Abstract
Purpose
Contrary to the extensive data accumulated regarding pancreatic carcinogenesis, the clinical and molecular features characteristic of advanced stage (stage III and IV) disease are unknown. A comprehensive study of pancreatic cancers from patients who have succumbed to their disease has the potential to greatly expand our understanding of the most lethal stage of this disease and identify novel areas for intervention.
Materials and Methods
Rapid autopsies were performed on 76 patients with documented pancreatic cancer. The histologic features of end stage disease were determined and correlated to the stage at initial diagnosis, patterns of failure (locally destructive v metastatic disease) and the status of the KRAS2, TP53, and DPC4 genes.
Results
At autopsy, 30% of patients died with locally destructive pancreatic cancer, and 70% died with widespread metastatic disease. These divergent patterns of failure found at autopsy (locally destructive v metastatic) were unrelated to clinical stage at initial presentation, treatment history, or histopathologic features. However, Dpc4 immunolabeling status of carcinoma tissues harvested at autopsy, a sensitive marker of DPC4 genetic status, was highly correlated with the presence of widespread metastasis but not with locally destructive tumors (P = .007).
Conclusion
Pancreatic cancers are represented by distinct genetic subtypes with significantly different patterns of failure. Determinations of DPC4 status at initial diagnosis may be of value in stratifying patients into treatment regimens related to local control versus systemic therapy.
INTRODUCTION
This year in the United States, an estimated 37,680 patients will be diagnosed with pancreatic cancer, and 34,290 patients will die of their disease.1 Three major reasons account for these dismal statistics. There are currently no known tumor markers that can be used to screen for and detect early pancreatic neoplasia. Thus, 80% of patients with pancreatic cancer have advanced stage disease at diagnosis and are not candidates for surgical intervention.2,3 A recent study indicated an alarming reluctance in the medical community to proceed to potentially curative surgery in patients with early-stage pancreatic cancer, despite numerous guidelines that support pancreatectomy as the primary treatment modality for localized disease, indicating the pervasiveness of negative attitudes regarding the efficacy of resection and/or treatment for this disease.4 The treatment options for pancreatic cancer are limited. Gemcitabine in combination with erlotinib is among the few options for patients with advanced disease, though the reported 1-year survival for this regimen is only 23%.5
Most molecular studies of pancreatic cancer have only utilized tissues collected from resection specimens.6–10 Thus, contrary to the wealth of data regarding early pancreatic carcinogenesis,11 advanced stage pancreatic cancers have not been studied. As a result, little is known about the mechanisms responsible for cancer progression—the very processes ultimately responsible for the vast majority of pancreatic cancer–related deaths.12,13 Mouse models have been utilized to study metastases,14,15 but their relevance to the cognate human condition is questionable. This is compounded by the fact that unlike colorectal cancers in which liver metastases are commonly resected for clinical benefit and thus available for medical research,16 the management of advanced pancreatic cancer often does not include surgical resection of distant metastases.10 The ability to study advanced pancreatic cancers from humans has the potential to greatly expand our understanding of the most lethal stage of this disease.
To address this critical issue, we established a rapid autopsy program for patients with end stage gastrointestinal malignancies with a focus on pancreatic cancer, called the Gastrointestinal Cancer Rapid Medical Donation Program.17 We now present the clinical and pathologic features at autopsy of the first 76 patients with pancreatic cancer who participated in this program with particular reference to the histopathologic findings and genetic status in relation to patterns of failure.
MATERIALS AND METHODS
Complete Materials and Methods can be found in the Appendix (online only).
RESULTS
Clinicopathologic Characteristics of Patients at Diagnosis
Seventy-six patients with documented infiltrating pancreatic carcinoma underwent rapid autopsy from the time period of 2003 to 2007 (Table 1). Forty-three patients (57%) were male, 73 patients (96%) were white, and the mean age at diagnosis for all patients was 62.6 ± 11.5 years. Twenty-two patients (29%) presented with stage I/II disease, 18 patients (24%) presented with stage III disease, and 36 patients (47%) presented with stage IV disease. The most common histology at diagnosis for these 76 patients was ductal (tubular) adenocarcinoma (89%).
Table 1.
Clinicopathologic Features of Patients at Initial Diagnosis
| Characteristic | Clinical Stage |
P | |||||
|---|---|---|---|---|---|---|---|
| I-II (n = 22) |
III (n = 18) |
IV (n = 36) |
|||||
| No. | % | No. | % | No. | % | ||
| Sex | |||||||
| Male (n = 43) | 8 | 36 | 13 | 72 | 22 | 61 | .05 |
| Female (n = 33) | 14 | 64 | 5 | 28 | 14 | 39 | |
| Mean (± SD) age at presentation, years | 64.7 ± 11.8 | 62.8 ± 10.9 | 60.2 ± 12.7 | .66 | |||
| Race (white:Hispanic) | 21:1 | 18:0 | 34:2 | .80 | |||
| Location | |||||||
| Head | 17 | 77 | 11 | 61 | 20 | 56 | .07 |
| Body | 1 | 4 | 6 | 33 | 7 | 19 | |
| Tail | 4 | 18 | 0 | 5 | 14 | ||
| Not specified | 0 | 1 | 6 | 4 | 11 | ||
| Mean (± SD) size, cm | 3.4 ± 1.6 | 4.0 ± 1.5 | 4.7 ± 2.6 | .52 | |||
| Histology | |||||||
| Duct adenocarcinoma | 19 | 86 | 16 | 89 | 33 | 92 | .87 |
| Colloid (mucinous noncystic) carcinoma | 1 | 4 | 1 | 6 | 1 | 3 | |
| Adenocarcinoma with signet ring features | 1 | 4 | 0 | 1 | 3 | ||
| Signet ring carcinoma | 0 | 1 | 6 | 1 | 3 | ||
| Adenosquamous carcinoma | 1 | 4 | 0 | 0 | |||
| Differentiation | |||||||
| Well | 4 | 18 | 0 | 0 | .30* | ||
| Moderate | 11 | 50 | 1 | 6 | 5 | 14 | |
| Poor | 7 | 32 | 2 | 11 | 8 | 22 | |
| Unknown | 0 | 15 | 83 | 23 | 64 | ||
| Lymph node status of resection specimen (stage I/II) | |||||||
| Positive | 14 | 64 | — | — | — | — | |
| Negative | 6 | 27 | |||||
| Unknown | 2 | 9 | |||||
| Margin status of resection specimen (stage I/II) | |||||||
| Positive | 6 | 27 | — | — | — | — | |
| Negative | 14 | 64 | |||||
| Unknown | 2 | 9 | |||||
| Adjuvant treatment (stage I/II) | |||||||
| Yes | 13 | 59 | — | — | — | — | |
| No | 9 | 41 | |||||
| Median disease-free survival, months (stage I/II) | 14.0 | ||||||
| Range | 1-36 | — | — | — | — | ||
| Treatment for recurrent/advanced stage disease | |||||||
| Yes | 18 | 82 | 16 | 89 | 28 | 78 | .69 |
| No | 4 | 18 | 2 | 11 | 8 | 22 | |
| Median overall survival, months | 24.0 | 12.0 | 6.0 | ||||
| Range | 8-23 | 3-62 | 1-30 | .09 | |||
Abbreviation: SD, standard deviation.
Only cases with known differentiation status were included.
All 22 patients who initially presented with stage I/II disease underwent surgical resection of their primary infiltrating carcinoma. Fourteen (64%) of these patients had lymph node metastases at the time of surgery, and six patients (27%) had a positive surgical margin. Thirteen (59%) of these 22 patients received adjuvant chemoradiotherapy. The median disease-free survival for these 22 patients was 14.0 months (range, 1 to 36 months), and the median overall postsurgical survival was 24.0 months (range, 8 to 23 months).
Among the 18 patients diagnosed with stage III (locally advanced) pancreatic cancer, 89% received first-line chemoradiotherapy or chemotherapy. The median overall survival for these patients was 12.0 months (range, 3 to 62 months). By contrast, among the 36 patients who presented with stage IV (metastatic) disease, 28 (78%) received chemotherapy and the median overall survival for these patients was only 6.0 months (range, 1 to 30 months).
Patterns of Failure at Autopsy
At autopsy, 20 (91%) of 22 patients who initially presented with stage I/II disease and underwent surgical resection had gross evidence of recurrent pancreatic cancer (Appendix Table A1, online only). One of the two patients with no evidence of disease at autopsy completed adjuvant chemoradiotherapy but died at 15 months of other causes. The second patient completed chemoradiotherapy to the pancreatic bed for a local recurrence 34 months after surgery and died 13 months later of other causes (overall survival, 47 months). Of the remaining 20 patients who underwent surgical resection and who had gross disease at autopsy, three (15%) had recurrent carcinoma within the pancreatic bed only, four (20%) had metastatic disease only, and the remaining 13 patients (65%) had both locally recurrent carcinoma and metastatic disease. Five (83%) of the six patients with a positive surgical margin recurred locally.
Fifty-four patients did not have their cancer surgically resected corresponding to the 18 patients initially diagnosed with stage III and the 36 patients initially diagnosed with stage IV pancreatic cancer. Thirteen (72%) of 18 patients initially diagnosed with stage III disease had evidence of metastatic disease at autopsy in addition to the locally advanced primary carcinoma, and the other five (28%) of these 18 patients did not have metastases at autopsy. Thirty-five (97%) of 36 patients diagnosed with stage IV pancreatic cancer had metastatic disease at autopsy. In one patient, peritoneal metastases were documented at the time of an attempted pancreaticoduodenectomy, yet no evidence of peritoneal disease was found at autopsy.
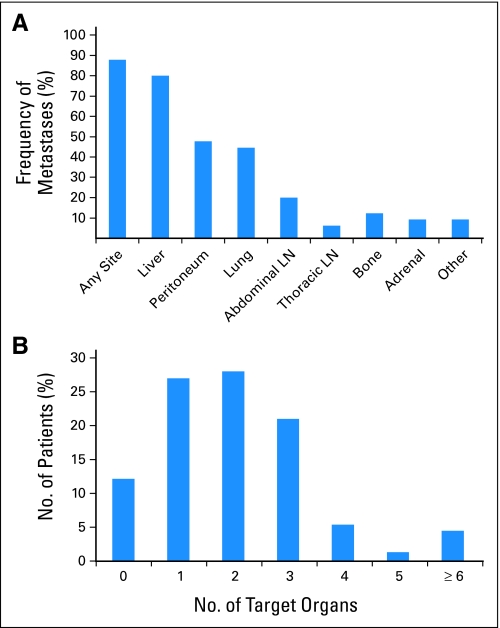
Overall, 65 (88%) of the 74 patients with disease at autopsy had documented metastatic disease. The extent of metastatic disease burden among these 65 patients varied dramatically, ranging from 1 to 10 documented metastases to more than 1,000 (Fig 1). This broad variation was not related to clinical stage at diagnosis, nor was it related to any other clinical or pathologic features of these patients' disease (Table 2). There was a trend toward increased metastatic burden with stage IV disease, but this was not statistically significant. A significant relationship was found among metastatic disease burden and mode of treatment regimen, but this likely reflects the differential usage of chemoradiotherapy in stage III versus stage IV disease. Review of the distribution of metastases indicated that the liver was the most common site of metastasis (52 patients; 80%), followed by the peritoneum (31 patients; 48%) and lungs (29 patients; 45%; Fig 2). Fifty-seven (88%) of 65 patients with metastases had metastatic disease in one to three organ sites, most commonly the liver alone or in combination with peritoneal and/or lung metastases, and eight patients (12%) had metastatic disease in four or more organ sites. Although the liver was the most frequent site of metastasis, 13 (20%) of 65 patients had documented metastatic disease that spared the liver. In these patients, the most frequent sites of metastasis were the lungs and/or peritoneum (11 patients), although in another two patients the adrenal glands or abdominal lymph nodes were the sole sites of metastasis. Eight patients (12%) had bone metastases although in these patients other sites were also affected.
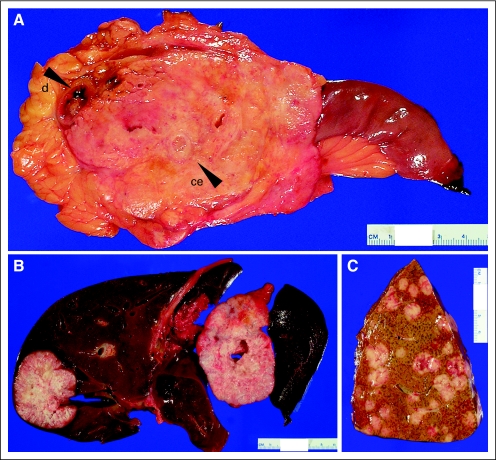
Fig 1.
(A) Example of locally advanced pancreatic cancer found at autopsy. The carcinoma measured 10 cm in greatest dimension and directly invaded the duodenum (d) and occluded the celiac artery (ce). The ultimate cause of death in this patient was bowel ischemia and peritonitis. (B) Example of a patient with limited metastatic burden at autopsy (≤ 10). Similar to that described for the carcinomas in (A), this patient also died of complications of locally advanced carcinoma (ascending cholangitis) even though at initial presentation limited metastatic disease was present. (C) Example of a patient with extensive metastatic burden at autopsy (> 1,000). The cause of death for this patient was hepatic failure secondary to massive tumor burden.
Table 2.
Relationship of Metastatic Burden at Autopsy to Clinicopathologic Features
| Characteristic | None (n = 9) | 1 to 10 (n = 13) | 11 to 99 (n = 26) | 100s to 1,000s (n = 26) | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (± SD) age, years | 62.0 ± 15.0 | 66.3 ± 8.5 | 61.3 ± 9.4 | 62.0 ± 13.1 | .61 | ||||
| Male:female | 4:5 | 10:3 | 17:9 | 11:15 | .12 | ||||
| Smoking history | |||||||||
| Never | 4 | 44 | 3 | 23 | 10 | 38 | 12 | 46 | .37 |
| Former | 2 | 22 | 7 | 54 | 11 | 42 | 7 | 27 | |
| Current | 3 | 33 | 1 | 8 | 4 | 15 | 3 | 12 | |
| Unknown | 0 | 2 | 15 | 1 | 4 | 4 | 15 | ||
| Stage at diagnosis | |||||||||
| I-II | 3 | 33 | 3 | 23 | 8 | 31 | 6 | 23 | .09 |
| III | 5 | 55 | 5 | 38 | 4 | 15 | 4 | 15 | |
| IV | 1 | 11 | 5 | 38 | 14 | 54 | 16 | 62 | |
| Tumor location | |||||||||
| Head/body | 7 | 77 | 12 | 92 | 20 | 77 | 21 | 81 | .80 |
| Tail | 1 | 11 | 1 | 8 | 3 | 12 | 4 | 15 | |
| Not specified | 1 | 11 | 0 | 3 | 12 | 1 | 4 | ||
| Mean (± SD) tumor size, cm (stage III/IV carcinomas only) | 5.3 ± 2.8 | 6.9 ± 3.8 | 5.5 ± 1.7 | 6.0 ± 3.0 | .58 | ||||
| Tumor differentiation | |||||||||
| Well | 0 | 1 | 8 | 0 | 0 | .19 | |||
| Moderate | 3 | 33 | 8 | 62 | 9 | 35 | 6 | 23 | |
| Poor | 6 | 66 | 4 | 31 | 16 | 62 | 18 | 69 | |
| Anaplastic | 0 | 0 | 1 | 4 | 2 | 8 | |||
| Histology | |||||||||
| Duct adenocarcinoma | 7 | 77 | 11 | 85 | 22 | 85 | 22 | 85 | .76 |
| Colloid (mucinous noncystic) carcinoma | 1 | 11 | 1 | 8 | 2 | 8 | 1 | 4 | |
| Adenosquamous carcinoma | 1 | 11 | 1 | 8 | 0 | 0 | |||
| Signet ring carcinoma | 0 | 0 | 1 | 4 | 1 | 4 | |||
| Anaplastic carcinoma | 0 | 0 | 1 | 4 | 2 | 8 | |||
| Treatment | |||||||||
| None | 2 | 22 | 4 | 31 | 2 | 8 | 3 | 12 | < .02 |
| Chemoradiation | 6 | 66 | 7 | 54 | 8 | 31 | 7 | 27 | |
| Chemotherapy | 1 | 11 | 2 | 15 | 16 | 62 | 16 | 62 | |
| Median overall survival, months | 17.0 | 10.0 | 11 | 8 | |||||
| Range | 3-53 | 1-48 | 1-62 | 2-27 | .69 | ||||
Abbreviation: SD, standard deviation.
Fig 2.
(A) Frequency of metastatic involvement by pancreatic cancer to various organ sites. The liver is the most common site of metastatic spread, followed by the peritoneum and lung. (B) Total number of target organs involved by metastatic disease. The majority of patients had metastatic disease limited to three or fewer organ sites, although in a subset of patients four or more target organs were affected. LN, lymph node.
Pathologic Features of Advanced Stage Pancreatic Cancers
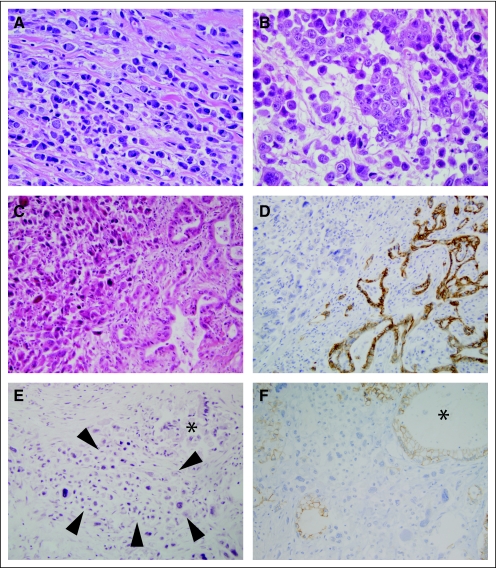
Histologic examination of harvested tissues confirmed the diagnosis of infiltrating carcinoma in all 74 patients with disease at autopsy. In 65 (88%) of these 74 patients, pathologic examination indicated the presence of infiltrating ductal (tubular) adenocarcinoma, in three (4%) of 74 patients adenosquamous carcinoma was present, in two (3%) of 74 patients signet ring carcinoma was found, and one (1%) of 74 patients had a colloid (mucinous noncystic) adenocarcinoma. In 71 of these patients, the pathologic diagnoses at initial presentation were concordant with the diagnosis at autopsy. However, in the remaining three of 74 patients, microscopic examination of harvested tissues indicated the presence of undifferentiated (anaplastic) carcinoma (defined as ≥ 30% undifferentiated features), suggesting dedifferentiation of these three carcinomas with disease progression. This observed frequency of undifferentiated carcinoma was significantly greater than that reported previously in a large surgical series of patients with stage I/II disease3 (9 of 1,423 patients with stage I/II disease [0.006%] v 3 of 74 patients with advanced disease [4%]; P = .018). Representative samples of the undifferentiated carcinoma in these three patients were immunolabeled with E-cadherin, a reliable marker of undifferentiated carcinoma,18 and all showed complete loss of E-cadherin expression (histology score = 0; Fig 3).
Fig 3.
Examples of high grade pancreatic cancer identified at rapid autopsy. (A) Diffusely infiltrative signet ring carcinoma. The single cells contain a prominent mucin vacuole that displaces the nucleus to the cell periphery. Note the lack of a desmoplastic response as is typically seen for conventional infiltrating pancreatic cancer. Signet ring carcinoma was present at initial presentation of this patient. (B) Undifferentiated anaplastic carcinoma. The cells show marked nuclear enlargement and pleomorphism with complete loss of cellular adhesion. This patient underwent surgical resection of a moderately differentiated duct (tubular) adenocarcinoma and completed adjuvant chemoradiotherapy but recurred 21 months postresection with undifferentiated carcinoma. (C) Pancreatic carcinoma showing both differentiated (right side) and undifferentiated (left side) morphologies. Similar to the patient described for (B), this patient also underwent surgical resection of a moderate to poorly differentiated duct (tubular) adenocarcinoma but recurred 4 months postresection. The overall survival for this patient was 23 months. (D) E-cadherin labeling of the carcinoma shown in (C) indicates the loss of E-cadherin expression in the undifferentiated carcinoma, in striking contrast to the moderately differentiated carcinoma present in the same section. (E) Small focus of anaplastic carcinoma (indicated by arrowheads) in an otherwise moderately differentiated adenocarcinoma. An asterisk indicates a focus of moderately differentiated carcinoma present in the same section. (F) E-cadherin labeling of this same region indicates loss of E-cadherin expression in this small focus of undifferentiated carcinoma (≈1% of the cancer volume), whereas E-cadherin expression is retained in the moderately differentiated component.
In light of the increased frequency of undifferentiated carcinoma features, all disease of the 71 patients with ductal adenocarcinoma, adenosquamous or colloid (mucinous noncystic) carcinoma were re-evaluated for undifferentiated features, and an additional nine carcinomas were identified with focal undifferentiated components that accounted for 1% to 10% of the neoplastic burden in these patients. In these nine patients, the undifferentiated component was again E-cadherin negative, whereas the differentiated components showed strong positive membranous labeling that was indistinguishable from labeling seen in carcinomas without an undifferentiated component (histology score 227.0 ± 64.4 v 211.0 ± 79.6; P = not significant; Fig 3). Overall, the frequency of undifferentiated morphology (any proportion) in advanced cancers was 12 (16%) of 74 patients. The presence of undifferentiated morphology was not correlated with any clinicopathologic parameters analyzed, including stage at diagnosis and extent of disease at autopsy (Appendix Table A2).
Molecular Features of Advanced-Stage Pancreatic Cancers
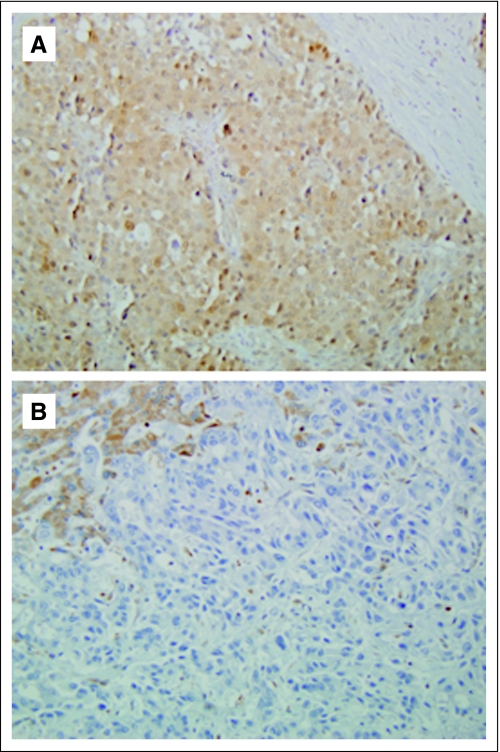
Genomic DNA was microdissected from representative sections of primary and/or metastatic cancer tissues and the sequence of the KRAS2 and TP53 genes was determined (Table 3). Activating mutations in the KRAS2 gene were identified in 56 (95%) of 59 patient's carcinomas analyzed. The most common KRAS2 gene mutation was G12D in 31 (55%) of 56 carcinomas, followed by G12V in 16 (29%) of 56 carcinomas. Inactivating mutations in the TP53 gene were identified in 46 (79%) of 58 patient's carcinomas analyzed, of which 24 (52%) were missense mutations, eight (17%) were nonsense mutations, and 10 (22%) were frameshift mutations. We also determined the genetic status of DPC4 by determining Dpc4 immunolabeling patterns, a strong marker of DPC4 genetic status.19,20 Of 65 carcinomas analyzed, 41 showed loss of Dpc4 immunolabeling indicating rates of inactivation of 63% in advanced disease. Overall, the prevalence of KRAS2, TP53, and DPC4 genetic alterations found are in keeping with previous reports for these three genes in primary infiltrating pancreatic cancers.21,22 KRAS2 and TP53 genetic status and Dpc4 immunolabeling patterns were then correlated with metastatic burden, and a striking relationship was found. For example, only two (22%) of nine locally advanced carcinomas with no documented metastatic disease at autopsy showed Dpc4 loss, whereas 16 (78%) of 22 carcinomas with 100s to 1,000s of metastases showed Dpc4 loss (P = .032; Appendix Fig A1). This association was even more significant when analyzed with respect to pattern of failure (locally destructive v metastatic, P = .007). A significant relationship among TP53 genetic status was also observed with respect to pattern of failure (P = .037), although this relationship was less robust than found for Dpc4. Dpc4 labeling and TP53 mutation status was concordant among the matched primary and metastatic carcinoma samples in all patients analyzed.
Table 3.
Relationship of Genetic Features to Patterns of Failure in Advanced Stage Pancreatic Cancer
| Metastatic Burden by Gene for Primary Carcinoma | Locally Destructive |
Locally Confined |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 |
1-10 |
11-99 |
100s-1,000s |
||||||
| No. | % | No. | % | No. | % | No. | % | ||
| KRAS2 (n = 59) | 6/7 | 86 | 11/11 | 100 | 19/21 | 90 | 20/20 | 100 | .283 |
| 17/18; 94% | 39/41; 95% | .672 | |||||||
| TP53 (n = 58) | 6/6 | 100 | 6/11 | 54 | 16/21 | 76 | 18/20 | 90 | .083 |
| 12/17; 71% | 34/41; 83% | .037 | |||||||
| DPC4 (n = 65) | 2/9 | 22 | 5/11 | 45 | 17/24 | 71 | 16/22 | 73 | .032 |
| 7/20; 35% | 33/46; 72% | .007 | |||||||
In consideration of the relationship of Dpc4 immunolabeling status and metastatic burden, we determined the Dpc4 immunolabeling status of the resected pancreatic cancers for 19 of 22 patients who underwent surgical resection at the time of initial diagnosis. Nine (47%) of 19 patients showed loss of Dpc4 immunolabeling of the resected primary carcinoma, and all nine patients metastatic disease at autopsy also showed Dpc4 loss. By contrast, among the 10 patients with intact Dpc4 labeling of their resected primary carcinoma, five patients recurrent disease at autopsy also showed intact Dpc4 labeling, three showed Dpc4 loss, and two patients had no evidence of disease at autopsy.
DISCUSSION
A better understanding of the cellular and molecular features of advanced disease will afford new opportunities for investigation, therapeutic intervention and clinical management of patients afflicted with pancreatic cancer. We now provide compelling evidence that advanced pancreatic cancer is not one disease, but instead is composed of distinct morphologic and genetic subtypes with significantly different patterns of metastasis. We acknowledge that our findings are based on a biased population in that they were predominantly white and male compared with the average population, and additional studies will be required using a more representative patient population to verify these findings. Nonetheless, they provide novel insight into the mechanisms of pancreatic cancer metastasis.
There are three clinical implications of these findings. First, contrary to common belief, not all patients with pancreatic cancer die of widespread metastatic disease. Twelve percent of patients had no evidence of metastasis, and this finding was not unique to patients who underwent treatment, nor was it specific to patients initially diagnosed at an early stage. This finding parallels other studies of disease burden at autopsy in pancreatic cancer patients in which 8% to 15% of patients died with locally advanced carcinoma and without metastatic disease, even in the absence of any treatment.23,24 Moreover, among the 88% of patients with metastasis at autopsy, a broad range of metastatic burden was seen that ranged from few metastases (1 to 10) to extensive and widespread metastatic disease (100s to 1,000s), and again this finding did not relate to clinicopathologic features of these patients at initial diagnosis or their treatment history. Overall, in the patients with locally advanced pancreatic cancer in association with no metastases or a limited metastatic disease burden (1 to 10 metastases), the causes of death were often related to complications of locally destructive growth. By contrast, in patients with a primary carcinoma that was relatively confined to the pancreas but with a significant metastatic disease burden (100s to 1,000s of metastases) death was more commonly related to organ failure and cachexia, an observation that has been previously reported.23–25 In one of the few studies to stringently evaluate causes of death related to pancreatic cancer, Nakahashi et al25 found that the number of patients with extensive metastatic disease leading to hepatic dysfunction and death was relatively small, and the presence of isolated hepatic metastases were often clinically insignificant in comparison to the complications that arose from locally destructive growth of the primary carcinoma. Thus, it is important to note that isolated metastases at initial diagnosis are not a harbinger of widespread metastatic disease, nor do they always pose the greatest threat to patient survival compared to that of the primary tumor or other factors such as cachexia.
The second clinical implication of our findings is that the genetic status of a pancreatic carcinoma can be used to predict widespread metastatic failure. For example, locally advanced carcinomas from patients with no documented metastatic disease uncommonly showed loss of Dpc4 expression (22%) as compared with carcinomas from patients with extensive metastatic burden (100s to 1,000s) in which the rates of Dpc4 loss approached 75%. A similar relationship has been found in patients with colon cancer.26 While these findings do not establish that Dpc4 plays a direct role in metastatic ability, an intriguing study by Michor et al27 who applied mathematical modeling to the dynamics of metastasis suppressor gene inactivation found, at a constant rate of cancer cell dissemination, a striking similarity among bi-allelic advantageous mutations in a primary carcinoma and the expected number of metastatic foci (none to > 1,000) that paralleled the metastatic burden seen in this cohort of patients. While more studies are needed to clarify the role of DPC4 and other molecular features of pancreatic cancers in relation to patterns of failure (locally destructive v distant metastasis), our observations do indicate that fundamental molecular differences exist in a primary pancreatic carcinoma that underlies aggressive behavior or may influence response to treatment.28,29–31 Our findings may also clarify previous findings correlating DPC4 status in surgically resected primary pancreatic cancers with patient outcome. Since loss of Dpc4 immunolabeling reflects an increased likelihood of the patient developing widespread metastasis, it should not be surprising that surgical series have reported a poorer survival in patients with pancreatic cancers with DPC4 inactivation.32,33 Thus, it may be conceivable that patients with DPC4 positive carcinomas would receive a greater clinical benefit from intensive local control by chemoradiotherapy compared to patients with DPC4 negative carcinomas in which systemic chemotherapy alone may be more appropriate.
The third clinical implication of our findings is that advanced pancreatic cancers more commonly have high grade histologic features than those reported for early-stage disease, a finding also reported by Kamisawa et al.24 This finding is reminiscent of epithelial-mesenchymal transition (EMT), a feature well described in a variety of human tumors and in mouse models of pancreatic carcinoma.34,35 High grade features were not correlated with stage at initial diagnosis nor any other clinicopathologic features, and suggest additional events occur in primary carcinomas that lead to dedifferentiation. EMT in pancreatic cancer has been suggested to be mediated by transforming growth factor-β signaling through the PI3K/PTEN pathway.29,31,36 Although functional Dpc4 does not appear necessary for TGF-β-mediated EMT,29 in mouse models of pancreatic cancer functional Dpc4 has been associated with an undifferentiated phenotype.34 However, four of seven patients' carcinomas with anaplastic morphology in this study had loss of Dpc4 immunolabeling, in support of claims that Dpc4 is not specifically required for this phenomenon.
In summary, we now show that information gathered from a rapid autopsy approach has value in identifying novel areas for investigation into pancreatic cancer biology and therapy. These data also suggest that advanced pancreatic cancer may be composed of distinct morphologic and genetic subtypes with different patterns of metastasis. Clinical trials that take into account molecular features such as Dpc4 status of pancreatic cancers may have a role in stratifying patients for different treatment regimens. For example, based on the recent observation that cyclophosphamide given in association with a pancreatic cancer vaccine enhances T-cell responses in pancreatic cancer patients,37 we have an open pancreas cancer study in the neoadjuvant/adjuvant setting using our granulocyte macrophage colony-stimulating factor allogeneic whole cell vaccine in combination with immune modulating doses of Cytoxan and chemoradiotherapy. Immunolabeling will be performed on all resected specimens to determine Dpc4 loss versus retention of protein expression. Should we find a relationship of Dpc4 status and treatment outcome, it is conceivable that a follow-up study could use this information to stratify patients to either chemoradiotherapy when an intact DPC4 gene is found versus systemic chemotherapy alone in the event of DPC4 loss.
Appendix
Materials and Methods
Tissue samples.
Tissues were collected in association with the Gastrointestinal Cancer Rapid Medical Donation Program. This program was approved by the Johns Hopkins institutional review board and deemed in accordance with the Health Insurance Portability and Accountability Act. Details of the program have been described in detail previously (Embuscado EE, Laheru D, Ricci F, et al: Cancer Biol Ther 4:548-554, 2005). Briefly, any patient with biopsy-proven or radiographic evidence of gastrointestinal cancer is eligible to participate. Patients are given informed consent and those who provide it undergo an autopsy as soon as possible after death, typically within 6 hours. Harvested samples of the primary carcinoma and/or metastatic deposits are formalin fixed for paraffin embedding and routine histologic examination, or are flash-frozen in liquid nitrogen and stored at −80°C. The documentation of metastatic disease is based on both gross and microscopic examination of any grossly visible and/or palpable deposits. Each patient is assigned a unique identifier and all clinicopathologic information related to the patients medical and surgical history, treatment, imaging studies, pathology and autopsy findings are entered into Excel (Microsoft Corp, Redmond, WA) spreadsheets.
Polymerase chain reaction and sequencing.
Neoplastic cells were microdissected from cancerous tissues using a PALM MicroLaser System (Carl Zeiss MicroImaging GmbH, Oberkochen, Germany) according to the manufacturers instructions. Genomic DNA was extracted from microdissected pancreatic cancers using QiAmp DNA Micro Kits (Qiagen, Valencia, CA) and whole genome amplified using an illustra Genomiphi V2 kit (GE Healthcare, Piscataway, NJ). Polymerase chain reaction (PCR) amplification of KRAS2 exons 1 and 2 and TP53 exons 5 to 9 was performed as described using whole genome amplified DNA (Embuscado EE, Laheru D, Ricci F, et al: Cancer Biol Ther 4:548-554, 2005; primer sequences available on request). PCR products were sequenced by use of a universal M13F primer that was incorporated into the forward primer of each primer pair (Agencourt Bioscience Corporation, Beverly, MA). Sequence data were analyzed with Sequencher 4.8 software (Gene Codes, Ann Arbor, MI). Verification of all mutations was accomplished by sequencing of a second PCR product derived independently from the original template.
Immunohistochemistry.
Immunohistochemistry was performed following standard methods previously described in detail (Embuscado EE, Laheru D, Ricci F, et al: Cancer Biol Ther 4:548-554, 2005; Winter JM, Ting AH, Vilardell F, et al: Clin Cancer Res 14:412-418, 2008) with appropriate dilutions of antibodies to E-cadherin or Dpc4 (prediluted anti-E-cadherin clone ECH-6, Cell Marque Corp, Hot Springs, AZ; 1:100 dilution anti-Dpc4 clone B8, Santa Cruz Biotechnology, Santa Cruz, CA) overnight using a DAKO automated stainer (DAKO, Carpinteria, CA). Immunohistochemical labeling of Dpc4 was scored as intact (positive) or lost (negative). Only sections in which internal controls (eg, lymphocytes, stromal cells) present on the same slide showed intact Dpc4 nuclear labeling were used. For E-cadherin, labeling was scored on an intensity scale of 0 to 3, with 0 corresponding to no membranous labeling of neoplastic epithelial cells, 1 to weak membranous labeling of neoplastic epithelium (labeling best seen at 10× objective or greater), 2 to unequivocal membranous labeling of epithelial cells, and 3 to intense membranous labeling. The percentage of labeled neoplastic epithelial cells was scored from 0 (complete absence) to 100% (all cells labeling). The labeling intensity and labeling percentage were used to generate a histology score ranging from 0 to 300, with histology score = intensity of immunolabel (range 0 to 3) × percentage of reactive cancer cells.
Statistics.
Summary data are expressed as the mean with or without standard deviation unless otherwise indicated. Fisher's exact tests (for count data) and Kruskal Wallace tests (for continuous data) were performed using STATA version (Stata Corp, College Station, TX) 9.0. P values of ≤ .05 were considered significant.
Fig A1.
(A) Positive Dpc4 immunolabeling in a locally advanced carcinoma that was associated with limited metastatic disease burden (< 10 documented metastase). (B) Loss of Dpc4 immunolabeling in a primary carcinoma associated with widespread metastatic disease (> 1,000 documented metastases).
Table A1.
Patterns of Recurrence After Surgical Resection for Pancreatic Carcinoma
| Case | Surgical Margins | Clinical Stage at Diagnosis | Disease Recurrence | DFS | Location of First Documented Recurrence | Sites of Recurrence Documented at Autopsy |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pancreatic Bed | Liver | Lung | Peritoneum | Abdominal Lymph Node | Thoracic Lymph Node | Bone | Other Sites | ||||||
| 16 | Negative | IIA | Yes | 14 | Pancreatic bed | Yes | Yes | ||||||
| 19* | Negative | IIB | No | 15 | |||||||||
| 20 | Positive | IIB | Yes | 5 | Liver | Yes | Yes | Yes | Yes | Yes | Yes | Adrenal | |
| 21 | Unknown | IIB | Yes | 21 | Liver | Yes | Yes | Yes | |||||
| 24 | Negative | IIB | Yes | 4 | Liver | Yes | Yes | ||||||
| 25 | Negative | IB | Yes | 18 | Pancreatic bed | Yes | |||||||
| 30 | Positive | III | Yes | 16 | Pancreatic bed | Yes | Yes | Yes | Yes | ||||
| 36 | Positive | III | Yes | 6 | Liver, peritoneum abdominal lymph nodes | Yes | Yes | Yes | Yes | Ovary rectum adrenal | |||
| 41 | Negative | III | Yes | 20 | Liver, peritoneum | Yes | Yes | Yes | Spleen, diaphragm | ||||
| 44 | Negative | III | Yes | 7 | Liver | Yes | Yes | Yes | Diaphragm | ||||
| 45 | Positive | III | Yes | 13 | Pancreatic bed, also liver, lung, lymph nodes | Yes | Yes | Yes | Yes | Yes | |||
| 48 | Negative | III | Yes | 26 | Lung | Yes | |||||||
| 54† | Negative | IB | Yes | 34 | Pancreatic bed | ||||||||
| 63 | Positive | III | Yes | 6 | Peritoneum | Yes | Yes | ||||||
| 64 | Negative | III | Yes | 2 | Liver | Yes | Yes | Yes | Yes | ||||
| 66 | Negative | IIA | Yes | 30 | Pancreatic bed and lungs | Yes | Yes | ||||||
| 67 | Negative | III | Yes | 14 | Peritoneum | Yes | Yes | ||||||
| 72 | Negative | IIA | Yes | 8 | Pancreatic bed | Yes | |||||||
| 74 | Unknown | IIB | Yes | 36 | Pancreatic tail (?recurrent IPMN), liver | Yes | Yes | Yes | Yes | Yes | |||
| 81 | Negative | I | Yes | 12 | Liver | Yes | Yes | Yes | Yes | ||||
| 82 | Positive | III | Yes | 1 | Peritoneum | Yes | Yes | Yes | Yes | ||||
| 83 | Negative | III | Yes | 3 | Lungs, spine | Yes | Yes | Yes | Yes | ||||
Abbreviations: DFS, disease-free survival; IPMN, intraductal papillary mucinous neoplasm.
This patient died 15 months after surgical resection from sepsis. No recurrence was found at autopsy.
This patient experienced disease recurrence within the pancreatic bed 34 months after surgical resection and was treated with chemoradiation. No residual disease was found at autopsy.
Table A2.
Clinicopathologic Features in Relation to Anaplastic Morphology at Autopsy
| Characteristic | Anaplastic Carcinoma (≥ 30%) | Carcinoma With Anaplastic Features (< 30%) | Carcinomas With No Anaplastic Morphology |
|---|---|---|---|
| No. of patients | 3 | 9 | 62 |
| Mean (± SD) age, years | 61.0 ± 5.0 | 59.2 ± 10.9 | 63.1 ± 11.7 |
| Male:female | 1:2 | 4:5 | 37:25 |
| Stage at diagnosis | |||
| I-II | 2 | 3 | 15 |
| III | 0 | 1 | 17 |
| IV | 1 | 5 | 30 |
| Tumor location | |||
| Head/body | 2 | 8 | 51 |
| Tail | 1 | 0 | 7 |
| Not specified | 0 | 1 | 4 |
| Median disease-free survival (stage I/II only) | 13.0 | 6.0 | 14.0 |
| Range | 4.0-21.0 | 5.0-7.0 | 1.0-26.0 |
| Mean (± SD) tumor size at autopsy, cm | 3.8 ± 2.8 | 4.0 ± 1.3 | 4.1 ± 2.2 |
| Tumor differentiation at autopsy | |||
| Well | 0 | 0 | 1 |
| Moderate | 1 | 1 | 16 |
| Poor | 2 | 3 | 12 |
| Unknown | 0 | 5 | 33 |
| Histology at autopsy | |||
| Duct adenocarcinoma | 3 | 9 | 54 |
| Colloid (mucinous noncystic) carcinoma | 0 | 0 | 5 |
| Adenosquamous carcinoma | 0 | 0 | 1 |
| Signet ring carcinoma | 0 | 0 | 2 |
| Metastatic burden | |||
| None | 0 | 0 | 9 |
| 1-10 | 0 | 0 | 13 |
| 11-99 | 1 | 5 | 20 |
| 100s to 1,000s | 2 | 4 | 20 |
| Mean (± SD) E-cadherin H score at autopsy (differentiated component only) | 233.3 ± 57.7 | 216.7 ± 79.2 | 211 ± 79.7 |
| Overall survival, months | 24.0 | 6.0 | 10.0 |
| Range | 11.0-24.0 | 3.0-23.0 | 1.0-62.0 |
Abbreviation: SD, standard deviation.
Footnotes
Supported by grants No. CA62924 and CA106610 from the National Institutes of Health (C.I.D.), The Joseph C. Monastra Fund for Pancreatic Cancer Research, The Jeff Zgonina Fund for Pancreatic Cancer Research, The George Rubis Endowment for Pancreatic Cancer Research, The Michael Rolfe Pancreatic Cancer Foundation, and the Sigma Beta Sorority.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Christine A. Iacobuzio-Donahue
Financial support: Christine A. Iacobuzio-Donahue
Provision of study materials or patients: Joseph Herman, John L. Cameron, Charles J. Yeo, Marian Raben, Manuel Hidalgo, Daniel Laheru
Collection and assembly of data: Christine A. Iacobuzio-Donahue, Baojin Fu, Shinichi Yachida, Mingde Luo, Hisashi Abe, Clark Henderson, Felip Vilardell, Zheng Wang, Jesse W. Keller, Marc K. Halushka, James R. Eshleman, Marian Raben, Ralph H. Hruban
Data analysis and interpretation: Christine A. Iacobuzio-Donahue, Baojin Fu, Shinichi Yachida, Mingde Luo, Hisashi Abe, Jesse W. Keller, Priya Banerjee, Joseph Herman, Charles J. Yeo, Marc K. Halushka, Alison P. Klein, Manuel Hidalgo
Manuscript writing: Christine A. Iacobuzio-Donahue, Priya Banerjee, Joseph Herman, Charles J. Yeo, James R. Eshleman, Ralph H. Hruban, Manuel Hidalgo, Daniel Laheru
Final approval of manuscript: Christine A. Iacobuzio-Donahue
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 3.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210-1211, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 6.Hahn SA, Seymour AB, Hoque AT, et al. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res. 1995;55:4670–4675. [PubMed] [Google Scholar]
- 7.Hruban RH, Goggins M, Parsons J, et al. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 8.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 9.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, et al. Highly expressed genes in pancreatic ductal adenocarcinomas: A comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614–8622. [PubMed] [Google Scholar]
- 10.Verslype C, Van Cutsem E, Dicato M, et al. The management of pancreatic cancer. Current expert opinion and recommendations derived from the 8th World Congress on Gastrointestinal Cancer, Barcelona, 2006. Ann Oncol. 2007;18(suppl 7):vii1–vii10. doi: 10.1093/annonc/mdm210. [DOI] [PubMed] [Google Scholar]
- 11.Hansel DE, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Annu Rev Genomics Hum Genet. 2003;4:237–256. doi: 10.1146/annurev.genom.4.070802.110341. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 13.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 14.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: A new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suemizu H, Monnai M, Ohnishi Y, et al. Identification of a key molecular regulator of liver metastasis in human pancreatic carcinoma using a novel quantitative model of metastasis in NOD/SCID/gammacnull (NOG) mice. Int J Oncol. 2007;31:741–751. [PubMed] [Google Scholar]
- 16.Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007;11:1057–1077. doi: 10.1007/s11605-006-0061-3. [DOI] [PubMed] [Google Scholar]
- 17.Embuscado EE, Laheru D, Ricci F, et al. Immortalizing the complexity of cancer metastasis: Genetic features of lethal metastatic pancreatic cancer obtained from rapid autopsy. Cancer Biol Ther. 2005;4:548–554. doi: 10.4161/cbt.4.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winter JM, Ting AH, Vilardell F, et al. Absence of e-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin Cancer Res. 2008;14:412–418. doi: 10.1158/1078-0432.CCR-07-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilentz RE, Su GH, Dai JL, et al. Immunohistochemical labeling for dpc4 mirrors genetic status in pancreatic adenocarcinomas: A new marker of DPC4 inactivation. Am J Pathol. 2000;156:37–43. doi: 10.1016/S0002-9440(10)64703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iacobuzio-Donahue CA, Song J, Parmiagiani G, et al. Missense mutations of MADH4: Characterization of the mutational hot spot and functional consequences in human tumors. Clin Cancer Res. 2004;10:1597–1604. doi: 10.1158/1078-0432.ccr-1121-3. [DOI] [PubMed] [Google Scholar]
- 21.Redston MS, Caldas C, Seymour AB, et al. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res. 1994;54:3025–3033. [PubMed] [Google Scholar]
- 22.Rozenblum E, Schutte M, Goggins M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 23.Mao C, Domenico DR, Kim K, et al. Observations on the developmental patterns and the consequences of pancreatic exocrine adenocarcinoma: Findings of 154 autopsies. Arch Surg. 1995;130:125–134. doi: 10.1001/archsurg.1995.01430020015001. [DOI] [PubMed] [Google Scholar]
- 24.Kamisawa T, Isawa T, Koike M, et al. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas. 1995;11:345–349. doi: 10.1097/00006676-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Nakahashi C, Oda T, Kinoshita T, et al. The impact of liver metastasis on mortality in patients initially diagnosed with locally advanced or resectable pancreatic cancer. Int J Gastrointest Cancer. 2003;33:155–164. doi: 10.1385/IJGC:33:2-3:155. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka T, Watanabe T, Kazama Y, et al. Chromosome 18q deletion and Smad4 protein inactivation correlate with liver metastasis: A study matched for T- and N- classification. Br J Cancer. 2006;95:1562–1567. doi: 10.1038/sj.bjc.6603460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michor F, Iwasa Y. Dynamics of metastasis suppressor gene inactivation. J Theor Biol. 2006;241:676–689. doi: 10.1016/j.jtbi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Ramaswamy S, Ross KN, Lander ES, et al. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 29.Vogelmann R, Nguyen-Tat MD, Giehl K, et al. TGFbeta-induced downregulation of E-cadherin-based cell-cell adhesion depends on PI3-kinase and PTEN. J Cell Sci. 2005;118:4901–4912. doi: 10.1242/jcs.02594. [DOI] [PubMed] [Google Scholar]
- 30.Kang Y, He W, Tulley S, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A. 2005;102:13909–13914. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deckers M, van Dinther M, Buijs J, et al. The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res. 2006;66:2202–2209. doi: 10.1158/0008-5472.CAN-05-3560. [DOI] [PubMed] [Google Scholar]
- 32.Tascilar M, Skinner HG, Rosty C, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115–4121. [PubMed] [Google Scholar]
- 33.Biankin AV, Morey AL, Lee CS, et al. DPC4/Smad4 expression and outcome in pancreatic ductal adenocarcinoma. J Clin Oncol. 2002;20:4531–4542. doi: 10.1200/JCO.2002.12.063. [DOI] [PubMed] [Google Scholar]
- 34.Bardeesy N, Cheng KH, Berger JH, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat Cell Biol. 2007;9:1000–1004. doi: 10.1038/ncb434. [DOI] [PubMed] [Google Scholar]
- 37.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: A pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]