomass of composition corresponding to the Redfield ratio (x= 4), the PQ value was approximately 10% higher than predicted by Eq. 2.
Chlamydomonas reinhardtii, NO2− as nitrogen source
NO3− did not support growth of C. reinhardtii, but NO2− was used as nitrogen source by C. reinhardtii although at a slower rate compared to NH4+ (Figure 3a). In contrast to when grown on NH4+, starch accumulated during the nitrogen sufficient phase, and no major increase in the specific starch concentration was seen after NO2− was depleted (Figure 3b). Although the specific chlorophyll a concentration was also low during the NO2− sufficient growth phase (Figure 3b), a decrease of the specific chlorophyll a concentration was still observed as the concentration of NO2− approached depletion (Figure 4a,b). The PQ value for growth on NO2− (Figure 3d) was higher (PQ = 1.15 ± 0.10) than for growth on NH4+ (Figure 2d), as would be expected for a more oxidised substrate. After NO2− was depleted, growth as well as the addition rates of CO2 and H2 ceased.
Figure 3.
Batch culture of Chlamydomonas reinhardtii grown on NO2− as nitrogen source. (a) Concentration of biomass dry weight (□), starch (○), NO2− (▿), and chlorophyll a (⋄). Symbols are experimental values, broken lines are calculated from Eqs. 11–13. Parameters used in Eqs. 11–13, qN,max = 0.01 mol C-mol−1 h−1, KN = 2.2 mM, qC,max = 0.08 C-mol C-mol−1 h−1, Smax = 0.6 C-mol C-mol−1, Yx/N = 7 C-mol mol−1. b Specific concentrations of chlorophyll a (⋄) and starch (○). Symbols are experimental values, broken line is calculated from Eqs. 11 and 12. c On-line measurements of pH, dissolved oxygen tension, headspace pressure, and number of pressure releases and additions of CO2, H2, and N2. d Estimated amounts of produced O2 and assimilated CO2 from Eqs. 5 and 6, respectively (broken lines), and PQ values estimated from Eq. 7 (solid line).
Figure 4.
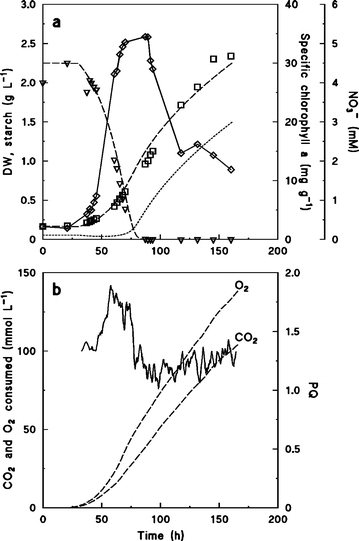
Batch culture of Chlorella sp. grown on NO3− as nitrogen source. (a) Concentration of biomass dry weight (□), NO3− (▿), and specific concentrations of chlorophyll a (⋄). Symbols are experimental values, broken lines are calculated from Eqs. 11–13. Predicted concentration of starch (Eq. 12) indicated by dotted line. Parameters used in Eqs. 11–13, qN,max = 0.01 mol C-mol−1 h−1, KN = 0.5 mM, qC,max = 0.05 C-mol C-mol−1 h−1, Smax = 1 C-mol C-mol−1, Yx/N = 5 C-mol mol−1. (b) Estimated amounts of produced O2 and assimilated CO2 from Eqs. 5 and 6, respectively (broken lines), and PQ values estimated from Eq. 7 (solid line).
Chlorella sp., NO3− as nitrogen source
Since C. reinhardtii did not grow on NO3−, growth experiments on this nitrogen source were carried out using Chlorella sp. (Figure 4). After a lag phase of approximately 30 h, Chlorella started to consume NO3− and the concentrations of biomass and chlorophyll a increased (Figure 4a). Starch measurements in this alga were highly irreproducible because its small cell size and solid cell wall resulted in incomplete cell disintegration and starch extraction. However, it was still clear that, after NO3− was depleted after 90 h, the biomass concentration continued to increase and more than 50% of the total biomass was produced during the nitrogen limited phase.
Estimation of PQ values from the titrations with CO2 and H2, showed that in the period when NO3− assimilated, PQ was high as would be expected from Eq. 1, but as soon as NO3− was depleted, and reducing power was no longer needed for reduction of nitrogen, the PQ value decreased.
When Chlorella was grown on NH4+ no major change in PQ values was observed when NH4+ was depleted (Table 2). Changes in chlorophyll a concentration were similar to the changes observed when NO3− was the nitrogen source, and the biomass concentration increased also during the nitrogen limited phase. Chlorella was also grown on NO2−, but in contrast to C. reinhardtii, the uptake rate of NO2− was of similar magnitude to the uptake rates of NH4+ and NO3−. Starch accumulated predominantly during the nitrogen limited phase, and a clear decrease in PQ as NO2− became depleted was observed (Table 2).
Table 2.
Ratio between PQ values during nitrogen sufficient and nitrogen depleted growth phases of different biomasses
| Biomass | PQN-sufficient/PQN-depleted | ||
|---|---|---|---|
| NH4+ | NO2− | NO3− | |
| CH2O(NH3)0.15a | 1.0 | 1.2 | 1.3 |
| CH1,8O0.5N0.2b | 1.05 | 1.35 | 1.45 |
| Chlamydomonas reinhardtii | 1.05 ± 0.04, n = 2 | n.d.c | n.d.d |
| Chlorella sp. | 0.95 ± 0.01, n = 2 | 1.14, n = 1 | 1.31 ± 0.11, n = 3 |
Experimental PQ values are means±standard deviations of values estimated either during the last 24 h before or the first 24 h after the nitrogen source was depleted. Number of replicate batch cultures indicated by n
aBiomass composition corresponding to the Redfield ratio (Redfield et al. 1963)
bBiomass composition corresponding to the average of microorganisms (Roels 1980)
cBiomass was not produced after depletion of NO2− in C. reinhardtii cultures
dC. reinhardtii did not grow on NO3−
Discussion
The batch cultures of Chlamydomonas reinhardtii and Chlorella sp. clearly demonstrated that growth of these species cannot simply be described by a lag phase, a growth phase, and a stationary phase. Large changes of biomass composition and kinetics of growth take place in response to changes in the availability of different nitrogen sources. Because the cultures were grown using the lumostat principle, where the light intensity on the surface of the culture automatically is maintained at the optimal intensity, which results in the maximal CO2 titration rate (Eriksen et al. 1996), growth was exponential throughout most of the nitrogen sufficient growth phase. Only at points close to the time the nitrogen source in the cultures was depleted did the light intensity reach its maximal value, after which the cultures might have begun to also experience light limitation.
Linear relationships were found between the amounts of CO2 and H2 added and the biomass concentration in the bioreactor. More H2 compared to CO2 and N2 was added per pulse because of the relatively low viscosity of H2 gas compared to other gasses (see e.g., Geankopolis 1978). The actual biomass concentrations were lower than expected from the gas titrations, probably as a result of cell lysis in the cultures. However, the estimation of PQ values based on on-line titrations with CO2 and H2 gas corresponded well to what was expected (see Tables 1 and 2). In Chlorella sp., growth on NO3− resulted in PQ values 1.3 times higher compared to biomass formation in the nitrogen depleted growth phase. In both species, growth on NH4+ resulted in PQ values almost identical to the PQ values during the nitrogen depleted growth phase. Growth on NO2− resulted in PQ values in between those of NO3− and NH4+ in Chlorella sp. (Table 2).
The PQ estimates depend on the distribution of oxygen and inorganic carbon in the culture and in the headspace. The major part of the total oxygen content in the system (approximately 97%) was present in the headspace due to the low solubility of oxygen in water. Therefore, an amount of oxygen corresponding to 2–4% of the oxygen produced by the algae was lost when excess pressure was released from the closed gas loop, and had to be accounted for in Eq. 5 in order to estimate the O2 production rate by the algae.
The distribution of inorganic carbon was very different from the distribution of O2 since the major part (81–86% of the total inorganic carbon) dissolved in the growth medium as HCO3−. Only 5% of the inorganic carbon was present as CO2 in the headspace gas, and less than 0.2% of the added CO2 was lost from the system via ventilations. Ventilation of excess pressure therefore had no significant effect on the estimations of CO2 uptake. In contrast, uptake of the nitrogen sources affected the CO2 titrations to a much greater extent. When NO2− or NO3− was the nitrogen source, approximately 8% of the added CO2 during the nitrogen sufficient growth phase was added to replenish the protons consumed during nitrogen reduction. When NH4+ was the nitrogen source, approximately 8% less CO2 than that removed by cells was added to maintain constant pH. Besides leading to underestimation of the CO2 uptake rate, uptake of NH4+ and the subsequent excretions of protons into the medium also had the consequence that inorganic carbon in the photobioreactor was depleted, unless the HCO3− concentration in the growth medium was higher than the NH4+ concentration at the beginning of the experiment.
CO2 also adsorbed reversibly onto the surface of the Pd catalyst, and during growth experiments, 4–8% of the total inorganic carbon in the systems was stored in the catalytic column. Low CO2 partial pressure resulted in a relatively high proportion of adsorbed CO2, since the column then was far from being saturated. The total amount of CO2 adsorbed onto the column increased by 40–60 μmol during NO3− or NO2− sufficient growth phases or decreased by 40–60 μmol during NH4+ sufficient growth phases. During the same periods, 25–35 mmol of organic carbon was produced, and the changes in the amount of adsorbed CO2 amounted to only 0.1%–0.25% of the amount of CO2 incorporated into biomass during nitrogen sufficient growth phases. The estimates of CO2 uptake were therefore not significantly influenced by the changes in the amount of CO2 adsorbed onto the catalytic column.
The strain of C. reinhardtii used in this study, which had been sub-cultured for several years in our laboratory, was deficient in its uptake of NO3− and NO2−. The strain was unable to grow on NO3−, despite the fact that four nitrate transport systems (some of which also transport NO2−) have been described in other strains of C. reinhardtii (Galván et al. 1996; Rexach et al. 2002). C. reinhardtii also had a very low affinity for NO2−. In order to obtain an accurate description of growth and NO2− uptake by Eqs. 8–13 (Figure 3a), a Ks value of 2–3 mM for NO2− was needed. This Ks value is much higher than reported Ks values of 1–30 μM for nitrite and nitrate transporters in C. reinhardtii (Galván et al. 1996; Rexach et al. 1999; Navarro et al. 2000). Because of the low NO2− affinity, NO2− was taken up at a relatively low rate 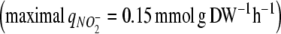 compared to the uptake rate of NH4+
compared to the uptake rate of NH4+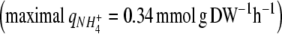 . However, the low uptake rate of NO2− gave the opportunity to compare growth of C. reinhardtii under nitrogen sufficiency on NH4+ and nitrogen limitation on NO2−. Because the CO2 assimilation rates during the growth phase were almost similar for both nitrogen sources (
. However, the low uptake rate of NO2− gave the opportunity to compare growth of C. reinhardtii under nitrogen sufficiency on NH4+ and nitrogen limitation on NO2−. Because the CO2 assimilation rates during the growth phase were almost similar for both nitrogen sources ( 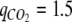 and 1.8 mmol g DW−1 h−1 for growth on NO2− and NH4+, respectively), the low NO2− uptake rate lead to a situation where the cells responded as if they had been nitrogen limited although NO2− was still present in the growth medium. Carbon was incorporated into starch simultaneously with growth of the non-starch biomass, and the specific concentration of chlorophyll a remained very low (Figure 2) compared to cells grown on NH4+.
and 1.8 mmol g DW−1 h−1 for growth on NO2− and NH4+, respectively), the low NO2− uptake rate lead to a situation where the cells responded as if they had been nitrogen limited although NO2− was still present in the growth medium. Carbon was incorporated into starch simultaneously with growth of the non-starch biomass, and the specific concentration of chlorophyll a remained very low (Figure 2) compared to cells grown on NH4+.
The uptake of CO2 in C. reinhardtii appeared to be regulated by the specific starch concentration in the cells. When the specific starch concentration reached approximately 0.6 g g−1, the increase in biomass dry weight ceased. With NH4+ as nitrogen source, the specific starch concentration was less than 0.1 g g−1 during the nitrogen sufficient growth phase (Figure 2b), and the cells were therefore able to synthesise and incorporate large amounts of starch after NH4+ was depleted. With NO2− as the nitrogen source, the specific starch concentration in the cells was already close to 0.6 g g−1 when NO2− was depleted (Figure 3b), and the culture therefore entered a stationary phase with no further increase in biomass dry weight.
The small amounts of starch also present in exponentially growing C. reinhardtii cells showed that the CO2 fixation capacity of the cells exceeded the need for reduced carbon for synthesis of non-starch biomass, and it is therefore reasonable to assume that the specific growth rate of light sufficient cultures of C. reinhardtii is restricted by the specific nitrogen uptake rate as described by Eq. 10.
Chlorella was able to utilise all three nitrogen sources tested, and all Chlorella cultures went through a nitrogen sufficient growth phase characterised by a high specific chlorophyll a concentration, and a nitrogen depleted growth phase characterised by an increasing content of what presumably is starch and a decreasing specific concentration of chlorophyll a. Pronounced decreases in PQ values were observed when NO2− and especially NO3− were depleted (Figure 4 and Table 2).
Segregation of batch growth into an exponential growth phase where active biomass is formed followed by a second growth phase where predominantly storage compounds are formed is a well-known phenomenon in algal cultures (Rigano et al. 2000; Ball 2002; Zhila et al. 2005). In this paper, we have presented a kinetic growth model describing the major effects of nitrogen limitation in C. reinhardtii and Chlorella and a methodology which enables these effects to be demonstrated on-line. The principle of simultaneous CO2 and H2 titrations to maintain constant pH and dissolved oxygen partial pressure, respectively, in gas-tight photobioreactors can be used for estimation of photosynthetic activity and PQ values, and to predict the composition of the produced biomass in cultures of algae. Titration of CO2 uptake and O2 production may also be developed into control and regulation tools for commercial algal cultures, e.g., to monitor the synthesis of lipids, poly-unsaturated fatty acids and other energy and carbon storage compounds, a class of microalgal products offering interesting biotechnological potentials (see e.g., Molina Grima et al. 1995; Zittelli et al. 1999; Kalacheva et al. 2002). Microalgal cultures have also been suggested for production of isotope labelled fine chemicals from 13CO2 or 14CO2 in closed photobioreactors (Delente et al. 1992; Behrens et al. 1994). In such systems, it would be possible to use estimates of PQ to control addition of the isotope labelled CO2 exclusively during the nitrogen limited growth phase, in order to cost-optimise the production of isotope labelled carbohydrates and lipids.
Acknowledgments
We thank Dr. Niels Iversen for help measuring nitrogen sources, Lars Jørgensen, DB Lab, for carrying out the biomass elemental composition analysis, and Gunnar Andersen for technical assistance.
Appendix
Distribution of CO2 in gas tight photobioreactor
To account for the effect of uptake of the nitrogen source on the total content of inorganic carbon in the photobioreactor, the distribution of inorganic carbon between the Pd catalyst, the gas phase, and the liquid medium was calculated. Since batch cultures were grown over relative long periods of time (200–300 h), the calculations were based on pseudo-steady-state conditions
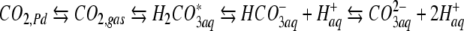 |
A1 |
The total amount of inorganic carbon in the photobioreactor, 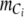 was the sum of all pools of inorganic carbon present in the system as described in Eq. A1
was the sum of all pools of inorganic carbon present in the system as described in Eq. A1
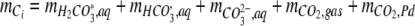 |
A2 |
where 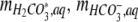 ,and
,and 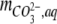 are the amounts of dissolved H2CO3 (including dissolved CO2), HCO3−, and CO32−, respectively,
are the amounts of dissolved H2CO3 (including dissolved CO2), HCO3−, and CO32−, respectively, 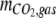 is the total amount of CO2 in the gas phase, and
is the total amount of CO2 in the gas phase, and 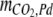 is the total amount of CO2 adsorbed to the Pd catalyst.
is the total amount of CO2 adsorbed to the Pd catalyst.
At pH 7.5, CO32− constituted only in the order of 0.1% of the total dissolved inorganic carbon, and the amounts of inorganic carbon in the different pools described in Eqs. A1 and A2 were essentially controlled by the concentration of HCO3− in the growth medium. Since 1 mol of H+ was consumed for each mol of NO3− or NO2− taken up by the cells, these protons were regenerated by addition of CO2, which dissolved as HCO3− and resulted in an equimolar increase of [HCO3−]. For each mol of NH4+ taken up, 1 mol of H+ was produced, and with NH4+ as the nitrogen source, less CO2 than taken up photosynthetically were therefore added to the photobioreactor resulting in an equimolar decrease of [HCO3−]. The amount of dissolved HCO3− was therefore estimated by
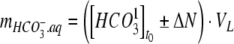 |
A3 |
where ΔN is the total decrease in concentration of the nitrogen source due to consumption by the algae, and VL is the volume of the liquid medium.
The amount of dissolved H2CO3* in the growth medium was described by
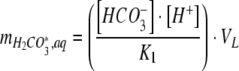 |
A4 |
where K1 is the equilibrium constant between H2CO3* and HCO3− + H+ (10−6.3 M, Stumm and Morgan 1995). The amount of dissolved CO32− is described by
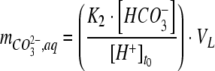 |
A5 |
where K2 is the equilibrium constant between HCO3− and CO32− + H+ (10−10.3 M, Stumm and Morgan 1995).
The relationship between the partial pressure of CO2 in the headspace, 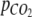 and the concentration of H2CO3* in the medium was described by Henry’s law
and the concentration of H2CO3* in the medium was described by Henry’s law
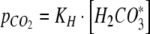 |
A6 |
where KH is Henry’s constant (3.0 • 103 kPa M−1, Atkins 1980). The total amount of CO2 in the headspace was calculated from 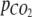 using the gas law
using the gas law
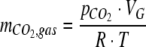 |
A7 |
where VG is the volume of the gas in the headspace and the closed gas loop, R is the gas constant, and T is the absolute temperature.
The amount of CO2 reversibly adsorbed onto the Pd catalyst was described by a Langmuir binding isotherm
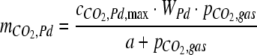 |
A8 |
where 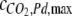 is the maximal surface-cover of CO2 on the Pd catalyst (60 μmol g−1), WPd is the mass of Pd catalyst in the catalytic column (25 g), and a is the half saturation constant (2.1 kPa). The parameters,
is the maximal surface-cover of CO2 on the Pd catalyst (60 μmol g−1), WPd is the mass of Pd catalyst in the catalytic column (25 g), and a is the half saturation constant (2.1 kPa). The parameters, 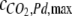 and a were estimated by measuring the increase of partial pressure after adding known amounts of CO2 to a closed chamber containing the Pd catalyst.
and a were estimated by measuring the increase of partial pressure after adding known amounts of CO2 to a closed chamber containing the Pd catalyst.
If the nitrogen uptake is measured or modelled, it is now possible to calculate the relationship between the overall change in total inorganic carbon content and nitrogen content in the photobioreactor, 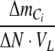
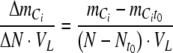 |
A9 |
With NO3− and NO2− as nitrogen sources, 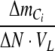 is negative. With NH4+ as nitrogen source,
is negative. With NH4+ as nitrogen source, 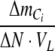 is positive. In the experiments described in this paper, ΔN was estimated from Eq. 13.
is positive. In the experiments described in this paper, ΔN was estimated from Eq. 13.
References
- Arvola L (1981) Spectrophotometric determination of chlorophyll a and phaopigments in ethanol extraction. Ann Bot Fenn 8:221–227
- Atkins PW (1980) Physical chemistry. Oxford University Press, Oxford
- Ball SG (2002) The intrinsic pathway of starch biosynthesis and degradation in the monocellular alga Chlamydomonas reinhardtii. Aust J Chem 55:49–59 [DOI]
- Behrens PW, Sicotte VJ, Delente J (1994) Microalgae as a source of stable isotopically labelled compounds. J Appl Phycol 6:113–121 [DOI]
- Coleman LW, Rosen BH, Schwartzbach SD (1988) Preferential loss of chloroplast proteins in nitrogen deficient Euglena. Plant Cell Physiol 29:1007-1014
- Delente JJ, Behrens PW, Hoeksema SD (1992) Closed photobioreactor and method of use. United States Patent 5,151,347
- Eriksen NT, Iversen JJL (1995) On-line determination of pigment composition and biomass in cultures of microalgae. Biotechnol Tech 9:49–54 [DOI]
- Eriksen NT, Geest T, Iversen JJL (1996) Phototrophic growth in the lumostat: a photo-bioreactor with on-line optimization of light intensity. J Appl Phycol 8:345–352 [DOI]
- Galván A, Quesada A, Fernández E (1996) Nitrate and nitrite are transported by different specific transport systems and by a bispecific transporter in Chlamydomonas reinhardtii. J Biol Chem 271:2088–2092 [DOI] [PubMed]
- Geankopolis CJ (1978) Transport processes and unit operations. Allyn and Bacon, Boston
- Kalacheva GS, Zhila NO, Volova TG (2002) Lipid and hydrocarbon compositions of a collection strain and a wild sample of the green microalga Botryococcus. Aquat Ecol 36:317-330 [DOI]
- Liu X, Gong JK, Collins AW, Grove LJ, Seyler JW (2001) Theoretical study of carbon dioxide coordination in palladium complexes. Appl Organomet Chem 15:95–98 [DOI]
- Molina Grima E, Pérez JAS, Camacho FC, Medina AR, Giménez AG, Alonso DL (1995) The productivity of polyunsaturaated fatty acids by microalgae: from strain selection to product purification. Process Biochem 30:711–719 [DOI]
- Navarro MT, Guerra E, Fernández E, Galván A (2000) Nitrite reductase mutants as an approach to understanding nitrate assimilation in Chlamydomonas reinhardtii. Plant Physiol 122:283–289 [DOI] [PMC free article] [PubMed]
- Nowakowski R, Grzeszczak P, Dús R (2002) AFM studies of the catalytic reaction of hydrogen with oxygen on thin Pd and Pt films under pressure ∼101 kPa. Surf Sci 507–510:813–818 [DOI]
- Redfield AC, Ketchum BH, Richards FA (1963) The influence of organisms on the composition of sea-water. In: Hill MN (ed) The sea: ideas and observations on progress in the study of the seas. Wiley, New York, pp 26–77
- Rexach J, Llamas A, Fernandéz E, Galván A (2002) The activity of the high-affinity nitrate transport system I (NRT2;1, NAR2) is responsible for the efficient signalling of nitrate assimilation genes in Chlamydomonas reinhardtii. Planta 215:606–611 [DOI] [PubMed]
- Rexach J, Montero B, Fernández E, Galván A (1999) Differential regulation of the high affinity nitrite transport systems III and IV in Chlamydomonas reinhardtii. J Biol Chem 274:27801–27806 [DOI] [PubMed]
- Richardson K, Beardall J, Raven JA (1983) Adaptation of unicellular algae to irradiance: An analysis of strategies. New Phytol 93:157–191 [DOI]
- Rigano VDM, Vona V, Cargagna S, Esposito S, Carillo P, Rigano C (2000) Effects of sulfate-starvation and re-supply on growth, NH4+ uptake and starch metabolism in Chlorella sorokiniana. Aust J Plant Physiol 27:335–342
- Roels JA (1980) Applications of macroscopic principles to microbial metabolism. Biotechnol Bioeng 22:2457–2514 [DOI] [PubMed]
- Starr RC (1978) The culture collection of algae at The University of Texas at Austin. J Phycol 14:47–100 [DOI]
- Stenholm H, Song S, Eriksen NT, Iversen JJL (1998) Indirect estimation of poly-ß-hydroxybutyric acid by cell carbon analysis. Biotechnol Techn 12:451–454 [DOI]
- Stumm W, Morgan JJ (1995) Aquatic chemistry. Wiley, New York
- Zhila NO, Kalacheva GS, Volova TG (2005) Effect if nitrogen limitation on the growth and lipid composition of the green alga Botryococcus braunii Kütz IPPAS H-252. Russ J Plant Physiol 52:357–365 [DOI]
- Zittelli GC, Lavista F, Bastianini A, Rodolfi L, Vincenzini M, Tredici MR (1999) Production of eicosapentaenoic acid by Nannochloropsis sp. cultures in outdoor tubular photobioreactors. J Biotechnol 70:299–312 [DOI]


