Abstract
Purpose
Smudge cells are ruptured chronic lymphocytic leukemia (CLL) cells appearing on the blood smears of CLL patients. Our recent findings suggest that the number of smudge cells may have important biologic correlations rather than being only an artifact of slide preparation. In this study, we evaluated whether the smudge cell percentage on a blood smear predicted survival of CLL patients.
Patients and Methods
We calculated smudge cell percentages (ratio of smudged to intact cells plus smudged lymphocytes) on archived blood smears from a cohort of previously untreated patients with predominantly early-stage CLL enrolled onto a prospective observational study. The relationship between percentage of smudge cells, patient survival, and other prognostic factors was explored.
Results
Between 1994 and 2002, 108 patients were enrolled onto the study and had archived blood smears available for review; 80% of patients had Rai stage 0 or I disease. The median smudge cell percentage was 28% (range, 1% to 75%). The percentage of smudge cells was lower in CD38+ versus CD38– patients (P = .019) and in Zap70-positive versus Zap70-negative patients (P = .028). Smudge cell percentage as a continuous variable was associated with prolonged survival (P = .042). The 10-year survival rate was 50% for patients with 30% or less smudge cells compared with 80% for patients with more than 30% of smudge cells (P = .015). In multivariate analysis, the percentage of smudge cells was an independent predictor of overall survival.
Conclusion
Percentage of smudge cells on blood smear is readily available and an independent factor predicting overall survival in CLL.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is one of the most common malignant lymphoid diseases. Each year, 15,000 to 19,000 individuals are diagnosed with this disease in the United States.1 The introduction of the automated CBC resulted in a considerable shift in the clinical stage at the time of CLL diagnosis, with approximately 70% of patients now diagnosed with early Rai/Binet stage disease.2 The prognosis of patients diagnosed with early clinical stage is highly variable; approximately 30% to 40% of patients experience a rapid progression of their disease, whereas others have a relatively indolent form of the disease with survival measured in decades.3,4 The shift toward early-stage disease at the time of diagnosis has increased interest in the identification of prognostic factors that would allow the recognition of early and intermediate clinical stage patients at risk for more rapid disease progression. A number of such factors have been identified, including immunoglobulin heavy chain (IgVH) mutational status, CD38 and Zap70 expression, and cytogenetic abnormalities identified by interphase fluorescence in situ hybridization (FISH).5 Despite this progress, many patients have limited access to these laboratory tests, which require highly sophisticated instruments and a high degree of technical expertise and are costly to perform. In addition, because of the technical complexity of some of the assays, a considerable effort is necessary to ensure reproducibility between the laboratories.6–8
Smudge cells are ruptured CLL B cells seen on routine blood smears of virtually all CLL patients. For nearly a century, smudge cells were thought to be merely an artifact of slide preparation.9,10 We have recently discovered that smudge formation is related to the content of the cytoskeletal protein vimentin present in leukemic cells.11 Vimentin is an intermediate filament protein critical for lymphocyte rigidity and integrity.12 We previously showed that CLL patients with high vimentin content have a low percentage of smudge cells.13 In addition, we found that high vimentin expression is associated with a shortened time to initial therapy in early-stage CLL.11 Because vimentin expression is a prognostic factor in early-stage CLL, we hypothesized that there could also be an association between smudge cell percentage and prognosis in patients with CLL. Indeed, we previously demonstrated that patients with a high percentage of smudge cells on a routine blood smear (eg, low vimentin) experience a prolonged time to first treatment.13
In the current study, we have calculated the percentage of smudge cells on a routine blood smear at diagnosis in an independent group of patients with predominantly early and intermediate Rai stage CLL enrolled onto a prospective observational trial and evaluated the impact of smudge cell percentage on overall survival (OS). We also investigated the relationship of the percentage of smudge cells with other well-established prognostic factors in CLL.
PATIENTS AND METHODS
Patients
Between January 1994 and October 2002, 159 patients with previously untreated B-cell CLL seen at the Mayo Clinic Rochester, NY and Jacksonville, FL campuses were enrolled onto a prospective study evaluating the prognostic importance of cytogenetic abnormalities in CLL, as previously reported.14 The study was approved by the Mayo Clinic Institutional Review Board, and all patients signed a written consent form to participate. Clinical data, including patient characteristics, disease course, and survival, and blood samples were collected at the time of study enrollment and periodically thereafter. The median time from diagnosis to study enrollment was 2.8 months, and the median follow-up time from diagnosis was 9.9 years (range, 0.8 to 23 years).
Peripheral-Blood Smear Examination
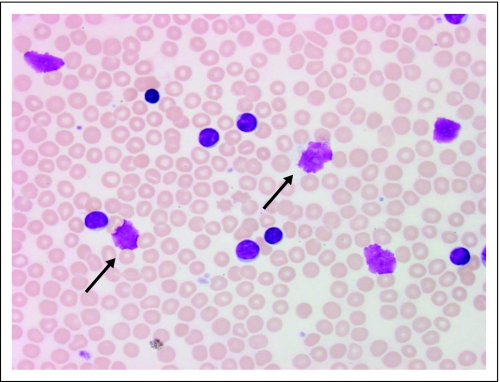
Archived Wright-Giemsa–stained blood smears made at the time of study entry for clinical purposes were reviewed. The blood smears were prepared from either EDTA anticoagulated blood or a finger stick. All blood smears were prepared using a semiautomatic device (Miniprep; Sedona Lab Products, Sedona, AZ) in which a simple spring mechanism pulls a drop of blood along a slide to ensure better smear uniformity. Slides were randomly assigned to five hematopathology technologists blinded to patients' clinical and outcome data. Each slide (one per patient) was evaluated once by one of the five technologists. Smudge cells were defined as broken cells with no intact cytoplasm and a disrupted nuclear membrane (Fig 1), and smudge cell percentage was estimated as previously described.13 On the basis of our previous findings that a 200-cell differential provides excellent interobserver reproducibility13 regardless of the method of slide preparation, a total of 200 lymphocytes and smudge cells were counted on each slide. The results were expressed as a percentage of the total lymphocytes (intact and smudged). In addition, to evaluate the interobserver reproducibility of smudge cell percentage estimate in the current cohort, we randomly selected 15 slides. These slides were randomly assigned to five technologists blinded to the first readout results and re-evaluated. The results from the first and the second readouts were then compared.
Fig 1.
Smudge cells on peripheral-blood smear of patient with chronic lymphocytic leukemia. The arrows show examples of smudge cells. The smudge cell percentage is estimated by counting 200 lymphocytes and/or smudge cells; the smudge cell number is then divided by total number of cells counted (smudge cells + intact lymphocytes) and multiplied by 100%.
Assessment of Other Prognostic Factors
CLL B cells were isolated by density gradient centrifugation from heparinized blood samples and either used immediately or suspended in RPMI 1640/20% fetal calf serum/10% dimethyl sulfoxide and stored at −80°C until used. CD38 expression in CD19+ cells was measured by flow cytometry using antibodies specific for CD38 and CD19, as previously described.14 IgVH mutation status and cytogenetic abnormalities by FISH panel testing were assessed using methods previously described.14–18 Patients with mutated IgVH genes using the VH 3-21 rearrangement were grouped with unmutated patients in categoric analysis.19 Zap70 expression was assessed as described by Rassenti et al.20
Statistical Considerations
Relationships between continuous variables were explored using the Spearman rank correlation coefficient. Differences between groups were evaluated using Fisher's exact test and Wilcoxon rank sum test for categoric and continuous variables, respectively. All tests were two-sided, and statistical significance was defined as P < .05. OS was calculated as the time from date of diagnosis of CLL to date of death from any cause or last follow-up. Differences in OS between prognostic groups were evaluated using the standard Kaplan-Meier method21 and log-rank statistics. Univariate and multivariate analyses were performed using Cox models.22
RESULTS
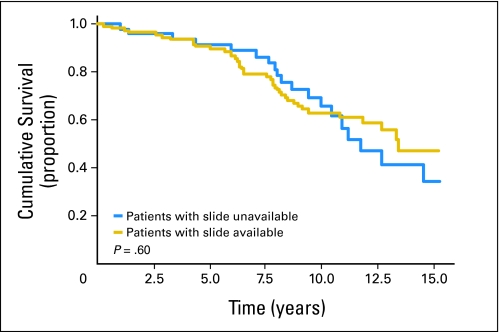
Of the 159 previously untreated patients with CLL accrued to the prospective observational study, 108 (68%) had archived blood smears available for review made within 1 week before study or on study entry. Clinical characteristics, including age, sex, Rai stage, CD38 expression, IgVH mutation status, and cytogenetic category, were not significantly different for the 51 patients who did not have archived slides available for review compared with patients with archived slides available (Appendix Table A1, online only). The proportion of patients with Zap70-positive disease was slightly higher in patients who had slides reviewed versus patients without available slides (58% v 40%, respectively; P = .04). Importantly, the estimated median OS of patients with archived slides versus those without archived slides was not significantly different (13.2 v 12.2 years, respectively; P = .6; Appendix Fig A1, online only). Detailed characteristics of 108 patients who had slides reviewed in this study are listed in Table 1. The median follow-up time from diagnosis was 9.9 years (range, 0.8 to 23 years), and there were 43 deaths during follow-up. The vast majority of patients (80%) had early-stage disease (Rai stage 0 or I) at study entry. Zap70 status, CD38 status, and cytogenetic analysis by FISH were available for all 108 patients. IgVH sequencing was attempted in all patients, with 89 patients (82%) classifiable as having either mutated or unmutated IgVH.
Table 1.
Patient Characteristics
| Characteristic | No. of Patients (N = 108) | % |
|---|---|---|
| Age, years | ||
| Median | 63 | |
| Range | 36-83 | |
| Male | 73 | 68 |
| Rai stage | ||
| 0 | 53 | 49 |
| I | 34 | 31 |
| II | 13 | 12 |
| III | 2 | 2 |
| IV | 6 | 6 |
| Zap70 ≥ 20% | 63 | 58 |
| IgVH unmutated* | 41 | 46 |
| CD38 ≥ 30% | 33 | 30 |
| FISH | ||
| del 13q14.2 | 41 | 38 |
| Normal | 30 | 28 |
| Trisomy 12 | 18 | 17 |
| del 11q22.3 | 7 | 6 |
| del 17p13.1 | 6 | 6 |
| Other | 3 | 3 |
Abbreviations: FISH, fluorescence in situ hybridization; IgVH, immunoglobulin heavy chain.
IgVH gene mutation status was attempted for all patients but classifiable for 89 patients.
Smudge Cell Percentage in CLL Patients
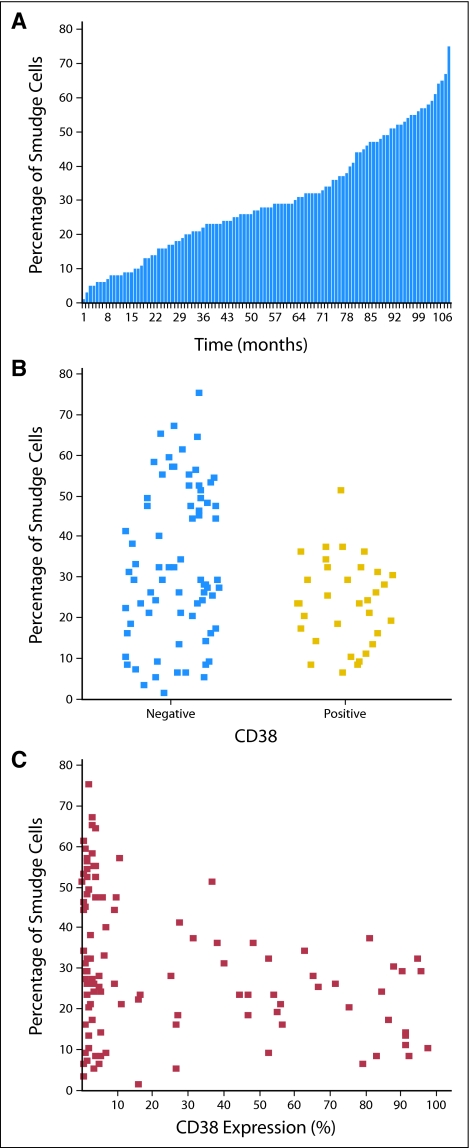
The median smudge cell percentage was 28% (range, 1% to 75%). The distribution of smudge cell percentages among patients is shown in Figure 2A. Percentage of smudge cells was lower in CD38+ patients than in CD38– patients (median, 23%; range, 6% to 51% v median, 29%; range, 1% to 75%, respectively; P = .019; Fig 2B). When analyzed as continuous variables, there was a weak inverse correlation between CD38 expression and smudge cell percentage (r = −0.31, P = .001; Fig 2C). The percentage of smudge cells was also lower in Zap70-positive patients than Zap70-negative patients (median, 27%; range, 1% to 75% v median, 34%; range, 5% to 67%, respectively; P = .028). When both factors were treated as continuous variables, there was a weak inverse correlation between Zap70 expression and percentage of smudge cells (r = −0.21, P = .024). Patients with 13q deletion as a sole abnormality, a favorable genetic finding, had a higher percentage of smudge cells than patients with normal karyotype and other cytogenetic abnormalities (median, 32%; range, 2% to 75% v median, 23%; range, 1% to 67%; P = .036). There was no difference in the percentage of smudge cells based on other FISH cytogenetic categories or IgVH mutation status. There was no correlation of smudge cell percentage with absolute lymphocyte count (r = 0.07, P = .45), which is a finding consistent with previous observations.13 We also did not see an association of smudge cell percentage with age or Rai stage. The median percentage of smudge cells was higher in females than males (26% v 32%, respectively; P = .023); however, sex was not a significant prognostic factor for survival in this cohort.
Fig 2.
Smudge cell percentage in 108 patients with chronic lymphocytic leukemia (CLL). (A) Distribution of smudge cell percentage in the entire cohort. The presence of smudge cells on peripheral-blood smears of CLL patients is a constant feature of CLL with significant interpatient variability. The median smudge cell percentage was 28% (range, 1% to 75%) in our study. Only four patients (4%) had a smudge cell percentage within the 1% to 5% range. (B) Percentage of smudge cells in CD38– and CD38+ patients. (C) Smudge cell percentage and CD38 expression as continuous variables. A distinct group of patients with a high percentage of smudge cells and CD38– disease can be identified.
There was a significant correlation of smudge cells percentage for 15 slides randomly selected for reassessment between the first and the second readout (r = 0.89, P < .001). The median percentage of smudge cells was not significantly different between the first and the second readout (24% v 25%, respectively; P = .68).
Percentage of Smudge Cells and Survival
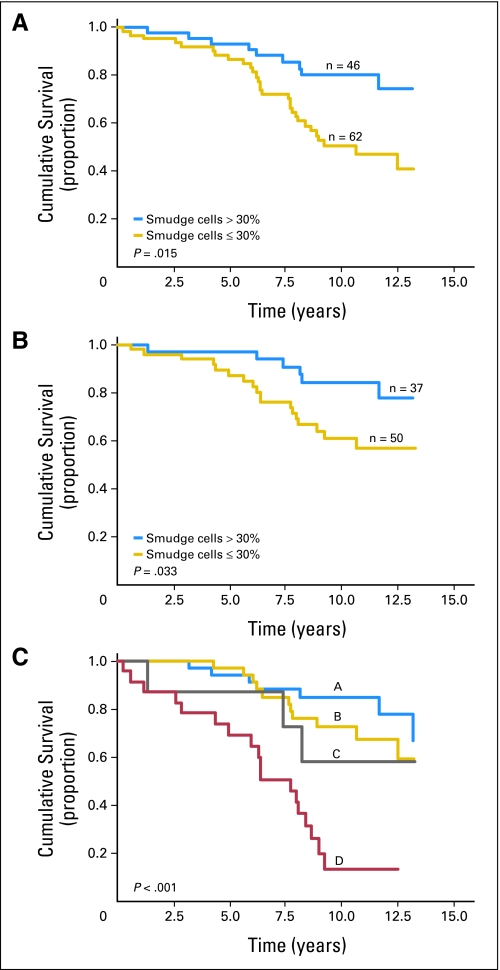
Smudge cell percentage as a continuous variable was associated with prolonged survival (P = .042). Next, we evaluated the relationship between smudge cells as a discrete variable and OS. We first applied the 30% threshold shown to best stratify outcome in an independent and previously reported patient series13 and observed a significant separation of survival curves. We next evaluated what level of smudge cells would best stratify survival in the current cohort. Remarkably, the 30% threshold was again confirmed using the minimal P value approach. Therefore, we used a 30% cutoff to classify patients as having low- or high-risk disease based on smudge cell percentage in all analyses. The 10-year survival estimate was 50% v 80% for patients with smudge cell percentage ≤ 30% v more than 30%, respectively (P = .015; Fig 3A). In patients with Rai stage of 0 to I (n = 87), 61% of patients with smudge cell percentage ≤ 30% were alive at 10 years compared with 84% of patients with more than 30% of smudge cells (P = .033; Fig 3B). A comparison of CD38 expression and smudge cell percentage revealed a distinct subgroup of patients with CD38-negative disease and a high percentage of smudge cells (Fig 2C). Because CD38 expression is an independent prognostic factor from smudge cell percentage in multivariate analysis, we wondered whether the prognostic value of smudge cells is related to the excellent prognosis seen in this distinct patient subgroup. Interestingly, the prognostic value of smudge cell percentage appears to be related to the ability to further risk stratify the CD38+ rather than CD38– patients (Fig 3C). The 10-year survival rate of patients with CD38+ disease and less than 30% smudge cells was only 13% compared with rates of 58%, 72%, and 85% for patients with CD38+ disease and ≥ 30% smudges cells, CD38– disease and less than 30% smudge cells, and CD38– disease and ≥ 30% smudge cells, respectively (P < .001).
Fig 3.
Overall survival (OS) based on the percentage of smudge cells on blood smear. (A) Kaplan-Meier estimates of OS based on the percentage of smudge cells in the entire cohort. The 10-year survival rate was 50% v 80% for patients with smudge cell percentage ≤ 30% v more than 30%, respectively (P = .015). (B) Kaplan-Meier estimates of OS based on the percentage of smudge cells in patients with Rai stage 0 and I disease only. The 10-year survival rate was 61% v 84% for patients with smudge cell percentage ≤ 30% v more than 30%, respectively (P = .033). (C) Kaplan-Meier estimates of OS based on smudge cell percentage and CD38 expression. The 10-year survival of patients with CD38+ disease and less than 30% smudge cells was only 13% compared with 58%, 72%, and 85% for patients with CD38+ disease and ≥ 30% smudge cells, CD38– disease and less than 30% smudge cells, and CD38– disease and ≥ 30% smudge cells, respectively (P < .001).
Multivariate Analysis for Survival
We then evaluated how the percentage of smudge cells impacts survival in relation to other commonly used prognostic factors such as Rai stage, Zap70 and CD38 expression, IgVH gene mutation status, and cytogenetic abnormalities detected by FISH. For the purposes of these analyses, a patient with a FISH diagnosis of del(17p13.1) or del(11q22.3) was categorized as having a poor FISH-based prognosis. Similarly, patients with a FISH classification of normal, del(13q14.2), or trisomy 12 were categorized as having a good/intermediate FISH-based prognosis. The prognostic value of each marker on univariate analysis is listed in Table 2. In multivariate analysis (Table 3), which included all prognostic markers that were significant in univariate analysis, smudge cell percentage was an independent prognostic factor.
Table 2.
Univariate Analysis for Overall Survival
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Smudge cell percentage: ≤ 30% | 2.1 | 1.1 to 4.1 | .018 |
| FISH: 11q- or 17p- | 1.9 | 0.9 to 4.1 | .11 |
| IgVH unmutated | 1.8 | 0.9 to 3.7 | .071 |
| Zap70: ≥ 20% | 1.9 | 1.1 to 4.4 | .017 |
| CD38: ≥ 30% | 3.4 | 1.8 to 6.2 | < .001 |
| Rai stage: III or IV | 4.8 | 1.9 to 11.8 | < .001 |
| Age: > 60 years | 1.8 | 0.9 to 3.6 | .083 |
| Sex: male | 1.2 | 0.6 to 2.5 | .51 |
Abbreviations: HR, hazard ratio; FISH, fluorescence in situ hybridization; IgVH, immunoglobulin heavy chain.
Table 3.
Multivariable Analysis for Overall Survival
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Smudge cell percentage: ≤ 30% | 2.9 | 1.1 to 3.9 | .038 |
| CD38: ≥ 30% | 2.7 | 1.2 to 6.3 | .012 |
| Rai stage: III or IV | 2.5 | 1.3 to 4.9 | .005 |
Abbreviation: HR, hazard ratio.
DISCUSSION
The appearance of smudge cells on a peripheral-blood smear is a characteristic feature of CLL, with virtually all patients demonstrating at least some degree of smudging.13,23 In contrast, smudge cells are a rare and inconsistent feature of other lymphoproliferative disorders.24 Since its description in 1896 by Gumprecht, smudge cells were thought to be just an artifact of slide preparation resulting from the fragility of CLL cells.9,10 The first suggestion that smudge cells may be biologically significant came from two intriguing observations in the 1950s demonstrating that smudge cell percentage was independent from the degree of lymphocytosis and seemed to be patient specific.23 We recently confirmed these two observations and demonstrated that the percentage of smudge cells remains constant over time in any given CLL patient.13 We also demonstrated that smudge cell formation is inversely correlated with CLL B cell content of vimentin, a cytoskeletal protein critical for rigidity and integrity of lymphocytes. The physiologic role of vimentin may extend beyond maintaining cell integrity; rearrangement of vimentin fibers was shown to participate in cell activation and signal transduction.25 High vimentin expression has been shown to be associated with poor prognosis and metastatic potential in breast26,27 and colon cancer.28
Because the percentage of smudge cells inversely correlates with vimentin expression,13 we hypothesized that the calculated smudge cell percentage on a blood smear would have prognostic value in CLL. Accordingly, patients with low vimentin expression would have a high percentage of smudge cells and a better prognosis, whereas those with high vimentin expression would have a low percentage of smudge cells and a worse prognosis. Indeed, we previously demonstrated that a high percentage of smudge cells correlated with a prolonged time to the initial therapy in a small cohort of 75 patients with early- and intermediate-stage CLL.13 Follow-up was insufficient to evaluate the relationship with OS in that series.
In the current study, we demonstrate that a low percentage of smudge cells predicts shortened OS as both a continuous and categoric variable in an independent sample of CLL patients participating in a prospective observational cohort study. The percentage of smudge cells was lower in high-risk patients as defined by Zap70 and CD38 expression and 13q deletion; however, there was only weak correlation between Zap70 and CD38 expression as a continuous variable and smudge cell percentage. The lack of strong association of smudge cell percentage with other prognostic factors suggests a distinct role for the cytoskeleton in CLL biology. The latter notion is further supported by the fact that, in multivariate analysis, the percentage of smudge cells was an independent predictor of OS.
The smudge cell percentage has two potential advantages over other recently identified prognostic markers. First, it is nearly universally accessible because microscopic evaluation of a blood smear is typically available even to patients in countries with limited resources. In the present study, counting smudge cells and calculating the percentage were performed by laboratory technologists and took, on average, 3 to 5 minutes per slide. Second, the smudge cell percentage can be retrospectively determined for patients participating in completed or ongoing studies based on review of archived slides even if no other biologic samples were stored. The difference in OS at 10 years for patients with a high versus low percentage of smudge cells was approximately 30%. Importantly, because smudge cell percentage is an independent variable and only weakly correlates with other prognostic factors, we believe that smudge cell percentage may be used for additional risk stratification in combination with other biologic prognostic factors.
The prognostic value of smudging may have direct biologic implications as to the role of cytoskeleton in survival and progression of CLL cells. In this regard, the question is whether fragile CLL B cells undergo in vivo rupture secondary to vascular shear stress similar to the process of smudging during slide preparation and whether this in vivo smudging plays any role in CLL homeostasis. Soluble CD20 levels are detectable in CLL, and presumably, CD20 shedding could be a result of leukemic cell rupture. Although this hypothesis seems to be contradicted by the fact that high levels of soluble CD20 are related to an adverse prognosis in CLL, it should be noted that CD20 levels correlate with measures of tumor volume like Rai stage or β2-microglobulin level. In contrast, we did not see a correlation of smudge cell percentage with Rai stage or lymphocytosis. Therefore, it is plausible that the relation of CD20 to tumor mass negates its correlation with smudge cell levels. Further studies of the relationship between smudge cell percentage and soluble CD20 may shed light on the link between these hypothetical markers of in vivo cell fragility. Agents targeting proteins involved in cytoskeleton function, including vimentin, are currently being developed,29–31 and further preclinical testing in CLL maybe warranted.
Our findings are subject to a number of limitations. First, because blood smears were not a mandatory component of the study, the smudge cell percentage could not be determined for all participants in the original cohort. Second, although we used the previously published threshold of a 30% smudge cell percentage to classify patients at risk of shorter survival in the present cohort, the optimal cut point to stratify patient risk will need to be defined in future studies using even larger cohorts.
Our study also has a number of important strengths. The individuals studied were from a well-defined cohort of CLL patients participating in a prospective observational trial. Nearly 90% of the patients studied had early-stage disease at study entry and thus represent the patient group for whom prognostic tools are most needed. Smudge cell percentage correlated with OS as a continuous variable and was an independent predictor of OS on multivariable analysis including the other well-established prognostic parameters. Other prognostic factors, including IgVH mutational status, Zap70 and CD38 expression, and cytogenetic abnormalities detected by FISH, were also characterized in this cohort, allowing us to investigate the relationship between these factors and the percentage of smudge cells.
In conclusion, the percentage of smudge cells on a routine blood smear is an independent prognostic factor in patients with CLL. Patients with a high percentage of smudge cells experience prolonged survival. Because minimal technical resources are required, the estimation of smudge cell percentage is a potentially universally available prognostic marker. Future studies of the cytoskeleton in CLL may shed light on the biology of this disease and identify new therapeutic targets.
Acknowledgment
We thank Susan McLean for her support in manuscript preparation and submission.
Appendix
Fig A1.
Kaplan-Meier estimate of overall survival (OS) of patients with slides available and not available for review. The estimated median OS of patients with archived slides and without archived slides was not significantly different between these two groups (13.2 v 12.2 years, respectively; P = .6).
Table A1.
Characteristics of Patients With Slides Available (n = 108) and Not Available (n = 51) for Review
| Characteristic | Slides Reviewed (n = 108) |
Slides Not Available (n = 51) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Age, years | .65 | ||||
| Median | 63 | 65 | |||
| Range | 36-83 | 41-87 | |||
| Male | 73 | 68 | 39 | 76 | |
| Rai stage | .44 | ||||
| 0 | 53 | 49 | 31 | 60 | |
| I | 34 | 31 | 14 | 27 | |
| II | 13 | 12 | 5 | 10 | |
| III | 2 | 2 | 0 | 0 | |
| IV | 6 | 6 | 1 | 2 | |
| Lymphocyte count, ×109/L | .54 | ||||
| Median | 17.8 | 16.9 | |||
| Range | 2.0-278.4 | 5.1-167 | |||
| Zap70 ≥ 20% | 63 | 58 | 20 | 40 | .04 |
| IgVH unmutated* | 41 | 46 | 15 | 47 | .84 |
| CD38 ≥ 30% | 33 | 30 | 13 | 26 | .45 |
| FISH | .66 | ||||
| del 13q14.2 | 41 | 38 | 30 | 58 | |
| Normal | 30 | 28 | 9 | 18 | |
| Trisomy 12 | 18 | 17 | 5 | 10 | |
| del 11q22.3 | 7 | 6 | 5 | 10 | |
| del 17p13.1 | 6 | 6 | 1 | 2 | |
| Other | 3 | 3 | 1 | 2 | |
Abbreviations: FISH, fluorescence in situ hybridization; IgVH, immunoglobulin heavy chain.
IgVH gene mutation status was attempted for all patients but classifiable for 126 patients.
Footnotes
Supported in part by Grant No. CA95241 from the National Cancer Institute, National Institutes of Health, and philanthropic support from the Donner Family Foundation.
Presented in part at the 12th Annual International Workshop for Chronic Lymphocytic Leukemia, September 14-16, 2007, London, United Kingdom.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Neil E. Kay, Hospira, Bayer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Grzegorz S. Nowakowski, James D. Hoyer, Tait D. Shanafelt, Clive S. Zent, Betsy LaPlant, Diane F. Jelinek, Neil E. Kay
Financial support: Diane F. Jelinek, Neil E. Kay
Administrative support: Grzegorz S. Nowakowski, Neil E. Kay
Provision of study materials or patients: Grzegorz S. Nowakowski, Tait D. Shanafelt, Clive S. Zent, Timothy G. Call, Thomas E. Witzig, Neil E. Kay
Collection and assembly of data: Grzegorz S. Nowakowski, James D. Hoyer, Tait D. Shanafelt, Nancy D. Bone, Betsy LaPlant, Renee C. Tschumper, Thomas E. Witzig, Neil E. Kay
Data analysis and interpretation: Grzegorz S. Nowakowski, James D. Hoyer, Tait D. Shanafelt, Clive S. Zent, Gordon W. Dewald, Diane F. Jelinek, Neil E. Kay
Manuscript writing: Grzegorz S. Nowakowski, Tait D. Shanafelt, Clive S. Zent, Gordon W. Dewald, Neil E. Kay
Final approval of manuscript: Grzegorz S. Nowakowski, James D. Hoyer, Tait D. Shanafelt, Timothy G. Call, Betsy LaPlant, Gordon W. Dewald, Renee C. Tschumper, Diane F. Jelinek, Thomas E. Witzig, Neil E. Kay
REFERENCES
- 1.Zent CS, Kyasa MJ, Evans R, et al. Chronic lymphocytic leukemia incidence is substantially higher than estimated from tumor registry data. Cancer. 2001;92:1325–1330. doi: 10.1002/1097-0142(20010901)92:5<1325::aid-cncr1454>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Call TG, Phyliky RL, Noel P, et al. Incidence of chronic lymphocytic leukemia in Olmsted County, Minnesota, 1935 through 1989, with emphasis on changes in initial stage at diagnosis. Mayo Clin Proc. 1994;69:323–328. doi: 10.1016/s0025-6196(12)62215-0. [DOI] [PubMed] [Google Scholar]
- 3.Montserrat E, Rozman C. Chronic lymphocytic leukaemia: Prognostic factors and natural history. Baillieres Clin Haematol. 1993;6:849–866. doi: 10.1016/s0950-3536(05)80179-9. [DOI] [PubMed] [Google Scholar]
- 4.Rai KR, Chiorazzi N. Determining the clinical course and outcome in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1797–1799. doi: 10.1056/NEJMe030032. [DOI] [PubMed] [Google Scholar]
- 5.Shanafelt TD, Geyer SM, Kay NE. Prognosis at diagnosis: Integrating molecular biologic insights into clinical practice for patients with CLL. Blood. 2004;103:1202–1210. doi: 10.1182/blood-2003-07-2281. [DOI] [PubMed] [Google Scholar]
- 6.Letestu R, Rawstron A, Ghia P, et al. Evaluation of ZAP-70 expression by flow cytometry in chronic lymphocytic leukemia: A multicentric international harmonization process. Cytometry B Clin Cytom. 2006;70:309–314. doi: 10.1002/cyto.b.20132. [DOI] [PubMed] [Google Scholar]
- 7.Wilhelm C, Neubauer A, Brendel C. Discordant results of flow cytometric ZAP-70 expression status in B-CLL samples if different gating strategies are applied. Cytometry B Clin Cytom. 2006;70:242–250. doi: 10.1002/cyto.b.20123. [DOI] [PubMed] [Google Scholar]
- 8.Peková S, Baran-Marszak F, Schwarz J, et al. Mutated or non-mutated? Which database to choose when determining the IgVH hypermutation status in chronic lymphocytic leukemia? Haematologica. 2006;91:ELT01. [PubMed] [Google Scholar]
- 9.Gumprecht F. Leucocytenzerfall in blute bal leukemic und bel sohwaren anamlan. Deutches Archlv Kinlache Medicin. 1896;5:523–548. [Google Scholar]
- 10.Macdonald D, Richardson H, Raby A. Practice guidelines on the reporting of smudge cells in the white blood cell differential count. Arch Pathol Lab Med. 2003;127:105. doi: 10.5858/2003-127-105-PGOTRO. [DOI] [PubMed] [Google Scholar]
- 11.Nowakowski GS, Lee Y, Bone ND, et al. Proteomic analysis of chronic lymphocytic leukemia cells identifies vimentin as a novel prognostic factor for aggressive disease. Blood. 2005;106:707. abstr. [Google Scholar]
- 12.Brown MJ, Hallam JA, Colucci-Guyon E, et al. Rigidity of circulating lymphocytes is primarily conferred by vimentin intermediate filaments. J Immunol. 2001;166:6640–6646. doi: 10.4049/jimmunol.166.11.6640. [DOI] [PubMed] [Google Scholar]
- 13.Nowakowski GS, Hoyer JD, Shanafelt TD, et al. Using smudge cells on routine blood smears to predict clinical outcome in chronic lymphocytic leukemia: A universally available prognostic test. Mayo Clin Proc. 2007;82:449–453. doi: 10.4065/82.4.449. [DOI] [PubMed] [Google Scholar]
- 14.Shanafelt TD, Witzig TE, Fink SR, et al. Prospective evaluation of clonal evolution during long-term follow-up of patients with untreated early-stage chronic lymphocytic leukemia. J Clin Oncol. 2006;24:4634–4641. doi: 10.1200/JCO.2006.06.9492. [DOI] [PubMed] [Google Scholar]
- 15.Jelinek DF, Tschumper RC, Geyer SM, et al. Analysis of clonal B-cell CD38 and immunoglobulin variable region sequence status in relation to clinical outcome for B-chronic lymphocytic leukaemia. Br J Haematol. 2001;115:854–861. doi: 10.1046/j.1365-2141.2001.03149.x. [DOI] [PubMed] [Google Scholar]
- 16.Jelinek DF, Tschumper RC, Stolovitzky GA, et al. Identification of a global gene expression signature of B-chronic lymphocytic leukemia. Mol Cancer Res. 2003;1:346–361. [PubMed] [Google Scholar]
- 17.Dewald G, Brockman S, Paternoster S, et al. Chromosome anomalies detected by interphase fluorescence in hybridization: Correlation with significant biological features of chronic lymphocytic leukemia. Br J Haematol. 2003;121:287–295. doi: 10.1046/j.1365-2141.2003.04265.x. [DOI] [PubMed] [Google Scholar]
- 18.Shanafelt TD, Jelinek D, Tschumper R, et al. Cytogenetic abnormalities can change during the course of the disease process in chronic lymphocytic leukemia. J Clin Oncol. 2006;24:3218–3219. doi: 10.1200/JCO.2006.06.1077. [DOI] [PubMed] [Google Scholar]
- 19.Tobin G, Thunberg U, Johnson A, et al. Chronic lymphocytic leukemias utilizing the VH3-21 gene display highly restricted Vlambda2-14 gene use and homologous CDR3s: Implicating recognition of a common antigen epitope. Blood. 2003;101:4952–4957. doi: 10.1182/blood-2002-11-3485. [DOI] [PubMed] [Google Scholar]
- 20.Rassenti LZ, Huynh L, Toy TL, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 23.Heinivaara O. Smudge cells in lymphocytic leukemia. Ann Med Intern Fenn. 1959;48:69–75. [PubMed] [Google Scholar]
- 24.Schleiffenbaum B, Fehr J. Value of the blood picture and flow cytometry immunotyping in the early diagnosis of low-grade lymphoma. Ther Umsch. 1996;53:117–122. [PubMed] [Google Scholar]
- 25.Ivaska J, Pallari HM, Nevo J, et al. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. 2007;313:2050–2062. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 26.Raymond WA, Leong AS. Vimentin: A new prognostic parameter in breast carcinoma? J Pathol. 1989;158:107–114. doi: 10.1002/path.1711580205. [DOI] [PubMed] [Google Scholar]
- 27.Domagala W, Lasota J, Dukowicz A, et al. Vimentin expression appears to be associated with poor prognosis in node-negative ductal NOS breast carcinomas. Am J Pathol. 1990;137:1299–1304. [PMC free article] [PubMed] [Google Scholar]
- 28.Ngan CY, Yamamoto H, Seshimo I, et al. Quantitative evaluation of vimentin expression in tumour stroma of colorectal cancer. Br J Cancer. 2007;96:986–992. doi: 10.1038/sj.bjc.6603651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velasco-Velázquez MA, Agramonte-Hevia J, Barrera D, et al. 4-Hydroxycoumarin disorganizes the actin cytoskeleton in B16–F10 melanoma cells but not in B82 fibroblasts, decreasing their adhesion to extracellular matrix proteins and motility. Cancer Lett. 2003;198:179–186. doi: 10.1016/s0304-3835(03)00333-1. [DOI] [PubMed] [Google Scholar]
- 30.Bargagna-Mohan P, Hamza A, Kim YE, et al. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem Biol. 2007;14:623–634. doi: 10.1016/j.chembiol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adjei AA, Hidalgo M. Intracellular signal transduction pathway proteins as targets for cancer therapy. J Clin Oncol. 2005;23:5386–5403. doi: 10.1200/JCO.2005.23.648. [DOI] [PubMed] [Google Scholar]