Abstract
Leptin is a key neuroendocrine hormone regulating food intake, metabolism, and fat accumulation, and it may also affect blood pressure and contribute to hypertension through sympathetic activation in the vasculature or at the renal level. Although previous studies have shown that the distribution of leptin is significantly different between males and females, as is the risk of hypertension between males and females, results regarding the role of leptin in the gender-specific regulation of blood pressure are controversial. Thus, we performed family-based association analyses in the National Heart, Lung, and Blood Institute Family Heart Study to test the hypothesis that LEPTIN gene variants and the plasma leptin level influence variability in blood pressure and the risk of hypertension differently by gender. We identified significant associations between LEPTIN single nucleotide polymorphisms with blood pressure and hypertension, but in postmenopausal women only. We also identified significant associations between plasma leptin levels and both blood pressure and hypertension in women. The current study supports a role for LEPTIN and plasma leptin levels in blood pressure regulation in women. It also provides insight into the gender differences in hypertension, as well as the differential distribution and activity of leptin in men and women.
Keywords: leptin, blood pressure, hypertension, association, gender
In developed and developing countries, hypertension has been identified as an expanding health crisis,1 and there are apparent gender differences in the mechanisms involved in the relationship of obesity and hypertension in men and women.2,3 Although numerous studies have been performed to identify the genetic background underlying hypertension and to seek quantitative trait loci controlling blood pressure,4-7 there is a paucity of reports addressing genes and their products that are involved in sex-specific regulation of blood pressure and sex-specific onset of hypertension. Understanding how such factors differentially influence the regulation of blood pressure in men and women can provide insight into the contributing pathways and may provide clues for treatment strategies targeted toward appropriate populations.
The LEPTIN gene (LEP) was originally identified as a murine obesity gene, and its human homologue is a neuroendocrine hormone functioning as an important regulator of food intake, neuroendocrine outflow, metabolism, and fat accumulation.8 As shown in experimental studies, leptin may also affect blood pressure and contribute to the occurrence of hypertension through sympathetic activation in the circulatory district or at the renal level,9,10 and microinjections of leptin have indicated that higher intracerebroventricular level of leptin lead to higher effects toward sympathetic activation.11 It is widely recognized that the leptin levels are several times higher in women than men,12,13 which suggests the possibility of greater effects on the sympathetic nervous system (SNS) in women.14 Although many studies have previously examined the association between leptin levels and blood pressure, the gender-specific effects of genetic variants in LEP as well as leptin levels in the regulation of blood pressure are still unsettled. Some previous studies report associations in gender-specific manners that are different from each other, whereas other studies do not detect gender differences.15-19 In the current study, we aim to evaluate whether DNA sequence variability in the upstream LEP promoter region, and within the LEP gene itself, as well as plasma leptin levels influence blood pressure and hypertension differentially by gender in a large, multicenter study: the National Heart, Lung, and Blood Institute Family Heart Study (NHLBI-FHS).
Methods
Subjects
The current association studies were based on the same data set used in a previous evaluation of LEP variants with body mass index (BMI) by Jiang et al.20 The data contain 695 subjects from 82 white families in the NHLBI-FHS study. The families contribute strong support to linkage evidence for BMI on chromosome 7q13,21 and the multipoint logarithm of odds score for this data set is 17.09 at 136.95 cM (Genetic Map Index web site; Center for Medical Genetics). All subjects gave informed consent. The study was approved by an institutional review committee, and the procedures followed were in accordance with institutional guidelines.
Phenotypes
The measurements of systolic blood pressure (SBP) and diastolic blood pressure (DBP) were performed in sitting position by random-zero sphygmomanometry in each field center of the NHLBI-FHS study, which included Forsyth County, NC; Minneapolis, Minn; Framingham, Mass; and Salt Lake City, Utah. Both SBP and DBP were measured 3 times, and the mean of the second and the third measurements was used for analysis. Hypertension is defined as SBP ≥140 mm Hg or DBP ≥90 mm Hg, or currently under hypertension medication. All subjects were fasting for 12 hours before the blood draws. The measurement of leptin was performed on EDTA plasma using Human Leptin RIA Kit (LINCO Research).
Stepwise multiple regression models were used to adjust for the effects of age and field center on the mean and the variance of SBP and DBP within sex, similar to our previous studies,21 which is hereafter referred to as regular adjustment in the current study. However, we further adjusted for the effects of BMI in addition to the factors mentioned above, which is hereafter referred to as BMI adjustment. The association analyses were based on the residual SBP and DBP, the distributions of which were standardized to a limit normal. In the later phenotype association studies between plasma leptin level and blood pressure/hypertension, regular and BMI adjustments were also applied to leptin levels. In the association studies between LEP single nucleotide polymorphisms (SNPs) and plasma leptin, only regular adjustments were applied to leptin levels. There are 105 subjects with measured SBP and DBP already taking antihypertensive medication (53 males and 52 females), and their blood pressures are recoded as missing in the quantitative association analysis to avoid the confounding effects of antihypertensive medications on blood pressure. Menopausal status was ascertained based on self-report in a reproductive questionnaire for women.
Genotyping and Quality Control
SNP selection, genomic DNA preparation, SNP typing methods, and bioinformatic searching for transcription factor binding sites (TFBSs) were described in detail in the previous study.20 In summary, 29 SNPs located within LEP, 159 824 bp upstream, and 67 728 bp downstream (between 127 508 743 and 127 752 645 bp on chromosome 7) were selected according to Assays-on-Demand SNP Genotyping Products, previous publications, or the human SNP database in the Celera Discovery System. TFBSs were obtained by using BLAST basing on TRANSFAC database. The locations of the SNPs are summarized in Table 1. Genomic DNA was extracted from whole blood, and the SNPs were genotyped using the ABI TaqMan Technology. The pedigree relationships were verified previously using the linkage STR marker panel by graphical representation of relationship errors.22 Additional quality control measures such as checks for Mendelian errors and Hardy–Weinberg equilibrium were performed. Double recombinants were detected using MERLIN23 and were set as missing genotypes.
Table 1.
SNP Summary
| SNP No. | Marker | Gene | SNP | Frequency | NCBI Location | SNP Type | rs No. | Haplotype Block |
|---|---|---|---|---|---|---|---|---|
| 1 | C1331361 | SND1 | T/C | 0.348 | 127508743 | Exon | rs322825 | Block 1 |
| 2 | C1331345 | G/A | 0.19 | 127526902 | Intergenic | rs6953698 | Block 1 | |
| 3 | C618722 | A/G | 0.456 | 127543188 | Intergenic | rs322785 | ||
| 4 | C618685 | G/C | 0.272 | 127576432 | Intergenic | rs53125 | ||
| 5 | H1331258 | A/G | 0.351 | 127634249 | Intergenic | rs11772985 | ||
| 6 | H1331250 | G/A | 0.16 | 127644562 | Intergenic | rs6947095 | Block 2 | |
| 7 | H1328090 | C/A | 0.389 | 127653349 | Intergenic | rs791601 | Block 2 | |
| 8 | C1328085 | T/G | 0.34 | 127664262 | 5'flank/TFBS | rs10249476 | Block 2 | |
| 9 | H1328084 | G/A | 0.411 | 127664449 | 5'flank/TFBS | rs1349419 | Block 2 | |
| 10 | H1328083 | G/A | 0.495 | 127664980 | 5'flank/TFBS | rs13245201 | Block 2 | |
| 11 | H1328082 | C/A | 0.345 | 127665334 | 5'flank/TFBS | rs12535708 | Block 2 | |
| 12 | H1328081 | C/T | 0.404 | 127665503 | 5'flank/TFBS | rs11770725 | Block 2 | |
| 13 | H1328080 | C/A | 0.341 | 127665571 | 5'flank | rs12535747 | Block 2 | |
| 14 | G2548A | G/A | 0.495 | 127666019 | Promoter | rs7799039 | Block 3 | |
| 15 | H6501175 | A/G | 0.446 | 127666584 | Promoter | rs6467166 | Block 3 | |
| 16 | H2944325 | G/A | 0.493 | 127666604 | Promoter | rs12536535 | Block 3 | |
| 17 | C1887T | C/T | 0.092 | 127666680 | Promoter | rs28954369 | Block 3 | |
| 18 | G1387A | A/G | 0.446 | 127667180 | Promoter | rs13228377 | Block 3 | |
| 19 | A19G | LEP | G/A | 0.342 | 127668585 | Exon1/5 UTR | rs2167270 | Block 4 |
| 20 | H1328078 | LEP | T/C | 0.407 | 127669087 | Intron | rs2278815 | Block 4 |
| 21 | H1432616 | LEP | G/T | 0.423 | 127674304 | Intron | rs12706831 | Block 4 |
| 22 | H1432615 | LEP | G/A | 0.426 | 127674375 | Intron | rs12706832 | Block 4 |
| 23 | H1328076 | LEP | A/T | 0.476 | 127675925 | Intron | rs10244329 | Block 5 |
| 24 | H3001671 | LEP | A/G | 0.477 | 127677298 | Intron | rs11763517 | Block 5 |
| 25 | H1328074 | LEP | G/A | 0.356 | 127678323 | Intron | rs11760956 | Block 6 |
| 26 | C1328073 | LEP | G/A | 0.363 | 127678676 | Intron | rs10954173 | Block 6 |
| 27 | C3001667 | A/G | 0.424 | 127686365 | Intergenic | rs2060715 | Block 6 | |
| 28 | C1574222 | RBM28 | G/A | 0.353 | 127737961 | 5' UTR | rs12850 | Block 7 |
| 29 | C1328040 | RBM28 | C/G | 0.306 | 127752645 | Intron | rs10279576 | Block 7 |
Family-Based Association Analysis
To test the association between LEP SNPs with SBP, DBP, and plasma leptin level, Quantitative Transmission Disequilibrium Test (QTDT) version 2.424,25 was used to test single SNP association, as well as haplotype association based on the 6 SNPs with the most significant marginal P values. We input estimated haplotypes from MERLIN23 for the haplotype analyses.
TRANSMIT26,27 version 2.5.4 was used to perform the Transmit Disequilibrium Tests (TDT) to test the association between LEP SNPs and hypertension. Asymptotic P values were reported in the results.
Additional powerful haplotype analyses were performed to test the association between LEP SNPs and hypertension using SPTDT (Sequential Peeling Transmission Disequilibrium Test).28 We performed SPTDT based on 2 sets of SNPs. The first set contains SNPs in blocks located at the 5′ TFBS and promoter region (SNPs 6 to 18 in blocks 2 to 3; Table 1), and the second set contains SNPs in blocks located at the intron/exon region, 3′UTR, and further downstream (SNPs 19 to 29 in blocks 4 to 7; Table 1). Haplotypes with frequencies <0.005 were excluded from the analysis, and 10 000 permutations were used to determine empirical P values.
Phenotype Association
Pearson correlations were estimated by SAS to explore the relationship between plasma leptin levels and blood pressures using the residuals of blood pressures and plasma leptin levels after regular adjustment. We further estimated the correlation between leptin levels and blood pressures, accounting for the effects of BMI, in SAS.
To test the association between leptin levels and hypertension, we used a logistic function in generalized estimating equations to adjust for the nonindependence of subjects within families. We first analyzed the residuals of leptin levels after regular adjustment as the independent variable and hypertension as the dependent variable in the model. We also investigated leptin levels additionally adjusted for BMI.
Because of potential interactions between endogenous hormones and LEP/plasma leptin in the regulation of blood pressure, all of the above genotype and phenotype association studies were stratified by gender and self-reported menopausal status. Phenotypes of the subjects who do not belong to the categories of interest were coded as missing.
Results
SNP Location and Characteristics
Twenty-nine SNPs within and around LEP were analyzed in the current study. Their location and characteristics are presented in Table 1, ordered by their NCBI bp location. The range of minor allele frequencies is from 0.092 to 0.495. The SNPs are in 7 linkage disequilibrium blocks identified by Haploview, using confidence-bound estimates for D′. A block is defined when ≥95% of SNP pairs meet the criteria of 1-sided upper 95% confidence bound of D′>0.98 and a lower bound of >0.7.
Phenotype Distribution
The characteristics of the sample are shown in Table 2. The level of plasma leptin is significantly higher in female subjects (P<0.05), and SBP and DBP are significantly higher in male subjects (P<0.05). Postmenopausal women have significantly higher SBP, DBP, plasma leptin levels, and hypertension prevalence compared with premenopausal women (P<0.05).
Table 2.
Phenotype Distribution by Gender and Menopausal Status (Mean±SD)
| Characteristic | Males | Females | Premenopausal Women | Postmenopausal Women |
|---|---|---|---|---|
| No. | 208 | 235 | 100 | 113 |
| Age, y | 48.99±13.73 | 49.94±12.45 | 38.62±6.36 | 60.10±6.89 |
| SBP, mm Hg | 115.85±12.97 | 111.48±16.47 | 104.62±12.40 | 117.96±17.26 |
| DBP, mm Hg | 70.57±9.61 | 66.70±9.56 | 64.73±8.89 | 68.13±10.05 |
| BMI, kg/m2 | 28.06±4.97 | 28.54±7.39 | 27.91±7.83 | 29.39±7.13 |
| Plasma leptin, ng/mL | 8.44±7.69 | 24.58±18.98 | 21.86±18.21 | 27.19±20.24 |
| Percentage with hypertension | 25.27% | 23.79% | 6.45% | 49.23% |
Mean±SD of age, SBP, DBP, BMI, and plasma leptin are based on subjects with SBP or DBP measurements not using antihypertensive medications. Hypertension is defined as SBP ≥140 mm Hg or DBP ≥90 mm Hg or currently taking antihypertensive medication.
Single SNP Association Studies Between LEP and Blood Pressure/Hypertension
QTDT analyses evidence significant associations between SBP and a 5′ TFBS SNP (P=0.0028), 3 SNPs within the promoter region (P<0.01), and 2 intronic SNPs (P<0.002), all in women only (Table 3). QTDT analysis also showed significant association between these SNPs and DBP, also in women. After further adjusting SBP and DBP for BMI, the associations remained significant (Figure 1). To account for multiple testing, we calculated the false discovery rates, and the corresponding q values are significant for SBP (q<0.05) and borderline significant for DBP (q≤0.15). No significant association was detected in men (P>0.05). Stratifying the data by menopausal status revealed that the significant associations are only in postmenopausal women. In the postmenopausal group, significance level of LEP SNPs is increased 2- to 80-fold regarding the associations of SBP, and the significance level is also slightly increased regarding the associations of DBP (Figure 2). The QTDT P values and false discovery rate q values for SBP in all women and postmenopausal women are shown in Table 3.
Table 3.
QTDT Single SNP Analysis Results of SBP
| SNP No. | SNP Name | Female Regular Adjustment P Value | Female Regular Adjustment FDR q | Postmenopausal Regular Adjustment P Value | Postmenopausal Regular Adjustment FDR q | Female BMI Adjustment P Value | Female BMI Adjustment FDR q | Postmenopausal BMI Adjustment P Value | Postmenopausal BMI Adjustment FDR q |
|---|---|---|---|---|---|---|---|---|---|
| 10 | rs13245201 | 0.0028 | 0.0203 | 0.0003 | 0.0029 | 0.0019 | 0.0145 | 0.0001 | 0.0007 |
| 14 | rs7799039 | 0.0085 | 0.0411 | 0.0008 | 0.0033 | 0.0068 | 0.0329 | 0.0003 | 0.0012 |
| 15 | rs6467166 | 0.0053 | 0.0307 | 0.0004 | 0.0029 | 0.0033 | 0.0191 | 4e-05 | 0.0006 |
| 16 | rs12536535 | 0.0009 | 0.0131 | 0.0002 | 0.0029 | 0.0016 | 0.0145 | 0.0002 | 0.0012 |
| 23 | rs10244329 | 0.0006 | 0.0131 | 0.0003 | 0.0029 | 0.001 | 0.0145 | 4e-05 | 0.0006 |
| 24 | rs11763517 | 0.0017 | 0.0164 | 0.0008 | 0.0033 | 0.002 | 0.0145 | 0.0001 | 0.0007 |
Figure 1.
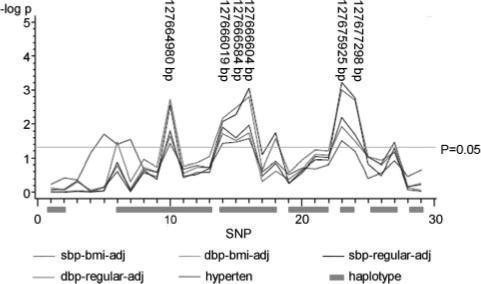
Single SNP association between LEP and blood pressure/hypertension in female subjects. sbp-bmi-adj indicates SBP BMI adjustment; dbp-bmi-adj, DBP BMI adjustment; sbp-regular-adj, SBP regular adjustment; dbp-regular-adj, DBP regular adjustment; hyperten, hypertension. The correspondence between SNP numbers and rs numbers is shown in Table 1.
Figure 2.
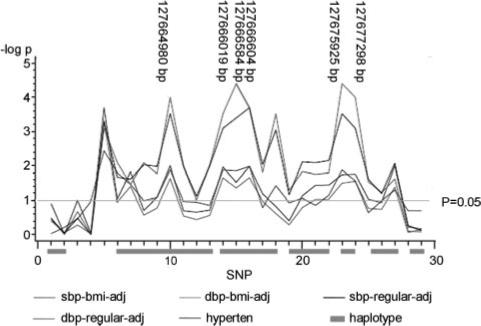
Single SNP association between LEP and blood pressure/hypertension in postmenopausal women. sbp-bmi-adj indicates SBP BMI adjustment; dbp-bmi-adj, DBP BMI adjustment; sbp-regular-adj, SBP regular adjustment; dbp-regular-adj, DBP regular adjustment; hyperten, hypertension. The correspondence between SNP numbers and rs numbers is shown in Table 1.
TDTs also identified significant associations between the above 6 SNPs with hypertension in women (Figure 1). Further analysis stratified by menopausal status supported the association of these SNPs in postmenopausal women, with improved significance levels (Figure 2).
LEP Haplotype Associations With Blood Pressure/Hypertension
The 6 LEP SNPs that consistently show association in women using marginal SNP association tests (rs13245201, rs7799039, rs6467166, rs12536535, rs10244329, and rs11763517) can be analyzed as a haplotype, which is also associated with SBP and DBP. The P values in the haplotype analysis for DBP are 0.0054 with regular adjustment, and 0.0076 with BMI adjustment in women, whereas the P values in the haplotype analysis for SBP are 0.0032 with regular adjustment, and 0.0036 with BMI adjustment in women. Among postmenopausal women, haplotype analysis also yielded higher significance of association with DBP (P=0.0018 with both regular and BMI adjustment), as well as higher significance of association with SBP (P=0.0007 with regular adjustment and P=0.0002 with BMI adjustment).
SPTDT results indicate that the SNPs located at LEP 5′ TFBS and promoter region (SNPs 6 to 18; ie, rs6 947 095 to rs13 228 377; Table 1) are jointly significantly associated with hypertension in women, and the global adjusted empirical P value is 0.0065. The SNPs located at blocks in LEP introns, exons, 3′UTR region, and further downstream (SNPs 19 to 28; ie, rs2 167 270 to rs10 279 576; Table 1) are also jointly significantly associated with hypertension in women, with the global empirical adjusted P value at 0.0020. The haplotype association is restricted to women, and no association is seen in men. Among postmenopausal women, SPTDT provides empirical P values as 0.0064 and 0.0043 regarding the above TFBS and promoter region (SNPs 6 to 18; Table 1), as well as LEP intron, exon, and 3′UTR regions (SNPs 19 to 28; Table 1), respectively.
Phenotype Association Studies
Plasma leptin levels are correlated with SBP and DBP in women (Table 4). However, after SBP and DBP are additionally adjusted by BMI, the correlation appreciably diminishes, suggesting that the relationship between plasma leptin levels and blood pressure is mediated by BMI. Generalized estimating equation logistic models reveal a significant association between plasma leptin levels and hypertension in women (P<0.001; β=0.4830; 95% CI, 0.2498 to 0.7162). However, no significant association remains after further BMI adjustment (P=0.1540; β=0.1293; 95% CI, 0.0485 to 0.3072; Table 4). No significant association was detected in men.
Table 4.
Pearson Correlation Coefficients and P Values of Association Between Leptin Level and SBP, DBP, and Hypertension
| Phenotype | Female Regular Adjustment |
Female BMI Adjustment |
Postmenopausal Regular Adjustment |
Postmenopausal BMI Adjustment |
|---|---|---|---|---|
| SBP | 0.2829 | 0.0324 | 0.3326 | 0.1579 |
| P<0.0001 | P=0.6235 | P=0.0004 | P=0.0996 | |
| DBP | 0.2575 | 0.1103 | 0.3498 | 0.2116 |
| P<0.0001 | P=0.0939 | P=0.0002 | P=0.0265 | |
| Hypertension | P<0.001 | P=0.1540 | P=0.0045 | P=0.3593 |
The association between plasma leptin levels and SBP, DBP, or hypertension is also localized in postmenopausal women. After accounting for BMI, the association either diminishes or is weakened appreciably (Table 4).
Single SNP Association Studies Between LEP and Plasma Leptin Levels
QTDT results suggest that there are no appreciable significant associations between the LEP SNPs and plasma leptin in men or women, regardless of menopausal status (Figure 3). The only marginally significant signals observed are at rs2060715 in postmenopausal women (P=0.0451) and rs28954369 in premenopausal women (P=0.0234).
Figure 3.
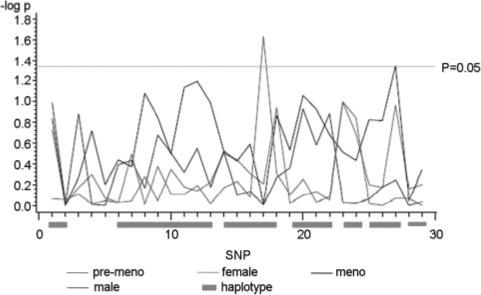
Single SNP association results between LEP and plasma leptin. Female indicates results from women; male, results from men; meno, results from postmenopausal women; pre-meno, results from premenopausal women. The correspondence between SNP numbers and rs numbers is shown in Table 1.
Discussion
The current study shows evidence of associations between SNPs in LEP with blood pressure and hypertension in postmenopausal women, and it also provides evidence of significant associations between plasma leptin levels with blood pressure and hypertension in women regardless of menopausal status. The associations were identified across a variety of approaches and persisted after false discovery rate correction for multiple comparisons. Moreover, the menopausal dependence of the associations is unique compared with previous studies.15-19
One interesting finding is that the association between LEP genotype and blood pressure is robust to the adjustment of blood pressure for BMI, but the association between plasma leptin levels and blood pressure diminishes or is weakened appreciably after adjustment for BMI. It is well known that plasma leptin levels are closely related to BMI (r=0.74 in female subjects in the current data), so adjusting blood pressure for BMI could account for the association with plasma leptin levels. Because LEP SNP variants are not associated with BMI in women,20 it stands to reason that adjusting for BMI does not ameliorate the association of blood pressure with LEP SNPs in women. Finally, we cannot rule out that the absence of association between plasma leptin levels and blood pressure after BMI adjustment may be caused by pleiotropic effects of genes other than LEP underlying BMI and blood pressure (Figure 4). Genes influencing BMI could also affect blood pressure through BMI. Thus, effects toward blood pressure regulation from genes underlying BMI would disappear after adjusting for BMI, leading to diminished associations of blood pressure.
Figure 4.
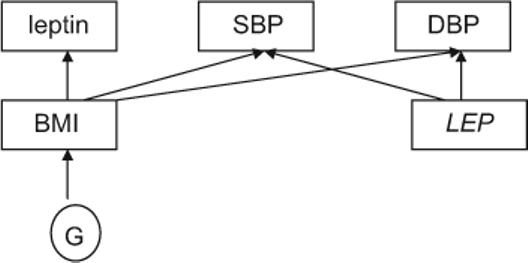
The relationship among LEP, leptin, blood pressures, and BMI in women. G indicates genes other than LEP.
Taking into account the role of leptin in obesity and insulin resistance, we studied the association between homeostasis model assessment index with blood pressure and LEP SNPs. We found that even if homeostasis model assessment is significantly associated with SBP and DBP, homeostasis model assessment is not associated with LEP SNPs among postmenopausal women (the only exception is rs10244329, with P=0.0403). Thus, the association between LEP SNPs and blood pressure might be independent from insulin resistance.
Numerous studies have suggested how leptin could regulate blood pressure. Haynes et al reported that leptin activates a sympathetic response affecting vascular and renal districts.29 This could be mediated through the interaction between leptin and insulin,30,31 by the upregulation of angiotensinogen,32,33 or by the interaction between leptin and the specific receptor in the hypothalamus, which leads to the decrease of the level of neuropeptide Y.34-37 Although the precise mechanisms of the associated SNP variants on the regulation of LEP expression and activity are not well understood, the influence of these SNP variants are probably through a pathway independent from that of plasma leptin, at least in women. Patterns of expression of LEP in the brain are different from those observed in the circulation. Levels of leptin in the SNS are directly related to the regulation of blood pressure and the occurrence of hypertension.10 Because heart rate is an index of the activity of the SNS,38 we studied the association between LEP SNPs and heart rate in postmenopausal women. The 6 SNPs for which we report association with blood pressure and hypertension all show significant or borderline significant association with heart rate, based on the sample for blood pressure association study (P=0.0263 to 0.1587). This provides another piece of evidence that the effects of LEP SNPs function on central nervous system through autocrine or paracrine pathways. However, additional studies are necessary to verify the above hypothesis. Moreover, studies to identify the actual functional variants are needed, and more studies are necessary to understand the mechanisms of how the variations in the promoter region influence transcription factor binding and promoter activity in a sex-specific manner in the SNS, as well as how intronic variation influences protein level and activity in the SNS.
Many studies report higher leptin levels in women than in men.12,13 The sexual dimorphism of leptin levels is thought attributable to the endogenous hormones. Testosterone functions as a regulator of leptin,39,40 and Elbers et al demonstrated that suppression of testosterone in men leads to an increase of leptin to levels similar to those of women.41 Furthermore, other leptin regulators such as proopiomelanocortin contain estrogen- and testosterone-responsive elements.42,43 However, additional studies are need to clarify the mechanisms of how endogenous hormones influence leptin levels in the SNS.
In the current study, we extend the previous reports of LEP variant influence on blood pressure by demonstrating that the effect is dependent on both sex and menopausal status. It is possible that previous studies (eg, Gaukrodger et al)15 might have failed to find association in the overall data because of the group specificity of the association. Previous studies demonstrating male-specific association of LEP variants with BMI,20 as well as the dependence on menopausal status, suggest a possible interaction between leptin and endogenous hormones.
Perspectives
In the current study, we identified significant or suggestive associations between LEP SNPs and leptin levels with blood pressure and hypertension in women, specifically among postmenopausal women. The results suggest a role for LEP variants in blood pressure regulation that appear to be subject to hormonal milieu and provide insight into the mechanisms involved in the gender differences of hypertension.
Acknowledgment
Special thanks to Andrew Van Brunt for proofreading the English of this article.
Sources of Funding
This study is supported by National Institutes of Health grants 5RO-1 DK068336-03 (adiposity quantitative trait loci in the NHLBI-FHS) and RO-1 HL068891-06 (epidemiological and genetic studies of BMI in the Family Heart Study).
Footnotes
Disclosures
None.
References
- 1.Francischetti EA, Genelhu VA. Obesity-hypertension: an ongoing pandemic. Int J Clin Pract. 2007;61:269–280. doi: 10.1111/j.1742-1241.2006.01262.x. [DOI] [PubMed] [Google Scholar]
- 2.Kagan A, Faibel H, Ben-Arie G, Granevitze Z, Rapoport J. Gender differences in ambulatory blood pressure monitoring profile in obese, overweight and normal subjects. J Hum Hypertens. 2006;21:128–134. doi: 10.1038/sj.jhh.1002118. [DOI] [PubMed] [Google Scholar]
- 3.Wilsgaard T, Schirmer H, Arnesen E. Impact of body weight on blood pressure with a focus on sex differences: the Tromso Study, 1986−1995. Arch Intern Med. 2000;160:2847–2853. doi: 10.1001/archinte.160.18.2847. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SC, Ellison C, Atwood LD, Pankow JS, Province MA, Leppert MF. Genome scans for blood pressure and hypertension: the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension. 2002;40:1–6. doi: 10.1161/01.hyp.0000022660.28915.b1. [DOI] [PubMed] [Google Scholar]
- 5.Caulfield M, Munroe P, Pembroke J, Samani N, Dominiczak A, Brown M, Benjamin N, Webster J, Ratcliffe P, O'Shea S, Papp J, Taylor E, Dobson R, Knight J, Newhouse S, Hooper J, Lee W, Brain N, Clayton D, Lathrop GM, Farrall M, Connell J. Genome-wide mapping of human loci for essential hypertension. Lancet. 2003;361:2118–2123. doi: 10.1016/S0140-6736(03)13722-1. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell GF, DeStefano AL, Larson MG, Benjamin EJ, Chen MH, Vasan RS, Vita JA, Levy D. Heritability and a genome-wide linkage scan for arterial stiffness, wave reflection, and mean arterial pressure: the Framingham Heart Study. Circulation. 2005;112:194–199. doi: 10.1161/CIRCULATIONAHA.104.530675. [DOI] [PubMed] [Google Scholar]
- 7.Shmulewitz D, Heath SC, Blundell ML, Han Z, Ratnendra Sharma, Salit J, Auerbach SB, Signorini S, Breslow JL, Stoffel M, Friedman JM. Linkage analysis of quantitative traits for obesity, diabetes, hypertension, and dyslipidemia on the island of Kosrae, Federated States of Micronesia. Proc Natl Acad Sci USA. 2006;103:3502–3509. doi: 10.1073/pnas.0510156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dijk G. The role of leptin in the regulation of energy balance and adiposity. J Neuroendocrinol. 2001;13:913–921. doi: 10.1046/j.1365-2826.2001.00707.x. [DOI] [PubMed] [Google Scholar]
- 9.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 10.Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: role of leptin and sympathetic nervous system. Am J Hypertens. 2001;14:103S–115S. doi: 10.1016/s0895-7061(01)02077-5. [DOI] [PubMed] [Google Scholar]
- 11.Satoh N, Ogawa Y, Katsuura G, Numata Y, Tsuji T, Hayase M, Ebihara K, Masuzaki H, Hosoda K, Yoshimasa Y, Nakao K. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes. 1999;48:1787–1793. doi: 10.2337/diabetes.48.9.1787. [DOI] [PubMed] [Google Scholar]
- 12.Hickey MS, Israel RG, Gardiner SN, Considine RV, McCammon MR, Tyndall GL, Houmard JA, Marks RHL, Caro JF. Gender differences in serum leptin levels in humans. Biochem Mol Med. 1996;59:1–6. doi: 10.1006/bmme.1996.0056. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81:3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan DE, Vaile JC, Petley GW, Phillips DI, Godsland IF, Owens P, Moore VM, Cockington RA, Robinson JS. Gender differences in the relationship between leptin, insulin resistance and the autonomic nervous system. Regul Pept. 2007;140:37–42. doi: 10.1016/j.regpep.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Gaukrodger N, Mayosi BM, Imrie H, Avery P, Baker M, Connell JMC, Watkins H, Farrall M, Keavney B. A rare variant of the leptin gene has large effects on blood pressure and carotid intima-medial thickness: a study of 1428 individuals in 248 families. J Med Genet. 2005;42:474–478. doi: 10.1136/jmg.2004.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han HR, Ryu HJ, Cha HS, Go MJ, Ahn Y, Koo BK, Cho YM, Lee HK, Cho NH, Shin C, Shin HD, Kimm K, Kim HL, Oh B, Park KS. Genetic variations in the leptin and leptin receptor genes are associated with type 2 diabetes mellitus and metabolic traits in the Korean female population. Clinical Genet. 2008;74:105–115. doi: 10.1111/j.1399-0004.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 17.Suter PM, Locher R, Hasler E, Vetter W. Is there a role for the ob gene product Leptin in essential hypertension? Am J Hypertens. 1998;11:1305–1311. doi: 10.1016/s0895-7061(98)00162-9. [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Chen C, Wang B, Stampfer MJ, Xu X. Leptin concentrations in relation to overall adiposity, fat distribution, and blood pressure in a rural Chinese population. Int J Obes Relate Metab Disord. 2001;25:121–125. doi: 10.1038/sj.ijo.0801480. [DOI] [PubMed] [Google Scholar]
- 19.El-Gharbawy AH, Kotchen JM, Grim CE, Kaldunski M, Hoffmann RG, Pausova Z, Hamet P, Kotchen TA. Gender-specific correlates of Leptin with hypertension-related phenotypes in African Americans. Am J Hypertens. 2002;15:989–993. doi: 10.1016/s0895-7061(02)03089-3. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y, Wilk JB, Borecki I, Williamson S, DeStefano AL, Xu G, Liu J, Ellison RC, Province M, Myers RH. Common variants in the 5′ region of the Leptin gene are associated with body mass index in men from the National Heart, Lung, and Blood Institute Family Heart Study. Am J Hum Genet. 2004;75:220–230. doi: 10.1086/422699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feitosa MF, Borecki IB, Rich SS, Arnett DK, Sholinsky P, Myers RH, Leppert M, Province MA. Quantitative-trait loci influencing body-mass index reside on chromosomes 7 and 13: the National Heart, Lung, and Blood Institute Family Heart Study. Am J Hum Genet. 2002;70:72–82. doi: 10.1086/338144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abecasis GR, Cherny SS, Cookson WOC, Cardon LR. GRR: graphical representation of relationship errors. Bioinformatics. 2001;17:742–743. doi: 10.1093/bioinformatics/17.8.742. [DOI] [PubMed] [Google Scholar]
- 23.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin-rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 24.Abecasis GR, Cardon LR, Cookson WOC. A general test of association for quantitative traits in nuclear families. Am J Hum Genet. 2000;66:279–292. doi: 10.1086/302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abecasis GR, Cookson WOC, Cardon LR. Pedigree tests of transmission disequilibrium. Eur J Hum Genet. 2000;8:545–551. doi: 10.1038/sj.ejhg.5200494. [DOI] [PubMed] [Google Scholar]
- 26.Clayton D. A generalization of the transmission/disequilibrium test for uncertain-haplotype transmission. Am J Hum Genet. 1999;65:1170–1177. doi: 10.1086/302577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clayton D, Jones H. Transmission/disequilibrium tests for extended marker haplotypes. Am J Hum Genet. 1999;65:1161–1169. doi: 10.1086/302566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu K, Zhang SL, Borecki I, Kraja A, Xiong C, Myers R, Province M. A haplotype similarity based transmission/disequilibrium test under founder heterogeneity. Ann Hum Genet. 2005;69:455–467. doi: 10.1046/j.1529-8817.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- 29.Haynes WG, Sivitz WI, Morgan DA, Walsh SA, Mark AL. Sympathetic and cardiorenal actions of leptin. Hypertension. 1997;30:619–623. doi: 10.1161/01.hyp.30.3.619. [DOI] [PubMed] [Google Scholar]
- 30.Reaven GM, Lithell H, Landberg L. Hypertension and associated metabolic abnormalities-the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 31.Tuominen JA, Ebeling P, Laquier FW. Serum leptin concentration and fuel homeostasis in healthy man. Eur J Clin Invest. 1997;27:206–211. doi: 10.1046/j.1365-2362.1997.940642.x. [DOI] [PubMed] [Google Scholar]
- 32.Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A, Hunt SC, Hopkins PN, Williams RR, Lalouel JM, Corvol P. Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992;71:169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 33.Tamura K, Umemura S, Iwamoto T, Yamaguchi S, Kobayashi S, Takeda K, Tokita Y, Takagi N, Murakami K, Fukamizu A. Molecular mechanism of adipogenic activation of the angiotensinogen gene. Hypertension. 1994;23:364–368. doi: 10.1161/01.hyp.23.3.364. [DOI] [PubMed] [Google Scholar]
- 34.Campfield LA, Smith FJ, Burn P. The OB protein (leptin) pathway-a link between adipose tissue mass and central neural networks. Horm Metab Res. 1996;28:619–632. doi: 10.1055/s-2007-979867. [DOI] [PubMed] [Google Scholar]
- 35.Campfield LA, Smith FJ, Burn P. OB protein: a hormonal controller of central neural networks mediating behavioral, metabolic and neuroendocrine responses. Endocrinol Metab. 1997;4:81–102. [Google Scholar]
- 36.Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV. Leptin: the tale of an obesity gene. Diabetes. 1996;45:1455–1462. doi: 10.2337/diab.45.11.1455. [DOI] [PubMed] [Google Scholar]
- 37.Wolf G. Neuropeptides responding to leptin. Nutr Rev. 1997;55:85–88. doi: 10.1111/j.1753-4887.1997.tb01602.x. [DOI] [PubMed] [Google Scholar]
- 38.Quilliot D, Bohme P, Zannad F, Ziegler O. Sympathetic-leptin relationship in obesity: effect of weight loss. Metabolism. 2008;57:555–562. doi: 10.1016/j.metabol.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 39.Tena-Sempere M, Manna PR, Zhang FP, Pinilla L, Gonzalez LC, Dieguez C, Huhtaniemi I, Aguilar E. Molecular mechanisms of leptin action in adult rat testis: potential targets for leptin-induced inhibition of steroido-genesis and pattern of leptin receptor messenger ribonucleic acid expression. J Endocrinol. 2001;170:413–423. doi: 10.1677/joe.0.1700413. [DOI] [PubMed] [Google Scholar]
- 40.Martin LJ, Mahaney MC, Almasy L, MacCluer JW, Blangero J, Jaquish CE, Comuzzie AG. Leptin's sexual dimorphism results from genotype by sex interactions mediated by testosterone. Obes Res. 2002;10:14–21. doi: 10.1038/oby.2002.3. [DOI] [PubMed] [Google Scholar]
- 41.Elbers JM, Asscheman H, Seidell JC, Frolich M, Meinders AE, Gooren LJ. Reversal of the sex differences in serum leptin levels upon cross-sex hormone administration in transsexuals. J Clin Endocrinol Metab. 1997;82:3267–3270. doi: 10.1210/jcem.82.10.4284. [DOI] [PubMed] [Google Scholar]
- 42.Matera C, Wardlaw SL. Aromatization is not required for androgen induced changes in the proopiomelanocortin gene expression in the hypothalamus. Mol Brain Res. 1994;27:275–280. doi: 10.1016/0169-328x(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 43.Majdoubi ME, Ramaswamy S, Sahu A, Plant TM. Effects of orchidectomy on levels of mRNAs encoding gonadotropin-releasing hormone and other hypothalamic peptides in the adult male rhesus monkey (Macaca mulatta). J Neuroendocrinol. 2000;12:167–176. doi: 10.1046/j.1365-2826.2000.00433.x. [DOI] [PubMed] [Google Scholar]


