Abstract
Although relatively uncommon individually, the various causes of hypophosphataemic rickets have provided an impetus for unravelling the mechanisms of phosphate homeostasis and bone mineralisation. Over the past 10 years, considerable advances have been made in establishing the gene mutations responsible for a number of the inherited causes and in understanding the mechanisms responsible for tumour-induced osteomalacia/rickets. The most exciting aspects of these discoveries have been the discovery of a whole new class of hormones or phosphatonins which are thought to control phosphate homoeostasis and 1 alpha-hydroxylase activity in the kidney, through a bone–kidney–intestinal tract axis. Although our understanding of the interrelationships is far from complete, it raises the possibilities of improved therapeutic agents in the long-term, and has resulted in improved diagnostic abilities in the short-term.
Keywords: Hypophosphataemic rickets, Phosphatonins, Renal tubular reabsorption of phosphate, FGF-23, PHEX, MEPE, Tumour-induced rickets, Polyostotic fibrous dysplasia
Introduction
Over the past decade, considerable advances have been made in our understanding of the pathogenesis of the various forms of hypophosphataemic rickets, although there are still many gaps in our knowledge. These advances have led to improved diagnosis and, in some situations, improved management. They have also gone some way in helping to unravel the mysteries surrounding bone mineralisation, the control of serum phosphorus concentrations and phosphorus homoeostasis. This paper briefly describes the control of serum phosphorus (Pi) concentrations in children and then discusses the advances made in elucidating the pathogenesis of various forms of hypophosphataemic rickets, concentrating on those associated with isolated renal tubular Pi loss.
The control of serum phosphorus
The normal range of serum Pi changes from the neonatal period through adolescence to reach adult values towards the end of puberty. Pi concentrations are highest in the neonatal period (1.88–2.4 mmol/l), but fall rapidly in the first few months of life and then more slowly to reach childhood levels (1.45–1.80 mmol/l). The concentrations then remain fairly constant until adolescence, when values fall to adult values (0.8–1.45 mmol/l). Unlike dietary calcium, ingested phosphate is generally efficiently absorbed (65–90% in children) from the gastrointestinal tract, although complex plant phosphate (phytate) is almost totally excreted. The majority of dietary Pi is absorbed by passive concentration-dependent processes, but the active metabolite of vitamin D (1,25-dihydroxyvitamin D(1,25(OH)2D)) does increase the intestinal Pi absorption marginally.
Serum Pi homeostasis is maintained largely through the control of renal phosphate reabsorption, and, in a steady state, urine Pi excretion reflects dietary intake. A number of factors influence the renal tubular reabsorption of Pi, such as dietary phosphorus content, and parathyroid hormone (PTH), growth hormone (insulin-like growth factor 1) and thyroid hormone concentrations, although these latter two hormones probably do not play a major role in the short-term control of serum Pi concentrations, but, rather, determine long-term concentrations.
The major rate-limiting factor in the renal reabsorption of Pi is the Pi transporter located at the brush border membrane of the proximal renal tubular cells. Eighty-five percent of Pi reabsorption normally takes place in the proximal renal tubule. There are three genes encoding for sodium-dependent phosphate co-transporters; NaPi-I–NaPi-III [11]. NaPi-IIa and NaPi-IIc are the major transporters in the proximal tubule and are regulated by PTH, which internalises the transporters and increases their catabolism, thus, reducing Pi reabsorption. Recently, it has been established that fibroblast growth factor-23 (FGF23) is also a potent regulator of NaPi-II activity. FGF23 is one of the newly discovered phosphatonins (hormones that control serum Pi concentration through stimulating phosphaturia) and will be discussed in more detail later. The co-transporter, NaPi-IIc, was originally thought to play a lesser role in normal Pi homeostasis, but this has been questioned recently.
As the renal handling of Pi plays such a vital role in the maintenance of serum Pi concentration, it is important in situations of both hyper- and hypophosphataemia to assess renal tubular Pi reabsorption (or, more appropriately, the tubular maximum for Pi reabsorption (TmP/GFR)) in order to establish the pathogenesis. This is easily done by measuring the renal phosphate handling on a morning fasting but untimed urine specimen (measuring urinary Pi and creatinine concentrations), together with the simultaneous measurement of serum Pi and creatinine. Tubular reabsorption is calculated as [1−(UPi×PCr)/(UCr×PPi)]×100, where UPi and PPi are the urine and serum Pi concentrations, respectively, and UCr and PCr are the corresponding creatinine concentrations. As appropriate renal tubular reabsorption is dependent on the serum Pi concentration, Walton and Bijvoet [24] created a nomogram to calculate the tubular maximum for Pi reabsorption (TmPi/GFR), which is similar in concept to the renal glucose threshold; in other words it is the serum Pi concentration at which Pi begins to appear in the urine. The nomogram described above is not applicable to children, but paediatric age-specific normal ranges have been developed by Alon and Hellerstein [1]. In normal children, the TmPi/GFR should be similar to the normal values of serum Pi at a particular age. A calculated TmPi/GFR below this range indicates inappropriate renal Pi loss (similar to that which occurs in hyperparathyroidism or renal tubular Pi leaks, e.g. Fanconi syndrome and isolated renal phosphate loss from various forms of hypophosphataemic rickets) and a value above the normal range suggests inappropriate renal Pi retention (as that occurs in hypoparathyroidism or dietary phosphate restriction).
Hypophosphataemic rickets
Table 1 provides a summary of the various forms of hypophosphataemic rickets. The list is not complete, but it does cover the major categories. Hypophosphataemic rickets due to decreased intestinal absorption either because of a low dietary intake or the prolonged ingestion of antacids will not be discussed further, as these do not involve a primary defect in renal Pi handling. This review concentrates on those conditions associated with isolated renal tubular Pi loss rather than diseases associated with complex renal tubular defects, such as in the Fanconi syndrome (OMIM 134600), Lowe syndrome (OMIM 309000) [2], Dent disease (OMIM 300009) [2] or as a result of ifosfamide nephrotoxicity [22].
Table 1.
A classification of the various forms of hypophosphataemic rickets
| Decreased gastrointestinal Pi absorption | Isolated renal phosphate leak | Renal tubular disorders associated with Pi leak |
|---|---|---|
| Decreased dietary intake: Breast-fed very-low-birthweight infants | Isolated phosphaturia: | Phosphaturia, calciuria plus acidosis: Distal renal tubular acidosis |
| Impaired intestinal absorption: Prolonged antacid use | X-linked hypophosphataemic rickets (XLH) | Phosphaturia, aminoaciduria, acidosis, glucosuria, plus electrolyte disturbances: |
| Autosomal dominant hypophosphataemic rickets (ADHR) | Fanconi syndrome (primary and secondary) | |
| Autosomal recessive hypophosphataemic rickets (ARHR) | Dent disease | |
| Tumour-induced rickets (TIO) | Lowe syndrome | |
| Polyostotic fibrous dysplasia | Ifosfamide toxicity | |
| Neurocutaneous syndromes | ||
| Phosphaturia plus calciuria: Hereditary hypophosphataemic rickets with hypercalciuria |
Numerous diseases are associated with rickets and increased renal Pi loss; however, it is the isolated phosphaturic entities that have attracted the most attention over the past decade, as the isolation of the genetic mutations responsible for their pathogenesis has resulted in a new understanding of the hormonal axis between bone mineralisation, renal Pi handling and vitamin D metabolism (Table 2).
Table 2.
The genetic abnormalities associated with the various forms of hypophosphataemic rickets
| Disease | Gene involved | Gene product | Serum FGF23 | Serum 1,25(OH)2D |
|---|---|---|---|---|
| X-linked hypophosphataemic rickets | PHEX | Inactivating mutation in PHEX (endopeptidase) | Generally increased | Within normal range or decreased |
| Autosomal dominant hypophosphataemic rickets | FGF23 | Activating mutation in FGF23 (phosphatonin) | Variable—may be increased during symptomatic disease | Decreased |
| Autosomal recessive hypophosphataemic rickets | DMP1 | Inactivating mutation in DMP1 (involved in mineralisation) | Increased | Within normal range |
| Hereditary hypophosphataemic rickets with hypercalciuria | SLC34A3 | Inactivating mutation in NaPi-IIc (renal Na-Pi cotransporter) | Not known | Elevated |
The best known and most common of the inherited isolated proximal tubular defects associated with rickets is X-linked hypophosphataemic rickets (XLH) (OMIM 307800). This disease usually presents clinically in the first two years of life with short stature, bowing of the legs, osteomalacia and rickets, hypophosphataemia, phosphaturia, normocalcaemia and normal or nearly-normal PTH with inappropriately low or normal 1,25-(OH)2D concentrations. Hypophosphataemia develops within the first few months of life and is, thus, a useful biochemical test in young infants who might be suspected of inheriting the abnormal gene from an affected parent. One of the most striking and distinctive features of XLH on bone histology (besides osteomalacia) is the presence of hypomineralised peri-osteocytic lesions in cortical bone. Although it was hypothesised that a mutation in one of the genes encoding for a renal Pi transporter would be found, this eventually proved not to be the case. Rather, mutations in the PHEX gene (phosphate-regulating gene with homologies to endopeptidases on the X-chromosome) were discovered in the majority of patients [23]. The encoded endopeptidase (PHEX) is membrane-bound and expressed mainly by osteoblasts in bone and odontoblasts in teeth, with no expression in the kidney [18]. This apparently surprising finding is in keeping with experiments using the mouse homologue of XLH, the Hyp mouse, in which convincing evidence for a circulating factor causing the renal Pi loss in the disease has been found [16]. It was, thus, hypothesised that PHEX is involved in the catabolism of a phosphaturic factor (phosphatonin) and the initial substrate candidate was thought to be FGF23. However, a number of laboratories have been unable to show that FGF23 is the substrate for PHEX; thus, it is now believed that PHEX may prevent the cleavage of an intermediate, such as matrix extracellular phosphoglycoprotein (MEPE), which controls circulating levels of FGF23 [6]. Inactivating mutations of PHEX, thus, result in an increase in circulating concentrations of FGF23 with resultant phosphaturia [28]. Although FGF23 concentrations are not invariably increased in XLH, an inverse relationship between FGF23 and the degree of hypophosphataemia has been found [25]. More recent evidence indicates that elevated FGF23 plays a central role in hypophosphataemia, disturbed vitamin D metabolism and the development of osteomalacia and rickets characteristic of XLH [27]. However, it is unclear whether the bone disease and, in particular, the peri-osteocytic lesions are a direct or indirect effect of FGF23.
These new findings concerning the pathogenesis of XLH may, in the long-term, offer a possibility of more effective therapeutic management of the disease. However, in the short-term, the new discoveries underline the evidence that current forms of treatment with calcitriol and phosphate supplements only partially address some of the defects in the disease (hypophosphataemia and low to normal 1,25(OH)2D concentrations), but do not alter the disturbed underlying mechanisms, such as elevated FGF23 and MEPE levels and the peri-osteocytic mineralisation defects. Further, they provide a possible scientific basis for the often poor response to treatment in subjects with XLH who may have radiographic evidence of healing of the rachitic lesions, but in whom osteomalacia persists, although at a reduced severity. These findings raise concerns about the possible role of treatment (calcitriol) in stimulating FGF23 concentrations and, thus, increasing renal Pi loss, inducing a vicious cycle of increasing Pi supplements, increasing secondary hyperparathyroidism and further Pi loss.
Autosomal dominant hypophosphataemic rickets (ADHR) (OMIM 193100) is a very rare cause of rickets, which presents with variable penetrance and age of clinical onset. Although superficially the disease presents in young children with similar features to XLH (renal phosphate wasting, rickets and lower limb deformities), in older female patients, features of muscle weakness and bone pain may predominate [3]. Furthermore, a number of older subjects have been reported to become asymptomatic with time. The importance of ADHR in clinical paediatrics was established when it became the first disease shown to be associated with abnormalities of FGF23. This discovery has resulted in an enormous flurry of research into the role of FGF23 and other putative phosphatonins in Pi homeostasis and the control of bone mineralisation. The mutations described in this condition have all been associated with amino acid substitutions at a protease cleavage site in the FGF23 molecule, which result in the mutant FGF23 being resistant to cleavage. The mutant FGF23 would, thus, be expected to have a longer circulating half-life than the wild type and be associated with higher serum concentrations. A recent report found that FGF23 values were, surprisingly, not consistently elevated in patients with ADHR and that serum concentrations fluctuated between normal and elevated values, depending on whether or not the individual subject was hypophosphataemic (symptomatic) [9].
Autosomal recessive hypophosphataemic rickets (ARHR) (OMIM 241520) is another rare form of hypophosphataemic rickets whose genetic mutation has recently been described [26]. Inactivating mutations of the dentin matrix protein-1 (DMP1) gene result in a phenotype similar to that seen in XLH. It appears that the biochemical features of hypophosphataemia, renal phosphate loss and inappropriately low 1,25(OH)2D, are due to the secondary elevation of FGF23 concentrations. The clinical presentation is, surprisingly, not found at birth, but affected individuals present later during childhood and even in adulthood. As with PHEX and FGF23, DMP1 is highly expressed in cells of the osteoblasts/osteocyte lineage. However, the interrelationships between these three proteins are unclear. It does appear that DMP1 normally decreases FGF23 concentrations, as FGF23 concentrations are elevated in ARHR [21].
Tumour-induced osteomalacia/rickets (TIO) is a paraneoplastic syndrome, characterised by small tumours of mesenchymal origin (generally diagnosed as haemangiopericytomas or non-ossifying fibromas) causing rickets and osteomalacia through the secretion of phosphatonins, which results in hypophosphataemia, renal phosphate wasting and rickets/osteomalacia. Although the disease has been described mainly in adult subjects, paediatric patients have been reported [4]. Several striking features are helpful in differentiating this condition from the other hypophosphataemic syndromes: clinical features include severe muscle weakness, marked bone demineralisation and severe osteomalacia, and biochemically, besides the typical features of hypophosphataemic rickets, 1,25(OH)2D concentrations are markedly suppressed. The majority of tumours are small and benign and may be found anywhere in soft-tissue or bone, which make detection very difficult. Several secreted factors have been proposed to be responsible for the syndrome. These include FGF23, MEPE and frizzled related protein-4 (FRP4) [27], with FGF23 being the most often studied. Serum levels of FGF23 are found to be elevated in most but not all patients with proven TIO. Removal of the tumour results in a rapid decline in FGF23 concentrations and a restoration of normal biochemistry and healing of the osteomalacia/rickets.
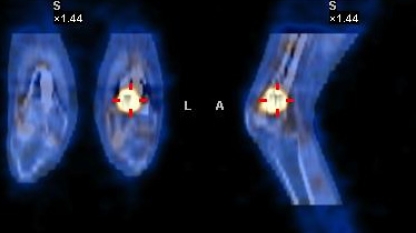
As mentioned earlier, the detection of a suspected tumour is notoriously difficult. However, the expression of somatostatin receptors by the tumours has resulted in the recent development of 111In-octreotide scintigraphy [7, 15], which, coupled with computed tomography (CT) scanning, can accurately detect and localise the tumour (Fig. 1). Further, in those patients in whom the tumour cannot be found or removed because of its situation, subcutaneous octreotide therapy may result in a reversal of the biochemical abnormalities.
Fig. 1.
Co-localisation of an osteomalacia-inducing tumour in the distal metaphysis of the left femur using 111In-octreotide scintigraphy and computed tomography (CT) scanning in a 26-year-old subject who had presented at the age of 16 years with severe hypophosphataemic rickets and multiple fractures
A number of other conditions, such as polyostotic fibrous dysplasia (including the McCune-Albright syndrome) and several neurocutaneous syndromes (e.g. linear nevus sebaceous syndrome), may also be associated with hypophosphataemic rickets. Recent evidence indicates that hypophosphataemia and rickets are probably due to the over-expression of FGF23 in the lesions [8, 19].
Hereditary hypophosphataemic rickets with hypercalciuria (HHRH) (OMIM 241530) is a recessively inherited disorder, whose genetic mutations have only recently been identified [14]. Mutations involve the SLC34A3 gene, which encodes for NaPi-IIc (Na-dependent Pi cotransporter found in the proximal renal tubule). The condition can be readily differentiated from other forms of rickets associated with renal phosphate wasting, as the disease is associated with hypercalciuria (>0.60 mmol calcium:mmol creatinine) and elevated 1,25(OH)2D concentrations. Unlike the other forms of hypophosphataemic rickets described above, HHRH is the only one in which there is a primary defect in Pi transport across the renal tubular cells. Thus, the body responds appropriately to the resultant hypophosphataemia, with stimulation of 1 alpha-hydroxylase activity and a consequent rise in 1,25(OH)2D. The latter increases both calcium and phosphate intestinal absorption, which results in hypercalciuria and the suppression of PTH. The importance of understanding the pathogenesis of these biochemical changes is that phosphate supplementation alone results in a correction of the biochemical perturbations. There is no need for calcitriol supplementation, as the 1,25(OH)2D concentrations are already elevated.
Tying the strands together
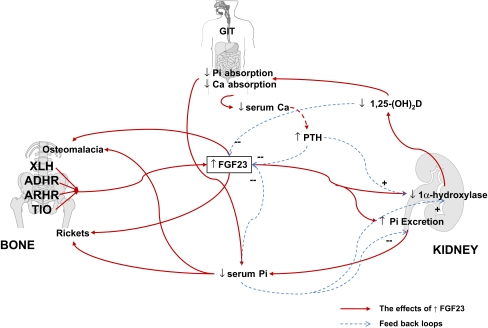
It is clear that FGF23 plays a central role in the pathogenesis of hypophosphataemia, depressed 1,25-(OH)2D concentrations, osteomalacia and rickets in the majority of the forms of rickets described above. Elevated concentrations of FGF23 internalise and reduce the NaPi-IIa and c cotransporters in the proximal renal tubule, reducing Pi reabsorption and suppressing 1 alpha-hydroxylase activity, thus, reducing 1,25-(OH)2D and serum Pi concentrations (Fig. 2) [11]. The resultant low serum Pi concentrations may be directly responsible for the rachitic changes at the growth plate, but it is likely that FGF23 has a direct effect on the mineralisation of newly formed osteoid at the trabecular bone surface, resulting in the features of osteomalacia [17]. However, the mechanisms of control of FGF23 and its role in normal Pi homoeostasis are unclear at present. Whether or not FGF23 is involved in the day to day control of serum Pi in response to dietary Pi changes remains controversial, as some studies have shown a relationship between FGF23 and dietary or serum Pi [5] and others have not [10, 12]. Both PTH and 1,25-(OH)2D increase FGF23 production, which helps to reduce the Pi load induced by increased intestinal Pi absorption and bone resorption associated with elevated levels of the two former hormones. It is of interest that the mRNA of all three factors (FGF23, PHEX and DMP1) involved in the pathogenesis of hypophosphataemic rickets are highly expressed in cells of the osteoblasts/osteocyte lineage. Thus, bone plays a central role in FGF23 control and plays an important part in the newly described kidney–intestine–bone hormonal axis controlling Pi homeostasis and bone mineralisation. It is likely that PHEX controls FGF23 production indirectly, possibly through its binding of MEPE [20], although this has not been confirmed [13]. DMP1 appears to suppress FGF23 production directly [17]. Although considerable advances have been made in understanding the control of these bone/matrix proteins, there is still much to be learned about the interplay of these various factors, how they are controlled and the role they play in normal mineral and bone homeostasis.
Fig. 2.
The effects of increased FGF23 on bone and mineral homeostasis. Abbreviations: ADHR=autosomal dominant hypophosphataemic rickets; ARHR=autosomal hypophosphataemic rickets; Ca=calcium; FGF23=fibroblast growth factor 23; Pi=inorganic phosphate; PTH=parathyroid hormone; TIO=tumour-induced osteomalacia; XLH=X-linked hypophosphataemic rickets; 1,25-(OH)2D=1,25 dihydroxyvitamin D; –, suppression; +, stimulation; ↑, increased; ↓, decreased
Conclusions
Although the individual forms of hypophosphataemic rickets are relatively uncommon in childhood (with the exception of X-linked hypophosphataemic rickets [XLH]), the recent discoveries of their genetic defects have resulted in a greater understanding of the complexities surrounding inorganic phosphate (Pi) homeostasis and bone mineralisation. Further, they have provided the paediatrician with a better understanding of the deficiencies in current treatment options, as, in the majority of conditions, the current therapy does not address the basic defects. On the other hand, we should be optimistic that new and more effective treatment options will emerge as our understanding of the complex processes involved become greater and new drugs are synthesised to alter the deranged homeostatic mechanisms. A further spin-off from the renewed interest in the syndromes has been the development of better diagnostic procedures for the detection of phosphatonin-secreting tumours.
Abbreviations
- ADHR
Autosomal dominant hypophosphataemic rickets
- ARHR
Autosomal recessive hypophosphataemic rickets
- DMP
Dentin matrix protein
- FGF23
Fibroblast growth factor-23
- FRP4
Frizzled related protein-4
- HHRH
Hereditary hypophosphataemic rickets with hypercalciuria
- MEPE
Matrix extracellular phosphoglycoprotein
- NaPi-I
Na-dependent phosphate cotransporter-1
- NaPi-II
Na-dependent phosphate cotransporter-2
- NaPi-III
Na-dependent phosphate cotransporter-3
- PHEX
Phosphate-regulating gene with homologies to endopeptidases on the X-chromosome
- Pi
Inorganic phosphate
- PTH
Parathyroid hormone
- TIO
Tumour-induced osteomalacia
- XLH
X-linked hypophosphataemic rickets
- 1,25(OH)2D
1,25-dihydroxyvitamin D
References
- 1.Alon U, Hellerstein S (1994) Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr Nephrol 8:250–251 [DOI] [PubMed]
- 2.Cho HY, Lee BH, Choi HJ, Ha IS, Choi Y, Cheong HI (2007) Renal manifestations of Dent disease and Lowe syndrome. Pediatr Nephrol [Epub ahead of print] [DOI] [PubMed]
- 3.Econs MJ, McEnery PT (1997) Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab 82:674–681 [DOI] [PubMed]
- 4.Eyskens B, Proesmans W, Van Damme B, Lateur L, Bouillon R, Hoogmartens M (1995) Tumour-induced rickets: a case report and review of the literature. Eur J Pediatr 154:462–468 [DOI] [PubMed]
- 5.Ferrari SL, Bonjour JP, Rizzoli R (2005) Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90:1519–1524 [DOI] [PubMed]
- 6.Garabedian M (2007) Regulation of phosphate homeostasis in infants, children, and adolescents, and the role of phosphatonins in this process. Curr Opin Pediatr 19:488–491 [DOI] [PubMed]
- 7.Hesse E, Moessinger E, Rosenthal H, Laenger F, Brabant G, Petrich T, Gratz KF, Bastian L (2007) Oncogenic osteomalacia: exact tumor localization by co-registration of positron emission and computed tomography. J Bone Miner Res 22:158–162 [DOI] [PubMed]
- 8.Hoffman WH, Jüppner HW, Deyoung BR, O’Dorisio MS, Given KS (2005) Elevated fibroblast growth factor-23 in hypophosphatemic linear nevus sebaceous syndrome. Am J Med Genet A 134:233–236 [DOI] [PubMed]
- 9.Imel EA, Hui SL, Econs MJ (2007) FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res 22:520–526 [DOI] [PubMed]
- 10.Ito N, Fukumoto S, Takeuchi Y, Takeda S, Suzuki H, Yamashita T, Fujita T (2007) Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab 25:419–422 [DOI] [PubMed]
- 11.Kronenberg HM (2002) NPT2a—the key to phosphate homeostasis. N Engl J Med 347:1022–1024 [DOI] [PubMed]
- 12.Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB (2003) Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64:2272–2279 [DOI] [PubMed]
- 13.Liu S, Brown TA, Zhou J, Xiao ZS, Awad H, Guilak F, Quarles LD (2005) Role of matrix extracellular phosphoglycoprotein in the pathogenesis of X-linked hypophosphatemia. J Am Soc Nephrol 16:1645–1653 [DOI] [PMC free article] [PubMed]
- 14.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM (2006) Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet 78:193–201 [DOI] [PMC free article] [PubMed]
- 15.Moran M, Paul A (2002) Octreotide scanning in the detection of a mesenchymal tumour in the pubic symphysis causing hypophosphataemic osteomalacia. Int Orthop 26:61–62 [DOI] [PMC free article] [PubMed]
- 16.Nesbitt T, Coffman TM, Griffiths R, Drezner MK (1992) Crosstransplantation of kidneys in normal and Hyp mice. Evidence that the Hyp mouse phenotype is unrelated to an intrinsic renal defect. J Clin Invest 89:1453–1459 [DOI] [PMC free article] [PubMed]
- 17.Qin C, D’Souza R, Feng JQ (2007) Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res 86:1134–1141 [DOI] [PubMed]
- 18.Quarles LD (2003) FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol Endocrinol Metab 285:E1–E9 [DOI] [PubMed]
- 19.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P (2003) FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest 112:683–692 [DOI] [PMC free article] [PubMed]
- 20.Rowe PSN, Garrett IR, Schwarz PM, Carnes DL, Lafer EM, Mundy GR, Gutierrez GE (2005) Surface plasmon resonance (SPR) confirms that MEPE binds to PHEX via the MEPE-ASARM motif: a model for impaired mineralization in X-linked rickets (HYP). Bone 36:33–46 [DOI] [PMC free article] [PubMed]
- 21.Silve C (2006) DMP1 and phosphate metabolism—matrix proteins go systemic. BoneKEy-Osteovision 3:30–35
- 22.Skinner R (2003) Chronic ifosfamide nephrotoxicity in children. Med Pediatr Oncol 41:190–197 [DOI] [PubMed]
- 23.The HYP Consortium (1995) A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nature Genet 11:130–136 [DOI] [PubMed]
- 24.Walton RJ, Bijvoet OL (1975) Nomogram for derivation of renal threshold phosphate concentration. Lancet 2:309–310 [DOI] [PubMed]
- 25.Weber TJ, Liu S, Indridason OS, Quarles LD (2003) Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res 18:1227–1234 [DOI] [PubMed]
- 26.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ (2001) Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int 60:2079–2086 [DOI] [PubMed]
- 27.White KE, Larsson TE, Econs MJ (2006) The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis: frizzled related protein-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23. Endocr Rev 27:221–241 [DOI] [PubMed]
- 28.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S (2002) Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87:4957–4960 [DOI] [PubMed]