Abstract
Acquisition of T cell responses during primary CMV infection in lung transplant recipients (LTRs) appear critical for host defense and allograft durability, with increased mortality in donor+/recipient− (D+R−) individuals. In 15 D+R− LTRs studied, acute primary CMV infection was characterized by viremia in the presence or absence of pneumonitis, with viral loads higher in the lung airways/allograft compared with the blood. A striking influx of CD8+ T cells into the lung airways/allograft was observed, with inversion of the CD4+:CD8+ T cell ratio. De novo CMV-specific CD8+ effector frequencies in response to pooled peptides of pp65 were strikingly higher in lung mononuclear cells compared with the PBMC and predominated over IE1-specific responses and CD4+ effector responses in both compartments. The frequencies of pp65-specific cytokine responses were significantly higher in lung mononuclear cells compared with PBMC and demonstrated marked contraction with long-term persistence of effector memory CD8+ T cells in the lung airways following primary infection. CMV-tetramer+CD8+ T cells from PBMC were CD45RA− during viremia and transitioned to CD45RA+ following resolution. In contrast, CMV-specific CD8+ effectors in the lung airways/allograft maintained a CD45RA− phenotype during transition from acute into chronic infection. Together, these data reveal differential CMV-specific CD8+ effector frequencies, immunodominance, and polyfunctional cytokine responses predominating in the lung airways/allograft compared with the blood during acute primary infection. Moreover, we show intercompartmental phenotypic differences in CMV-specific memory responses during the transition to chronic infection.
Cytomegalovirus, a member of the β-herpesvirus family, is a major complicating opportunistic infection in solid organ transplant (SOT)3 recipients, particularly in lung transplant recipients (LTRs) as well as hematopoietic cell transplant recipients (1, 2). Active CMV infection is associated with acute cellular rejection episodes in SOT recipients through unclear mechanisms, with CMV pneumonitis and viremia recognized as risk factors for the development of chronic lung allograft rejection or the bronchiolitis obliterans syndrome in LTRs (3-5). The observation of enhanced susceptibility to CMV in LTRs may be due in part to the lung being a major reservoir for latent virus, with activation occurring posttransplant in the setting of sustained immunosuppression and/or a naive host (6). In this regard, donor+/recipient− (D+R−) SOT recipients are at highest risk for active CMV infection, with this serologically mismatched group noted to have increased mortality among LTRs (7, 8). Thus, it is important to understand the adaptive host T cell response to CMV in D+R− LTRs, as these responses might be critical not only for host defense but perhaps long-term allograft durability. Moreover, adaptive immune responses might also impact clinicopathology during active viral infection (9).
Despite maintenance immunosuppression in SOT recipients, most often with calcineurin inhibitor-based multidrug therapy, several groups have reported the development of CMV-specific CD4+ and CD8+ T cell responses in the peripheral blood of D+R− SOT recipients with primary infection (10, 11). We recently reported the long-term persistence of CMV-specific T cell responses in the blood, as well as in the lung allograft in a small number of subjects, following primary CMV infection in LTRs (12). However, the acquisition of de novo CMV-specific T cell responses in a human organ allograft during primary infection has not been prospectively investigated in a population of susceptible patients. To this end, lung transplantation offers a distinct opportunity as the only solid organ allograft that can be readily sampled for the development of viral-specific responses over time using lung mononuclear cells (LMNC) obtained by routine bronchoalveolar lavage (BAL) for comparison to viral-specific responses in the blood. Moreover, D+R− LTRs demonstrate a predictably high incidence of primary CMV infection, providing a unique opportunity to study the development of viral T cell memory in humans (13). For these reasons, we prospectively studied the acquisition of CMV-specific T cell memory in 15 D+R− LTRs with primary CMV infection posttransplant.
Several recent studies in mice have shown the lung airways and lung parenchyma to be major sites for trafficking of primary effector and effector memory T cells (14-16). In our human model of primary CMV infection, we hypothesized that significant differences would be detectable in the primary CMV-specific T cell responses in the LMNC compared with PBMC during acute primary infection. In this article, we report our findings of marked differences in CMV-specific T cell effector responses in the lung airways/allograft compared with the blood in terms of effector frequencies, function, immunodominance, and phenotype during the transition from acute primary CMV infection into chronic infection, with CMV-specific CD8+ T effector responses in the lung airways/allograft predominating during acute infection.
Materials and Methods
Subjects and tissue samples
Lung transplant recipients in the Johns Hopkins Lung Transplantation Clinic (Baltimore, MD) were identified based on immediate pretransplant CMV serology mismatch status (D+R−), and study candidates consented for study participation using an Institutional Review Board-approved consent form and enrolled in a prospective study over a 3-year period. All patients were treated with standard maintenance, three-drug immunosuppression at the time of analyses (see Table I), and although calcineurin inhibitor doses were variable among patients, all patients had therapeutic levels at the time of study. All patients undergo routine surveillance bronchoscopy at 1, 3, 6, 9, 12, 18, and 24 mo posttransplant as well as any symptomatic clinical episodes, including the detection of CMV viremia. All patients were treated with routine antiviral prophylaxis therapy for CMV (ganciclovir and/or valganciclovir) for the initial 3 mo posttransplant. Plasma CMV viral load was determined by the Johns Hopkins Hospital Clinical Virology Laboratory using Cobas CMV DNA quantitative PCR (Roche) before the discontinuation of antiviral therapy to confirm undetectable levels, and then every 2 wk. CMV disease episodes included CMV viremia and/or clinicopathologic evidence of end organ CMV disease, such as pneumonitis. CMV viremia was defined by plasma viral load (quantitative PCR). CMV pneumonitis is diagnosed based on a combination of clinical and radiographic findings, immunohistochemical criteria on transbronchial biopsy specimens, and/or evidence of viral replication in the BAL fluid using a CMV early Ag detection assay and/or BAL CMV viral load according to standard clinical definitions (17, 18).
Table I.
Characteristics of LTRs and Primary CMV Infection
| LTR | Age (years) | Gender | Primary Diagnosis | Immunosuppressiona | CMV Onsetb | Viremia | Pneumonitis |
|---|---|---|---|---|---|---|---|
| 21 | 27 | F | Pulmonary veno-occlusive disease | TAC 4/3.5; MMF 0.54; Pred 151 | 155 | + | + |
| 22 | 60 | F | Idiopathic pulmonary fibrosis | TAC 3.52; MMF 12; Pred 101 | 157 | + | + |
| 23 | 59 | M | Idiopathic pulmonary fibrosis | CSA 100/75; MMF 12; Pred 101 | 257 | + | + |
| 24 | 31 | F | Cystic fibrosis | TAC 1.52; MMF 0.53; Pred 101 | 96 | + | − |
| 25 | 34 | F | Primary pulmonary hypertension | TAC 42; MMF 0.52; Pred 101 | 129 | + | + |
| 28 | 33 | F | Cystic fibrosis | TAC 62; MMF 0.52; Pred 51 | 210 | + | − |
| 29 | 62 | F | Chronic obstructive pulmonary disease | TAC 2.52; MMF 0.53; Pred 101 | 168 | + | + |
| 30 | 61 | F | Idiopathic pulmonary fibrosis | CSA 175/100; RAPA 11; Pred 51 | 94c | − | − |
| 31 | 55 | M | Cystic fibrosis | TAC 32; AZA 501; Pred 51 | 248 | + | − |
| 32 | 60 | F | Idiopathic pulmonary fibrosis | TAC 2/1.5; MMF 0.52; Pred 7.51 | 149 | + | + |
| 33 | 51 | F | Idiopathic pulmonary fibrosis | TAC 22; MMF 0.53; Pred 7.51 | 186 | + | − |
| 34 | 59 | F | Chronic obstructive pulmonary disease | TAC 42; MMF 0.252; Pred 101 | 174 | + | + |
| 35 | 27 | M | Cystic fibrosis | TAC 12; MMF 0.52; Pred 7.51 | 219 | + | − |
| 36 | 49 | F | Idiopathic pulmonary fibrosis | TAC 42; MMF 0.252; Pred 101 | 167 | + | + |
| 37 | 56 | F | Obliterative bronchiolitis | TAC 52; MMF 0.54; Pred 101 | 133 | + | + |
Full-sized numbers indicate dosage and superscript numbers indicate times per day: AZA, azathioprine (dose in milligrams); CSA, cyclosporine (dose in milligrams); MMF, mycophenolate mofetil (dose in grams); Pred, prednisone (dose in milligrams); RAPA, sirolimus (dose in milligrams); TAC, tacrolimus (dose in milligrams).
Days posttransplant.
LTR no. 30 never developed viremia that was captured by routine screening but was noted to have de novo CMV-specific T cell responses at 94 days.
Cell preparation and Ag stimulation
PBMC were isolated from heparinized blood samples by density gradient centrifugation using Ficoll-Paque (Amersham Biosciences). BAL cells were obtained from BAL fluid (BAL LMNC). Pooled, overlapping, 15-aa peptides for pp65 (SwissProt accession no. P06725; 138 peptides) and IE1 (SwissProt accession no. P13202; 120 peptides) were gifts from Dr. D. Stroncek (Department of Transfusion Medicine, Clinical Center, National Institutes of Health, Bethesda, MD). PBMC and LMNC were cultured in round-bottom tissue culture tubes (Sarstedt) in the presence or absence of pp65 or IE1 pooled peptides (1 μg/ml) or the control staphylococcal enterotoxin B (Toxin Technology) at 1 μg/ml.
Flow cytometry
All stimulations for intracellular cytokine staining (ICS) were performed for 6 h at 37°C except in experiments using CMV-specific tetramers. Brefeldin A at 10 μg/ml (Sigma-Aldrich) was added for the final 4 h of culture as previously described (12). The following fluorochrome-labeled Abs were purchased from BD Biosciences: FITC-conjugated anti-TNF-α, PE-cyanine 7-conjugated anti-TNF-α, PE-conjugated anti-IL-2, PE-conjugated anti-CD4, PerCP-cyanine 5.5-conjugated anti-CD8, AmCyan-conjugated anti-CD8, allophycocyanin-conjugated anti-IFN-γ, PE-Alexa Fluor 700-conjugated anti-CD3, PerCP-conjugated anti-CD45RA, and appropriately conjugated isotype controls. The following PE-conjugated CMV-specific tetramers were purchased from Beckman Coulter: A1-VTEHDTLLY, A2-NLVPMVATV, B7-TPRVTGGGAM, B8-ELRRKMMYM, and B35-IPSINVHHY. Cell fluorescence was analyzed using a FACSCalibur or FACSAria (BD Biosciences) cytometer. Data were analyzed using FlowJo software (Tree Star).
Results
Primary CMV infection in lung transplant recipients is characterized by viremia in the presence or absence of pneumonitis
Because primary CMV infection in D+R− LTRs is associated with increased morbidity and mortality, we sought to assess the major features of active CMV infection in these individuals along with CMV-specific immunity. In a prospective D+R− LTR cohort, we identified primary CMV infection in 15 individuals as shown in Table I. All patients were receiving three-drug, calcineurin inhibitor-based immunosuppression therapy and had primary CMV infection within the first year posttransplant following routine discontinuation of viral prophylaxis, typically at 3 mo posttransplant. Subsequently, the development of de novo plasma viremia was detected in 14 of 15 LTRs using frequent blood CMV DNA viral load surveillance, with a mean duration of 26 days of viremia during primary infection. Upon detection of viremia, LTRs then underwent diagnostic bronchoscopy to assess for viral pneumonitis, with 9 of 15 LTRs (60%) having demonstrable evidence of pneumonitis using standard transbronchial biopsy tissue staining, rapid CMV viral culture, and standard clinicopathologic criteria. Thus, the presence of viremia, most often accompanied by a fatigue/malaise clinical syndrome, is very commonly detectable in primary CMV infection in the presence or absence of viral pneumonitis in LTRs. All 15 subjects were found to have seroconverted following primary infection, as assessed by positive CMV IgG using a specific ELISA. In addition to rapid CMV viral culture in BAL fluid, we also assessed the CMV DNA viral load in the BAL fluid supernatants at the time of primary CMV diagnosis and found that the mean CMV viral load in BAL supernatants was significantly increased over the paired plasma viral loads from the same day, as shown in Fig. 1.
FIGURE 1.
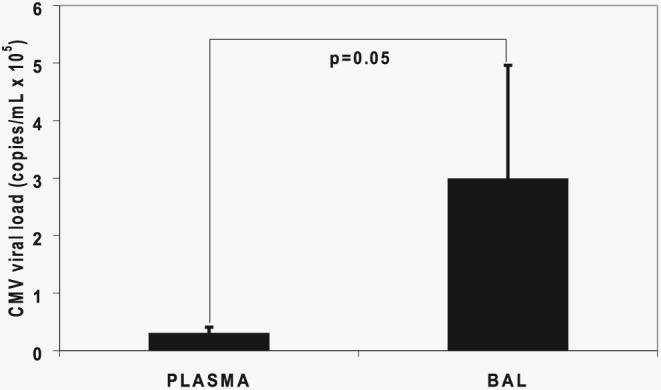
CMV viral loads are increased in the lung allograft compared with the plasma during primary infection. CMV viral loads in the plasma and BAL fluid were determined for the cohort of LTRs by quantitative PCR. Bars represent mean ± SEM viral loads in the respective compartments, and the p value was calculated by Wilcoxon signed-rank test.
A pulmonary CD8+ T cell influx with inversion of the CD4+:CD8+ ratio occurs during primary CMV infection
Next, to assess adaptive CMV-specific immunity during primary CMV infection, we first examined LMNC obtained from BAL fluid at diagnostic bronchoscopy for evidence of alterations in T cell subset populations. We compared LMNC from a pre-CMV infection time point (typically 3 mo posttransplant, before discontinuation of antiviral prophylaxis) to those obtained at the diagnosis of primary CMV infection as shown in a representative LTR in Fig. 2A. The lymphoid gates at pre-CMV infection time points were typically a small percentage (<5%) of total BAL cells. In striking contrast, however, during primary CMV infection a massive expansion of the lymphoid gate was observed with a predominant CD8+ > CD4+ T cell influx, resulting in an inversion of the LMNC CD4+:CD8+ ratio (Fig. 2, A and B), but not a significant change in the PBMC CD4+:CD8+ ratio (data not shown). To determine the relative changes in BAL CD4+ and CD8+ T cells, we compared these frequencies pre-CMV and during primary infection (Fig. 2C). Although both T cell subsets increased during primary infection, only the CD8+ population increased significantly across the cohort, consistent with our findings above. Together, these data demonstrate an increased CD8+ T cell influx into the lung airways/allograft with inversion of the pulmonary CD4+:CD8+ ratio during acute primary CMV infection.
FIGURE 2.
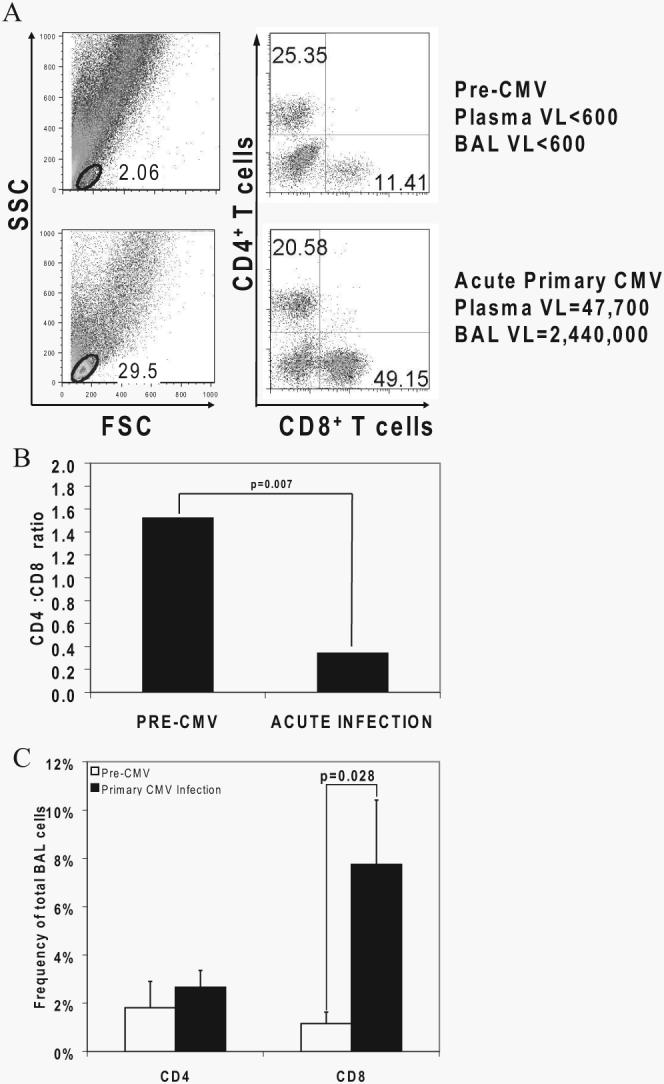
Massive CD8+ T cell expansion occurs in the lung allograft during primary CMV infection with inversion of the CD4+:CD8+ T cell ratio at the time of diagnosis. A, Representative plots of LMNC from LTR no. 29 at pre-CMV and primary CMV time points showing frequencies of CD3+CD4+ and CD3+CD8+ T cells from the lymphoid gates determined by forward scatter (FSC) and side scatter (SSC). VL, Viral load. B, Bars represent geometric means of the CD4+:CD8+ T cell ratio of LMNC from pre-CMV and acute primary CMV infection time points in 10 LTRs, and the p value was calculated by Wilcoxon signed-rank test. C, Bars represent frequencies of CD3+CD4+ and CD3+CD8+ T cells in BAL cells from 10 LTRs at pre-CMV and acute primary CMV infection time points, with p values calculated by Wilcoxon signed-rank test.
De novo CMV-specific CD8+ effector T cell responses directed toward pp65 Ag predominate during acute primary infection, with increased frequencies in the lung airways/allograft compared with the blood
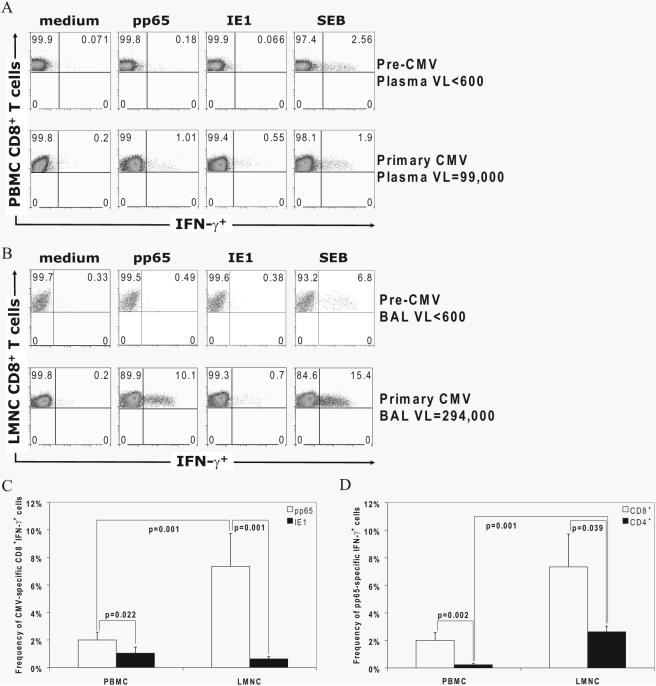
Given the marked influx of CD8+ T cells into the lung airways/allograft during primary CMV infection, we next asked whether de novo CMV-specific effector responses (IFN-γ, TNF-α, and IL-2) were detectable in the LMNC and PBMC. To do this, we performed in vitro restimulation using pooled overlapping peptides of the major immunodominant Ags pp65 or IE1, followed by ICS. As shown in a representative LTR in Fig. 3, A and B, de novo CMVspecific CD8+IFN-γ+ effector responses were detectable to pp65 and/or IE1 in both tissue compartments at a diagnosis of primary infection in all 15 LTRs, except in the PBMC from LTR no. 36 (data not shown), with no detectable effector responses at pre-CMV infection time points in 10 LTRs tested (data not shown). However, CD8+IFN-γ+ effector pp65-specific frequencies were increased compared with IE1-specific responses in PBMC and were increased even more strikingly in LMNC, where pp65-specific responses predominated (Fig. 3C). In contrast, we did not find a significant difference between IE1-specific CD8+IFN-γ+ effector responses between the LMNC and the PBMC (p = 0.875 by Wilcoxon signed-rank test). Moreover, we did not detect a significant difference in CD8+IFN-γ+ effector frequencies between LTRs with pneumonitis compared with those without pneumonitis during primary infection (data not shown). Together, these data indicate that the lung airways/allograft are major tissue sites for the trafficking of CD8+IFN-γ+ pp65-specific effectors more than for IE1-specific effectors during acute primary infection regardless of whether viral pneumonitis is present.
FIGURE 3.
Acquisition of de novo CMV-specific effector T cell responses in the LMNC and PBMC is predominated by pp65-specific CD8+IFN-γ+ effectors in the lung airways/allograft during acute primary CMV infection. A and B, Representative plots of PBMC (A) or LMNC (B) from LTR no. 23 at pre-CMV and acute primary CMV time points are shown. Cells were cultured in medium alone or in the presence of either pp65- or IE1-pooled peptides or the positive control staphylococcal enterotoxin B (SEB) followed by ICS as detailed in Materials and Methods. Quadrant numbers indicate frequencies of populations, with gating on CD3+CD8+ T cells. VL, Viral load. C and D, Frequencies of CD3+CD8+IFN-γ+ pp65-specific and IE1-specific effector responses in LMNC vs PBMC (C) and frequencies of pp65-specific CD3+CD8+IFN-γ+ or CD3+CD4+ IFN-γ+ T cells were determined following in vitro restimulation with pooled pp65 or IE1 peptides. Bars represent mean ± SEM frequency of IFN-γ+ LMNC or PBMC from 15 LTRs, with p values calculated by Wilcoxon signed-rank test.
Having found pp65-specific responses to be immunodominant in CD8+ effector responses over IE1, we next compared these responses to pp65-specific CD4+ effector responses in the lung airways/allograft and blood (we did not detect significant IE1-specific CD4+ effector responses; data not shown). Similar to CD8+ effector responses, pp65-specific CD4+IFN-γ+ frequencies were significantly higher in LMNC compared with those detected in PBMC at primary CMV diagnosis, as shown in Fig. 3D. Additionally, pp65-specific CD8+IFN-γ+ effector frequencies were significantly higher in both the lung airways/allograft and blood compared with pp65-specific CD4+IFN-γ+ cells. Collectively, these data show that pp65-specific CD8+ effector responses in the lung airways/allograft predominate over other T cell effector responses measured during acute primary CMV infection.
CMV-specific CD8+IFN-γ+ effector responses in the lung airways and blood do not correlate with respective viral loads at the time of primary CMV diagnosis
Next, we examined whether compartmental pp65-specific CD8+IFN-γ+ effector responses from our primary infection cohort correlated with respective tissue CMV viral loads. Interestingly, we found no correlation between pp65-specific CD8+IFN-γ+ effector responses in either the blood (Fig. 4A) or the lung airways/allograft (Fig. 4B) with respective viral loads at the time of primary CMV diagnosis (rs = −0.289 and 0.119 for lung airways/allograft and blood, respectively, by Spearman's rank correlation test). Thus, while BAL viral loads were increased compared with plasma viral loads across our primary CMV cohort, a correlative relationship between compartmental CD8+ effector responses and viral loads within individuals could not be elucidated.
FIGURE 4.
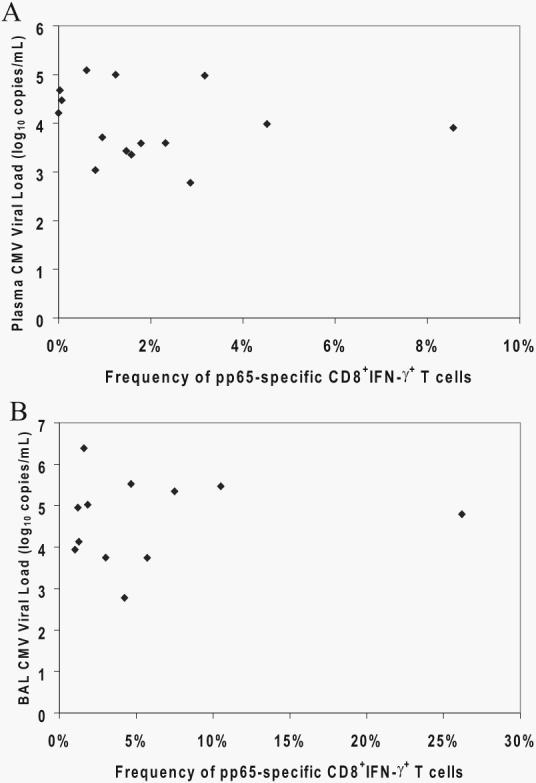
CMV viral loads in the plasma and BAL fluid do not correlate with compartmental frequencies of pp65-specific CD8+IFN-γ+ effectors at primary CMV diagnosis. CMV viral loads in the plasma (A) and BAL fluid (B) were determined by quantitative PCR. Frequencies of pp65-specific CD8+ PBMC (A) and LMNC (B) were determined by ICS for IFN-γ. Values for each of 15 or 12 LTRs, respectively, were subjected to scatterplot analyses in the blood (A) and lung (B).
Increased frequencies of cytokine-producing pp65-specific CD8+ effectors are detected in the lung airways/allograft over the blood during acute primary infection
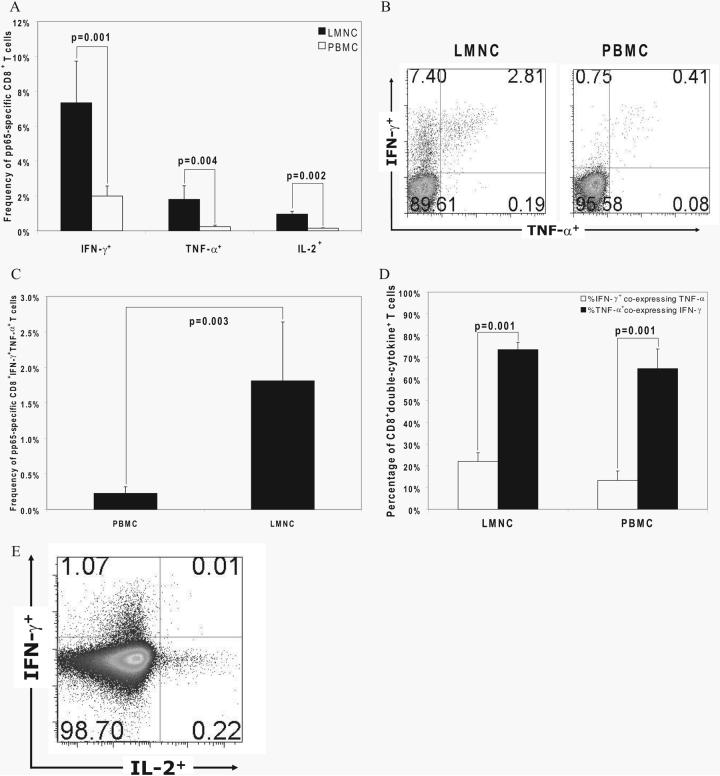
The quality of the T cell effector response may be a critical factor in the host defense to pathogens. We found a hierarchical difference in CMV-specific CD8+ effector responses with frequencies of IFN-γ+ > TNF-α+ > IL-2+ cells in response to pooled pp65 peptides, and significantly higher frequencies for each cytokine detected in LMNC compared with PBMC, as shown in Fig. 5A.In addition, we asked whether there were discernible quantitative and qualitative differences between these tissue sites in regard to poly-functional cytokine production. The most common double-positive CD8+ effector cells detected produced IFN-γ and TNF-α, with these cells demonstrably IFN-γbright as shown in a representative LTR in Fig. 5B. We analyzed the frequencies of CD8+IFN-γ+TNF-α+ T cells as a percentage of total CD8+ T cells from LMNC and PBMC, and found these double-cytokine+ cells to be significantly increased in LMNC during primary infection (Fig. 5C). Next, because a higher number of naive CD8+ T cells may be present in the blood, we compared double-cytokine+ cells as a percentage of CD8+cytokine+ effector populations (IFN-γ+ and TNF-α+, respectively) and found that a significantly higher percentage of TNF-α+ cells coexpressed IFN-γ in both compartments (Fig. 5D). Thus, while the majority of TNF-α+ cells coexpress IFN-γ+ and these double-cytokine+ cells are detected at higher frequencies in the lung airways/allograft, the polyfunctional cytokine quality of these cells on a per cell basis is similar between both tissue compartments. In contrast, we found that pp65-specific CD8+ IL-2+ T cells less frequently coexpressed IFN-γ or TNF-α as shown in Fig. 5E, resulting in low frequencies of triple-positive cells. Nonetheless, as with double-positive effectors, rare triple-positive cells were more frequently detected in LMNC than in PBMC (data not shown). Taken together, the data show increased frequencies of both single and polyfunctional cytokine-producing CMV-specific CD8+ effectors in the lung airways/allograft compared with the blood during acute primary infection.
FIGURE 5.
Hierarchal production of effector cytokines by CD8+ T cells in LMNC and PBMC, with a higher polyfunctional capacity in LMNC. A, Production of IFN-γ, TNF-α, and IL-2 by CD8+ T cells was determined by ICS after in vitro restimulation with pooled pp65 peptides. Bars represent mean ± SEM frequency of cytokine+CD8+ T cells in LMNC and PBMC. B, Representative plot of CD8+ LMNC and PBMC from LTR no. 23 showing frequency of IFN-γ and TNF-α production to pp65. Quadrant numbers indicate frequencies of populations, with gating on CD3+CD8+ T cells. C, Frequencies of CD8+IFN-γ+TNF-α+ LMNC and PBMC from 15 LTRs following in vitro restimulation with pooled pp65 peptides. Bars represent mean ± SEM frequency of CD8+ T cells that coproduce IFN-γ and TNF-α, with p value calculated by Wilcoxon signed-rank test. D, Percentage of CD8+ double-cytokine+ T cells (IFN-γ+ and TNF-α+ detected following gating on IFN-γ+ or TNF-α+ cells in the LMNC or PBMC. E, Representative plot of CD8+ PBMC from LTR no. 34 showing the frequency of IFN-γ and IL-2 production to pp65. Quadrant numbers indicate frequencies of populations with gating on CD3+CD8+ T cells. All p values were determined by Wilcoxon signed-rank test.
The pp65-specific CD8+ effector response contracts in the transition from acute primary infection into chronic infection with long-term persistence of CMV-specific effector memory CD8+ T cells in lung airways/allograft and blood
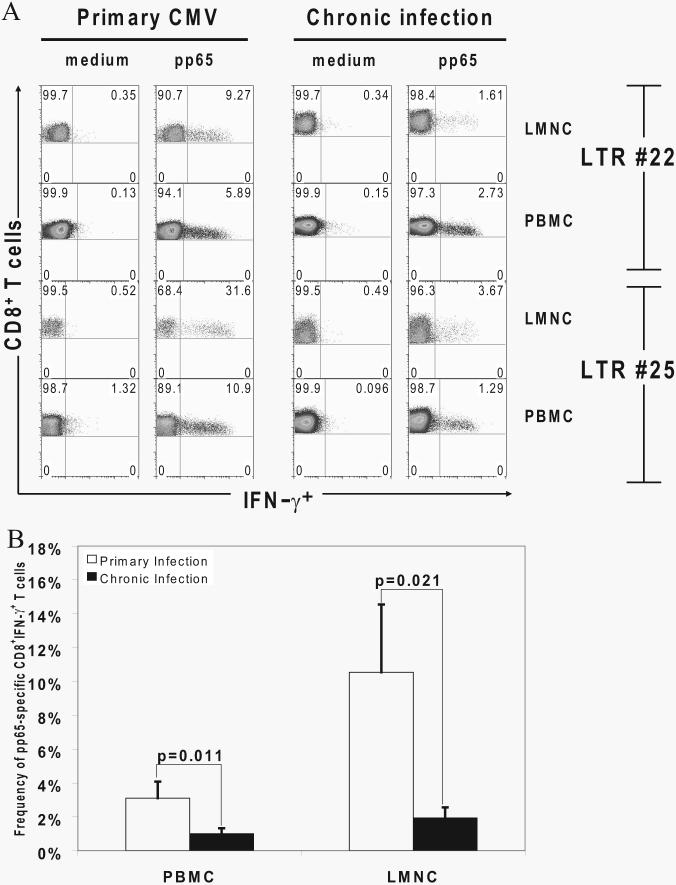
Multiple studies in murine models of infection have demonstrated that the CD8+ T cell response undergoes significant contraction during the transition of the primary effector phase into the memory phase (19, 20). Therefore, we compared CMV-specific CD8+ effector responses in the lung allograft and PBMC during acute primary infection to effector memory responses during chronic infection following the resolution of primary infection. We observed a marked contraction ranging from ∼2- to 10-fold in the frequencies of pp65-specific CD8+IFN-γ+ effector cells in both tissue compartments as shown in two representative LTRs in Fig. 6A. Cumulative data on pp65-specific CD8+IFN-γ+ effector frequencies from nine LTRs during acute primary infection and chronic infection (3–6 mo later) demonstrated significant contraction of pp65 responses in both tissue compartments, as shown in Fig. 6B. Thus, the immunodominant CD8+ effector response contracts in the transition from acute primary infection into chronic infection.
FIGURE 6.
Primary pp65-specific CD8+ effector responses contract in the transition from active to chronic infection but persist long term. A, Representative plots of CD8+IFN-γ+ effector T cell responses in the presence of absence of pooled pp65 peptides by ICS from LMNC and PBMC in LTRs no. 22 and no. 25 during active primary infection compared with latency 1 mo (LTR no. 22) or 3 mo (LTR no. 25) later. Gating is on CD3+CD8+ T cells. B, Contraction of pp65-specific CD8+IFN-γ+ effector T cell frequencies in LMNC and PBMC from nine LTRs during acute primary infection and 3–6 mo postinfection. All p values were determined by Wilcoxon signed-rank test.
CMV-specific CD8+ T cells are CD45RA− during primary infection and transition to CD45RA+ during latent infection in the PBMC, but not in the lung airways/allograft
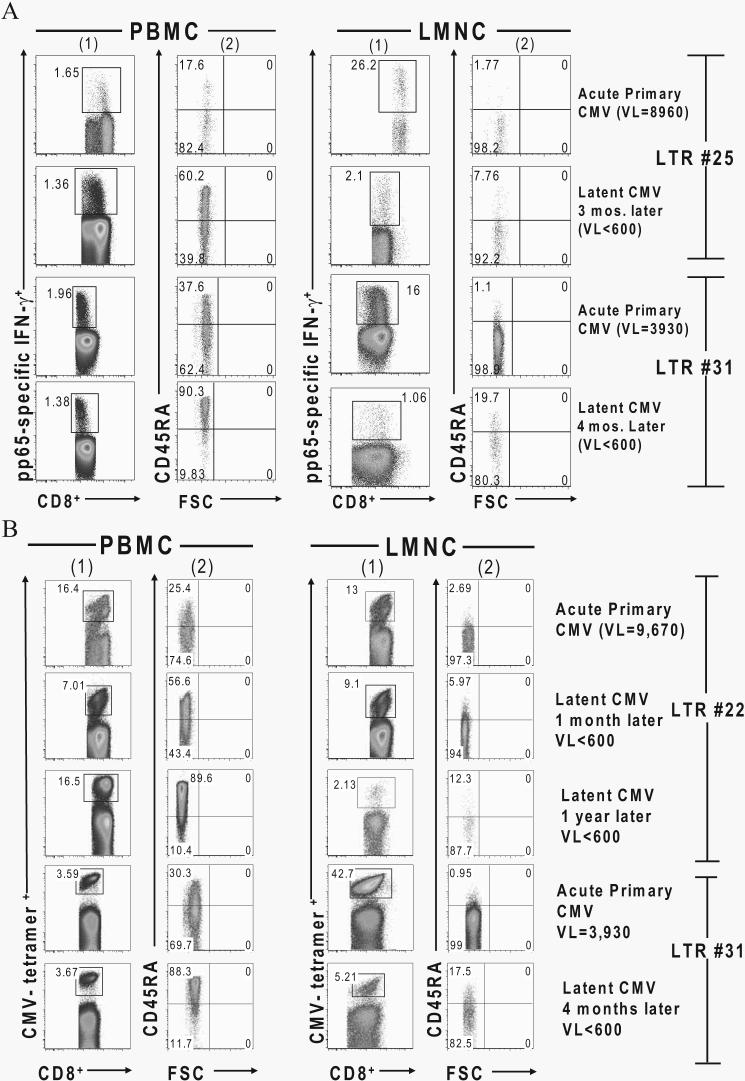
Several recent studies assessing isoforms of the common leukocyte Ag CD45 (CD45RA and CD45RO) have found the majority of long-term, CMV-specific effector memory CD8+ T cells to be CD45RA+ (21-24). However, the majority of these studies were performed in PBMC from individuals during latent infection. To address whether there are changes in surface CD45 isoform expression in CMV-specific CD8+ T cells during the transition from acute primary into latency in either the blood or the lung airways, we assessed CD8+IFN-γ+ effectors and MHC class I CMV tetramer+CD8+ T cells over time. As shown in two representative LTRs in Fig. 7A, primary effector CD8+IFN-γ+ T cells in the PBMC obtained during viremia demonstrated a predominantly CD45RA− phenotype. However, following resolution of viremia, effector memory CD8+IFN-γ+ T cells shifted to a predominantly CD45RA+ phenotype in the blood. In contrast, both primary effector and effector memory CD8+IFN-γ+ T cells demonstrated a predominant CD45RA− phenotype in the lung airways during both acute and latent infection despite a negative BAL CMV viral culture and a viral load that was often undetectable by quantitative PCR at later time points. As an additional control, we examined total CD3+CD8+ T cells in the BAL fluid of non-lung transplant patients (13 patients with sarcoidosis) and found that 94–97% of these cells were of the CD45RA−CD45RO+ phenotype (data not shown). Similarly in Fig. 7B, during primary infection and viremia the majority of CMV tetramer+CD8+ T cells in the blood from LTRs no. 22 and no. 31 were CD45RA− and transition to CD45RA+ during latent infection in the absence of viremia. In sharp contrast, CMV tetramer+CD8+ T cells in the lung allograft are CD45RA− (CD45RO+; data not shown) during acute primary infection, with the majority of these cells maintaining this phenotype into latent infection. In total, we simultaneously analyzed five LTRs for LMNC/PBMC paired-sample tetramer responses during acute and chronic infection (1–3 mo following resolution of viremia/active infection) for CD45RA+ surface expression and found a significant mean increase in the percentage of CD8+CD45RA+tetramer+ cells in PBMC from 23.06 ± 4.24% (SEM) to 72.5 ± 7.59% (p = 0.043 by Wilcoxon signed-rank test) in acute vs chronic infection, respectively. In contrast, CD8+CD45RA+tetramer+ cells in LMNC only changed modestly from 3.84 ± 1.50% during acute infection to 10.14 ± 2.52% in chronic infection (p = 0.08) (data not shown). Similar results were obtained when we analyzed CD8+IFN-γ+ effectors for CD45RA expression, as seen in Fig. 7A. We should also mention that, as an additional control, we also assessed the memory marker CCR7 and found that the vast majority of CMV-specific effectors were CCR7− in both the lung airways/allograft and blood both during and following acute infection (data not shown). Together, these data show that CMV-specific CD8+ T cells from the blood, but not the lung airways, transition from a CD45RA− phenotype to a CD45RA+ phenotype as viremia/acute infection resolves in both compartments, indicating distinct phenotypic differences between CMV-specific CD8+ effector memory T cells in LMNC compared with PBMC during chronic infection.
FIGURE 7.
CMV-specific CD8+ T cells transition from a CD45RA− phenotype in the PBMC during viremia/acute infection to CD45RA+ during latent infection but remain CD45RA− in the lung allograft. A, Representative plots of gated pp65-specific CD8+ IFN-γ+ effector T cells (columns 1) in LMNC and PBMC from LTRs no. 25 and no. 31 during acute primary infection and at latent infection time points following in vitro restimulation with pooled pp65 peptides. CD45RA surface expression by FSC is shown for respective effector gates in columns 2. B, Representative plots of CD8+ CMV tetramer+ T cells in LMNC and PBMC from LTRs no. 22 (both HLA-A2 and HLA-B7 pp65 tetramers) and no. 31 (HLA-A2 pp65 tetramer) at the indicated time points during the transition from acute primary CMV infection into chronic infection. Gating is on CD3+CD8+ tetramer+ T cells. VL, Viral load.
Discussion
Several reports have documented the detection of CMV-specific T cell responses in the blood during primary infection following renal and liver transplantation (10, 11) as well as the lung allograft and blood in a small number of LTRs (25). However, this is the first report, to our knowledge, to assess the acquisition of CMV-specific, CD8+ effector responses in a solid organ allograft or peripheral effector site such as the lung airways in a larger prospective cohort of D+R− LTRs during primary viral infection in humans. Our results provide evidence that CMV-specific T cell responses significantly differ between the blood and lung airways/allograft during the primary viral response, with a CD8+ effector response directed toward pp65 in the lung airways being the immunodominant response over pp65-specific responses in the blood and pp65-specific CD4+ effector responses at the time of diagnosis of primary CMV. Moreover, we demonstrate that the immunodominance of pp65-specific CD8+ effector responses over IE1-specific responses is most strikingly found in the lung airways/allograft but also in the blood during primary infection, consistent with a previous report showing higher blood pp65 responses in neonates with primary CMV infection (26). In contrast, IE1-specific responses were similar in both tissue compartments, indicating that the CD8+ predominance of effector cells in nonlymphoid tissues such as the lung airways can be Ag dependent.
Although our data show the lung airways to be a major site for CMV-specific T cell responses during acute primary infection, the factors governing these responses remain incompletely understood. During primary CMV infection we detected an influx of CD8+ T cells greater than that of CD4+ T cells into the lung airways resulting in the inversion of the physiologic CD4+:CD8+ ratio of ∼1-2:1 in LMNC recovered from the BAL fluid as previously described (27), which suggests an important role for CD8+ T cells during primary infection. These findings contrast with those of an earlier report on BAL T cell subsets during active vs inactive CMV infection in which no differences were found although only one of eight patients that developed active CMV in this study had primary infection, which might account for these differences (28). Although this CD8+ T cell influx was comprised of significant frequencies of pp65-specific de novo CMV effectors greater than those of IE1-specific de novo CMV effectors, we recognize that our studies may underestimate the total frequency of CMV-specific CD8+ effectors, because other viral Ags very likely contribute to a broad antiviral CD8+ T cell response as previously reported in both human and murine CMV infection (29-32). This may, in part, account for why the immunodominant pp65-specific CD8+IFN-γ+ effector frequencies did not significantly correlate with pneumonitis clinicopathology during acute infection, as other Ag-specific responses might play an important role(s). Our observation that pp65-specific responses for both CD8+ and CD4+ effector T cells are increased in the lung airways/allograft during primary infection is consistent with viral-specific CD8+ effectors trafficking to the lung in various murine models of infection (14, 15). Our results differ from a recent report in which similar frequencies of CMV- and EBV-specific CD8+ T cells were detected in the lung airways compared with the circulation; however, those studies were not performed during active infection (33). One potential explanation for a high influx of CMV-specific effector cells into the lung airways may be Ag load, as increased viral loads were detected in the BAL fluid compared with the plasma during primary infection. These findings are consistent with recent reports indicating that the sampling of a lung allograft site for viral replication may be a more sensitive measure of active CMV infection (34, 35). Alternatively, higher effector frequencies in the lung airways/allograft may be due to higher levels of Ag presentation in the mediastinal lymph nodes that drain the lung rather than the viral load in the organ itself (36, 37). Another factor for high effector recruitment into the lung airways may be the tissue site itself, as recent murine studies have demonstrated preferential localization of CD8+ and CD4+ effector/effector memory T cells in the lung airways and lung parenchyma (16, 38, 39). It is plausible that effector/effector memory cells are recruited to the lung in response to specific chemokine signals or gradients, although specific pathways that have not yet been elucidated. Thus, while the factors regulating the high influx of pp65-specific CD8+ effector T cells into the human lung airways/allograft during primary infection remain incompletely understood, these effector cells predominate in frequency over those in the blood during acute primary infection.
Recent evidence has revealed a remarkable heterogeneity in the quality of T cell memory responses, with polyfunctional Th1 and CD8+ T cells demonstrating enhanced durability (40). Thus, it remains an important question as to whether the quality of the immune response is as critical for long-term protection as the magnitude of the response. Our results show a hierarchy in CMV-specific CD8+ effector responses with IFN-γ+ > TNF-α+ > IL-2+ cells, consistent with a recent murine study by La Gruta et al. of influenza-specific responses (41). Interestingly, although we detected low frequencies of CD8+IL-2+ T cells in response to pooled pp65 peptides in the majority of patients, which was perhaps due to calcineurin inhibitor therapy in our subjects, a recent report by Casazza et al. on blood-derived, CMV-specific CD4+ T cells also found polyfunctional Th1 profiles in the absence of significant IL-2 production (42). It is possible that the factors contributing to increased pp65-specific effector frequencies in the lung airways/allograft mentioned above (i.e., Ag load and tissue site), might also influence enhanced polyfunctional cytokine quality in effector sites such as the lung. Collectively, our findings demonstrate higher functional cytokine quality of CMV-specific CD8+ T cells trafficking to the lung airways compared with the peripheral blood during acute primary infection; however, it remains to be determined whether the polyfunctional effector memory cells demonstrate enhanced durability in the human lung airways during transition into chronic infection.
The surface expression of the CD45 isoforms CD45RA and CD45RO as phenotypic markers indicative of T cell Ag experience (e.g., CD45RA+ on naive cells vs CD45RO+ on memory cells) (43) has undergone significant modifications in recent years with the observation that memory T cells can express CD45RA (23, 44). In our studies, the CD45RA−CD45RO+ phenotype in CMV-specific cells in PBMC correlates with the presence of CMV viremia, with in vivo transition to CD45RA+ in these cells in the absence of viremia and the resolution of acute infection as reported in acute EBV infection (45). In contrast, the vast majority of CMV-specific CD8+ T cells in the lung airways maintain a CD45RA−CD45RO+ phenotype during both primary and chronic infection. These findings are consistent with earlier studies in both BAL-obtained LMNC and the lung parenchyma of patients with sarcoidosis, which reported that lung T cells demonstrated a CD45RO+ phenotype thought to be due to the lung being a site for memory T cells (46, 47). However, it is now clear that CMV-specific CD8+ memory cells can be CD45RA+ and, therefore, other factors such as Ag or tissue site may play a role in the CD45RA−CD45RO+ phenotype. Because the CD45RO+ phenotype persists in CD8+ lung effectors even with undetectable CMV replication, this suggests that this surface phenotype may not be solely due to Ag. Furthermore, as the CD45RO+ phenotype is also the predominant phenotype present in airway CD8+ T cells from nontransplant patients, it is unlikely that the airway CD45RO+ phenotype in transplant recipients is due to activated T cells trafficking through a foreign graft. Alternatively, the concept that the tissue site may play a role in the CD45RO+ phenotype is consistent with recent evidence in the mouse by Kohlmeier et al., who demonstrated that T cells undergo profound phenotypic changes consistent with an activated effector phenotype soon after arrival in the lung airways, even in the absence of cognate Ag (48). Taken together, our data show that CMV-specific effector memory cells in the lung airways can differ from those cells in the PBMC in regard to CD45 isoform expression. Understanding such intercom-partmental differences in memory cells may provide insights into the significance of markers such as the CD45 isoforms.
Previous studies in bone marrow transplant recipients and murine CMV infection support the notion that CMV-specific CD8+ T cell responses confer protection and limit disease, particularly in the lungs of the host (49-51). Moreover, data from HIV/CMV coinfected individuals indicates that insufficient CD4+ T cell help may result in the loss of CD8+ effector memory cells and, subsequently, the loss of control of CMV infection (52). Interestingly, we did not find a correlation between CMV-specific effectors and compartmental viral loads at the time of diagnosis of primary CMV. Several potential factors may explain these findings. First, there is the caveat that only one time point can usually be sampled from the lung airways at primary infection, and the kinetics of Ag load and effector responses may be out of phase in vivo. Interestingly, a study by Westall et al. (25) reported that CMV-specific CD8+ T cells preceded the detection of BAL viral load in one patient before primary infection, whereas we did not detect effector responses in the absence of active infection before the discontinuation of antiviral prophylaxis in our studies. These differences may be due to variability in antiviral prophylaxis therapies, viral detection methods, and CMV-specific effector assays (e.g., ICS vs tetramer staining) between the two studies. Second, other cells, such as NK cells, may play a role in controlling viral replication during human primary infection, as these cells are known to play a critical role in murine CMV host resistance based on the presence or absence of the Ly49H NK receptor (53). Additionally, it is possible that other CD8+ effector functions, such cytotoxic capacity, may represent a better correlate of protection or viral control compared with IFN-γ+ cells during primary infection, although this was not assessed in our studies because cells were limited. Nonetheless, while other factors may play protective roles in the control of viral replication during primary infection, it is likely that CMV-specific CD8+ effector T cells are critical for the establishment of long-term protection, although the mechanisms remain incompletely understood. Finally, the fact that effector CD8+ T cells were often observed in the lung airways/allograft of patients with CMV pneumonitis also raises the possibility that these cells may play a dual role and also contribute to immunopathology during primary infection as previously suggested (54). In this regard, we could not correlate CMV-specific CD8+ effector frequencies with the presence or absence of pneumonitis in our cohort, although the size of our cohort might have limited our ability to detect a difference. Of note, however, we did not observe any protective correlation between the acquisition of IE1-specific CD8+ effector responses and the attenuation of primary infection severity (e.g., absence of pneumonitis) as recently reported in the reactivation of CMV infection in recipient+ heart and lung transplant recipients (55). Thus, the roles of CMV-specific CD8+ effector/effector memory T cells in host defense and immunopathology are complex, and the cellular/molecular mechanisms regulating these roles have not been fully elucidated.
Overall, we report the acquisition of de novo CMV-specific effector responses in high-risk D+R− LTRs with significant inter-compartmental effector differences evident during acute primary infection. We find that CMV-specific CD8+ effectors predominate in the lung airways/allograft during primary infection in terms of frequency, immunodominance toward pp65, and functional cytokine quality, and we report differences in effector memory phenotype in regard to CD45 isoform expression during transition into chronic infection. Understanding the acquisition and regulation of CMV-specific CD8+ memory responses in susceptible hosts and particularly in human allografts may improve treatment strategies and long-term allograft durability in LTRs and other SOT recipients following transplantation. Finally, the identification of functional and phenotypic immune correlates of protection during and after primary infection in our model may provide important insights for ongoing CMV vaccine efforts, which are regarded as a public health priority (56).
Acknowledgments
We thank the entire Johns Hopkins Lung Transplant clinical team, in particular Susan Miller, Theresa Cooke, and Sharon Allan for assistance with the patients in this study.
Footnotes
This work was supported by U.S. Public Health Service Grants HL-068682 and AI-072537 (to J.F.M.).
Abbreviations used in this paper: SOT, solid organ transplant; BAL, bronchoalveolar lavage; D+R−, donor+/recipient−; ICS, intracellular cytokine staining; LMNC, lung mononuclear cell; LTR, lung transplant recipient.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N. Engl. J. Med. 1998;338:1741–1751. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 2.Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am. J. Respir. Crit. Care Med. 2004;170:22–48. doi: 10.1164/rccm.200309-1322SO. [DOI] [PubMed] [Google Scholar]
- 3.Rubin RH. Cytomegalovirus in solid organ transplantation. Transpl. Infect. Dis. 2001;3(Suppl 2):1–5. doi: 10.1034/j.1399-3062.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 4.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J. Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 5.Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC. Bronchiolitis obliterans syndrome and early human cytomegalovirus DNAaemia dynamics after lung transplantation. Transplantation. 2003;75:2064–2068. doi: 10.1097/01.TP.0000069234.04901.A3. [DOI] [PubMed] [Google Scholar]
- 6.Balthesen M, Messerle M, Reddehase MJ. Lungs are a major organ site of cytomegalovirus latency and recurrence. J. Virol. 1993;67:5360–5366. doi: 10.1128/jvi.67.9.5360-5366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamora MR. Cytomegalovirus and lung transplantation. Am. J. Transplant. 2004;4:1219–1226. doi: 10.1111/j.1600-6143.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 8.Trulock EP, Christie JD, Edwards LB, Boucek MM, Aurora P, Taylor DO, Dobbels F, Rahmel AO, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J. Heart Lung Transplant. 2007;26:782–795. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Barry SM, Johnson MA, Janossy G. Cytopathology or immunopathology? The puzzle of cytomegalovirus pneumonitis revisited. Bone Marrow Transplant. 2000;26:591–597. doi: 10.1038/sj.bmt.1702562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, Ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-γ-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101:2686–2692. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 11.La Rosa C, Limaye AP, Krishnan A, Longmate J, Diamond DJ. Longitudinal assessment of cytomegalovirus (CMV)-specific immune responses in liver transplant recipients at high risk for late CMV disease. J. Infect. Dis. 2007;195:633–644. doi: 10.1086/511307. [DOI] [PubMed] [Google Scholar]
- 12.Shlobin OA, West EE, Lechtzin N, Miller SM, Borja M, Orens JB, Dropulic LK, McDyer JF. Persistent cytomegalovirus-specific memory responses in the lung allograft and blood following primary infection in lung transplant recipients. J. Immunol. 2006;176:2625–2634. doi: 10.4049/jimmunol.176.4.2625. [DOI] [PubMed] [Google Scholar]
- 13.Ison MG, Fishman JA. Cytomegalovirus pneumonia in transplant recipients. Clin. Chest Med. 2005;26:691–705. doi: 10.1016/j.ccm.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 15.Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J. Immunol. 2004;172:6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- 16.Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J. Clin. Invest. 2005;115:3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preiksaitis JK, Brennan DC, Fishman J, Allen U. Canadian society of transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am. J. Transplant. 2005;5:218–227. doi: 10.1111/j.1600-6143.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- 18.Humar A, Michaels M, AST ID Working Group on Infectious Disease Monitoring American Society of Transplantation recommendations for screening, monitoring, and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am. J. Transplant. 2006;6:262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 19.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8+ T cells after infection. Nat. Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 20.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 21.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 22.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 23.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wills MR, Okecha G, Weekes MP, Gandhi MK, Sissons PJ, Carmichael AJ. Identification of naive or Ag-experienced human CD8+ T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8+ T cell response. J. Immunol. 2002;168:5455–5464. doi: 10.4049/jimmunol.168.11.5455. [DOI] [PubMed] [Google Scholar]
- 25.Westall G, Kotsimbos T, Brooks A. CMV-specific CD8 T-cell dynamics in the blood and the lung allograft reflect viral reactivation following lung transplantation. Am. J. Transplant. 2006;6:577–584. doi: 10.1111/j.1600-6143.2005.01212.x. [DOI] [PubMed] [Google Scholar]
- 26.Gibson L, Piccinini G, Lilleri D, Revello MG, Wang Z, Markel S, Diamond DJ, Luzuriaga K. Human cytomegalovirus proteins pp65 and immediate early protein 1 are common targets for CD8+ T cell responses in children with congenital or postnatal human cytomegalovirus infection. J. Immunol. 2004;172:2256–2264. doi: 10.4049/jimmunol.172.4.2256. [DOI] [PubMed] [Google Scholar]
- 27.The BAL Cooperative Group Steering Committee Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. Am. Rev. Respir. Dis. 1990;141:S169–S202. doi: 10.1164/ajrccm/141.5_Pt_2.S169. [DOI] [PubMed] [Google Scholar]
- 28.Stephan F, Bernaudin JF, Cesari D, Fajac A, Grenet DD, Caubarrere I, Stern M. Blood and alveolar lymphocyte subsets in pulmonary cytomegalovirus infection after lung transplantation. BMC Infect. Dis. 2001;1:15. doi: 10.1186/1471-2334-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elkington R, Walker S, Crough T, Menzies M, Tellam J, Bharadwaj M, Khanna R. Ex vivo profiling of CD8+-T-cell responses to human cytomegalovirus reveals broad and multispecific reactivities in healthy virus carriers. J. Virol. 2003;77:5226–5240. doi: 10.1128/JVI.77.9.5226-5240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manley TJ, Luy L, Jones T, Boeckh M, Mutimer H, Riddell SR. Immune evasion proteins of human cytomegalovirus do not prevent a diverse CD8+ cytotoxic T-cell response in natural infection. Blood. 2004;104:1075–1082. doi: 10.1182/blood-2003-06-1937. [DOI] [PubMed] [Google Scholar]
- 31.Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J. Immunol. 2006;176:3760–3766. doi: 10.4049/jimmunol.176.6.3760. [DOI] [PubMed] [Google Scholar]
- 32.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Bree GJ, van Leeuwen EM, Out TA, Jansen HM, Jonkers RE, van Lier RA. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J. Exp. Med. 2005;202:1433–1442. doi: 10.1084/jem.20051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC. Human cytomegalovirus load in plasma and bronchoalveolar lavage fluid: a longitudinal study of lung transplant recipients. J. Infect. Dis. 2004;190:1076–1083. doi: 10.1086/422327. [DOI] [PubMed] [Google Scholar]
- 35.Chemaly RF, Yen-Lieberman B, Chapman J, Reilly A, Bekele BN, Gordon SM, Procop GW, Shrestha N, Isada CM, Decamp M, Avery RK. Clinical utility of cytomegalovirus viral load in bronchoalveolar lavage in lung transplant recipients. Am. J. Transplant. 2005;5:544–548. doi: 10.1111/j.1600-6143.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 36.Mueller SN, Jones CM, Smith CM, Heath WR, Carbone FR. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J. Exp. Med. 2002;195:651–656. doi: 10.1084/jem.20012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat. Immunol. 2002;3:265–271. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]
- 38.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 39.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 40.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J. Exp. Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.La Gruta NL, Turner SJ, Doherty PC. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J. Immunol. 2004;172:5553–5560. doi: 10.4049/jimmunol.172.9.5553. [DOI] [PubMed] [Google Scholar]
- 42.Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, Koup RA. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 2006;203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merkenschlager M, Beverley PC. Evidence for differential expression of CD45 isoforms by precursors for memory-dependent and independent cytotoxic responses: human CD8 memory CTLp selectively express CD45RO (UCHL1) Int. Immunol. 1989;1:450–459. doi: 10.1093/intimm/1.4.450. [DOI] [PubMed] [Google Scholar]
- 44.Song K, Rabin RL, Hill BJ, De Rosa SC, Perfetto SP, Zhang HH, Foley JF, Reiner JS, Liu J, Mattapallil JJ, et al. Characterization of subsets of CD4+ memory T cells reveals early branched pathways of T cell differentiation in humans. Proc. Natl. Acad. Sci. USA. 2005;102:7916–7921. doi: 10.1073/pnas.0409720102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roos MT, van Lier RA, Hamann D, Knol GJ, Verhoofstad I, van Baarle D, Miedema F, Schellekens PT. Changes in the composition of circulating CD8+ T cell subsets during acute Epstein-Barr and human immunodeficiency virus infections in humans. J. Infect. Dis. 2000;182:451–458. doi: 10.1086/315737. [DOI] [PubMed] [Google Scholar]
- 46.Agostini C, Trentin L, Zambello R, Bulian P, Siviero F, Masciarelli M, Festi G, Cipriani A, Semenzato G. CD8 alveolitis in sarcoidosis: incidence, phenotypic characteristics, and clinical features. Am. J. Med. 1993;95:466–472. doi: 10.1016/0002-9343(93)90328-m. [DOI] [PubMed] [Google Scholar]
- 47.Fazel SB, Howie SE, Krajewski AS, Lamb D. Increased CD45RO expression on T lymphocytes in mediastinal lymph node and pulmonary lesions of patients with pulmonary sarcoidosis. Clin. Exp. Immunol. 1994;95:509–513. doi: 10.1111/j.1365-2249.1994.tb07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohlmeier JE, Miller SC, Woodland DL. Cutting edge: Antigen is not required for the activation and maintenance of virus-specific memory CD8+ T cells in the lung airways. J. Immunol. 2007;178:4721–4725. doi: 10.4049/jimmunol.178.8.4721. [DOI] [PubMed] [Google Scholar]
- 49.Reusser P, Riddell SR, Meyers JD, Greenberg PD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. [PubMed] [Google Scholar]
- 50.Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83:1971–1979. [PubMed] [Google Scholar]
- 51.Holtappels R, Podlech J, Geginat G, Steffens HP, Thomas D, Reddehase MJ. Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates. J. Virol. 1998;72:7201–7212. doi: 10.1128/jvi.72.9.7201-7212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bronke C, Palmer NM, Jansen CA, Westerlaken GH, Polstra AM, Reiss P, Bakker M, Miedema F, Tesselaar K, van Baarle D. Dynamics of cytomegalovirus (CMV)-specific T cells in HIV-1-infected individuals progressing to AIDS with CMV end-organ disease. J. Infect. Dis. 2005;191:873–880. doi: 10.1086/427828. [DOI] [PubMed] [Google Scholar]
- 53.Daniels KA, Devora G, Lai WC, O'Donnell CL, Bennett M, Welsh RM. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 2001;194:29–44. doi: 10.1084/jem.194.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grundy JE, Shanley JD, Griffiths PD. Is cytomegalovirus interstitial pneumonitis in transplant recipients an immunopathological condition? Lancet. 1987;2:996–999. doi: 10.1016/s0140-6736(87)92560-8. [DOI] [PubMed] [Google Scholar]
- 55.Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, Proesch S, Reinke P, Volk HD, Lehmkuhl H, Kern F. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J. Exp. Med. 2005;201:1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R, National Vaccine Advisory Committee Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 2004;39:233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]