Abstract
Objective
To determine the stability and functional significance of blood-brain barrier (BBB) integrity in patients with mild to moderate Alzheimer disease (AD).
Methods
Thirty-six patients (mean age 71 ± 7 years) with mild to moderate AD (Mini-Mental State Examination [MMSE] 19 ± 5) participated in a biomarker study involving clinical assessments, brain imaging, and CSF and plasma collection over 1 year. BBB integrity was assessed with the CSF-albumin index (CSF-AI).
Results
BBB disruption was present in an important subgroup of patients (n = 8/36, 22%) at all time points measured. CSF-AI was highly reproducible over 1 year with an intraclass correlation of 0.96. Age, sex, and APOE status did not correlate with CSF-AI. Vascular factors (blood pressure, Hachinski ischemia score, MR-derived white matter hyperintensity, body mass index) were not strongly associated with CSF-AI levels (p = 0.066). CSF/plasma IgG ratio correlated with CSF-AI in a manner indicating that peripheral IgG has greater access to the CNS in patients with an impaired BBB. Further evidence for the physiologic significance of the CSF-AI was noted in the form of correlations with rates of disease progression, including annual change on MMSE (r2 = 0.11, p = 0.023), annual Clinical Dementia Rating sum-of-boxes change (r2 = 0.29, p = 0.001), and annual ventricular volume change (r2 = 0.17, p = 0.007).
Conclusions
Blood-brain barrier (BBB) impairment is a stable characteristic over 1 year and present in an important subgroup of patients with Alzheimer disease. Age, gender, APOE status, vascular risk factors, and baseline Mini-Mental State Examination score did not explain the variability in BBB integrity. A role for BBB impairment as a modifier of disease progression is suggested by correlations between CSF-albumin index and measures of disease progression over 1 year.
The pathophysiology of Alzheimer disease (AD) remains incompletely understood, although the “amyloid cascade hypothesis” forms the basis of much research, much literature, and many clinical trials.1,2 One hypothesis considered previously was the notion that blood-brain barrier (BBB) impairment might play an important role in the pathogenesis of AD.3 However, clinical investigations in this area suggested that BBB impairment was associated more with cerebrovascular disease than with AD,4 and the notion that BBB dysfunction is physiologically important in AD has fallen out of favor. However, the recent interest in immunotherapy for AD5 provides one reason to re-evaluate the BBB issue, as the intact BBB is a potential barrier to effective therapy with anti-amyloid immunoglobulin. At the same time, a dysfunctional BBB may represent a risk factor for immune-mediated toxicity including autoimmune encephalitis, which was seen in 6% of AD patients receiving active immunization against β-amyloid.6 Another reason to revisit the role of the BBB in AD is the incomplete current understanding of AD pathogenesis, specifically the variability in rate of disease progression. We consequently included CSF-albumin index (CSF-AI) in a series of AD patients participating in a longitudinal biomarker study and examined the stability and functional significance of this index of BBB integrity.
METHODS
We analyzed the relationships between biochemical markers of BBB integrity, clinical risk factors, CNS IgG synthesis, APOE genotype, MR-derived white matter hyperintensities (WMH), and volume changes over 1 year in 36 patients with mild to moderate AD to determine the stability and functional significance of the BBB.
Study participants
The subject population for this longitudinal biomarker study comprised patients in the NIA-Layton Aging and Alzheimer's Disease Center of Oregon Health and Science University with a diagnosis of probable AD. Participants were diagnosed with probable AD by National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria7 and Clinical Dementia Rating of 0.5 or 1 establishing mild to moderate AD. All patients from the biomarker study with biochemical and quantifiable MRI data were included, yielding 36 patients with mild to moderate probable AD. All participants provided informed consent in accord with the Institutional Review Board for human study at Oregon Health and Science University.
Data collection
Thirty-six patients (12 women; mean age 71 ± 7 years) were assessed at baseline and 3 and 12 months. Clinical evaluation included medical history, physical exam, Mini-Mental State Examination (MMSE),8 Clinical Dementia Rating (CDR),9 Hachinski ischemia score,10 Geriatric Depression Scale,11 and lumbar CSF and blood analysis. MRI was performed at baseline and 12 months.
MRI acquisition and analysis
MRI was performed with a 1.5 T scanner using methods as described previously.12 Hippocampus, temporal lobe, total brain, WMH, and total intracranial and ventricle volumes were measured in cubic centimeters at baseline and 1 year. Annualized rates of atrophy were calculated by dividing the difference in volume by the time elapsed between images.
Biochemical assays
Lumbar punctures were performed in the morning under standardized conditions at L3 to L4 or L4 to L5 interspace, immediately aliquoted, and snap frozen at -70 °C until assayed, at which point samples had normal cell count and glucose levels. Blood samples collected at the same visit as lumbar puncture were analyzed with the CSF to quantify albumin and IgG antibody ratios.13 CSF-albumin and IgG index were calculated as previously described.14 Genotyping of APOE isoforms was determined by methods also described elsewhere.15 All biochemical and genetic analyses were performed and the results recorded without knowledge of patients' clinical or MR-derived information.
Statistical methods
An intraclass correlation coefficient from a random effects analysis-of-variance was used to determine the reliability of CSF-AI measurements over 1 year. Linear regression models were used to test for associations between baseline CSF-AI and clinical, biochemical, and imaging measures within the entire sample. All analyses were adjusted for age. Two-sample t tests were used to assess whether average rates of change in CDR sum of boxes (SOB), MMSE, and ventricular volume differed between those with an intact BBB (CSF-AI < 9.0) vs patients with BBB impairment (CSF-AI ≥ 9.0). Linear regression was used to assess whether baseline CSF-AI was associated with the rate of change in clinical, biochemical, and imaging measures after adjusting for baseline of each. SPSS (v. 15; Chicago, IL, 2006) was used for all analyses.
RESULTS
Subject characteristics are summarized in the table.
Table.
Baseline demographic, genetic, biochemical, and radiographic characteristics for cohort as a whole, then dichotomized by blood-brain barrier integrity
| AD, n = 36 | BBB impairment,* n = 8 | BBB intact,† n = 28 | p Value‡ | |
|---|---|---|---|---|
| Age, y | 71 (7) | 73 (8) | 70 (7) | 0.285 |
| Female, (%) | 12 (33) | 1 (13) | 11 (39) | 0.165 |
| APOE ε4 carriers, (%) | 27 (75) | 6 (75) | 21 (75) | 1.0 |
| APOE ε4 non-carriers, (%) | 9 (25) | 2 (25) | 7 (25) | 1.0 |
| BP systolic, mm Hg | 142.4 (23.3) | 136.7 (30.0) | 143.3 (21.3) | 0.490 |
| BP diastolic, mm Hg | 77.00 (11.7) | 76.3 (7.8) | 78.7 (13.4) | 0.690 |
| BMI | 26.7 (4.4) | 29.1 (5.5) | 26.2 (4.2) | 0.108 |
| MMSE | 20 (5) | 18 (5) | 20 (5) | 0.494 |
| CDR | 0.8 (0.2) | 1.0 (0) | 0.8 (0.2) | 0.048§ |
| CDR sum of boxes | 5.7 (1.4) | 6.3 (1.3) | 5.6 (1.4) | 0.229 |
| CSF-albumin index | 7.2 (3.7) | 12.9 (3.3) | 5.6 (1.7) | <0.0001§ |
| Albumin, CSF, μM | 29.2 (15.2) | 53.1 (14.8) | 22.5 (6.5) | <0.0001§ |
| Albumin, serum, μM | 4,032 (359.0) | 4,075 (166.8) | 4,020 (398.9) | 0.712 |
| CSF IgG index | 0.461 (0.0599) | 0.500 (0.0535) | 0.450 (0.0577) | 0.035§ |
| IgG, CSF, μM | 3.2 (2.0) | 6.1 (2.4) | 2.4 (0.8) | <0.0001§ |
| IgG, serum, μM | 949.0 (213.1) | 939.3 (271.9) | 951.4 (199.1) | 0.889 |
| Intracranial volume, cm3 | 1,285.98 (145.07) | 1,298.93 (132.08) | 1,282.28 (150.64) | 0.779 |
| Ventricle volume, cm3 | 63.3 (26.4) | 87.5 (27.3) | 58.1 (23.5) | 0.005§ |
| Hippocampus volume, cm3 | 1.2 (0.2) | 1.2 (0.1) | 1.17 (0.2) | 0.433 |
| Temporal lobe volume, cm3 | 112.5 (20.2) | 110.9 (20.2) | 113.2 (21.1) | 0.794 |
| Total brain volume, cm3 | 925.7 (129.0) | 908.0 (102.3) | 930.7 (136.9) | 0.667 |
| WMH, cm3 | 10.3 (13.8) | 9.7 (7.2) | 8.6 (8.5) | 0.741 |
| Hachinski ischemia score | 0.6 (0.9) | 1.0 (1.3) | 0.5 (0.8) | 0.200 |
Categorical data (APOE status, gender) expressed as number (%); continuous variables expressed as mean (SD).
CSF albumin index ≥ 9.0.
CSF albumin index < 9.0.
Comparison of means between groups (BBB impairment vs BBB intact).
Statistical significance.
AD = Alzheimer disease; BBB = blood-brain barrier; BP = blood pressure; BMI = body mass index; CDR = Clinical Dementia Rating; MMSE = Mini-Mental State Examination; WMH = white matter hyperintensities.
Stability of CSF-albumin index (CSF-AI)
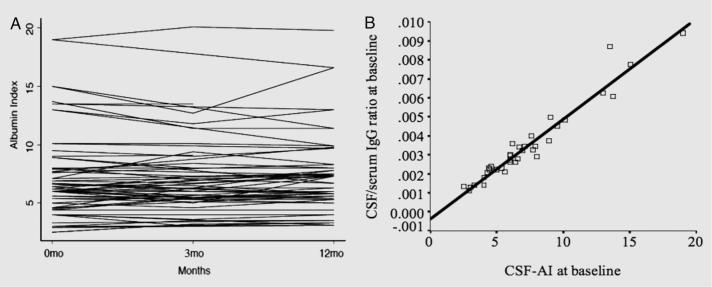
CSF-AI is highly reproducible over 1 year with an intraclass correlation of 0.96 where >0.75 indicates excellent reproducibility.16 Figure 1A illustrates the within-patient stability of the CSF-AI on repeated measures at baseline, 3, and 12 months. The reproducibility of the CSF-AI diminishes the possibility that these results are driven by contamination of CSF by blood during traumatic taps. The CSF-AI was elevated above the accepted upper limit of 9.017 in 22% of patients at each time point, indicating sustained BBB disruption in a subpopulation of AD patients.
Figure 1. CSF-albumin index in Alzheimer disease.
(A) Stability of CSF-albumin index (CSF-AI): within-patient CSF-AI on repeated measures over 1 year (baseline, 3 and 12 months). A level line indicates an identical value at each time point. (B) Functional significance of blood-brain barrier integrity in Alzheimer disease: relationship between baseline measures of CSF-AI and IgG levels (r2 = 0.94, p < 0.0001).
CSF-AI and vascular risk factors
Vascular risk factors explained approximately 31% of the variability in CSF-AI (p = 0.066). Systolic blood pressure was the most significant correlate (p = 0.026) with a 1-unit increase in systolic blood pressure being associated with a 0.067-unit decrease in CSF-AI; however, this reverse association was due to one outlier with a low systolic blood pressure and a high albumin index. When this subject was removed from analysis, the blood pressure association with albumin was positive and insignificant. Additionally, both baseline MR-derived white matter (p = 0.054) and body mass index (BMI) (p = 0.083) were close to statistically significant. Both had positive trends with increased WMH and increased BMI being associated with increased CSF-AI. These associations were not influenced by the outlier discussed above. Hachinski ischemia score (p = 0.48) and age (p = 0.63) were not associated with CSF-AI.
CSF-AI and intrathecal immunoglobulin levels
The CSF IgG index, a measurement of intrathecal antibody synthesis, was not elevated in any patients (range = 0.4 to 0.6, mean = 0.46; <0.7 is considered normal) at any time point.14 In the absence of significant intrathecal antibody synthesis, the CSF/serum ratio of IgG is expected to be dependent on the integrity of the BBB, as the majority of IgG is synthesized in the periphery. In keeping with this assumption, the CSF-AI was strongly correlated with the CSF/serum ratio of IgG (r2 = 0.94, p < 0.0001) (figure 1B).
CSF-AI, disease stage, and rate of degeneration
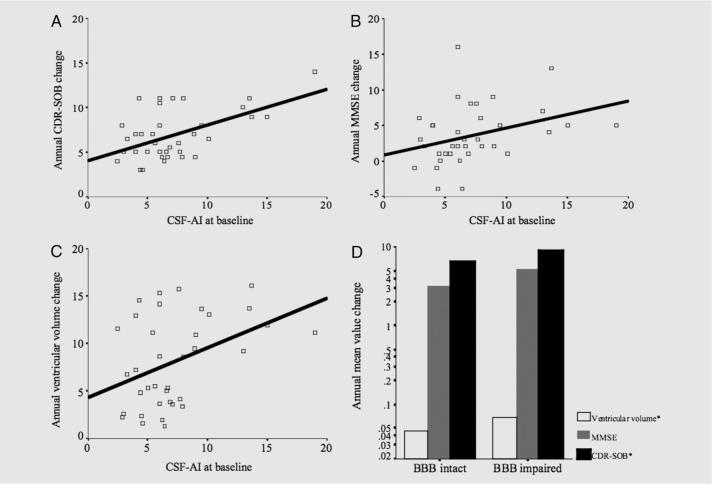
The variability in CSF-AI across patients was not explained by age (p = 0.66), gender (p = 0.19), or APOE ε4 carrier status (p = 0.60). Significant correlations between CSF-AI and baseline CDR and ventricle size were appreciated, but CSF-AI did not correlate with baseline MMSE or other brain volumes. However, multiple clinical and brain imaging indexes of rate of degeneration were correlated with CSF-AI, including rate of CDR-SOB change (r2 = 0.29, p = 0.001), rate of MMSE change (r2 = 0.11, p = 0.023), and rate of ventricle size increase (r2 = 0.17, p = 0.007) (figure 2, A through C). Rates of MMSE (p = 0.02) and CDR-SOB (p = 0.005) change maintained correlation with CSF-AI after controlling for each respective baseline measure (e.g., rate of MMSE change vs CSF-AI controlled for MMSE baseline measure). After dichotomizing patients by BBB intact and impaired at baseline (CSF-AI cut-off of 9.0), differences were appreciated between rates of disease progression in the two groups (CDR-SOB p = 0.013, ventricular volume p < 0.0001) (figure 2D).
Figure 2. Functional significance of blood-brain barrier (BBB) integrity in Alzheimer disease (AD): Relationship between baseline CSF-albumin index (CSF-AI) and measures of disease progression.
Scatter plot and regression line describing relationships between baseline CSF-AI and (A) annual rate Clinical Dementia Rating (CDR) sumof-boxes (SOB) change in points/year (r2 = 0.29, p = 0.001), (B) annual rate of Mini-Mental State Examination (MMSE) change in points/year (r2 = 0.112, p = 0.023), and (C) annual ventricular volume change in cm3 (r2 = 0.17, p = 0.007). (D) Clustered bar graph comparing subjects with BBB intact (CSF-AI < 9.0) against subjects with BBB impairment (CSF-AI ≥ 9.0) in terms of log-transformed annual mean value change in CDR-SOB (*p = 0.017, mean difference of 2.57), MMSE (p = 0.196, mean difference of 2.07 points), and ventricular volume (*p < 0.0001, mean difference 5.58 cm3).
Pathology
Brain autopsy was offered for all patients as part of their participation in the Oregon Alzheimer's Disease Center. Thirteen of the participants in this biomarker study came to brain autopsy: four with BBB impairment and nine with intact BBB, according to CSF-AI. All 13 patients met pathologic criteria for AD. None was pathologically diagnosed with vascular dementia.
DISCUSSION
These data demonstrate that CSF-AI is a stable characteristic of patients with mild to moderate AD and is increased in an important minority of such patients. The functional significance of BBB integrity in AD is suggested by correlations with CSF/plasma IgG ratio and with rates of disease progression. We did not find a relationship between vascular risk and BBB impairment as others have reported, perhaps because this group of patients was selected to minimize the presence of cerebrovascular disease by virtue of a diagnosis of probable AD and a Hachinski ischemia score of ≤4. The imaging data provide further evidence against the hypothesis that BBB impairment in these patients is due to occult vascular disease. The available brain pathology also fails to find significant cerebrovascular disease in this subject population, regardless of BBB status. Although BBB impairment may indeed be more common in vascular dementia than in AD,4 the relatively high prevalence of BBB impairment in these AD patients raises the possibility that BBB dysfunction may be more common in AD than is generally believed. If that is so, then the causes and consequences of BBB dysfunction in AD are of interest.
The cause remains speculative, although an effect of amyloid angiopathy on the BBB may be the most obvious candidate. None of these patients had clinically evident hemorrhages during follow-up. Brain MRI failed to disclose vascular complications of amyloid angiopathy, although MRI sequences appropriate for detecting remote microhemorrhages were not included. The subset of available neuropathology reports on this population further supports absence of significant hemorrhagic complications of amyloid angiopathy, although vascular amyloid was a common finding, as expected in most cases of AD. Amyloid angiopathy therefore remains a viable explanation for the BBB impairment seen in these patients, although the variation in BBB integrity was not obviously associated with variation in vascular amyloid.
A less obvious (but equally plausible) contributor to BBB impairment in AD is high plasma homocysteine. Whereas the relationships between hyperhomocysteinemia and dementia are well established, a relationship between homocysteine and BBB impairment is less well known. One recent study of 30 patients with hyperhomocysteinemia and amnestic mild cognitive impairment demonstrated a significant elevation of CSF-AI in patients compared with 35 healthy control subjects.18 As homocysteine is known to be toxic to vascular endothelial cells, the authors speculate that elevated homocysteine levels directly promote BBB impairment and tested this possibility by lowering hyperhomocysteinemia in the patients and then reassessing BBB status. Reducing homocysteine concentrations with B-vitamin therapy by approximately 50% significantly reduced CSF-AI by 11% over 9 months.18 This report therefore suggests that hyperhomocysteinemia may perturb BBB integrity in AD.
The consequences of BBB dysfunction are also not entirely clear, and aside from these data, one could even construct scenarios under which BBB permeability might actually be beneficial for patients with AD. For example, BBB permeability might provide a pathway for β-amyloid clearance or might permit endogenous anti-amyloid antibody to enter the CNS. However, the data here argue that BBB impairment in AD is associated with adverse consequences, as increased degrees of BBB impairment are associated with increased rates of neurodegeneration. Whether this is a causal association or noncausal correlation cannot be determined from these data, but it is clear that the data do not show any clinical benefit from increased BBB permeability in AD.
In addition to effects on the natural history of AD, BBB dysfunction may also potentially modify responses to treatment with large molecules like immune globulin. Previous trials of active immunization were halted because of encephalitis in a small but significant minority of patients,19 leading to current trials of passive immunization, which may be intrinsically safer. However, preliminary findings of MRI changes in AD patients receiving passive immunization show that even passive immunization may be risky.20 Furthermore, if the complication of CNS inflammation in immune therapy trials could be linked to BBB integrity at baseline, then it might be possible to diminish this very serious risk of immunotherapy by screening patients with the CSF-AI and foregoing immunotherapy in patients with BBB impairment.
Acknowledgments
Supported by the NIH-Ruth L. Kirschstein National Research Service Award (G.B.), VA Advanced Research Development Award (J.Q.), and NIA-AG08017, PHS M01 RR00034, and the Dana Foundation.
Footnotes
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J. Has the amyloid cascade hypothesis for Alzheimer's disease been proved? Curr Alzheimer Res. 2006;3:71–73. doi: 10.2174/156720506775697098. [DOI] [PubMed] [Google Scholar]
- 3.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 4.Blennow K, Wallin A, Fredman P, Karlsson I, Gottfries CG, Svennerholm L. Blood-brain barrier disturbance in patients with Alzheimer's disease is related to vascular factors. Acta Neurol Scand. 1990;81:323–326. doi: 10.1111/j.1600-0404.1990.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee M, Bard F, Johnson-Wood K, et al. Abeta42 immunization in Alzheimer's disease generates Abeta N-terminal antibodies. Ann Neurol. 2005;58:430–435. doi: 10.1002/ana.20592. [DOI] [PubMed] [Google Scholar]
- 6.Bayer AJ, Bullock R, Jones RW, et al. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 7.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Dooneief G, Marder K, Tang MX, Stern Y. The Clinical Dementia Rating scale: community-based validation of “profound” and “terminal” stages. Neurology. 1996;46:1746–1749. doi: 10.1212/wnl.46.6.1746. [DOI] [PubMed] [Google Scholar]
- 10.Moroney JT, Bagiella E, Desmond DW, et al. Meta-analysis of the Hachinski Ischemic Score in pathologically verified dementias. Neurology. 1997;49:1096–1105. doi: 10.1212/wnl.49.4.1096. [DOI] [PubMed] [Google Scholar]
- 11.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 12.Mueller EA, Moore MM, Kerr DC, et al. Brain volume preserved in healthy elderly through the eleventh decade. Neurology. 1998;51:1555–1562. doi: 10.1212/wnl.51.6.1555. [DOI] [PubMed] [Google Scholar]
- 13.Link H, Tibbling G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within the central nervous system in multiple sclerosis. Scand J Clin Lab Invest. 1977;37:397–401. doi: 10.1080/00365517709091498. [DOI] [PubMed] [Google Scholar]
- 14.Tibbling G, Link H, Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest. 1977;37:385–390. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg SM, Briggs ME, Hyman BT, et al. Apolipoprotein E epsilon 4 is associated with the presence and earlier onset of hemorrhage in cerebral amyloid angiopathy. Stroke. 1996;27:1333–1337. doi: 10.1161/01.str.27.8.1333. [DOI] [PubMed] [Google Scholar]
- 16.Rosner B. Fundamentals of biostatistics. 6th ed Duxbury; Pacific Grove, CA: 2006. [Google Scholar]
- 17.Herndon RM. The cerebrospinal fluid. Kluwer Academic Publishers; 1989. [Google Scholar]
- 18.Lehmann M, Regland B, Blennow K, Gottfries CG. Vitamin B12-B6-folate treatment improves blood-brain barrier function in patients with hyperhomocysteinaemia and mild cognitive impairment. Dement Geriatr Cogn Disord. 2003;16:145–150. doi: 10.1159/000071002. [DOI] [PubMed] [Google Scholar]
- 19.Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 20.Fox NC, Black RS, Gilman S, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]