Abstract
Corticosteroids have been shown to exert beneficial effects in the treatment of acute myocardial infarction, but the precise mechanisms underlying their protective effects are unknown. Here we show that high-dose corticosteroids exert cardiovascular protection through a novel mechanism involving the rapid, non-transcriptional activation of endothelial nitric oxide synthase (eNOS). Binding of corticosteroids to the glucocorticoid receptor (GR) stimulated phosphatidylinositol 3-kinase and protein kinase Akt, leading to eNOS activation and nitric oxide–dependent vasorelaxation. Acute administration of pharmacological concentrations of corticosteroids in mice led to decreased vascular inflammation and reduced myocardial infarct size following ischemia and reperfusion injury. These beneficial effects of corticosteroids were abolished by GR antagonists or eNOS inhibitors in wild-type mice and were completely absent in eNOS-deficient (Nos3−/−) mice. The rapid activation of eNOS by the non-nuclear actions of GR, therefore, represents an important cardiovascular protective effect of acute high-dose corticosteroid therapy.
Corticosteroids are potent anti-inflammatory agents which are used in the treatment of asthma1, hypersensitivity reactions2 and autoimmune diseases3. Corticosteroids inhibit the inflammatory response through binding to the glucocorticoid receptor (GR)4. The GR is a steroid hormone nuclear receptor5, which when bound to corticosteroids, modulates the expression of target genes by binding to DNA sequences containing the glucocorticoid response elements (GRE)6. Recent studies, however, suggest that some of the anti-inflammatory effects of corticosteroids occur via non-GRE-mediated effects on prostaglandin synthesis7,8 and nuclear factor-κB (NF-κB) activation9. Thus, the precise role of nuclear and non-nuclear GR in mediating the immunosuppressive effects of corticosteroids is still unknown.
In cardiovascular disease, corticosteroids exert both beneficial and harmful effects. High-dose corticosteroid therapy protects the myocardium from ischemic injury10,11. However, the subsequent development of cardiac rupture, which has been attributed to the genomic inhibitory effects of GR on wound healing and cardiomyocyte remodeling12, has limited the use of corticosteroids in acute myocardial infarction13. Indeed, corticosteroids are no longer used in acute myocardial ischemia because of its potential adverse effects on the cardiovascular system; many of which are observed with chronic use and are probably mediated by genomic mechanisms. Therefore, defining the nuclear and non-nuclear actions of GR could have important therapeutic implications; particularly in the development of selective GR modulators, which could distinguish between the beneficial and harmful effects of corticosteroids.
An important endogenous mediator of cardiovascular protection is endothelium-derived nitric oxide (NO)14. NO produced by endothelial NO synthase (eNOS) possesses anti-inflammatory, anti-atherogenic and anti-ischemic properties15,16. Transgenic mice overexpressing eNOS show decreased leukocyte accumulation and reduced vascular lesion formation following vascular injury17. Conversely, mice with targeted disruption of eNOS (Nos3−/− mice) exhibit increased vascular inflammation18 and larger cerebral infarctions19 following ischemic insult. These studies suggest that factors that increase eNOS activity could have beneficial effects in cardiovascular disease.
Non-transcriptional activation of eNOS by corticosteroids
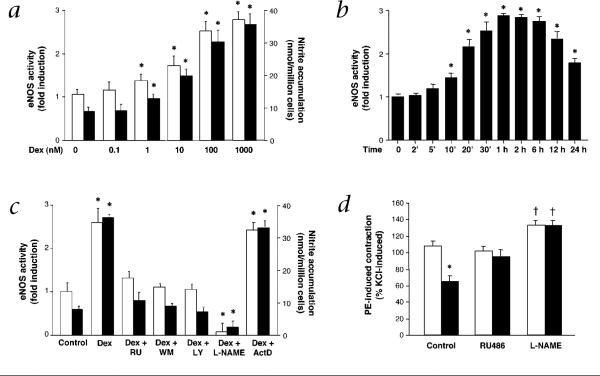
Treatment of human endothelial cells with the corticosteroid, dexamethasone (Dex), stimulated eNOS activity in a concentration-dependent manner, with a maximal 2.7-fold increase occurring at a concentration of 100 nM (Fig. 1a). The increase in eNOS activity by Dex was significant after 10 min of stimulation and peaked at 30−60 minutes before gradually decreasing at 24 hours (Fig. 1b). This increase was completely blocked by cotreatment with the GR antagonist, RU486. Furthermore, the activation of eNOS by Dex was inhibited by the PI3K inhibitors, wortmannin and LY294002 and by the eNOS inhibitor, NG-nitro-l-arginine methylester (L-NAME), but not by the transcriptional inhibitor, actinomycin D (Fig. 1c). These results correlated with changes in NO production as measured by nitrite accumulation (Fig. 1a and c) and were comparable with the effects of other corticosteroids such as corticosterone and hydrocortisol (data not shown). Indeed, the activation of eNOS by Dex correlated with Dex-induced NO-dependent vasorelaxation (Fig. 1d).
Fig. 1.
Non-transcriptional activation of eNOS by corticosteroids. a, Concentration-dependent effect of Dex on eNOS activity (□) and NO production (■) as measured by nitrite levels at 30 min. Values are means ± s.e.m.; n = 3; *, P < 0.05 compared with untreated condition. b, Time-dependent effect of Dex (100 nM) on eNOS activity. Values are means ± s.e.m.; n = 3; *, P < 0.05 compared with time 0. c, Effects of Dex with and without RU486 (RU), wortmannin (WM), LY294002 (LY), L-NAME or actinomycin D (ActD) on eNOS activity (□) and NO production (■) at 30 min. Values are means ± s.e.m.; n = 4; *, P < 0.05 compared with control. d, Effects of saline (□) or Dex (1 μM) (■) on phenylephrine (PE; 1 μM)-induced vascular contraction (% KCl-induced contraction), with and without RU486 or L-NAME. Values are means ± s.e.m.; n = 4−6; *, P < 0.05 compared with saline; †, P < 0.05 compared with control.
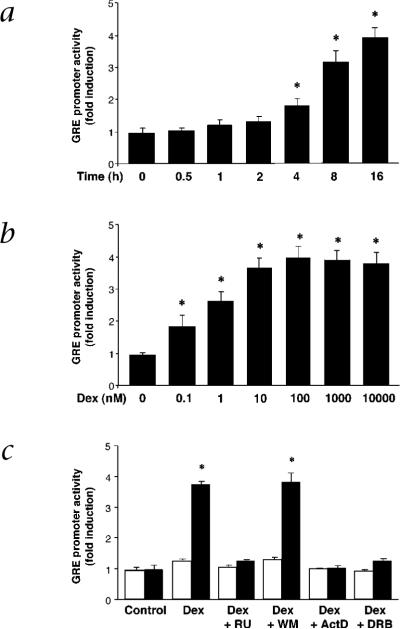
Using a minimal promoter construct containing GRE consensus sequences6, we found that Dex induced GRE-mediated gene transcription starting at about four hours after stimulation (Fig. 2a). Furthermore, relatively lower concentrations of Dex were able to maximally induce GRE promoter activity (10 nM) (Fig. 2b). The effect of Dex on GRE promoter activity was mediated by the nuclear effects of GR given that RU486, actinomycin D and the RNA polymerase inhibitor 5,6-dichlorobenzimidazole riboside completely blocked Dex-induced GRE promoter activity (Fig. 2c). However, Dex-induced GRE promoter activity was not blocked by wortmannin. These findings suggest that, in contrast to GRE-mediated effects, the activation of eNOS involves the rapid, non-transcriptional effects of GR.
Fig. 2.
Activation of GRE-mediated gene transcription by corticosteroids. a, Time-dependent effect of Dex (100 nM) on GRE promoter activity. b, Concentration-dependent effect of Dex on GRE promoter activity (fold induction) at 16 h. Values are means ± s.e.m.; n = 4; *, P < 0.05 compared with time 0 or untreated condition. c, Effects of Dex with and without RU, WM, ActD or DRB on pTAL-Luc (□) and pGRE-Luc (■) activities at 16 h. Values are means ± s.e.m.; n = 3; *, P < 0.05 compared with control (untreated).
Activation of PI3K/Akt pathway by corticosteroids
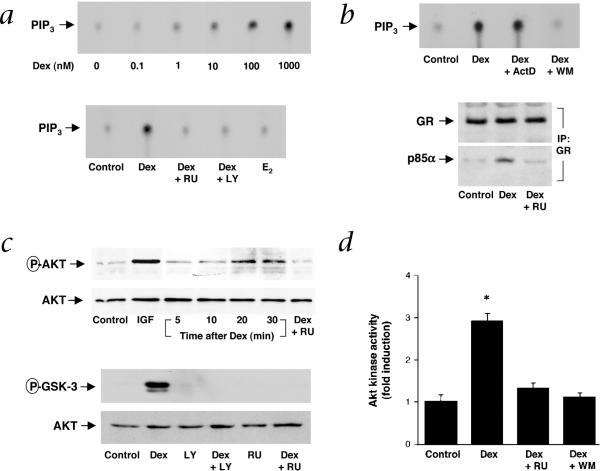
PI3K catalyzes the formation of phosphatidylinositol 3,4,5-triphosphate (PtdIns-3,4,5-P3)20, which leads to the recruitment of pleckstrin homology (PH)-containing proteins such as phosphatidyl-dependent protein kinases21 and protein kinase Akt (ref. 22). Stimulation with Dex increased GR-associated PI3K activity by more than four-fold in a concentration-dependent manner, an effect which was blocked by RU486, LY294002 and wortmannin, but not by actinomycin D (Fig. 3a and b). As a control, estrogen, which stimulates estrogen receptor (ER)-associated PI3K activity23, had no effect on GR-associated PI3K activity (Fig. 3a), suggesting specificity in steroid hormone receptor interaction with PI3K. Indeed, similarly to ER, GR associates with the regulatory subunit of PI3K, p85α, in a ligand-dependent manner (Fig. 3b).
Fig. 3.
Activation of PI3K/Akt pathway by corticosteroids. a, Thin layer chromatography blots show concentration-dependent effect of Dex with and without RU or LY, and 17β-estradiol (E2; 100 nM), on GR-associated PI3K activity. Experiments performed 3 times with comparable results. b, Effect of ActD and WM on Dex-stimulated PI3K activity and RU on Dex-induced GR-p85α interaction. Following treatment with Dex (100 nM, 20 min), endothelial-cell lysates were immunoprecipitated with GR antibody and then immunoblotted for GR and p85α. 3 experiments yielded similar results. c, Effect of Dex (time-course) or insulin growth factor (IGF; 50 ng/ml, 5 min), with and without RU and LY (at 20 min time point) on Akt and GSK-3 phosphorylation. Experiments performed at least 3 times with similar results. d, Effects of Dex with and without RU or WM on Akt kinase activity. Values are means ± s.e.m.; n = 3, *, P < 0.05 compared with control.
To determine whether corticosteroids can activate Akt, we measured Akt phosphorylation and, in turn, the ability of Akt to phosphorylate its downstream target, glycogen synthase kinase (GSK)-3 (ref. 24). In a delayed manner, Dex increased Akt phosphorylation with peak phosphorylation occurring between 20 and 30 minutes, which was blocked by cotreatment with RU486 (Fig. 3c). As a control, insulin growth factor (IGF), a known activator of PI3K, rapidly increased Akt phosphorylation within five minutes. Phosphorylation of Akt by Dex was associated with phosphorylation of GSK-3, an effect that was completely blocked by LY294002 and RU486. Furthermore, treatment of endothelial cells with Dex increased Akt activity by three-fold, as measured by a peptide containing Akt phosphorylation consensus sequence (Fig. 3d). These findings indicate that corticosteroids can functionally activate downstream targets of PI3K such as Akt and GSK-3. Indeed, Akt, which is activated in response to vascular endothelial growth factor (VEGF), insulin and shear stress, has been shown to directly phosphorylate and activate eNOS via PI3K (refs. 25,26).
Vascular protective effects mediated by PI3K and eNOS
To investigate the physiological significance of Dex on eNOS activity, we studied its effect on ischemia and reperfusion (I/R) injury in mouse cremasteric microvessels. Vascular inflammation following I/R is marked by decreased leukocyte rolling velocity and increased leukocyte adhesion to the vascular endothelium; both processes are inhibited by NO (ref. 27). This model of vascular inflammation, therefore, is useful in determining whether the activation of eNOS by corticosteroids is physiologically important.
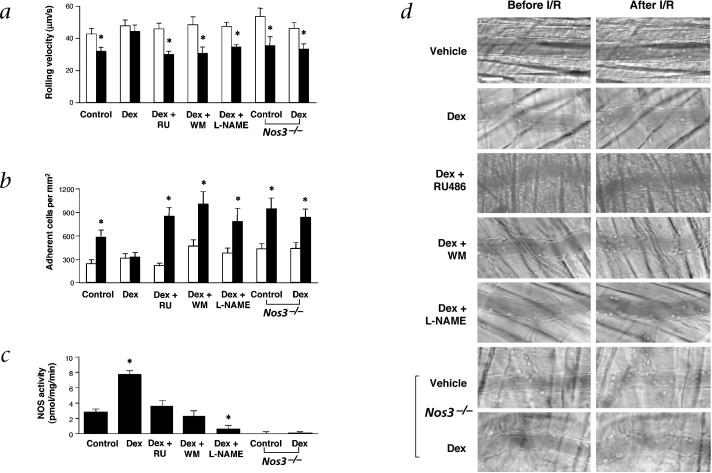
Under baseline conditions, leukocytes roll but few adhere to the endothelium of cremaster venules. Treatment with Dex did not affect leukocyte rolling velocity or the number of adherent leukocytes before ischemia. In wild-type mice following I/R, the leukocyte rolling velocity was reduced by 26% (from a mean velocity of 42 ± 3 to 30 ± 2 μm/s; P < 0.05) (Fig. 4a) and the number of adherent leukocytes was increased by 3.2-fold (from 240 to 578 cells/mm2; P < 0.01) (Fig. 4b). However, acute treatment with Dex completely prevented the decrease in leukocyte rolling velocity and blocked the increase in leukocyte adhesion following I/R (Fig. 4a and b).
Fig. 4.
Vascular protective effects of corticosteroids. a–c, Effects of acute administration of Dex with and without RU, WM, or L-NAME on leukocyte rolling velocity (a) and leukocyte adherence (b); before (□) and after (■) I/R in wild-type and Nos3−/− mice, and cremasteric NOS activity (c). Values are means ± s.e.m.; n = 8−19 venules per group; *, P < 0.05 compared with vehicle (control). d, Representative video images showing the same venules before and after I/R with the indicated treatments in wild-type and Nos3−/− mice. Scale bar, 40 μm.
Treatment with Dex increased NOS activity in the cremaster by 3-fold (Fig. 4c). However, the expression of eNOS and neuronal NOS (nNOS) was unchanged and the expression of inducible NOS (iNOS) was not detected under these conditions23. To demonstrate that the vascular protective effect of corticosteroids was due to increases in NOS activity, the cremasters of Dex-treated mice were superfused with L-NAME during I/R. Cotreatment with L-NAME inhibited Dex-stimulated eNOS activity below basal levels. Following I/R, L-NAME blocked the effects of Dex by decreasing median leukocyte rolling velocity by 25% (Fig. 4a) and increasing leukocyte adhesion by 2.1-fold (Fig. 4b). Consistent with these findings, Nos3−/− mice treated with Dex showed no vascular protective effects compared with control mice as median leukocyte rolling velocity was reduced by 27% and firm leukocyte adhesion was increased by 2.3-fold following I/R.
To determine the importance of GR and PI3K in eNOS activation by corticosteroids in vivo, RU486 and wortmannin were administered to Dex-treated mice. Following I/R, RU486 and wortmannin completely blocked Dex-stimulated cremasteric NOS activity (Fig. 4c). Furthermore, the median leukocyte rolling velocities were similarily decreased by 35% (Fig. 4a) and the numbers of adherent leukocytes in both groups were increased by 2.2-fold (Fig. 4b). Treatment with RU486 alone did not increase NOS activity or have any effect on leukocyte rolling velocity and adhesion compared with control, suggesting that physiological or endogenous levels of corticosteroids had little or no effect on basal cremasteric NOS activity and leukocyte adhesion after I/R. Representative pairs of videomicrographs in wild-type and Nos3−/− mice for each treatment condition are shown (Fig. 4d).
Myocardial protective effects mediated by eNOS
To determine whether the beneficial effects of corticosteroids extend to tissue protection, we used a murine model of transient myocardial ischemia to assess whether acute administration of corticosteroids can limit myocardial infarct size, and if so, whether this effect requires eNOS. Transient ischemia was accomplished by occluding the left anterior descending artery (LAD) for 30 minutes followed by reperfusion for 24 hours. Under anesthesia, the baseline heart rate before LAD occlusion was significantly lower in the Dex-treated group than in the control group; however, this difference in heart rate was no longer apparent during and after I/R (Table 1).
Table 1.
Physiological parameters and myocardial infarct sizes in C57Bl/6 mice
| Control (n = 8) |
Dex (n = 8) |
Dex + RU486 (n = 8) |
Dex + L-NAME (n = 8) |
|
|---|---|---|---|---|
| HR (pre) | 395 ± 9 | 346 ± 16* | 425 ± 13 | 374 ± 13 |
| HR (dur) | 423 ± 12 | 403 ± 20 | 468 ± 17* | 401 ± 12 |
| HR (post) | 436 ± 17 | 408 ± 9 | 468 ± 13 | 405 ± 9 |
| Heart/BWT (%) | 0.48 ± 0.01 | 0.46 ± 0.01 | 0.51 ± 0.01 | 0.48 ± 0.02 |
| LV/BWT (%) | 0.35 ± 0.01 | 0.32 ± 0.01* | 0.39 ± 0.018 | 0.36 ± 0.02 |
| RR (% LV) | 35.6 ± 1.7 | 36.2 ± 3.3 | 40.5 ± 2.5 | 34.5 ± 2.9 |
| IF/LV (%) | 19.2 ± 2.1 | 12.2 ± 2.4* | 20.7 ± 2.0 | 18.5 ± 2.8 |
Control, untreated; HR, heart rate before (pre), during (dur) and after (post) 30 min of ischemia; BWT, body weight; LV, left ventricle; RR, region at risk for ischemia; IF, infarct area. Values are means ± s.e.m.
P < 0.05 versus control group.
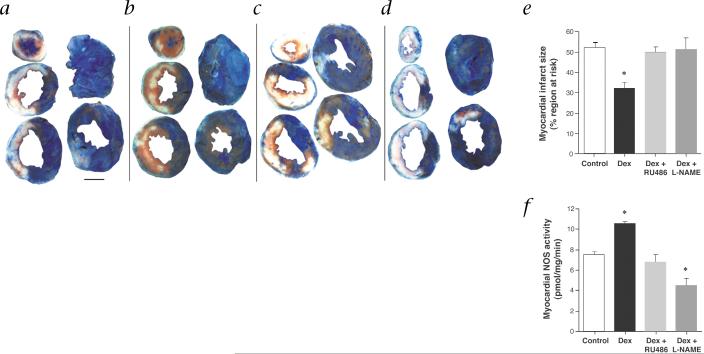
Phthalo-blue staining demonstrated that the size of the region at risk for ischemia was similar for all treatment groups. However, infarct size as a fraction of risk region was 38% smaller in Dex-treated mice compared with control mice (Table 1; Fig. 5a–e). Dex-treated mice also exhibited decreased tissue edema as suggested by the reduced ratio of LV mass to body weight. The myocardial protective effects of Dex were completely reversed by cotreatment with RU486 or L-NAME, indicating a GR-mediated NO-dependent mechanism. Administration of L-NAME alone, however, had no appreciable effect on infarct size at the concentration used. When NOS activity was measured in heart tissues from similarly treated mice, we found that treatment with Dex increased basal NOS activity by 39%, which was completely blocked by cotreatment with RU486 and L-NAME (Fig. 5f).
Fig. 5.
NO-mediated cardioprotective effects of corticosteroids. a–d, TTC- and phthalo blue–stained, short-axis tissue sections of hearts removed from representative mice that were injected before coronary occlusion with PBS (control; a), Dex (b), Dex + RU486 (c), or Dex + L-NAME (d). White areas, infarcted tissue; blue areas, non-ischemic tissue; red areas, salvaged (viable) tissue within the risk region. Scale bar, 2 mm. e, Myocardial infarct size as a percentage of region at risk for infarction in C57Bl/6 mice treated with PBS (control), Dex, Dex + RU486, or Dex + L-NAME. Values are means ± s.e.m.; n = 8 per group; *, P < 0.05 compared with control. f, Corresponding NOS activities in hearts from mice treated similarly as in e as determined 90 min after the administration of Dex. Values are means ± s.e.m.; n = 5 per group; *, P < 0.05 compared with control.
Discussion
Our finding that dexamethasone exerts cardiovascular protective effects is consistent with previous studies showing that acute corticosteroid therapy reduced myocardial ischemic injury in animals10,11 and improved short-term survival in humans following acute myocardial infarction28. Similar to these earlier studies, we found that pharmacological concentrations of corticosteroids were required to acutely protect the myocardium from ischemic injury, whereas a lower or more physiologically-relevant concentration of dexamethasone (0.2 mg/kg) had no protective effects. Indeed, the peak serum dexamethasone level following high-dose administration corresponded to the concentrations required to maximally stimulate PI3K and eNOS in vitro (data not shown). This concentration is approximately 15-fold higher than what is required to maximally induce GRE-mediated responses5,6. These high plasma concentrations of corticosteroids have been observed in patients receiving high-dose corticosteroids for rheumatoid arthritis29 and congenital adrenal insufficiency30. However, it can still be argued whether corticosteroids could be beneficial in the clinical setting of acute myocardial infarction given previous experiences with many adverse side effects, especially when administered chronically.
The extent of myocardial tissue damage resulting from I/R injury is directly related to the extent of the inflammatory response attributed, in part, to leukocyte recruitment31. Although the inhibitory effects of corticosteroids on endothelial-leukocyte adhesion have been shown to be mediated by GR (ref. 4), the mechanism by which GR inhibits endothelialleukocyte interaction is not known. We found that acute administration of dexamethasone induced vascular relaxation in vitro and protected the cremaster microcirculation from I/R injury through a NO-dependent mechanism. Indeed, dexamethasone had no vascular protective effects in Nos3−/− mice, indicating that eNOS is the NOS isoform mediating these protective effects. Our results therefore suggest that the mechanism by which corticosteroids reduce myocardial infarction size is predominantly through NO-mediated vascular protection.
Although the activation of PI3K and eNOS by corticosteroids requires GR, no GRE-mediated gene transcription is necessary for PI3K activation. This non-transcriptional effect of GR is similar to that of the estrogen receptor ERα, which interacts with and activates PI3K in a ligand-dependent manner23. An important downstream target of PI3K is protein kinase Akt, which has been shown to directly phosphorylate and activate eNOS (refs. 25,26). Indeed, overexpression of a dominant-negative Akt mutant in endothelial cells blocks estrogen- and VEGF-stimulated eNOS activity (refs. 23,26). Notably, when high-dose corticosteroids were given to animals and humans with acute myocardial infarction, they caused rapid and transient decrease in blood pressure and systemic vascular resistance10,32. It is possible that this previously unrecognized rapid vascular effect of corticosteroids might have been mediated by NO.
Although GR regulates gene expression by DNA-binding33, recent studies suggest that many anti-inflammatory effects of corticosteroid do not require binding to GRE (refs. 33,34). For example, corticosteroids inhibit prostaglandin synthesis7,8 and activation of pro-inflammatory transcription factor NF-κB by non-GRE-dependent mechanisms9. Furthermore, transgenic mice expressing a mutant GR (GRDim), which cannot dimerize or bind GRE, exhibit intact immunosuppressive responses to corticosteroids35. Mice deficient in GR, however, do not exhibit normal anti-inflammatory response to corticosteroids and die shortly after birth due to inadequate lung maturation36. Thus, the non-DNA binding effects of GR are necessary for survival and seem to be also important for mediating some of the anti-inflammatory responses to corticosteroids.
In summary, the non-transcriptional activation of eNOS by GR represents a physiologically important signaling pathway by which corticosteroids exert their rapid, anti-inflammatory effects. Because NO also has vasodilatory14, anti-thrombotic37 and anti-proliferative properties16, the beneficial effects of corticosteroids in cardiovascular disease may extend beyond their anti-inflammatory properties. In addition, by linking GR to PI3K, the biological effects of corticosteroids are considerably broadened since PI3K is known to mediate various cellular functions20. It remains to be determined how GR recruits and activates PI3K and whether selective GR modulators, which preferentially activate this pathway, could provide even greater benefits in cardiovascular disease.
Methods
Cell cultures
Human vascular endothelial cells were cultured in phenol red-free Medium 199 (Gibco BRL, Life Technologies, Gaithersburg, Maryland) as described15. Steroid hormones and growth factors were removed from FBS by charcoal-stripping.
eNOS activity and NO production assay
eNOS activity and NO production were measured at 20 min after stimulation. eNOS activity was determined by conversion of [3H]arginine to [3H]citrulline38 and NO production was determined by a modified nitrite assay using 2,3-diaminonaphthalene39.
Vascular ring bioassay
After acute administration of Dex (20 mg/kg, intraperitoneally (i.p.)) or saline, mice were killed and aortic rings were quickly mounted on an isometric myograph (610M, Danish Myo Technology, Aarhus, Denmark) in physiological solution (composition, mM: NaCl, 118; KCl, 4.6; NaHCO3, 25; MgSO4, 1.2; KH2PO4, 1.2; CaCl2, 1.25; glucose, 10; EDTA, 0.025; pH 7.4 at 37 °C) with or without Dex (1 μM). After equilibration, contractile responses (that is, wall tension) were recorded as described40. Aortic rings were exposed successively to a 100 mM KCl-depolarizing solution, and after washout, exposed to cumulative concentrations of phenylephrine (0.1 nM – 1 μM). In some studies, aortic rings were immersed in RU486 (1 μM) for 10 min before Dex treatment or in L-NAME (100 μM) for 30 min before phenylephrine treatment. There was no difference in KCl-induced maximal contraction between control and Dex-treated aortic rings, with or without RU486. All of the animal experimentation protocols were approved and performed according to institutional guidelines.
PI3K assay
The GR antibody (1 μg, Santa Cruz Biotechnology, Santa Cruz, California) was added to equal amounts of cell lysates (500 μg) in 1 ml of lysis buffer for at least 1 h at 4 °C with gentle rocking. Afterwards, 40 μl of 1:1 Protein A-agarose was added and the entire mixture was rocked gently for another 1 h at 4 °C. The mixture was then centrifuged at 13,000g for 5 min at 4 °C. PI3K activity in the immunoprecipitate was assayed as described41.
Akt phosphorylation and kinase assay
Phosphorylation of Akt (Ser473) and GSK-3α/β (Ser21/9) was determined by immunoblotting with specific antibodies that recognize the phosphorylated forms of these proteins (Cell Signaling Technology, Beverly, Massachusetts). The Akt kinase activity was determined using the PKB/Akt kinase assay kit (Upstate Biotechnology, Lake Placid, New York).
Transfection assay
Using the minimal promoter construct, pTAL-Luc, a GRE consensus sequence (5′-GGTACATTTTGTTCTAGAACAAAATGTACCGGTACATTTTGTTCT-3′)6 was inserted upstream of the firefly luciferase reporter (pGRE-Luc, Clontech, Palo Alto, California). Bovine aortic endothelial cells were transfected with pTAL-Luc or pGRE-Luc (4 μg of each) using the Lipofectamine reagent system (Gibco BRL)39. The relative luciferase activity (fold induction) was normalized to either β-galactosidase activity (cotransfected pCMV.β-gal) or protein concentration.
Ischemia and reperfusion model of vascular injury
8−12-wk-old, 20−26 g, male C57Bl/6 mice (Hilltop, Scottdale, Pennsylvania) or Nos3−/− mice42 were injected with 40 mg/kg of Dex i.p. (American Regent Laboratories, Shirley, New York) 1 h before experiments. Cremaster muscle from anesthetized mice was studied by intravital microscopy as described23. Ischemia was induced by applying pressure to supplying arteries just sufficient to stop blood flow for 30 min. In some experiments, RU486 (160 mg/kg, i.p.) was given 30 and 10 min before ischemia, and wortmannin (100 nM) or L-NAME (0.1 mM) was applied to the cremaster muscle during the ischemic period only. The pressure was released for reperfusion, and the same vessels were recorded in each animal before and after I/R. The velocities of 25 leukocytes were measured in 8−19 venules per condition before and after I/R. As there is a linear relation between leukocyte rolling velocity and wall-shear rate43, rolling velocities were corrected for shear rates. The adhesion of leukocytes was examined in the same vessel before and after I/R.
Ischemia and reperfusion model of myocardial infarction
Myocardial infarction was studied in 28−32 g male C57Bl/6 mice by occluding the left anterior descending coronary artery (LAD) for 30 min as described44. Mice were injected with PBS (100 μl, i.p., control) or Dex (16 mg/kg, i.p.) 1 h before LAD occlusion. In some experiments, RU486 (140 mg/kg, i.p.) was administered in 2 equally divided doses, 30 min and 10 min before injection of Dex. In other experiments, L-NAME (15 mg/kg, intravenously (i.v.)) was administered 50 min after injection of Dex (that is, 10 min before LAD occlusion). After 24 h of reperfusion, the hearts were perfused with 2−3 ml of 0.9% sodium chloride and 3−4 ml of 1.0% 2,2,5-triphenyltetrazolium chloride (TTC) in phosphate buffer (pH 7.4, 37 °C). After TTC staining, the LAD was re-occluded and the hearts were perfused with 2−3 ml of 10% phthalo blue to define the non-ischemic zone. The left ventricle was cut into 5−7 transverse slices, which were fixed in 10% neutral buffered formalin. Each slice was weighed and photographed from both sides using a microscope equipped with a high-resolution digital camera (DVC-1300, DVC, Austin, Texas). Images were analyzed by computer-assisted planimetry.
Acknowledgments
This study was supported by the National Institutes of Health grants HL70274 and HL48743 (to J.K.L.), HL62602 (to M.A.M.), HL54136 (to K.L.), HL58582 (to B.A.F.), HL67574 (to C.M.H.), the American Heart Association Established Investigator Grants (to J.K.L. and B.A.F.) and Bugher Foundation Award (to J.K.L.), and the Mary Horrigan Connors Center for Women's Health. T.S. is supported by the Scuola Superiore di Studi e di Perfezionamento ‘S. Anna’ and the University of Pisa. J.C.P. is a recipient of an American Heart Association New England Affiliate Beginning Grant-in-Aid Award. F.P.L. is a recipient of a grant from the Deutsche Forschungsgemeinschaft. M.C.R. is a recipient of a Swiss National Science Foundation Fellowship.
Footnotes
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Barnes PJ. Inhaled glucocorticoids for asthma. N. Engl. J. Med. 1995;332:868–875. doi: 10.1056/NEJM199503303321307. [DOI] [PubMed] [Google Scholar]
- 2.McGregor AM. Immunoendocrine interactions and autoimmunity. N. Engl. J. Med. 1990;322:1739–1741. doi: 10.1056/NEJM199006143222409. [DOI] [PubMed] [Google Scholar]
- 3.Kirwan JR. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The Arthritis and Rheumatism Council Low-Dose Glucocorticoid Study Group. N. Engl. J. Med. 1995;333:142–146. doi: 10.1056/NEJM199507203330302. [DOI] [PubMed] [Google Scholar]
- 4.Cronstein BN, Kimmel SC, Levin RI, Martiniuk F, Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: The glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA. 1992;89:9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangelsdorf DJ, et al. The nuclear receptor superfamily: The second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker PB, Gloss B, Schmid W, Strahle U, Schutz G. In vivo protein-DNA interactions in a glucocorticoid response element require the presence of the hormone. Nature. 1986;324:686–688. doi: 10.1038/324686a0. [DOI] [PubMed] [Google Scholar]
- 7.Flower RJ, Blackwell GJ. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature. 1979;278:456–459. doi: 10.1038/278456a0. [DOI] [PubMed] [Google Scholar]
- 8.Lewis GP, Piper PJ. Inhibition of release of prostaglandins as an explanation of some of the actions of anti-inflammatory corticosteroids. Nature. 1975;254:308–311. doi: 10.1038/254308a0. [DOI] [PubMed] [Google Scholar]
- 9.Brostjan C, et al. Glucocorticoid-mediated repression of NFκB activity in endothelial cells does not involve induction of IκBα synthesis. J. Biol. Chem. 1996;271:19612–19616. doi: 10.1074/jbc.271.32.19612. [DOI] [PubMed] [Google Scholar]
- 10.Libby P, Maroko PR, Bloor CM, Sobel BE, Braunwald E. Reduction of experimental myocardial infarct size by corticosteroid administration. J. Clin. Invest. 1973;52:599–607. doi: 10.1172/JCI107221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spath JA, Jr, Lane DL, Lefer AM. Protective action of methylprednisolone on the myocardium during experimental myocardial ischemia in the cat. Circ. Res. 1974;35:44–51. doi: 10.1161/01.res.35.1.44. [DOI] [PubMed] [Google Scholar]
- 12.Hammerman H, Schoen FJ, Braunwald E, Kloner RA. Drug-induced expansion of infarct: Morphologic and functional correlations. Circulation. 1984;69:611–617. doi: 10.1161/01.cir.69.3.611. [DOI] [PubMed] [Google Scholar]
- 13.Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can. J. Cardiol. 2000;16:505–511. [PubMed] [Google Scholar]
- 14.Loscalzo J. Nitric oxide and vascular disease. N. Engl. J. Med. 1995;333:251–253. doi: 10.1056/NEJM199507273330410. [DOI] [PubMed] [Google Scholar]
- 15.De Caterina R, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J. Clin. Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida A, Sasaguri T, Kosaka C, Nojima H, Ogata J. Induction of the cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1) by nitric oxide-generating vasodilator in vascular smooth muscle cells. J. Biol. Chem. 1997;272:10050–10057. doi: 10.1074/jbc.272.15.10050. [DOI] [PubMed] [Google Scholar]
- 17.Kawashima S, et al. Endothelial NO synthase overexpression inhibits lesion formation in mouse model of vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2001;21:201–207. doi: 10.1161/01.atv.21.2.201. [DOI] [PubMed] [Google Scholar]
- 18.Moroi M, et al. Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J. Clin. Invest. 1998;101:1225–1232. doi: 10.1172/JCI1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Z, et al. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-l-arginine. J. Cereb. Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 21.Stephens L, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 22.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 23.Simoncini T, et al. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 25.Dimmeler S, et al. Activation of nitric oxide synthase in endothelial cells by Akt- dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 26.Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubes P, Suzuki M, Granger DN. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA. 1991;88:4651–465. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barzilai D, et al. Use of hydrocortisone in the treatment of acute myocardial infarction. Summary of a clinical trial in 446 patients. Chest. 1972;61:488–491. doi: 10.1378/chest.61.5.488. [DOI] [PubMed] [Google Scholar]
- 29.Wenting-Van Wijk MJ, Blankenstein MA, Lafeber FP, Bijlsma JW. Relation of plasma dexamethasone to clinical response. Clin. Exp. Rheumatol. 1999;17:305–312. [PubMed] [Google Scholar]
- 30.Charmandari E, Johnston A, Brook CG, Hindmarsh PC. Bioavailability of oral hydrocortisone in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Endocrinol. 2001;169:65–70. doi: 10.1677/joe.0.1690065. [DOI] [PubMed] [Google Scholar]
- 31.Frangogiannis NG, et al. Cytokines and the microcirculation in ischemia and reperfusion. J. Mol. Cell. Cardiol. 1998;30:2567–2576. doi: 10.1006/jmcc.1998.0829. [DOI] [PubMed] [Google Scholar]
- 32.Vyden JK, et al. Effects of methylprednisolone administration in acute myocardial infarction. Am. J. Cardiol. 1974;34:677–686. doi: 10.1016/0002-9149(74)90157-x. [DOI] [PubMed] [Google Scholar]
- 33.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: Many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 34.Karin M. New twists in gene regulation by glucocorticoid receptor: Is DNA binding dispensable? Cell. 1998;93:487–490. doi: 10.1016/s0092-8674(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 35.Reichardt HM, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 36.Cole TJ, et al. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–16021. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 37.Radomski MW, Palmer RM, Moncada S. An l-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. USA. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laufs U, et al. Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J. Clin. Invest. 2000;106:15–24. doi: 10.1172/JCI9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 40.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 41.Walsh JP, Caldwell KK, Majerus PW. Formation of phosphatidylinositol 3-phosphate by isomerization from phosphatidylinositol 4-phosphate. Proc. Natl. Acad. Sci. USA. 1991;88:9184–9187. doi: 10.1073/pnas.88.20.9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shesely EG, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 1996;93:13176–131781. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damiano ER, Westheider J, Tozeren A, Ley K. Variation in the velocity, deformation, and adhesion energy density of leukocytes rolling within venules. Circ. Res. 1996;79:1122–1130. doi: 10.1161/01.res.79.6.1122. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z, Zingarelli B, Szabo C. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation. 2000;101:1019–1026. doi: 10.1161/01.cir.101.9.1019. [DOI] [PubMed] [Google Scholar]