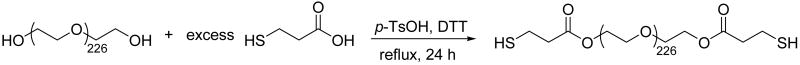Abstract
Methods to assemble polymeric hydrogels on the basis of noncovalent protein-glycosaminoglycan interactions have been previously demonstrated by us and others and hold promise in the development of receptor-responsive hydrogel materials; improvements in the mechanical properties of such systems would broaden their utility. Thus, in situ crosslinkable and degradable heparin-containing hydrogels were designed for the binding and controlled release of growth factors. Specifically, maleimide-functionalized high molecular weight heparin (HMWH) was synthesized via straightforward chemical methods that permitted facile and controllable modification of carboxylates in HMWH with maleimide groups via control of catalyst and reaction conditions, as assessed via 1H NMR spectroscopy. These modified heparins were crosslinked into hydrogels via reaction with various thiol-functionalized PEGs. The gelation times and elastic moduli of the gels, as assessed through oscillatory rheometry, could be tuned by via control of the functionality of HMWH, the concentration of hydrogel, the identity of the PEG-based crosslinker, as well as the molar ratio between maleimide and thiol groups. The capability of the hydrogels to bind to growth factors was investigated with immunochemical assays. Preliminary studies indicate the controlled release of basic fibroblast growth factor (bFGF) from these materials and suggest their broader use in the design of responsive materials.
Keywords: Heparin, Hydrogel, Growth factor, Controlled release, Protein release
1. Introduction
The assembly and covalent production of polymeric hydrogels has remained a popular area of basic and applied research with aims toward the production of drug and protein delivery systems, augmentation agents, and tissue engineering scaffolds. The physical similarity of hydrogels to many native soft tissues, for instance their high water content and their often comparable mechanical properties, has been a primary factor in the wide popularity of such hydrogel materials [1-3]. A variety of synthetic and naturally derived polymers have been employed in investigations of this kind, including poly(ethylene glycol) (PEG), poly(vinyl alchol), poly(N-isopropylacrylamide), gelatin, collagen, and alginate. Manipulation of the bulk properties and degradation of these scaffolds are key issues in their effectiveness, as well as biocompatibility and the ability to contain and release drugs in a controlled manner to induce desired biological responses [4]. Thus, strategies to integrate synthetic and biomolecular materials into delivery vehicles has remained an important goal; the scope of the bioconjugates for given applications is essentially infinite, providing scores of ways to tailor hydrogel properties for desired applications.
For potential applications in affinity-based protein delivery, polysaccharide-derivatized hydrogels have gained increasing prominence owing to the importance of protein-polysaccharide interactions in the extracellular matrix (ECM). Heparin-functionalized hydrogels have been particularly widely used in such applications, owing to the importance of the highly anionic, sulfated glycosaminoglycans (GAGs) heparin and heparan sulfate in many biological processes [5]. Heparin has the highest electronegative charge of any known biological molecule, which mediates its important biological roles as a multivalent binding agent for many proteins [6-9], including antithrombin III (to mediate thromobosis) and growth factors (to protect against degradation and potentiate receptor binding). Accordingly, heparin has been incorporated covalently into numerous diverse drug delivery vehicles in which its interactions with proteins have permitted the controlled release of growth factors [10-17]. For example, Tae et al. has previously described a system in which heparin was modified with a dihydrazide and crosslinked to the N-hydroxysuccinimidyl ester of PEG-bis-butanoic acid; the resulting system showed controllable release of bioactive vascular endothelial growth factor (VEGF). Another example from Nakamura et al. shows heparin modified with cinnamate groups to allow for ultraviolet photocrosslinking. This material also showed successful controlled release of basic fibroblast growth factor (bFGF) and neovascularization in rat models.
In addition, our group and others have been investigating the noncovalent assembly of heparinized materials as a route to responsive, reversible, and injectable drug delivery systems, with main interests in protein delivery and the production of ECM-mimetic materials [11,18,19]. Poly(ethylene glycol) star polymers functionalized with heparin-binding peptides can be mixed directly with heparin to form viscoelastic solutions with tunable properties [20], or can be mixed with star poly(ethylene glycol)-heparin conjugates to form noncovalent hydrogels materials capable of growth factor delivery via hydrogel erosion [11,18,21]. Such erosion strategies, although passive, may offer unique opportunities for improving growth factor activity via the co-release of growth factor with the heparinized macromolecules. In an extension of this work, we have also recently reported that the PEG-heparin conjugates are competent for the formation of elastic hydrogels via the interaction with dimeric, heparin-binding growth factors (such as vascular endothelial growth factor, VEGF) [22]. Importantly, these hydrogels are capable of receptor-mediated VEGF release and hydrogel erosion upon exposure to the VEGF receptor VEGFR-2, and given the primary role of the VEGFR-2 in controlling the proliferation and migration of vascular endothelial cells, these results suggest important strategies for the targeted delivery of drugs in vascular and wound-healing therapies.
Of particular interest in the current work is the expansion of these unique noncovalent assembly strategies with covalent crosslinking methods, for the formation mechanically tunable, biodegradable, heparinized hydrogels that may show receptor-responsive rheology and delivery profiles. Toward these ends, we have investigated methods to reliably functionalize heparin with chemically reactive groups at controlled degrees of substitution, and have demonstrated the rapid in-situ crosslinking of this multifunctional heparin with thiol-derivatized PEGs of various molecular weights and polymer structures. The rheological properties of these hydrogels can be tuned through variations in heparin functionality and hydrogel composition (polymer concentration and molar ratio of crosslinkers), and the gels can be used as a controlled delivery vehicle for growth factors with activities useful for tissue regeneration and vascularization (here, basic fibroblast growth factor, bFGF) [9,23]. As in other heparinized materials, the mainly electrostatic interactions between heparin and bFGF acts as an anchor to prevent burst disassociation of bFGF, additionally, the charged and hydrophilic character of heparin and PEG result in good hydrophilicity of the gel. The extent of swelling of these networks can be controlled by the extent of crosslinking and polymer composition. Our future studies will confirm the potential of additional noncovalent crosslinks for modifying the rheological and responsive behaviors of these covalently crosslinked materials and will assess the biological activities of these matrices in vivo.
2. Materials and methods
2.1 Materials
Recombinant Human basic FGF (bFGF) was obtained from R & D Systems (Minneapolis, MN). Pluronic F127 (average Mw 12,700 g/mol, 50 wt% PEO) was obtained from BASF Corporation (Parsippany, NJ). Deionized water (18 MΩ) was used in all experiments (Barnstead NANOpure Diamond ultrapure water systems; Barnstead Inc, Dubuque, IO). Poly(ethylene glycol) (average Mn 10,000 g/mol and average Mn 4,600 g/mol), heparin sodium salt (Grade I-A from porcine intestinal mucosa (HMWH)), N-(2-aminoethyl) maleimide, trifluoroacetate salt (AEM), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC hydrochloride), 1-hydroxybenzotriazole hydrate (HOBT hydrate), N-hydroxy succinidine (NHS), 2-(N-morpholino)ethanesulfonic acid (MES), 3-mercaptopropionic acid, p-toluenesulfonic acid (TsOH), dithiothreitol (DTT), and toluene were obtained from Sigma-Aldrich (St. Louis, MO). All of the chemicals were used as received.
2.2 Methods
2.2.1 Synthesis of maleimide-functionalized HMWH
To a 20 mL vial were added HMWH (20 mg) dissolved in 10 mL 0.1M MES buffer. EDC·HCl (3 eq. to COOH groups in HMWH), HOBT or NHS (3 eq. to COOH groups), AEM (1 eq. to COOH groups) were dissolved in 10 mL MES buffer, and then were added to the HMWH solution. The reaction mixture was stirred at r.t. for 5 h. Various pH values of the MES buffer (pH 5.5 – 6.0) were studied. The product was dialyzed, using a SpectraPor dialysis membrane (MWCO 1000), first against 1 M NaCl and then against D.I. water, and then lyophilized to give a white solid (45 mg, 90% yield). 1H NMR (400 MHz, D2O): δ = 7.90 (2H, CO-CH=CH-CO, m), δ = 3.90-3.50 (4H, CH2-CH2-NH, m), δ = 5.43-3.38 (72 H, heparin, m).
2.2.2 Synthesis of PEG-thiol (and Pluronic F127) derivatives
The synthesis of thiol functionalized PEG (average Mn 10,000 g/mol) is given as an example. PEG (average Mn 10,000 g/mol) was dried by removing residual water by azeotropic distillation with toluene before use. Under a flow of nitrogen, PEG (10 g, 2mmol), mercaptopropionic acid (4.24 g, 40 mmol), p-toluenesulfonic acid (76 mg, 0.4 mmol), DTT (154 mg, 1 mmol) were dissolved in toluene (200 mL). The reaction mixture was refluxed with stirring for 24 h. Toluene was removed under reduced pressure and the polymer was precipitated 3 times in cold acetone. After drying in vacuo, 9.0 g (90%) of white solid was recovered and stored at -20 °C for future use. The thiol functionality of the linear PEGs as determined via 1H NMR spectroscopy was 1.95. PEG-thiol derivatives: 1H NMR (CDCl3): δ = 4.27 (4H, CH2-O-C(O), m), 3.74-3.50 (bs, (CH2-CH2-O)n), 2.80-2.69 (8H, CH2-CH2-SH, m). Pluronic F127-thiol derivatives: 1H NMR (CDCl3): δ = 4.27 (4H, CH2-O-C(O), m), 3.74-3.28 ((CH2-CH2-O)n, (-O-CH2-CH-(CH3)-O)n, m,), 2.80-2.69 (8H, CH2-CH2-SH, m), 1.10-0.96 (m, (-O-CH2-CH-(CH3)-O)n)).
2.2.3 Hydrogel preparation
Four different concentrations of HMWH-PEG (3%, 4%, 5%, 6%) were prepared with maleimide:thiol molar ratio of 2:1. The functionality of maleimide in HMWH was 12, and the functionality of thiol in PEG was 1.95. HMWH and PEG were dissolved separately in PBS solutions and mixed uponvortexing to form hydrogels at the desired concentrations given above. Changes in viscoelasticity upon mixing were immediately apparent. For experiments to investigate growth factor binding and delivery, bFGF was added to the solution of PEG. The resulting solutions were incubated for 12 h with solutions of HMWH to allow association of bFGF with HMWH, as well as complete reaction between HMWH and PEG, although rheology experiments (below) indicate that hydrogel formation was complete within 180 minutes.
2.2.4 1H NMR spectroscopy
A Bruker DRX-400 NMR spectrometer (Bruker Daltonics, Billerica, MA) was used to collect all NMR spectra under standard quantitative conditions. CDCl3 or deuterium oxide were used as the NMR solvent and TMS or DSS as the reference.
2.2.5 Bulk rheology experiments
The time for onset of gelation and evolution of elasticity was determined via rheometry experiments at a constant temperature of 25 °C in a constant (0.02%) strain mode. The measurements were obtained with an AR-2000 rheometer (TA Instruments, New catstle, DE) using a 25.0 mm diameter parallel plate geometry and a 0.5 mm gap. In the time-sweep experiments, the moduli were measured at a constant frequency of 5 rad/s for 3 h. Freequency sweeps were performed on samples immediately after the time sweep at frequencies from 0.1 rad/s to 100 rad/s. All experiments were repeated in duplicate.
2.2.6 bFGF release experiments
bFGF release experiments were performed at 4°C in 24-well polystyrene assay plates (Corning Inc., Corning, NY), blocked with PBS containing 5% BSA and 0.05% Tween 20 (Sigma, St. Louis, MO). 280 μL of hydrogel was placed on the bottom of each well and 2 mL PBS was added carefully over each gel. 1 mL PBS was taken out of the well at 1, 2, 6 and 24 h and then each following day, followed by replacement with 1mL fresh PBS. The amount of bFGF in each sample was measured with a bFGF Quantikine kit (R&D Systems, Minneapolis, MN). The determined cumulative release of bFGF is based on the initial loading of bFGF in the gel. All experiments were repeated in duplicate.
3. Results and Discussion
3.1 Synthesis of maleimide-functionalized HMWH
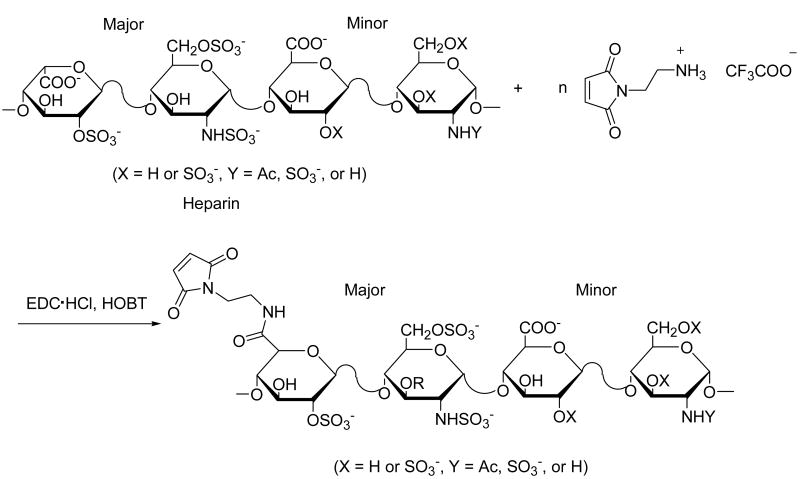
As in our earlier studies, the heparin intended for hydrogel formation was modified with maleimide groups, as the selectivity of the maleimide group near neutral pH for sulfhydryl reagents over amines (∼1000-fold difference in reaction rates) [24] offers advantages in the conjugation of maleimide-functionalized heparins to a variety of peptides and polymers. In our previous studies, functionalization of LMWH with a single maleimide group was of interest, and our synthetic modification strategy consisted of deacetylation of LMWH via hydrazine treatment, followed by reaction of the liberated free amines with an NHS ester-maleimide bifunctional reagent [18]. In the current studies, the potential for a higher degree of functionalization of heparin was desired, and therefore carboxylic acids of the heparin were modified with aminoethyl maleimide (AEM), using the general synthetic method shown in Scheme 1. The HMWH employed in these investigations had an average molecular weight of about 15 kDa, and therefore an average number of carboxylates of approximately 25 [5], providing a range of accessible degrees of functionalization and subsequent control of the rheological properties of the hydrogels. The maleimide-functionalized HMWH can then be readily employed in crosslinking reactions between thiolated polymers (see below).
Scheme 1.
Schematic of the synthesis of maleimide-functionalized HMWH.
Of the three primary methods employed for the conjugation of heparin to polymers and proteins – reaction at the reducing terminus, modification of free amines, or coupling to carboxylates – reaction at the reducing terminus is preferable, as it most closely mimics the covalent linkage of the GAG to proteoglycans in the ECM [25]. Indeed, in surface plasmon resonance (SPR) studies of the binding of heparin with various proteins, protein affinity was shown to be highest when heparin was immobilized in this manner, with decreasing affinity observed if heparin was immobilized via conjugation of amines or carboxylates [26]. Our early attempts to modify heparin via reaction at the reducing terminus were unsuccessful (only 15-20% conversion) [18], which lead us to choose reaction at the reactive sidegroups of the GAG chain. While modification of the carboxylates instead of amines has been shown to reduce protein binding by heparin [26], previous studies reported by Tae et al. and Cai et al. indicated that HMWH modified in this manner is still useful in the controlled release of growth factors [12,13]. These previous reports, coupled with our interests in producing heparins with various degrees of functionalization, provided further motivation for the selection of Scheme 1 as a strategy for the modification of HMWH.
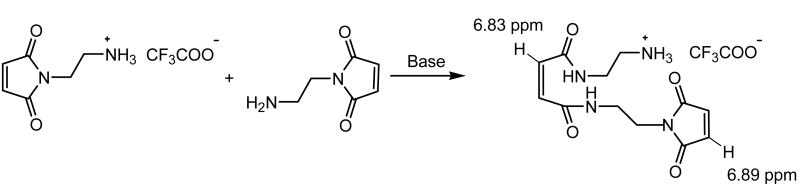
The activation of carboxylates for reaction with amines has been commonly achieved with N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC hydrochloride) and N-hydroxy succinimide (NHS) as catalysts in the amidation [27]. The O-acylisourea group generated via activation by the carbodiimide is converted into the NHS-activated carboxylic acid group, which is less susceptible to hydrolysis than the O-acylisourea and improves conversion. The use of these strategies for the modification of heparin has therefore been widely employed [14, 15]. Our initial attempts to obtain maleimide-functionalized HMWH upon reaction with AEM/EDC/NHS yielded quite variable results, however, because of two side reactions. Of primary difficulty was the ring opening reaction of AEM under the conditions employed for the amidation and neutralization of the AEM trifluoroacetate salt even when the pH was as low as 5. Under these conditions, free amines of AEM reacted with the maleimide of another AEM [28] (Scheme 3), which was confirmed via 1H NMR with the observation of the ring-opened product at 6.83 ppm along with the intact maleimide groups at 6.89 ppm, with a molar ratio between them of approximately 1/1. Despite the lower stability of alkyl maleimides toward hydrolysis relative to that of the cyclohexyl maleimides [29], hydrolysis of the maleimide was not indicated in these experiments, as the expected peaks at 5.60 ppm and 6.10 ppm were not observed under these reaction conditions. Although this side reaction did not preclude the maleimide functionalization of the HMWH, it complicated the ability to select a desired degree of functionalization in the modified HMWH product and motivated our efforts to identify a more reliable chemical modification strategy. A second side reaction that limited the conversion of carboxylates was the retention of NHS-activated carboxylates in the HMWH. Although NHS-activated carboxylic acid groups are readily hydrolyzed in the presence of weak base [30], under the conditions we employed to reduce the extent of ring opening, the NHS-activated carboxylic acid was not fully hydrolyzed, with the retention of the NHS-activated esters evident in 1H NMR spectrum at 2.70 ppm.
Scheme 3.
Schematic of the side reaction of AEM.
We therefore employed EDC and 1-hydroxybenzotriazole hydrate (HOBT) as the catalyst (Scheme 1), the resulting HOBT-activated carboxylic acid group could be hydrolyzed under desired mild conditions that minimized the ring opening side reactions of the AEM. Characterization of the modified HMWH via 1H NMR clearly shows the protons from the maleimide at 6.9 ppm, and the substantial decrease of any ring-opened product (the molar ratio between the maleimide and ring-opened product is greater than 9/1). 1H NMR also shows the complete absence of HOBT-activated carboxylates. Thus, the degree of functionalization (functionality) of the HMWH could be readily controlled, and easily evaluated via integration of the 1H NMR spectrum. An additional reaction variable that significantly affected the final functionality of the modified HMWH was the initial pH of the buffer, owing to its impact on changes in the hydrolysis and nucleophilicity of the AEM, although no significant ring opening was observed at pH value up to 7.0. Reactions were conducted at intermediate pH values (5.5-6.0) that minimized ring-opening and maximized nucleophilicity. Interestingly, small changes in pH permitted significant and controlled variation in the functionality, with representative results displayed in Table 1. As shown in the table, functionalization of heparin with ∼10 maleimide groups was observed at pH values of 6.0, while a reduction in pH value to 5.8 and 5.5 resulted in functionalities of ∼ 8 and ∼ 5, respectively.
Table 1.
Effect of the pH of MES buffer on the observed degree of functionalization of maleimide groups on HMWH.
| pH value | 5.5 | 5.8 | 6.0 |
| Functionality | 5.3 | 8.3 | 9.6 |
Molar ratio: COOH/AEM/EDC·HCl/HOBT = 1/1/3/3, r.t.
3.2 Synthesis of thiol-functionalized PEG
We have been interested in the formation of hydrogels containing both heparins and PEGs via noncovalent and covalent strategies owing to the molecular recognition properties of heparin, as mentioned above, and the hydrophilic and generally nonfouling properties of PEG [31]. A multitude of PEG-based hydrogel systems have been developed employing mainly free radical crosslinking [32, 33] as well as chemical transformations such as Michael addition across acrylates and vinyl sulfones [34]. PEG–based hydrogels have also prepared via γ-radiation or UV photopolymerization [35, 36]. Of relevance to our studies, thiol-functionalized star and linear PEGs are commercially available and provide a simple strategy for producing covalently crosslinked hydrogels via the above Michael addition reactions and also upon reaction with maleimide-functionalized HMWH. Thiol-maleimide conjugation chemistry has been widely used in the preparation of polymer-protein conjugates [37], as well as protein-lipid conjugates [38,39], and was therefore a logical choice for the efficient in situ formation of hydrogels. The multifunctionality possible for the maleimide-functionalized HMWH provided significant versatility for our investigations, as it permits the use of thiolated PEG crosslinks of various compositions and molecular masses to control the degradation and rheological properties of resulting hydrogels.
The straightforward synthetic route employed for the end-functionalization of commercially available PEGs with thiol groups is illustrated in Scheme 2. Formation of esters at the chain ends of the PEG was achieved via the reaction of hydroxyl-functionalized PEG with mercaptopropionic acid in toluene under reflux conditions, with p-toluene sulfonic acid as the catalyst [40]. DTT was employed during the reaction as a reducing agent to prevent disulfide bond formation. The thiolated polymer was easily isolated at 90% yield and characterization of the polymer via 1H NMR confirmed that the functionality of the PEG-thiol polymer was nearly 2. The thiolated polymer was expected to be competent for hydrogel formation in situ, upon reaction with the multifunctional maleimide-functionalized HMWH (f > 2). The ester linkages in the thiolated PEG also serve as degradation sites for the polymeric hydrogels in vivo, as previously indicated in other hydrogel systems. For example, work by Hubbell and coworkers recently demonstrated the biodegradability of these networks via slow hydrolysis of ester bonds adjacent to sulfide linkages, which leads to degradation on the order of days to weeks [41, 42]. Consistent with these repots, preliminary in vivo evaluation of the degradation behavior of the hydrogels here indicated degradation within 2 weeks (not shown).
Scheme 2.
Schematic of the synthesis of thiol functionalized PEG (average Mn 10,000 g/mol).
3.3 Hydrogel formation
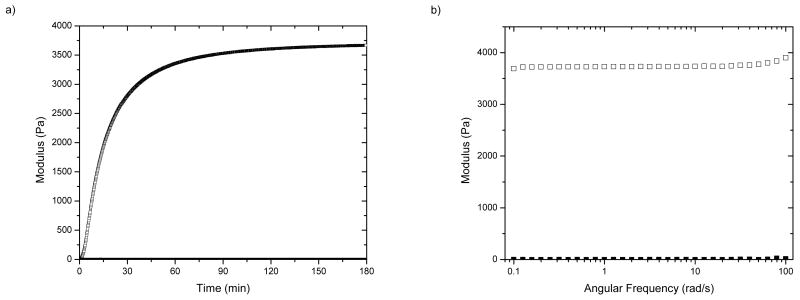
A primary goal of these investigations was the establishment of methods for the reliably tunable chemical modification of HMWH and the formation of hydrogels of various heparin content and rheological properties. A variety of methods have been employed for the covalent immobilization of heparin into gels, including crosslinking of dihydrazide-modified heparin with PEG [13], cinnamate-modified photopolymerized heparin [14], copolymerization of styrylated heparin with albumin [43] and collagen [15]. An advantage of the chemical strategies here is their rapid reaction times and selectivity, which will permit the incorporation of additional peptides and proteins with minimal reaction of free amines on the protein. The utility of these hydrogels in a variety of potential applications would be facilitated by rapid crosslinking, which would enable the injection of in-situ forming hydrogels, the fabrication of hydrogel particles via emulsion-based approaches, or the fabrication of gels of various shapes and aspect ratios. We therefore investigated, via oscillatory rheometry, the kinetics of formation of the hydrogels upon mixing of HMWH of different functionalities with thiolated star PEGs (average Mn 10,000 g/mol) or linear PEGs (produced as above, average Mn 4600 or 10,000 g/mol). Solutions of HMWH and thiolated PEG were co-injected into the plate gap of the rheometer, and both G′ and G″ were measured as a function of time at constant frequency (ω = 5 rad/s). All experiments were conducted at room temperature unless otherwise specified. Representative results for hydrogel formation between HMWH (f = 12) and a linear PEG (average Mn 10,000 g/mol) at a total polymer concentration of 6wt% are shown in Figure 1a; the ratio of maleimide:thiol in these and subsequent studies is approximately 2:1. As shown in the figure, hydrogels are formed rapidly as expected, with a maximum elastic modulus of 3700 Pa achieved within 180 minutes under these conditions. (G′ values greater than 10,000 Pa are possible with variations in polymer composition and concentration (below).) The elastic modulus increased rapidly without any increase in the loss modulus, indicating the highly elastic and expected character of the hydrogels. Hydrogel formation time ranged from immediate (i.e., too rapidly to measure via rheometry) for HMWH of high functionality (f = 12) at high polymer concentrations (10 wt%), to 1 hour for HMWH of low functionality (f = 4) and lower polymer concentration (3 wt%). As expected, faster gelation times were observed for HMWH crosslinked with the star-PEGs. Frequency sweep measurements of the hydrogels were also conducted, and representative results are shown in Figure 1b. As shown in the data, the storage modulus (G′) exceeds the loss modulus (G″) at all frequencies, confirming the existence of a crosslinked elastic network in these polymeric hydrogel materials, as expected.
Figure 1.
Oscillatory rheometry characterization of HMWH-PEG (average Mn 10,000 g/mol) hydrogels (6 wt%). HMWH (f = 12), molar ratio maleimide/thiol = 2/1. (a) Storage (G′, open symbol) and loss (G″, solid symbol at bottom) moduli as a function of gelation time. (b) Frequency sweep measurements of the hydrogel, showing both storage (open symbol) and loss (closed symbol) moduli. Data shown is the average of two sets of measurements with an average error of 10%.
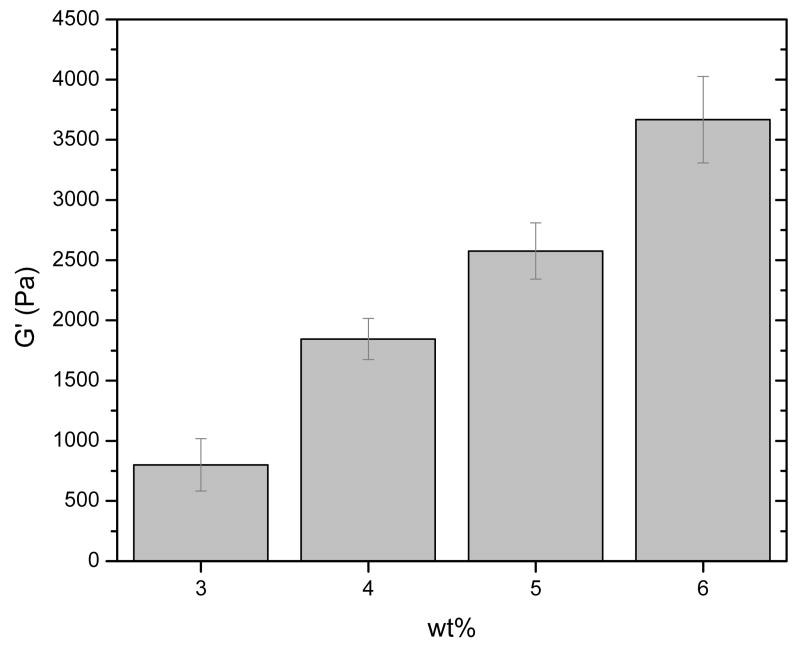
The impact on elastic properties by variables such as hydrogel concentration, polymer molecular mass, polymer hydrophobicity, and temperature was also investigated, as is illustrated in more detail in Figures 2 and 3. Figure 2 illustrates the impact of polymer concentration on the resulting elastic moduli of various gels produced via reaction of HMWH (f = 12) with PEG (average Mn 10,000 g/mol). As shown in the data, elastic moduli ranging from 800Pa to 3700Pa are readily obtained with small changes in hydrogel concentration ranging from 3 to 6 wt%. Variations in the functionality of HMWH also had measurable effects on G′ in hydrogels in which other variables were kept constant (data not shown), as expected. For example, in 6 wt% hydrogels comprising HMWH and PEG (average Mn 10,000 g/mol), the G′ of a HMWH (f = 6)-PEG hydrogel was 2120 Pa, while the G′ of a HMWH (f = 12)-PEG hydrogel increased to 3700 Pa.
Figure 2.
Comparison of storage moduli (G′) of HMWH-PEG (average Mn 10,000 g/mol) hydrogels at different concentrations. HMWH (f = 12), molar ratio maleimide/thiol = 2/1.
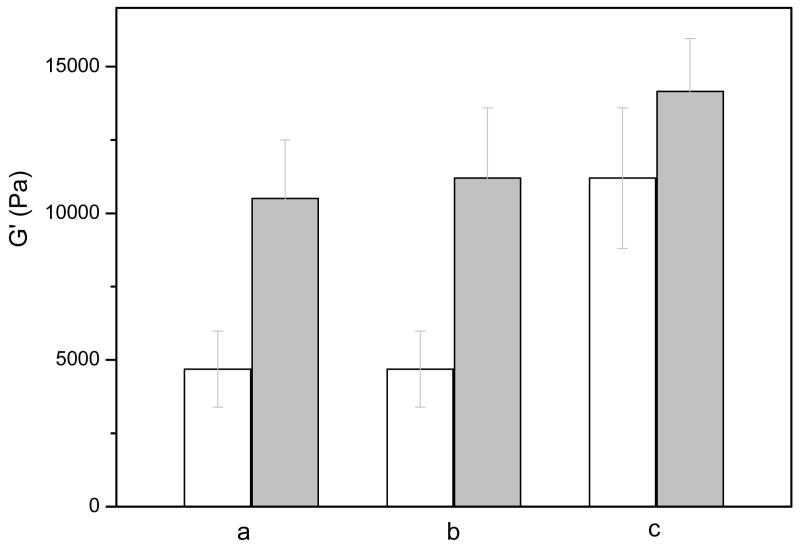
Figure 3.
Comparison of storage moduli (G′) of HMWH-Polymer hydrogels under different conditions. These experiments were conducted with 10 wt% hydrogels comprising HMWH (f = 8) and a molar ratio of maleimide/thiol = 1.7 /1. The polymer component was varied as given below. (a) HMWH-PEG (average Mn 10,000 g/mol) and HMWH-PEG (average Mn 4,600 g/mol), 25 °C; (b) HMWH-PEG (average Mn 10,000 g/mol) and HMWH-Pluronic F127, 25 °C; (c) HMWH-Pluronic F127 at 25 °C and 37 °C. The relatively large error in this particular set of measurements likely arises from a small amount of dimerization between PEG polymer end groups, as assessed via 1H NMR, during the elapsed time between the two sets of measurements.
The data in Figure 3 illustrate the impact of additional variables on the mechanical properties of the hydrogels; these gels were made at 10wt% polymer concentration in order to further increase the range of compositions investigated. In these studies, the functionality of the HMWH is 8, and the maleimide:thiol ratio is 1.7:1. As shown in Figure 3, an increase in the elastic modulus was observed upon reduction in the MW between crosslinks (reduction of PEG MW), as expected. Specifically, the G′ of a HMWH-PEG (average Mn 10,000 g/mol) hydrogel was 4690 Pa, while the G′ of HMWH-PEG (average Mn 4,600 g/mol) increased to 10500 Pa (Figure 3a). Such changes in hydrogel properties with changes in crosslink density have been regularly employed to alter the delivery properties of various hydrogels. For example, Wettering et al. have also studied protein drug delivery from gels in which PEG with different Mn were employed. The crosslink density of the hydrogels was controlled by variations in the molecular weights of PEG, and had marked impact on the rate of drug diffusion [42]. Similar impact is expected for the heparin-based hydrogels here.
Given the high charge density of the heparin employed in these networks, swelling of the hydrogels is significant, particularly for the hydrogels of lower polymer concentration and lower heparin functionality. For example, the 3 wt% HMWH(f = 6)-PEG(average Mn 10,000 g/mol) hydrogel was observed to swell 50%, as estimated via changes in volume, when incubated in PBS solution after hydrogel formation. Therefore, we were also interested in strategies that would reduce swelling of the hydrogels without the need for an extremely high functionality of HMWH, as a higher extent of modification of carboxylate groups could negatively impact the ability of HMWH to sequester and protect growth factors. Toward these ends, PEO-b-PPO-b-PEO triblocks have been initially employed as the crosslinking polymers, to increase hydrophobicity (and reduce swelling) and provide temperature sensitivity. The thiolated PEO-b-PPO-b-PEO triblock copolymers were produced via the strategies shown in Scheme 2, via the use of an FDA-approved commercially available PEO-b-PPO-b-PEO (Pluronic F127) with a average molecular weight of 12,700 g/mol and comprising 50% PEO. An aqueous solution of Pluronic F127 of 20 wt% has a sol–gel transition near room temperature [44], suggesting the potential for similar temperature-responsive behavior in our studies. As shown in Figure 3b, in 10wt% HMWH (f = 8.3)-PEG (10,000) hydrogels, the measured G′ is 6690 Pa, while for HMWH (f = 8.3)-Pluronic F127-based gels, the G′ increased to 10,970 Pa. (Figure 3b). The Pluronic F127-containing hydrogels were also sensitive to increasing temperature, with the G′ increasing to 12,160 Pa (from 10,970 Pa) when the temperature was increased to 37 °C (Figure 3c). These results are consistent with expected behavior of such materials based on previous literature reports. For example, Choi et al. studied the thermally responsive swelling of PEO-b-PPO-b-PEO/heparin composite nanocapsules, which exhibited a significant volume transition over a temperature range of 25∼33 °C [45]. These results suggest the opportunities to optimize heparin functionality and mechanical properties via tuning of the length of the PPO block in these hydrogel systems. Furthermore, taken together, this set of results indicates the flexibility of these approaches for producing heparin-based hydrogel formulations with a range of mechanical properties that could then be further tuned by additional noncovalent assembly and that are relevant to multiple soft tissue engineering and drug delivery applications [46].
The ultimate goal of these investigations is the illustration of a combination of covalent and noncovalent crosslinks in the assembly of hydrogels and the resulting impact on their mechanical properties and responsive release profiles. The initial stages of our studies suggest the ability to reliably tune the moduli of these hydrogels with properties that could be augmented with secondary, noncovalent crosslinking with dimeric, heparin-binding growth factors. The interactions of growth factors and heparin have appropriate kinetics to support the formation of elastic networks [22], and it is reasonable to predict that these interactions should also provide structural crosslinks and augment mechanical properties of crosslinked hydrogels. Indeed, the ability of noncovalent peptide-GAG interactions to provide measurable and beneficial mechanical properties to covalently crosslinked systems has been demonstrated in studies of star PEG-heparin-binding peptide (HBP) conjugates covalently crosslinked with dithiol-modified peptides and noncovalently crosslinked via the interactions of heparin with the HBP termini of the multi-arm PEG. [19]. In these systems, the noncovalent crosslinks provide a more rapid crosslinking motif that is temperature sensitive and that significantly increases the mechanical integrity of the hydrogels, with moduli of the range of those reported here. Although the structural impact of GF binding to HMWH-PEG hydrogels is not of primary interest in our investigations, these previous studies support the feasibility of our approach and, in concert with our recent work [22], suggest that removal of the noncovalent crosslinks could cause measurable changes of hydrogel properties. Such crosslink removal under pathological or wound conditions could therefore contribute to the targeted delivery and degradation of these networks in desired therapeutic settings.
3.4 Growth factor release
Given our ultimate goal of noncovalent immobilization and controlled release of growth factors via receptor-ligand interactions, the growth factor delivery profiles of the heparinized hydrogels are of interest. Toward these ends, the release of bFGF, which is known to induce the proliferation of a wide range of cells including endothelial cells, fibroblasts, smooth muscle cells, and chondrocytes [47], from hydrogels of various compositions was investigated in in vitro assays. In these assays, hydrogels of various polymer concentrations ranging from 3 wt% to 6 wt% were investigated; the heparin:GF ratio in the hydrogels was also varied from 27,250:1 to 109,000:1. In these initial studies, growth factor-loaded hydrogels were incubated in buffer, and aliquots of the GF-containing buffer were removed at various timepoints over the course of one week, followed by analysis via immunochemical assay to determine the amount of growth factor released. The cumulative release of bFGF from the hydrogels as a function of time is shown in Figure 4.
Figure 4.
Comparison of bFGF release from HMWH-PEG (average Mn 10,000 g/mol) hydrogels of differing compositions. In these hydrogels, HMWH (f = 12) was reacted with dithiol PEG at a molar ratio of maleimide/thiol = 2/1. (a) represents a 3 wt% hydrogel with G′ = 800Pa and HMWH/bFGF = 54,500/1; (b) represents a 4 wt% hydrogel with G′ = 1700Pa and HMWH/bFGF = 54,500/1; (c) represents a 3 wt% hydrogel with G′ = 800Pa and HMWH/bFGF = 109,000/1; (d) represents a 6 wt% hydrogel with G′ = 3700Pa and HMWH/bFGF = 27,250/1.
As shown in Figure 4, the release of growth factor from the hydrogels shows very little to slight burst release, with only 20-30% of the total bFGF released from the hydrogel during the course of 7 days. Both the polymer concentration and the heparin:GF ratio are indicated to effect the release of bFGF in these experiments. Comparisons of the 3 wt% and 4 wt% hydrogels via rheometry reveal that the G′ is increased 2-fold (from 800 Pa to 1700 Pa). Assessments of bFGF release from these two hydrogels under conditions in which the heparin:GF ratio is the same (54,500:1) clearly shows that the 3 wt% hydrogel of lower concentration and modulus exhibits a higher rate of release, releasing nearly 35% of the loaded bFGF in contrast to the approximately 20% release of bFGF from the 4 wt% gel. The similarity of the burst release in these two hydrogels suggests that the heparin:GF stoichiometry plays a larger role in the initial release than the mechanical properties of the hydrogel; additional investigation of these types of delivery vehicles is required to substantiate this trend. The impact of heparin:bFGF molar ratio on release is also illustrated in the data shown in Figure 4. Specifically, the 3 wt% hydrogels with different molar ratios show different rates of bFGF release, with the hydrogel of the higher molar ratio of heparin:bFGF (109,000:1, Figure 4, line c) yielding a lower burst release and a lower overall rate of bFGF release than the hydrogel of a lower heparin:bFGF ratio (54,500:1, Figure 4, line a). The data in Figure 4 also illustrate the dependence of release on polymer concentration. A 6wt% hydrogel carrying the lowest heparin:GF ratio shows the slowest rate of release of the hydrogels (Figure 4, line d), indicating that both heparin:GF ratio and polymer concentration can be tuned to modulate GF release.
The well controlled release from the hydrogels results in large part from the high heparin:GF ratios employed during these experiments, consistent with previous reports by us and others. For example, early theoretical and experimental studies by Sakiyama et al. showed that heparin:bFGF ratios of only 1000:1 are required to reduce the release of bFGF to 1.4% of that observed via passive diffusion from fibrin-based gels [10]. Similarly, covalently crosslinked PEG-LMWH hydrogels with LMWH:GF ratios of 13,000:1 were shown to eliminate burst release and show sustained and slow delivery of approximately 10% of loaded bFGF over the course of 16 days, in contrast to gels with lower heparin content, which showed increasing burst and overall release (80%) [18,47]. Controlled release of VEGF from heparin-PEG hydrogels over the course of 3 weeks in gels with a heparin:GF ratio of 2,000:1 has also been demonstrated in the related work of Tae et al.; the release profiles obtained from these formulations were further shown to support vascularization in in vivo studies [13]. The release profiles observed in our experiments are also comparable to those reported by Pikea et al. for release of bFGF from heparinized, hyaluronic acid-based hydrogels of similar growth factor loading and heparin content [48]. While it may be possible that available thiols in the bFGF [49] promote covalent attachment of bFGF to the gels via the reaction of free cysteines to the maleimide-functionalized heparin, as suggested in this report [48], the steady increase in the amount of bFGF released over the course of these experiments suggests that such immobilization is not a primary factor in the release profiles observed here.
Importantly, the rates of release shown here are similar to those reported for other heparin-containing delivery systems that have demonstrated utility for stimulation of endothelial cell growth in vitro and neovascularization in vivo [13,14,17,47,48], suggesting their potential use in such applications. The broad utility of heparin in binding multiple proteins, coupled with the potential impact of such binding on mechanical properties [19], offers strategies for tailoring the properties of the gels via the inclusion of multiple heparin-binding proteins. Furthermore, the demonstrated potential for selective release of noncovalent protein-based crosslinks via interactions with cell surface receptors [22], coupled with expanding opportunities to design proteins and peptides with specific binding and biological function, will offer new strategies in the design of materials with controlled and responsive protein delivery profiles and mechanical behaviors. Such investigations are underway and will be the subject of future reports.
4. Conclusions
These studies illustrate the potential for PEG-heparin hydrogels of various moduli and growth factor delivery profiles to be easily produced. Straightforward chemical methods employing EDC/HOBT catalysts were identified to minimize deleterious side reactions that reduce functionality and reproducibility of the reaction, and slight variations in pH were illustrated to be potential variables for controlling the final functionality of the heparin. The hydrogels formed via the reaction of thiolated PEGs and maleimide-functionalized heparin showed rates of formation that could be tuned depending on the composition of the gel, a feature that may prove useful for applications requiring injectable or preformed hydrogels. The rheological properties of these hydrogels can be readily tuned over ranges of 100's Pa to ∼12,000 Pa with slight changes in polymer concentration, composition, and HMWH functionality, while still offering opportunities for construction of hydrogels with properties useful for various drug delivery and soft tissue engineering applications. Finally, the controlled release of bFGF was studied as a function of hydrogel modulus and heparin/bFGF molar ratio, with results illustrating the potential for controlled release of the bFGF over various timescales appropriate for different wound healing and vascular therapies. The combination of these hydrogel synthesis methods and our previously demonstrated GF-mediated hydrogel assembly and receptor-targeted erosion strategies offers significant opportunities in the controlled release and delivery-mediated erosion of an expanded set of heparinized materials.
Acknowledgments
This work has been supported by grants from the National Institutes of Health (1RO1 EB00317201), the National Science Foundation (DGE-0221651), and the Arnold & Mabel Beckman Foundation. Le Zhang, Tuna Yucel, Sung Hye Kim are thanked for helpful discussions and assistance with experimental protocols.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 3.Brandl F, Sommer F, Goepferich A. Rational design of hydrogels for tissue engineering: Impact of physical factors on cell behavior. Biomaterials. 2007;28:134–146. doi: 10.1016/j.biomaterials.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Lee KY, Mooney DJ. Hydrogels for Tissue Engineering. Chemical Reviews. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 5.Capila I, Linhardt RJ. Heparin – Protein interactions. Angew Chem Int Ed. 2002;41:390–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Fromm JR, Hileman RE, Caldwell EEO, Weiler JM, Linhardt RJ. Pattern and spacing of basic amino acids in heparin binding sites. Arch Biochem Biophys. 1997;343(1):92–100. doi: 10.1006/abbi.1997.0147. [DOI] [PubMed] [Google Scholar]
- 7.Kamei K, Wu XF, Xu XY, Minami K, Huy NT, Takano R, Kato H, Hara S. The analysis of heparin-protein interactions using evanescent wave biosensor with regioselectively desulfated heparins as the ligands. Anal Biochem. 2001;295(2):203–213. doi: 10.1006/abio.2001.5193. [DOI] [PubMed] [Google Scholar]
- 8.Fromm JR, Hileman RE, Weiler JM, Linhardt RJ. Interaction of fibroblast growth factor-1 and related peptides with heparan sulfate and its oligosaccharides. Arch Biochem Biophys. 1997;346(2):252–262. doi: 10.1006/abbi.1997.0299. [DOI] [PubMed] [Google Scholar]
- 9.Nugent M, Iozzo R. Fibroblast growth factor-2. The International Journal of Biochemistry & Cell Biology. 2000;32:115–120. doi: 10.1016/s1357-2725(99)00123-5. [DOI] [PubMed] [Google Scholar]
- 10.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. Journal of Controlled Release. 2000;65(3):389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Furst EM, Kiick KL. Manipulation of hydrogel assembly and growth factor delivery via the use of peptide-polysaccharide interactions. J Control Release. 2006;114(2):130–142. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai S, Liu Y, Shu XZ, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Tae GY, Scatena1 M, Stayton P, Hoffman A. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J Biomater Sci Polymer Edn. 2006;17:187–197. doi: 10.1163/156856206774879090. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura M, Ishihara, Obara K, Masuoka K, Ishizuka T, Kanatani Y, Takase B, Matsui T, Hattori H, Sato T, Kariya Y, Maehara1 T. Controlled release of fibroblast growth factor-2 from an injectable 6-O-desulfated heparin hydrogel and subsequent effect on in vivo vascularization. J Biomed Mat Res. 2006;78A(Part A):364–37. doi: 10.1002/jbm.a.30688. [DOI] [PubMed] [Google Scholar]
- 15.Wissink MJB, Beernink R, Pieper JS, Poot AA, Engbers GHM, Beugeling T, van Aken WG, Feijen J. Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation. Biomaterials. 2001;22:151–163. doi: 10.1016/s0142-9612(00)00164-2. [DOI] [PubMed] [Google Scholar]
- 16.Liu LS, Ng CK, Thompson AY, Poser JW, Spiro RC. Hyaluronate-heparin conjugate gels for the delivery of basic fibroblast growth factor (FGF-2) J Biomed Mater Res. 2002;62:128–135. doi: 10.1002/jbm.10238. [DOI] [PubMed] [Google Scholar]
- 17.Chinen N, Tanihara M, Nakagawa M, Shinozaki K, Yamamoto E, Mizushima Y, Suzuki Y. Action of microparticles of heparin and alginate crosslinked gel when used as injectable artificial matrices to stabilize basic fibroblast growth factor and induce angiogenesis by controlling its release. J Biomed Mater Res. 2003;67A(Part A):61–68. doi: 10.1002/jbm.a.10061. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi N, Kiick KL. Polysaccharide-Poly(ethylene glycol) Star Copolymer as a Scaffold for the Production of Bioactive Hydrogels. Biomacromolecules. 2005;6:1921–1930. doi: 10.1021/bm050003+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seal BL, Panitch A. Viscoelastic Behavior of Environmentally Sensitive Biomimetic Polymer Matrices. Macromolecules. 2006;39(6):2268–227. [Google Scholar]
- 20.Seal BL, Panitch A. Physical Polymer Matrices Based on Affinity Interactions between Peptides and Polysaccharides. Biomacromolecules. 2003;4:1572–1582. doi: 10.1021/bm0342032. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi N, Chae B, Zhang L, Kiick KL, Furst EM. Rheological Characterization of Polysaccharide-Poly(ethylene glycol) Star Copolymer Hydrogels. Biomacromolecules. 2005;6:1931–1940. doi: 10.1021/bm0500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi N, Zhang L, Chae B, Palla CS, Furst EM, Kiick KL. Growth Factor Mediated Assembly of Cell Receptor-Responsive Hydrogels. Journal of the American Chemical Society. 2007;129:3040–3041. doi: 10.1021/ja0680358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zetter B. Angiogenesis and Tumor Metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 24.Hermanson GT. Bioconjugate Techniques. Academic Press; New York: 1996. [Google Scholar]
- 25.Nadkarni VD, Pervin A, Linhardt RJ. Directional Immobilization of Heparin onto Beaded Supports. Analytical Biochemistry. 1994;222:59–67. doi: 10.1006/abio.1994.1454. [DOI] [PubMed] [Google Scholar]
- 26.Osmond RI, Kett WC, Skett SE, Coombe DR. Protein–heparin interactions measured by BIAcore 2000 are affected by the method of heparin immobilization. Anal Biochem. 2002;310:199–207. doi: 10.1016/s0003-2697(02)00396-2. [DOI] [PubMed] [Google Scholar]
- 27.Olde Daminka LHH, Dijkstraa PJ, van Luyn MJA, van Wachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17(8):765–773. doi: 10.1016/0142-9612(96)81413-x. [DOI] [PubMed] [Google Scholar]
- 28.Keller O, Rudinger J. Preparation and some properties of maleimido acids and maleoyl derivatives of peptides. Helv Chim Acta. 1975;58:531–541. doi: 10.1002/hlca.19750580224. [DOI] [PubMed] [Google Scholar]
- 29.Hashida S, Imagawa M, Inoue S, Ruan KH, Ishikawa E. More useful maleimide compounds for the conjugation of Fab' to horseradish peroxidase through thiol groups in the hinge. J Appl Biochem. 1984;6(12):56–63. [PubMed] [Google Scholar]
- 30.Hosoda H, Fukuda K, Gotoh Y. Enzyme labeling in steroid enzyme immunoassays. Comparison of the p-nitrophenyl ester and N-succinimidyl ester methods. Chem Pharm Bull (Tokyo) 1991;39(9):2373–2377. doi: 10.1248/cpb.39.2373. [DOI] [PubMed] [Google Scholar]
- 31.Hooftman G, Herman S, Schacht E. Review: poly(ethylene glycol)s with reactive endgroups. II. Practical consideration for the preparation of protein-PEG conjugates. J Bioact Compat Polymers. 1996;11:135–159. [Google Scholar]
- 32.Sawhney AS, Pathak CP, Hubbell JA. Bioerodible hydrogels based on photopolymerized poly (ethylene glycol)-co-poly (α-hydroxy acid) diacrylate macromers. Macromolecules. 1993;26:581–583. [Google Scholar]
- 33.Elbert DL, Hubbell JA. Conjugate addition reactions combined with free-radical cross-linking for the design of materials for tissue engineering. Biomacromolecules. 2001;2:430–441. doi: 10.1021/bm0056299. [DOI] [PubMed] [Google Scholar]
- 34.Elbert DL, Pratt AB, Lutolf MP, Halstenberg S, Hubbell JA. Protein delivery from materials formed by self-selective conjugate addition reactions. J Control Release. 2002;76:11–25. doi: 10.1016/s0168-3659(01)00398-4. [DOI] [PubMed] [Google Scholar]
- 35.Andreopoulos FM, Deible CR, Stauffer MT, Weber SG, Wagner WR, Beckman EJ, et al. Photoscissable hydrogel synthesis via rapid photopolymerization of novel PEG-based polymers in the absence of photoinitiators. J Am Chem Soc. 1996;118:6235–6240. [Google Scholar]
- 36.Williams CG, Kim TK, Taboas A, Malik A, Manson P. In Vitro Chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Engineering. 2003;9(4):679–689. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 37.Harris JM, Kozlowski A. Poly(ethylene glycol) derivatives with proximal reactive groups. US Patent: 6, 664, 331. 2003 December 16;
- 38.Kirpotin D, Park JW, Hong K, Zalipsky S, Li W, Carter P, Benz C, Papahadjopoulos D. Sterically Stabilized Anti-HER2 Immunoliposomes: Design and Targeting to Human Breast Cancer Cells in Vitro. Biochemistry. 1997;36:66–75. doi: 10.1021/bi962148u. [DOI] [PubMed] [Google Scholar]
- 39.Maruyama K, Takahashi N, Tagawa T, Nagaike K, Iwatsuru M. Immunoliposomes bearing polyethyleneglycol-coupled Fab' fragment show prolonged circulation time and high extravasation into targeted solid tumors in vivo. FEBS Lett. 1997;413:177–180. doi: 10.1016/s0014-5793(97)00905-8. [DOI] [PubMed] [Google Scholar]
- 40.Baskar G, Venkatesan S, Dhathathreyan A, Mandal A. Two-Dimensional Surface Properties of 2-Methoxy Ethyl Oleate at the Air/Water Interface. J Am Oil Chem Soc. 1999;76(7):853–858. [Google Scholar]
- 41.Schoenmakers RG, van de Wetering P, Elbert DL, Hubbell JA. The effect of the linker on the hydrolysis rate of drug-linked ester bonds. J Control Release. 2004;95:291–300. doi: 10.1016/j.jconrel.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 42.van de Wetering P, Metters AT, Schoenmakers RG, Hubbell JA. Poly(ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteins. J Control Release. 2005;102:619–627. doi: 10.1016/j.jconrel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 43.Matsuda T, Magoshi T. Preparation of Vinylated Polysaccharides and Photofabrication of Tubular Scaffolds as Potential Use in Tissue Engineering. Biomacromolecules. 2002;3:942–950. doi: 10.1021/bm0200229. [DOI] [PubMed] [Google Scholar]
- 44.Alexandridis P, Hatton TA. Poly(ethylene oxide)–poly(propylene oxide)–poly (ethylene oxide) blockcopolymer surfactants in aqueous solutions and at interfaces: thermodynamics, structure, dynamics, and modeling. Colloid Surf A. 1995;96:1–46. [Google Scholar]
- 45.Gajewiak J, Cai S, Shu XZ, Prestwich GD. Aminooxy Pluronics: Synthesis and Preparation of Glycosaminoglycan Adducts. Biomacromolecules. 2006;7(6):1781–1789. doi: 10.1021/bm060111b. [DOI] [PubMed] [Google Scholar]
- 46.Saltzman WM. Tissue engineering: engineering principles for the design of replacement organs and tissues. Oxford University Press; US: 2004. p. 121. [Google Scholar]
- 47.Wissink MJB, Beernink R, Poot AA, Engbers GHM, Beugeling T, van Aken WG, Feijen J. J Controlled Release. 2000;64:103–114. doi: 10.1016/s0168-3659(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 48.Pikea DB, Cai S, Pomraninga KR, Firpoc MA, Fisher RJ, Shub XZ, Prestwich GD, Peattie RA. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials. 2006;27:5242–5251. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 49.Eriksson AE, Cousens LS, Weaver LH, Matthews BW. Three dimensional structure of human basic fibroblast growth factor. Proc Natl Acad Sci. 1991;88:3441–3445. doi: 10.1073/pnas.88.8.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]