Abstract
Purpose
We sought to characterize the pharmacokinetics (PK) and determine a tolerable dose of oral sorafenib in patients with hepatic or renal dysfunction.
Patients and Methods
Patients were assigned to one of nine cohorts: cohort 1, bilirubin ≤ upper limit of normal (ULN) and AST ≤ ULN and creatinine clearance (CC) ≥ 60 mL/min; cohort 2, bilirubin more than ULN but ≤ 1.5× ULN and/or AST more than ULN; cohort 3, CC between 40 and 59 mL/min; cohort 4, bilirubin more than 1.5× ULN to ≤ 3× ULN (any AST); cohort 5, CC between 20 and 39 mL/min; cohort 6, bilirubin more than 3× ULN to 10× ULN (any AST); cohort 7, CC less than 20 mL/min; cohort 8, albumin less than 2.5 mg/dL (any bilirubin/AST); and cohort 9, hemodialysis. Sorafenib was administered as a 400-mg dose on day 1 for PK, and continuous daily dosing started on day 8.
Results
Of 150 registered patients, 138 patients were treated. With the exception of cohorts 6 and 7, at least 12 patients per cohort were assessable, and the dose level with prospectively defined dose-limiting toxicity in less than one third of patients by day 29 was considered tolerable. No significant associations between the sorafenib PK and cohort were found.
Conclusion
We recommend the following empiric sorafenib starting doses by cohort: cohort 1, 400 mg twice a day; cohort 2, 400 mg twice a day; cohort 3, 400 mg twice a day; cohort 4, 200 mg twice a day; cohort 5, 200 mg twice a day; cohort 6, not even 200 mg every third day tolerable; cohort 7, not defined; cohort 8, 200 mg each day; and cohort 9, 200 mg each day.
INTRODUCTION
Raf, which is an essential serine/threonine kinase constituent of the mitogen-activated protein kinase signaling pathway and a downstream effector of the central signal transduction mediator Ras, is activated in a wide range of human malignancies and is therefore recognized as a strategic target for therapeutic drug development.1 Sorafenib (formerly BAY 43-9006), an orally active multikinase inhibitor with effects on tumor cell proliferation and tumor angiogenesis, was initially identified as a Raf kinase inhibitor.2 It also inhibits vascular endothelial growth factor receptors 1, 2, and 3; platelet-derived growth factor receptor β; FMS-like tyrosine kinase 3; c-Kit protein; and RET receptor tyrosine kinase.2,3 Sorafenib is approved by the United States Food and Drug Administration and other global health authorities for the treatment of renal and hepatocellular carcinomas and has also demonstrated activity in a number of other malignancies.4–6 As in the development of other new drugs, sorafenib was initially tested in patients with adequate hepatic and renal function, and the recommended continuous daily dose is 400 mg twice a day for such patients.7–9 The most common side effects include hand-foot skin reactions, diarrhea, fatigue, rash, and hypertension.4–9 Sorafenib is primarily metabolized in the liver (predominantly by CYP3A4). Urinary excretion is a minor (< 20%) route of elimination. We hypothesized that the pharmacokinetics and tolerability of sorafenib would be affected by hepatic dysfunction, but not by renal dysfunction. Because patients with malignancies often present with abnormal hepatic or renal function, the objectives of this study were to characterize the pharmacokinetics and to determine tolerable starting doses of sorafenib in such patients.
PATIENTS AND METHODS
Eligibility Criteria
Patients were eligible if they had pathologically documented solid tumors, multiple myeloma, or non-Hodgkin's lymphoma for which standard curative or palliative measures did not exist. Patients had to be ≥ 18 years of age with an Eastern Cooperative Oncology Group performance status of 0 to 2. Required laboratory values at entry included an absolute neutrophil count ≥ 1,500/μL and platelet count ≥ 75,000/μL. Prior therapies (including chemotherapy, surgery, and radiation) had to be completed at least 4 weeks before enrollment. The study had to be approved by the institutional review board of each participating institution, and all patients gave written informed consent.
Exclusion Criteria
Patients who were pregnant or lactating were excluded. Concomitant medications known to cause hepatic or renal toxicity were not allowed on study. Other exclusion criteria included gastrointestinal tract disease resulting in an inability to take oral medication, HIV-positive patients on antiretroviral therapy, therapeutic anticoagulation, evidence of biliary or renal obstruction, and treatment with cytochrome P450 enzyme-inducing antiepileptic drugs, rifampin, or St John's wort.
Cohort Definitions
No widely accepted guidelines defining organ dysfunction in patients with cancer were available when this study was designed. Our aim was to use laboratory values that are routinely available in clinical practice, such as serum total bilirubin, AST, albumin, and creatinine. The creatinine clearance was estimated using the Cockcroft and Gault formula.10 The study included a cohort with normal organ function (cohort 1) and eight cohorts with increasing severity of hepatic dysfunction (cohorts 2, 4, 6, and 8) or renal dysfunction (cohorts 3, 5, 7, and 9) as defined in Table 1. Patients who fit into more than one of these cohorts (eg, elevated bilirubin and decreased creatinine clearance) were not eligible for this study, except for cohort 8, for which patients had creatinine clearance ≥ 60 mL/min and any bilirubin/AST (including bilirubin/AST ≤ upper limit or normal [ULN]).
Table 1.
Cohort Definitions
| Cohort Description | Hepatic | Renal |
|---|---|---|
| Normal organ function | Cohort 1: Total bilirubin ≤ ULN and AST ≤ ULN and CrCl ≥ 60 mL/min | |
| Mild dysfunction | Cohort 2: Bilirubin > ULN but ≤ 1.5× ULN and/or AST > ULN | Cohort 3: CrCl between 40 and 59 mL/min |
| Moderate dysfunction | Cohort 4: Bilirubin > 1.5× ULN to ≤ 3× ULN and any AST | Cohort 5: CrCl between 20 and 39 mL/min |
| Severe dysfunction | Cohort 6: Bilirubin > 3× ULN to 10× ULN and any AST | Cohort 7: CrCl < 20 mL/min |
| Very severe dysfunction | Cohort 8: Albumin < 2.5 mg/dL, any bilirubin, and any AST | Cohort 9: Hemodialysis, any CrCl |
Abbreviations: ULN, upper limit of normal; CrCl, creatinine clearance.
Treatment
The study was conducted in two parts. In part 1, all patients received a single oral test dose of 400 mg of sorafenib on day 1 to assess the pharmacokinetics by type and severity of organ impairment. Part 2 started on day 8 and was designed as a phase I dose-escalation study to evaluate the toxicity of continuous oral dosing of sorafenib by cohort. Cohort and dose level assignment occurred before the start of treatment on day 8 after a discussion with the study chair, which included a review of the eligibility and exclusion criteria and cohort assignment in parts 1 and 2. The initial dose (level 1) of sorafenib depended on cohort: cohort 1, 400 mg twice a day; cohorts 2 and 3, 200 mg twice a day; cohorts 4 and 5, 200 mg each day; and cohorts 6 through 9, 200 mg every other day. If dose-limiting toxicity (DLT) was reached at dose level 1 in cohorts 6 through 9, further patients were to be treated with sorafenib 200 mg every third day (dose level −1). Escalation to the next dose level occurred in a new group of patients if the maximum-tolerated dose (MTD) had not been reached and all patients in a cohort had been treated for at least 3 weeks (days 8 through 29). The sorafenib dose was escalated by cohort in 200-mg steps to 400 mg twice a day. Sorafenib was manufactured by Bayer Pharmaceuticals (West Haven, CT) and distributed by the National Cancer Institute Pharmaceutical Management Branch as 200-mg tablets.
Required Data
Patient registration and data collection were managed by the Cancer and Leukemia Group B (CALGB) Statistical Center. Before registration and weekly during the first month of treatment, details of history and physical examination, complete blood cell counts with differential, and pertinent chemistry results for hepatic and renal function were collected. The chemistry values determining cohort assignment were required twice within 10 days before registration. Data were prospectively recorded to calculate the Child-Pugh score.11 During months 2 and 3, history and physical examinations were done every 4 weeks and laboratory studies were done every 2 weeks. The Common Terminology Criteria for Adverse Events, version 3.0, and the Response Evaluation Criteria in Solid Tumors from the National Cancer Institute were used.12 Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chair.
DLT
Because there are no widely accepted rules to define DLT in patients with abnormal liver or renal function tests at entry on trial, our definitions were empiric, but they were prospectively defined in the protocol. For cohorts 2, 4, 6, and 8, an increase in AST or alkaline phosphatase ≥ 2.5× baseline or an increase in total bilirubin ≥ 1.5× baseline were considered dose-limiting hepatic toxicity unless unequivocally caused by tumor progression as evidenced by imaging studies, and a reduction of creatinine clearance more than 10 mL/min was defined as DLT. For cohorts 3, 5, 7, and 9, a reduction of creatinine clearance more than 20 mL/min was considered dose-limiting renal toxicity, and elevations in AST or alkaline phosphatase ≥ 5× ULN or total bilirubin ≥ 2.5× ULN were defined as DLT. Other grade 3 or worse nonhematologic toxicity was defined as DLT. Grade 3 or worse nausea, vomiting, diarrhea, or anorexia despite optimal supportive care were also considered DLT. Grade 4 neutropenia and grade 4 thrombocytopenia were defined as DLT.
Dose-Escalation Rules
Dose escalation to the next dose level occurred in groups of patients when the MTD was not reached and after patients in a cohort had been treated for at least 3 weeks with continuous daily dosing of sorafenib. If none of three patients developed DLT, three patients were entered at the next dose level. If two or more patients of three experienced DLT, dose escalation was stopped and this dose level was the highest administered dose. If one of three patients developed DLT, at least three more patients were entered at this dose level. If none of these three additional patients experienced DLT, we proceeded to the next dose level. If one or more of these three additional patients experienced DLT, then dose escalation was stopped, and this dose was the highest administered dose. The MTD was prospectively defined as the dose level at which no more than one of six patients had DLT. The objective was to assess patients for DLT in the first 4 weeks on protocol. Therefore, patients who discontinued protocol therapy within the first 4 weeks for reasons other than toxicity were replaced and not considered in the determination of MTD. We planned to treat 12 patients in cohort 1 and at least six patients in each of the other cohorts. The protocol allowed enrollment of more than six patients per dose level to clarify the toxicity pattern.
Pharmacokinetics
Blood samples were obtained at baseline and at 1, 2, 3, 4, 6, and 24 hours after the 400-mg test dose of sorafenib on day 1. After centrifugation, the plasma was separated and frozen and then shipped to the CALGB coordinating office at the Ohio State University. The plasma concentrations of sorafenib and its N-oxide, desmethyl, and N-oxide desmethyl metabolites were measured by high-performance liquid chromatography with tandem mass spectrometry by Bayer Pharmaceuticals (Hamden, CT).13 The fraction of sorafenib not bound to plasma proteins was measured in vitro by Bayer Pharmaceuticals (Wuppertal, Germany) with C14-labeled sorafenib in patients' plasma (sample at 4 hours after test dose) according to a published method.14 Sorafenib pharmacokinetic parameters were estimated using Adapt II software15 and maximum a posteriori Bayesian estimation methods. A two-compartment linear model parameterized using clearances and incorporating first order absorption with an absorption delay was applied to estimate the total apparent oral clearance of sorafenib. Prior parameter distributions were provided by Bayer Pharmaceuticals (West Haven, CT). The area under the curve (AUC) was calculated using the equation: AUC = dose/apparent clearance. The N-oxide metabolite AUC0-24 was determined by the linear trapezoidal rule.
Statistical Analyses
Statistical analyses were performed by CALGB statisticians. The marginal distributions of the pharmacokinetic parameters were summarized quantitatively using standard measures of central tendencies, location, and spread (eg, mean, median, standard deviation). The distribution of AUC is depicted graphically using dot plots rather than box plots because of the small number of patients within each cohort.16 The discrepancies among the distributions of AUC of sorafenib with respect to factors (eg, cohorts) were assessed using the Kruskal-Wallis test.17 Inference for the associations between AUC and continuous measurements (eg, creatinine clearance) were carried out using the Spearman rank correlation test.17 The exact two-sided P values were approximated using B = 10,000 permutation replicates for the rank tests. The statistical analyses and plots were produced using the statistical computing environment R (version 2.7.1)18 statistical environment including package coin19 for generating the conditional permutation P values.
RESULTS
Between January 2005 and February 2007, 150 patients were enrolled. Of these, 12 patients never started therapy and were excluded from all analyses. The demographics of the 138 treated patients were as follows: male sex, n = 95, female sex, n = 43; median age, 60 years (range, 21 to 85 years); white, n = 106, African America, n = 21, Asian, n = 6, other, n = 5; and performance status of 0, n = 32, 1, n = 83, 2, n = 23. Of the 138 patients in part 1, 124 patients proceeded into part 2 and 14 did not (13 with hepatic and one with renal dysfunction) because of their deteriorating clinical condition. The distribution of patients in the various cohorts by part was as follows: cohort 1, part 1, n = 12, part 2, n = 16; cohort 2, part 1, n = 14, part 2, n = 12; cohort 3, part 1, n = 18, part 2, n = 16; cohort 4, part 1, n = 21, part 2, n = 17; cohort 5, part 1, n = 14, part 2, n = 13; cohort 6, part 1, n = 13, part 2, n = 11; cohort 7, part 1, n = 5, part 2, n = 4; cohort 8, part 1, n = 24, part 2, n = 18; and cohort 9, part 1, n = 17, part 2, n = 17. Seventeen patients (10 with hepatic and seven with renal dysfunction) changed cohort assignment between part 1 and part 2 because of changes in their hepatic or renal function tests.
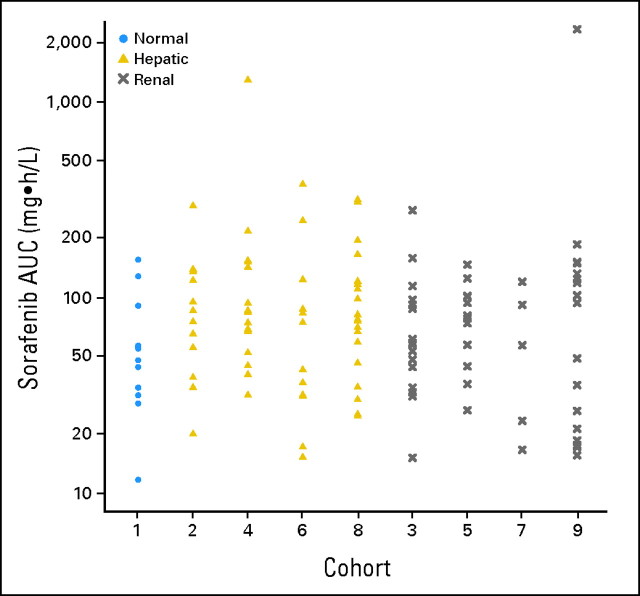
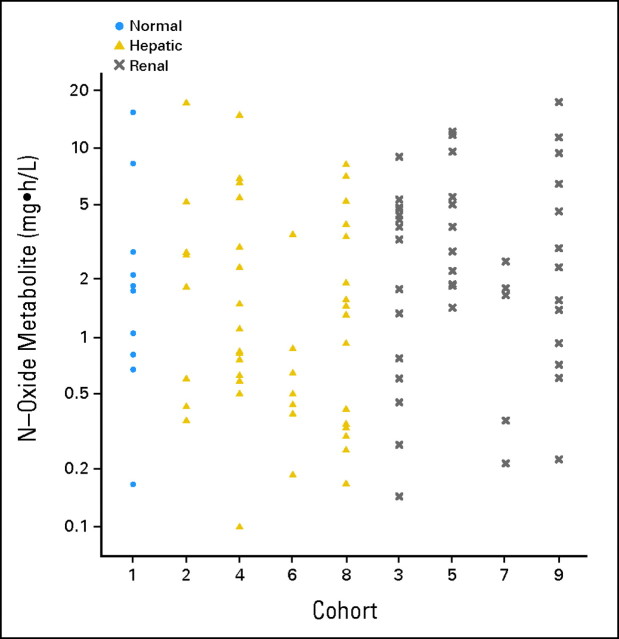
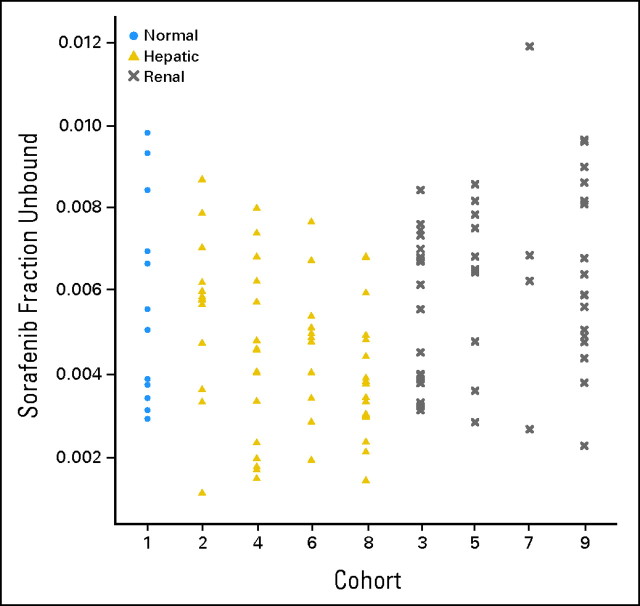
Pharmacokinetic results for sorafenib were available for 124 (90%) of the 138 treated patients. The AUC of the major metabolite, N-oxide-sorafenib, could be estimated in 100 patients (72%). Selected pharmacokinetic results for sorafenib and its N-oxide metabolite are summarized by cohort in Table 2. Figures 1 and 2 graphically depict the observed AUCs of sorafenib and its N-oxide metabolite by cohort. There was no (P > .05) significant evidence that the AUCs of sorafenib or its N-oxide metabolite were associated with cohort. Of the 72 patients in the hepatic cohorts, the Child-Pugh scores for 62 patients were available, and 53 of these patients had pharmacokinetic data. The analysis of the sorafenib AUC against the Child-Pugh scores and the bilirubin concentrations for the patients in the hepatic cohorts demonstrated a lack of significant relationships. Although there was no significant relationship between the AUC of the N-oxide metabolite and the Child-Pugh scores, higher bilirubin concentrations were associated (Spearman correlation test, P < .022) with lower AUCs of the metabolite in the hepatic cohorts. For the 54 patients in the renal cohorts, no significant relationships were identified between creatinine clearance and the AUC of sorafenib or the N-oxide metabolite. Because sorafenib is bound to plasma proteins (albumin, α- and β-globulin, and low-density lipoprotein), the unbound fractions (Fig 3) were compared between the patients with normal hepatic and renal function (cohort 1), hepatic dysfunction (cohorts 2, 4, 6, and 8), and renal dysfunction (cohorts 3, 5, 7, and 9), and a significant (Kruskal-Wallis test, P < .001) association was found; however, this was not explained by the albumin or bilirubin concentrations. Patients with hepatic dysfunction were significantly (Kruskal-Wallis test, P < .0009) younger than patients with renal dysfunction; however, there were no significant associations between age and the AUCs of sorafenib or the N-oxide metabolite.
Table 2.
Pharmacokinetic Results
| Variable and Statistic | Cohort 1 | Hepatic Cohorts |
Renal Cohorts |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 3 | 5 | 7 | 9 | ||
| Sorafenib AUC, mg·h/L | |||||||||
| No. | 12 | 13 | 16 | 12 | 19 | 18 | 12 | 5 | 17 |
| Mean | 61.6 | 99.8 | 166.8 | 97.3 | 106.4 | 80.2 | 80.4 | 61.7 | 212.4 |
| SD | 42.7 | 71.1 | 304.5 | 109.3 | 85.5 | 60.8 | 36.0 | 44.3 | 553.9 |
| Median | 51.3 | 85.5 | 79.0 | 58.5 | 77.1 | 59.9 | 79.4 | 56.9 | 93.7 |
| N-oxide metabolite AUC, mg·h/L | |||||||||
| No. | 10 | 8 | 15 | 7 | 16 | 15 | 11 | 5 | 13 |
| Mean | 3.5 | 3.9 | 3.1 | 0.9 | 2.3 | 3.0 | 5.3 | 1.3 | 4.6 |
| SD | 4.8 | 5.7 | 4.0 | 1.1 | 2.6 | 2.5 | 4.1 | 1.0 | 5.3 |
| Median | 1.8 | 2.3 | 1.1 | 0.5 | 1.4 | 3.3 | 3.8 | 1.7 | 2.3 |
| Sorafenib fraction unbound | |||||||||
| No. | 12 | 13 | 16 | 12 | 19 | 18 | 12 | 5 | 17 |
| Mean | 0.0058 | 0.0055 | 0.0043 | 0.0046 | 0.0038 | 0.0055 | 0.0059 | 0.0068 | 0.0064 |
| SD | 0.0025 | 0.0020 | 0.0021 | 0.0017 | 0.0015 | 0.0018 | 0.0021 | 0.0033 | 0.0022 |
| Median | 0.0053 | 0.0058 | 0.0043 | 0.0048 | 0.0034 | 0.0059 | 0.0065 | 0.0062 | 0.0059 |
| Sorafenib AUC of fraction unbound, mg·h/L | |||||||||
| No. | 12 | 13 | 16 | 12 | 19 | 18 | 12 | 5 | 17 |
| Mean | 0.4 | 0.6 | 1.0 | 0.4 | 0.4 | 0.5 | 0.5 | 0.5 | 1.6 |
| SD | 0.3 | 0.6 | 2.5 | 0.5 | 0.3 | 0.4 | 0.3 | 0.6 | 4.5 |
| Median | 0.2 | 0.4 | 0.3 | 0.3 | 0.3 | 0.4 | 0.3 | 0.4 | 0.4 |
Abbreviations: AUC, area under the curve; SD, standard deviation.
Fig 1.
Dot plot of the area under the curve (AUC) of sorafenib by cohort.
Fig 2.
Dot plot of the area under the curve (AUC) of the main metabolite by cohort.
Fig 3.
Dot plot of the fraction of sorafenib unbound to plasma proteins by cohort.
Of the 16 patients in cohort 1 treated in part 2 of the study, 14 patients were assessable and two patients had DLT (one grade 3 abdominal pain and one grade 3 rash). Table 3 contains the phase I experience for the hepatic cohorts. The main reason why patients were not assessable was their inability to take sorafenib continuously for 3 weeks as a result of progressive malignant disease with clinical deterioration. When two of two patients in cohort 6 at the starting dose level 1 developed DLT, nine patients were entered on dose level −1; six of these patients were assessable and three had DLT. Thus in cohort 6, even 200 mg of sorafenib every third day was intolerable based on our definitions of DLT. The events of DLT in the hepatic cohorts included increase in total bilirubin ≥ 1.5× baseline (n = 10); grade 3 diarrhea despite optimal supportive care (n = 1); grade 3 fatigue (n = 1); grade 3 fatigue and creatinine clearance reduction more than 10 mL/min (n = 1); and grade 3 hypertension (n = 1). One of the patients with an increase in total bilirubin ≥ 1.5× baseline had previously treated small-cell lung cancer and experienced fatal tumor lysis syndrome while taking sorafenib. Table 3 also summarizes the phase I experience for the renal cohorts. Patients with an estimated creatinine clearance less than 20 mL/min were infrequently encountered in clinical practice, and only four patients were enrolled in cohort 7. The DLT in the renal cohorts consisted of two events of grade 3 hypertension and one event each of the following: grade 3 abdominal pain; grade 3 fatigue; grade 3 nausea and vomiting despite optimal supportive care; grade 3 hand-foot skin reaction; grade 3 congestive heart failure; and grade 4 hemorrhage into brain metastasis. Dose level 4 of sorafenib 400 mg twice a day was not reached in either hepatic or renal cohorts 6 through 9.
Table 3.
Phase I Experience by Cohort and Dose Level
| Cohort and Variable | Dose Level |
|||
|---|---|---|---|---|
| −1 | 1 | 2 | 3 | |
| Hepatic cohorts | ||||
| Cohort 2 | — | — | ||
| No. of patients entered | 5 | 7 | ||
| No. of patients assessable | 5 | 7 | ||
| No. of patients with DLT | 1 | 1 | ||
| Dose of sorafenib, mg | 200 bid | 400 bid* | ||
| Cohort 4 | — | |||
| No. of patients entered | 6 | 10 | 1 | |
| No. of patients assessable | 6 | 6 | 1 | |
| No. of patients with DLT | 1 | 1 | 1 | |
| Dose of sorafenib, mg | 200 qd | 200 bid* | 400 bid | |
| Cohort 6 | ||||
| No. of patients entered | 9 | 2 | ||
| No. of patients assessable | 6 | 2 | ||
| No. of patients with DLT | 3 | 2 | ||
| Dose of sorafenib, mg | 200 q3d | 200 qod | 200 qd | 200 bid |
| Cohort 8 | ||||
| No. of patients entered | — | 4 | 9 | 6 |
| No. of patients assessable | 4 | 6 | 5 | |
| No. of patients with DLT | 0 | 1 | 3 | |
| Dose of sorafenib, mg | 200 qod | 200 qd* | 200 bid | |
| Renal cohorts | ||||
| Cohort 3 | ||||
| No. of patients entered | 6 | 10 | — | |
| No. of patients assessable | 6 | 9 | ||
| No. of patients with DLT | 0 | 2 | ||
| Dose of sorafenib, mg | 200 bid | 400 bid* | ||
| Cohort 5 | ||||
| No. of patients entered | 3 | 6 | 4 | |
| No. of patients assessable | 3 | 6 | 3 | |
| No. of patients with DLT | 0 | 1 | 2 | |
| Dose of sorafenib, mg | 200 qd | 200 bid* | 400 bid | |
| Cohort 7 | ||||
| No. of patients entered | 4 | |||
| No. of patients assessable | 4 | |||
| No. of patients with DLT | 1 | |||
| Dose of sorafenib, mg | 200 qod | 200 qd | 200 bid | |
| Cohort 9 | ||||
| No. of patients entered | 3 | 6 | 8 | |
| No. of patients assessable | 3 | 5 | 6 | |
| No. of patients with DLT | 0 | 0 | 2 | |
| Dose of sorafenib, mg | 200 qod | 200 qd* | 200 bid | |
Abbreviations: DLT, dose-limiting toxicity; bid, twice a day; qd, each day; q3d, every 3 days; qod, every other day.
Recommended starting dose level.
A wide variety of neoplasms were represented in this phase I study; the categories with at least 10 accruals included renal (n = 32), colorectal (n = 23), and hepatocellular (n = 17) carcinomas. Partial responses were observed in four patients: one each with papillary thyroid cancer (cohort 2, dose level 2), hepatocellular cancer (cohort 8, dose level 1), prostate cancer (cohort 9, dose level 2), and renal cancer (cohort 9, dose level 3).
DISCUSSION
Part 1 of this study tried to identify differences in the pharmacokinetics after a single oral test dose of 400 mg of sorafenib in patients with varying degrees of hepatic or renal dysfunction. In contradiction to our a priori hypothesis, no significant associations between the AUC of sorafenib or its major metabolite and cohort were apparent.
Part 2 of the study was successful because the phase I results summarized in Table 3 support the notion that patients with moderate to severe hepatic and renal dysfunction (cohorts 4 through 9) cannot tolerate the dose of 400 mg twice a day that is recommended for patients with normal organ function. Our study was intended to inform the clinician about the starting dose of sorafenib in patients with hepatic or renal dysfunction based on readily available laboratory information, and we recommend the following empiric starting doses by cohort (Tables 1 and 3 for reference): cohort 1, 400 mg twice a day; cohort 2, 400 mg twice a day; cohort 3, 400 mg twice a day; cohort 4, 200 mg twice a day; cohort 5, 200 mg twice a day; cohort 6, not even 200 mg every third day was tolerable; cohort 7, not defined; cohort 8, 200 mg each day; and cohort 9, 200 mg each day. We would like to emphasize that clinicians should consider dose escalation in an individual patient if the drug is well tolerated.
Treatment with sorafenib was associated with dose-limiting elevation in bilirubin concentrations over baseline in 10 patients with hepatic dysfunction, but not in patients with renal dysfunction. This may have been due to the inhibition of uridine disphosphate-glucuronosyl-transferase (UGT1A1) by sorafenib. Otherwise, sorafenib was well tolerated, and no unexpected toxicities were encountered. Whereas the majority of patients with renal dysfunction were assessable, many patients with hepatic dysfunction were unable to complete the first 3 weeks of continuous dosing and were deemed unassessable per protocol. This is consistent with studies classifying prognostic comorbidity in longitudinal studies. For instance, the commonly used Charlson risk index assigns higher points for liver disease than for renal disease.20 The worst possible risk for complications in the Charlson index is a metastatic solid tumor plus moderate or severe liver disease (6 + 3 points; very high risk ≥ 5 points). Abou-Alfa et al21 reported no significant differences in sorafenib pharmacokinetics between patients with Child-Pugh A and B scores. We were able to corroborate this finding.
A limitation of this study is that the set of definitions for hepatic and renal dysfunction is still based on standard clinical laboratory values (ie, bilirubin and creatinine) rather than more involved measurements of hepatic and renal function; however, the current definitions have evolved considerably from prior CALGB phase I and pharmacokinetic organ dysfunction trials with paclitaxel, gemcitabine, irinotecan, and erlotinib that were conducted in far fewer cohorts and patients.22–26 In the study of erlotinib,26 our findings in a cohort of three patients suggested that low albumin was an indicator of more severe liver dysfunction than hyperbilirubinemia. Cohort 8 of the current study confirmed that low albumin warrants special consideration.
Supplementary Material
Acknowledgment
We thank John Wright, MD, at the National Cancer Institute, for his contribution.
Appendix
The following institutions participated in this study: Memorial Sloan-Kettering Cancer Center, New York, NY—Clifford Hudis, MD, supported by Grant No. CA77651; University of Chicago Medical Center, Chicago, IL—Gini Fleming, MD, supported by Grant No. CA41287; Georgetown University Medical Center, Washington, DC—Minetta Liu, MD, supported by Grant No. CA77597; Ohio State University Medical Center, Columbus, OH—Clara D Bloomfield, MD, supported by Grant No. CA77658; Wake Forest University School of Medicine, Winston-Salem, NC—David D. Hurd, MD, supported by Grant No. CA03927; University of North Carolina, Chapel Hill, NC—Thomas C. Shea, MD, supported by Grant No. CA47559; Dartmouth Medical School, Lebanon, NH—Marc S. Ernstoff, MD, supported by Grant No. CA04326; Roswell Park Cancer Institute, Buffalo, NY—Ellis Levine, MD, supported by Grant No. CA02599; University of Maryland Cancer Center, Baltimore, MD—Martin Edelman, MD, supported by Grant No. CA31983; University of California, San Diego, CA—Joanne Mortimer, MD, supported by Grant No. CA11789; Whittingham Cancer Center, Norwalk, CT—Richard C. Frank, MD; and University of Iowa Hospitals, Iowa City, IA—Gerald Clamon, MD, supported by Grant No. CA47642.
Footnotes
Written on behalf of the Cancer and Leukemia Group B.
Supported by National Cancer Institute Grants No. CA31946 (to the Cancer and Leukemia Group B; Richard L. Schilsky, MD, Chair), CA33601 (to the CALGB Statistical Center; Stephen George, PhD), CA77651, CA41287, CA77597, CA03927, CA47559, CA04326, CA02599, CA77658, CA31983, CA11789, CA47642, and CA47577.
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Ghassan Abou-Alfa, Bayer Pharmaceuticals (C); Mark J. Ratain, Onyx Pharmaceuticals (C) Stock Ownership: Mark J. Ratain, University of Chicago patent filing Honoraria: Ghassan Abou-Alfa, Bayer Pharmaceuticals; Jimmy Hwang, Onyx Pharmaceuticals; Mark J. Ratain, Onyx Pharmaceuticals Research Funding: Ghassan Abou-Alfa, Bayer Pharmaceuticals; Miguel A. Villalona-Calero, Bayer Pharmaceuticals; Fred Millard, Bayer Pharmaceuticals; Mark J. Ratain, Bayer Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Antonius A. Miller, Daryl J. Murry, Kouros Owzar, Donna R. Hollis, Ghassan Abou-Alfa, Lionel D. Lewis, Mark J. Ratain
Administrative support: Antonius A. Miller, Donna R. Hollis
Provision of study materials or patients: Antonius A. Miller, Ghassan Abou-Alfa, Apurva Desai, Jimmy Hwang, Miguel A. Villalona-Calero, E. Claire Dees, Lionel D. Lewis, Marwan G. Fakih, Martin J. Edelman, Fred Millard, Richard C. Frank, Raymond J. Hohl, Mark J. Ratain
Collection and assembly of data: Antonius A. Miller, Kouros Owzar, Donna R. Hollis
Data analysis and interpretation: Antonius A. Miller, Daryl J. Murry, Kouros Owzar, Donna R. Hollis, Erin B. Kennedy, Ghassan Abou-Alfa, Mark J. Ratain
Manuscript writing: Antonius A. Miller, Daryl J. Murry, Kouros Owzar, Donna R. Hollis, Ghassan Abou-Alfa, Lionel D. Lewis, Martin J. Edelman, Mark J. Ratain
Final approval of manuscript: Antonius A. Miller, Daryl J. Murry, Kouros Owzar, Donna R. Hollis, Erin B. Kennedy, Ghassan Abou-Alfa, Apurva Desai, Jimmy Hwang, Miguel A. Villalona-Calero, E. Claire Dees, Lionel D. Lewis, Marwan G. Fakih, Martin J. Edelman, Fred Millard, Richard C. Frank, Raymond J. Hohl, Mark J. Ratain
REFERENCES
- 1.Beeram M, Patnaik A, Rowinsky EK. Raf: A strategic target for therapeutic development against cancer. J Clin Oncol. 2005;23:6771–6790. doi: 10.1200/JCO.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 2.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the Raf/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 3.Carlomagno F, Anaganti S, Guida T, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326–334. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 4.Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Hotte SJ, Hirte HW. BAY 43-9006: Early clinical data in patients with advanced solid malignancies. Curr Pharm Des. 2002;8:2249–2253. doi: 10.2174/1381612023393053. [DOI] [PubMed] [Google Scholar]
- 8.Clark JW, Eder JP, Ryan D, et al. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–5480. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 9.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 10.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 11.Pugh RH. Transection of the esophagus for bleeding esophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Zhao M, Rudek MA, He P, et al. A rapid and sensitive method for detection of sorafenib in human plasma using a liquid chromatography/tandem mass spectrometry assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;846:1–7. doi: 10.1016/j.jchromb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Schuhmacher J, Kohlsdorfer C, Bühner K, et al. High-throughput determination of the free fraction of drugs strongly bound to plasma proteins. J Pharm Sci. 2004;93:816–830. doi: 10.1002/jps.10588. [DOI] [PubMed] [Google Scholar]
- 15.D'Argenio DZ, Schumitzky A. Los Angeles, CA: Biomedical Simulations Resource; 1997. ADAPT II User's Guide: Pharmacokinetic/ pharmacodynamic systems analysis software. [Google Scholar]
- 16.Tukey JW. Reading, MA: Addison-Wesley Publishing Co Inc; 1977. Exploratory Data Analysis. [Google Scholar]
- 17.Hajek J, Sidak Z, Sen PK. Theory of Rank Tests. ed 2. San Diego, CA: Academic Press; 1999. [Google Scholar]
- 18.R Development Core Team: R. Vienna, Austria: R Foundation for Statistical Computing; 2008. A language and environment for statistical computing. http://www.R-project.org. [Google Scholar]
- 19.Hothorn T, Hornik K, van de Wiel MA, et al. A LEGO system for conditional inference. Am Stat. 2006;60:257–263. [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 22.Venook AP, Egorin MJ, Rosner GL, et al. Phase I and pharmacokinetic trial of paclitaxel in patients with hepatic dysfunction: Cancer and Leukemia Group B 9264. J Clin Oncol. 1998;16:1811–1819. doi: 10.1200/JCO.1998.16.5.1811. [DOI] [PubMed] [Google Scholar]
- 23.Venook AP, Egorin MJ, Rosner GL, et al. Phase I and pharmacokinetic trial of gemcitabine in patients with hepatic or renal dysfunction: Cancer and Leukemia Group B 9565. J Clin Oncol. 2000;18:2780–2787. doi: 10.1200/JCO.2000.18.14.2780. [DOI] [PubMed] [Google Scholar]
- 24.Venook AP, Enders Klein C, Fleming G, et al. A phase I and pharmacokinetic study of irinotecan in patients with hepatic or renal dysfunction or with prior pelvic radiation: CALGB 9863. Ann Oncol. 2003;14:1783–1790. doi: 10.1093/annonc/mdg493. [DOI] [PubMed] [Google Scholar]
- 25.Ratain MJ, Miller AA, McLeod HL, et al. The Cancer and Leukemia Group B pharmacology and experimental therapeutics committee: A historical perspective. Clin Cancer Res. 2006;12(suppl):3612s–3616s. doi: 10.1158/1078-0432.CCR-06-9008. [DOI] [PubMed] [Google Scholar]
- 26.Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of erlotinib for solid tumors in patients with hepatic or renal dysfunction: Cancer and Leukemia Group B study 60101. J Clin Oncol. 2007;25:3055–3060. doi: 10.1200/JCO.2007.11.6210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.