Abstract
Background
Suboptimal drug exposure attributable to physician-directed dosage reductions of pegylated interferon and/or ribavirin are associated with decreased sustained virologic response rates. However, data are limited with regard to suboptimal drug exposure that is attributable to missed doses by patients with chronic hepatitis C virus (HCV) infection. We examined the relationship between adherence to pegylated interferon and ribavirin therapy, measured by pharmacy refill, and HCV suppression during the initial 12 weeks of therapy.
Methods
We conducted a cohort study involving 188 patients with chronic HCV infection who were treated with pegylated interferon plus ribavirin. Adherence was calculated using pharmacy refill data and could exceed 100%. The primary outcome was decrease in HCV load at 12 weeks; early virologic response was a secondary outcome. Mixed-effects regression models estimated the association between adherence and HCV suppression during the initial 12 weeks. Subanalyses were performed among patients who received optimal weight-based dosages.
Results
The mean decrease in HCV load at 12 weeks was 0.66 log IU/mL greater for patients with ⩾85% adherence than for those with <85% adherence (3.23 vs. 2.57 log IU/mL; P = 04). When patients who received a suboptimal ribavirin dosage were excluded, the decrease in viral load was 1.00 log IU/mL greater for persons with <85% adherence (3.32 vs. 2.32 log IU/mL; P = 01). Early virologic response was more common among patients with ⩾85% adherence than it was among those with <85% adherence to treatment with pegylated interferon (73% vs. 29%; P = 02) and ribavirin (73% vs. 55%; P = 08).
Conclusions
Adherence of ⩾85% to pegylated interferon and ribavirin treatment was associated with increased HCV suppression. Decreases in HCV load became greater when patients with ⩾85% adherence to their regimen continued to receive their recommended weight-based ribavirin dosage.
Combination therapy with pegylated IFN alfa–2a or pegylated IFN alfa–2b plus ribavirin has led to substantial improvements in sustained virologic response rates among patients with chronic hepatitis C virus (HCV) infection [1–3]. However, the treatment requires a moderately complex regimen that includes weekly subcutaneous injections of pegylated IFN, twice-daily oral administrations of ribavirin, and frequent monitoring of adverse effects and laboratory results. Therefore, to date, relatively selective populations have been the focus of treatment efforts. However, given the potential for benefit, providers are being encouraged to offer therapy more widely [4]. Unfortunately, as seen with other chronic viral infections that require long-term treatment, particularly HIV infection, adherence to therapy can be poor, which may cause a reduction in treatment response [5, 6].
Decreased drug exposure because of physician-directed reductions in the dosage of pegylated IFN and/or ribavirin (primarily because of hematologic adverse effects) has been shown to result in poorer virologic response rates [7–10]. However, data are limited with regard to suboptimal drug exposure because of missed doses by patients with chronic HCV infection [11]. In particular, there is little information on the relationship between adherence to combination HCV therapy and virologic outcomes during the initial 12 weeks of therapy, the critical period in which a 2-log decrease in HCV RNA level must be observed to justify continuation of treatment [12, 13]. As a result, the levels of adherence to pegylated IFN and ribavirin therapy that are required for maximal virologic suppression remain unknown. These data are critical for clinicians who are evaluating patients who are not responding to HCV treatment, for determining risk factors for poor adherence, and for designing interventions to improve adherence to HCV therapy.
A pharmacy refill measure of adherence, which compares actual refills with expected refills, might be particularly advantageous for monitoring adherence to HCV therapy, because it is a reliable and valid indicator of actual patient adherence [14]. Pharmacy refill data are relatively simple to collect, do not require patient recall, and permit individual determination of adherence to pegylated IFN and ribavirin therapy [14].
We therefore evaluated the relationship between adherence to a pegylated IFN and ribavirin regimen, measured using pharmacy refill data, and viral suppression during the initial 12 weeks of HCV therapy. In addition, given the established importance of weight-based dosing, we conducted subanalyses among patients who maintained optimal weight-based pegylated IFN and ribavirin dosages throughout observation.
PATIENTS AND METHODS
Study design and patients
We performed a retrospective cohort study among patients with chronic HCV infection at the Philadelphia Veterans Affairs Medical Center (PVAMC) who were treated with pegylated IFN alfa–2a or pegylated IFN alfa–2b plus ribavirin. Patients were eligible for inclusion if they: (1) received at least 1 pharmacy refill of pegylated IFN plus ribavirin, (2) had a quantitative HCV load measured prior to initiation of HCV therapy, and (3) underwent quantitative HCV load determination 12 weeks after treatment initiation. Exclusion criteria included the initiation of treatment at another facility and a change in the form of pegylated IFN received. A list of patients who received pegylated IFN plus ribavirin from 1 January 2001 (the approximate date on which pegylated IFN was introduced at the PVAMC) through 31 December 2006 was obtained from the PVAMC’s pharmacy database. All eligible patients were included. Approval was obtained from the Institutional Review Boards of the University of Pennsylvania and the PVAMC.
Main outcome measures
The primary outcome was the decrease from baseline in HCV load (Versant HCV RNA 3.0 Quantitative Assay; Bayer Diagnostics) at 12 weeks after treatment initiation, because current guidelines recommend viral load testing at this time to determine response to therapy and treatment continuation [12, 13]. Early virologic response, defined as a 2-log decrease in viral load from baseline at 12 weeks, was a secondary outcome [1]. To reduce the misclassification of outcomes attributable to variability in HCV load, we required that the week 12 viral load measurement had been obtained between weeks 11 and 13 after treatment initiation.
Data collection
Baseline data were abstracted from the patients’ electronic records from visits closest to but before the start of therapy. HCV loads at week 12 were abstracted from electronic records from visits that occurred from week 11 through week 13.
To obtain refills, patients with chronic HCV infection at PVAMC must initiate contact with the PVAMC pharmacy. Pegylated IFN and ribavirin are dispensed monthly, and the dispensing of refills of pegylated IFN and ribavirin is not linked (i.e., ribavirin is not automatically filled when a refill for pegylated IFN is requested and vice versa). Information on filled prescriptions is recorded electronically. This database was accessed to determine: (1) prescription fill dates, (2) the number of pegylated IFN syringes and ribavirin pills dispensed with each prescription, (3) the prescribed frequency of administration, and (4) medication dosages.
Pegylated IFN alfa–2a dosages were categorized as either 180 μg/week or <180 μg/week, and pegylated IFN alfa–2b dosages were categorized as <1.1, 1.2–1.4, or >1.4 μg/kg/week, to allow for clinician rounding of patient body weight. For patients infected with HCV genotypes 1 or 4, the recommended dosage of ribavirin was considered to be 1000 mg/day for patients ≤75 kg and 1200 mg/day for patients >75 kg; for patients infected with HCV genotypes 2 or 3, the recommended dosage was considered to be 800 mg/day, irrespective of weight [12, 13]. Patients’ progress notes were examined for reductions in pegylated IFN and ribavirin dosages during the initial 12 weeks of therapy. Patients who were not prescribed the recommended dosages either at baseline or during follow-up were classified as having received suboptimal dosages of these medications.
Data analysis
Adherence to treatment with pegylated IFN and ribavirin was calculated separately over a 12-week period by dividing the days’ supply of medications dispensed by the days between the first and last prescription fills (expressed as a percentage) [15]. Therefore, for 30-day prescriptions of anti-HCV medications, such as those dispensed at the PVAMC, 4 prescription fills would cover the 12 contiguous weeks of HCV therapy (figure 1). We did not assess adherence on a month-by-month basis within the 12-week treatment interval, because shorter adherence intervals do not reflect a stable measure of an individual’s medication-taking behavior [16]. For patients who were identified from progress notes as having had their ribavirin dosage reduced but who did not have a prescription rewritten, we averaged the number of pills taken daily during the 30-day prescription period, so that these patients would not be misclassified as having poor adherence to ribavirin treatment. Depending on refill rate, adherence could exceed 100% if patients refilled medications early. Adherence was not truncated at 100%, because we wished to evaluate trends in virologic outcomes at the highest observed levels of adherence and because to do so would have misclassified drug exposure for patients who took extra doses.
Figure 1.
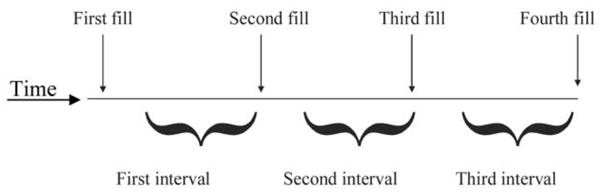
Schematic depiction of the calculation of pharmacy refill adherence for a patient with a 30-day supply of medication per prescription. The number of days of prescribed doses were summed for the 3 intervals; this sum constituted the numerator of the refill-defined adherence variable. The number of days between the first and fourth refills were calculated; this constituted the denominator of the refill-defined adherence variable.
Adherence to pegylated IFN treatment was compared with adherence to ribavirin treatment with use of the paired Student’s t tests. We cross-classified adherence to both medications by adherence level. Virologic outcomes were determined for each of 7 adherence strata: (1) <80%, (2) 81%–84%, (3) 85%–89%, (4) 90%–99%, (5) 100%–109%, (6) 110%–119%, and (7) ⩾120%.
For the primary analysis, we used a mixed-effects regression model to estimate the association between adherence and change in HCV load between baseline and 12 weeks [17]. Potential confounding effects that we evaluated included age, race, and time receiving HCV therapy. The final model included an effect for time, adherence, the interaction between time and adherence, and anti-HCV medication. Analyses were separately stratified by HCV genotype and baseline HCV load. We then examined decreases in HCV load between patients with good adherence and those with poor adherence, excluding patients who received suboptimal pegylated IFN and ribavirin dosages. We compared median adherence to anti-HCV medications between patients with and patients without various characteristics (i.e., HIV infection, depression, posttraumatic stress disorder, and history of alcohol abuse) using Wilcoxon rank-sum tests.
For analyses of the relation between treatment adherence and virologic response, P values were specified a priori to be 1-sided, because it was biologically implausible that increased adherence would increase HCV loads. All other P values were 2-sided. Assuming an SD of HCV load of 0.75 log IU/mL and a 20% rate of nonadherence, 130 patients provided 80% power to detect a ⩾0.5 log IU/mL difference in viral load decrease at week 12 between patients with good and patients with poor adherence.
RESULTS
Patient characteristics
A total of 337 individuals were prescribed treatment with pegylated IFN plus ribavirin during the observation period. Of these, 149 were excluded because they did not meet eligibility criteria (figure 2). Compared with patients who were included in the study, individuals who were excluded were less likely to have a history of alcohol abuse (76 [51%] of 149 vs. 126 [67%] of 188; P = 003) but had a similar prevalence of HIV coinfection (9 [6%] of 149 vs. 15 [8%] of 188; P = 2), depression (52 [35%] of 149 vs. 60 [32%] of 188; P = 6), and posttraumatic stress disorder (28 [19%] of 149 vs. 41 [22%] of 188; P = 5). There were no differences in age, sex, race, history of injection drug use, HCV genotype, and median HCV load. The final study sample included 188 patients (table 1). Included patients were predominantly men, and almost one-half were African American. Two-thirds had a history of alcohol abuse, 32% had depression, and 22% had posttraumatic stress disorder. Approximately 80% were infected with HCV genotype 1, and 57% had a high (>800,000 IU/mL) baseline HCV load.
Figure 2.
Selection of chronic hepatitis C virus (HCV)–infected patients for inclusion in the study.
Table 1.
Baseline characteristics of the study population.
| Patient adherence to pegylated IFN and ribavirin therapy |
||||
|---|---|---|---|---|
| Characteristic | All patients (n = 188) | <85% (n = 22) | ⩾85% (n = 166) | P |
| Age, median years (IQR) | 52 (48–56) | 53 (49–56) | 52 (48–56) | .5 |
| Male sex | 181 (96.3) | 22 (100.0) | 159 (95.8) | .3 |
| Race | ||||
| African American | 91 (48.4) | 13 (59.1) | 78 (47.0) | .5 |
| White | 92 (48.9) | 9 (40.9) | 83 (50.0) | |
| Other | 5 (2.7) | 0 (0.0) | 5 (3.0) | |
| Employment status | ||||
| Employed | 108 (57.4) | 10 (45.5) | 98 (59.0) | .1 |
| Unemployed | 56 (29.8) | 7 (31.8) | 49 (29.5) | |
| Disabled | 18 (9.6) | 5 (22.7) | 13 (7.8) | |
| Retired | 6 (3.2) | 0 (0.0) | 6 (3.6) | |
| Obesity | ||||
| BMI, mean value (IQR) | 29.3 (26.3–32.5) | 29.0 (26.6–30.6) | 29.6 (26.2–32.7) | >.5 |
| HCV transmission risk factora | ||||
| Injection drug use | 124 (66.0) | 12 (54.5) | 112 (67.5) | .2 |
| Unprotected heterosexual sex | 40 (21.3) | 3 (13.6) | 37 (22.3) | .4 |
| Men who have sex with men | 4 (2.1) | 0 (0.0) | 4 (2.4) | .5 |
| Transfusion | 25 (13.3) | 4 (18.2) | 21 (12.7) | .5 |
| Intranasal cocaine use | 70 (37.2) | 10 (45.5) | 60 (36.1) | .4 |
| Other | 30 (16.0) | 3 (13.6) | 27 (16.3) | >.5 |
| HCV load >800,000 IU/mL | 107 (56.9) | 13 (59.1) | 94 (56.6) | >.5 |
| HCV genotype | ||||
| 1 or 4 | 152 (80.9) | 19 (86.4) | 133 (80.1) | .5 |
| 2 or 3 | 36 (19.1) | 3 (13.6) | 33 (19.9) | |
| Fibrosis stage | ||||
| 0–3 | 99 (52.7) | 10 (45.5) | 89 (53.6) | >.5 |
| 4–6 | 34 (18.1) | 5 (22.7) | 29 (17.5) | |
| Unknownb | 55 (29.3) | 7 (31.8) | 48 (28.9) | |
| Chronic hepatitis B virus coinfection | 4 (2.1) | 1 (4.5) | 3 (1.8) | .2 |
| HIV coinfection | 15 (8.0) | 3 (13.6) | 12 (7.2) | >.5 |
| History of psychiatric diagnosis | ||||
| Depression | 60 (31.9) | 7 (31.8) | 53 (31.9) | >.5 |
| Posttraumatic stress disorder | 41 (21.8) | 6 (27.3) | 35 (21.1) | >.5 |
| Schizophrenia | 10 (5.3) | 3 (13.6) | 7 (4.2) | .06 |
| Schizoaffective disorder | 1 (0.5) | 0 (0.0) | 1 (0.6) | >.5 |
| Anxiety disorder | 18 (9.6) | 2 (9.1) | 16 (9.6) | >.5 |
| Bipolar disorder | 12 (6.4) | 2 (9.1) | 10 (6.0) | >.5 |
| Any of the above psychiatric diagnoses | 114 (60.6) | 7 (31.8) | 53 (31.9) | >.5 |
| Alcohol abuse history | 126 (67.0) | 14 (63.6) | 112 (67.5) | >.5 |
| Active methadone use | 10 (5.3) | 1 (4.5) | 9 (5.4) | >.5 |
| No. of medicationsc | ||||
| 0–5 | 123 (65.4) | 10 (45.5) | 113 (68.1) | .04 |
| ⩾6 | 65 (34.6) | 12 (54.5) | 53 (31.9) | |
| No. of outpatient visits in year before HCV therapy initiation | ||||
| 0–10 | 59 (31.4) | 7 (31.8) | 52 (31.3) | >.5 |
| >10 | 129 (68.6) | 15 (68.2) | 114 (68.7) | |
NOTE. Data are no. (%) of patients, unless otherwise indicated. BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); HCV, hepatitis C virus; IQR, interquartile range.
Patients may have had >1 risk factor.
No biopsy was performed.
Number of medications prescribed for any condition in addition to pegylated IFN plus ribavirin (standing and as needed prescriptions).
Medication dosing
One hundred thirty-five patients (72%) received treatment with pegylated IFN alfa–2a; 133 (99%) of these patients initiated treatment with the recommended dosage of 180 μg/week and maintained this dosage through week 12. Fifty-three patients (28%) received treatment with pegylated IFN alfa–2b; 38 (72%) of these patients received ⩾1.4 μg/kg/week at the start of treatment and maintained this dosage through week 12. The remaining 15 patients (28%) received 1.2–1.4 μg/kg/week at the start of treatment and continued to receive this suboptimal dosage through week 12. None of the patients who had suboptimal pegylated IFN exposure received a suboptimal ribavirin dosage.
Among 152 patients infected with HCV genotype 1 or 4, 7 (5%) were prescribed a suboptimal ribavirin dosage. By week 12, 9 additional patients had had their ribavirin dosage decreased; therefore, 16 (11%) of 152 patients infected with genotype 1 or 4 had received a suboptimal ribavirin dosage during the initial 12 weeks of treatment. None of the patients who were prescribed a suboptimal ribavirin dosage received a suboptimal pegylated IFN dosage. All patients infected with HCV genotypes 2 or 3 received the recommended ribavirin dosage throughout the initial 12 weeks of treatment.
Adherence to pegylated IFN and ribavirin treatment
Adherence to pegylated IFN treatment was 73%–142% (median adherence, 104%), with 138 patients (73%) having at least 100% adherence; adherence to ribavirin treatment was 65%–156% (median adherence, 103%), with 128 patients (68%) having at least 100% adherence. Mean adherence to pegylated IFN during the initial 12 weeks of treatment was only slightly higher than that for ribavirin (105% vs. 102%; P = 02). Cross-classification of adherence levels to pegylated IFN and ribavirin showed that adherence level to one medication corresponded in most cases to a similar level of adherence to the other medication (table 2). Thirteen patients (7%) had ⩾90% adherence to pegylated IFN but had <85% adherence to ribavirin. Mean adherence to pegylated IFN alfa–2a was not significantly different than mean adherence to pegylated IFN alfa–2b (105% vs. 104%; P = 4).
Table 2.
Levels of adherence to pegylated IFN and ribavirin treatment among 188 patients with chronic hepatitis C virus infection.
| No. of patients, by pegylated IFN adherence |
||||||
|---|---|---|---|---|---|---|
| Variable | <80% | 80%–84% | 85%–89% | 90%–99% | ⩾100% | Total |
| Ribavirin adherence | ||||||
| <80% | 2 | 0 | 0 | 2 | 7 | 11 |
| 80%–84% | 0 | 5 | 0 | 0 | 4 | 9 |
| 85%–89% | 0 | 0 | 6 | 0 | 0 | 6 |
| 90%–99% | 0 | 0 | 0 | 28 | 4 | 32 |
| ⩾100% | 0 | 0 | 0 | 5 | 125 | 130 |
| Total | 2 | 5 | 6 | 35 | 140 | 188 |
NOTE. Adherence to one medication corresponded in most cases to a similar level of adherence to the other medication. Thirteen patients (7%) had ⩾90% adherence to pegylated IFN but <85% adherence to ribavirin.
Adherence and viral suppression
Mean decreases in HCV load from baseline to 12 weeks in each of the 7 strata for adherence to pegylated IFN were as follows: (1) <80%, 1.43 log IU/mL; (2) 81%–84%, 2.75 log IU/mL; (3) 85%–89%, 4.02 log IU/mL; (4) 90%–99%, 3.44 log IU/mL; (5) 100%–109%, 3.07 log IU/mL; (6) 110%–119%, 3.63 log IU/mL; and (7) ⩾120%, 2.80 log IU/mL (figure 3A). Mean viral load decreases in the 7 strata for adherence to ribavirin were as follows: (1) <80%, 2.27 log IU/mL; (2) 81%–84%, 3.19 log IU/mL; (3) 85%–89%, 4.02 log IU/mL; (4) 90%–99%, 3.42 log IU/mL; (5) 100%–109%, 3.17 log IU/mL; (6) 110%–119%, 3.36 log IU/mL; and (7) ⩾120%, 2.65 log IU/mL (figure 3B). Viral load decreases were lower for both medications at adherence levels <85%, but at adherence levels ⩾85%, the decreases in viral load appeared to be constant. Therefore, adherence ⩾85% was classified as “good” adherence. One hundred seventy-nine patients (95%) had good adherence to pegylated IFN treatment, and 166 (88%) had good adherence to ribavirin treatment.
Figure 3.
Mean decrease in hepatitis C virus (HCV) load at 12 weeks among 188 patients, by level of adherence to pegylated IFN (A) and ribavirin (B) therapy. The bars on each data point represent 95% CIs.
Overall, the mean decrease in HCV load at 12 weeks of therapy was 0.66 log IU/mL greater for patients with good adherence than it was for patients with poor adherence (3.23 vs. 2.57 log IU/mL; P = 04) (table 3). Patients who had poor adherence had a higher mean HCV load at 12 weeks of treatment than did patients with good adherence (3.48 log IU/mL [95% CI, 2.96–4.00 log IU/mL] vs. 2.67 log IU/mL [95% CI, 2.51–2.82 log IU/mL]; P = 007). Similarly, among patients infected with HCV genotypes 1 or 4, mean viral load decrease was 0.67 log IU/mL greater for those with good adherence than it was for those with poor adherence (2.95 log IU/mL vs. 2.28 log IU/mL; P = 05). Patients infected with genotype 1 or 4 who had poor adherence had higher mean viral loads at week 12 than did those with good adherence (3.79 log IU/mL [95% CI, 3.21–4.37 log IU/mL] vs. 2.95 log IU/mL [95% CI, 2.77–3.13 log IU/mL]; P = 01). The sample sizes for patients infected with genotypes 2 or 3 were insufficient for subanalyses. When we stratified results by baseline HCV load (table 3), viral load decreases remained greater for patients with good adherence.
Table 3.
Mean decrease in hepatitis C virus (HCV) load from baseline at week 12 of pegylated IFN plus ribavirin therapy for patients with good (⩾85%) and poor (<85%) treatment adherence.
| Mean decrease in HCV load at week 12, log IU/mL |
||||
|---|---|---|---|---|
| Variable | Patients with <85% adherence | Patients with ⩾85% adherence | Difference, log IU/mL | P |
| All patients | 2.57 | 3.23 | 0.66 | .04 |
| Genotype 1 or 4 infectiona | 2.28 | 2.95 | 0.67 | .05 |
| Baseline HCV RNA level | ||||
| >800,000 IU/mL | 2.77 | 3.45 | 0.68 | .09 |
| ⩾800,000 IU/mL | 2.26 | 2.95 | 0.69 | .1 |
Sample size of patients infected with HCV genotypes 2 or 3 were insufficient for analyses.
When we performed a sub-analysis that excluded the 16 patients who received suboptimal ribavirin dosages during the initial 12 weeks of HCV therapy, the difference in HCV load decrease was even greater between patients with good and patients with poor adherence. The mean decrease in HCV load at 12 weeks of therapy was 1.00 log IU/mL greater for patients with good adherence than for patients with poor adherence (3.32 log IL/mL vs. 2.33 log IU/mL; P = 01). Among patients infected with HCV genotypes 1 or 4, the mean viral load decrease at 12 weeks was 1.09 log IU/mL greater for patients with good adherence than for patients with poor adherence (3.04 log IU/mL vs. 1.95 log IU/mL; P = 01). In contrast, when we excluded the 17 patients who received a suboptimal pegylated IFN dosage during follow-up, the differences in viral load decrease between patients with good and patients with poor adherence were similar to those observed for patients who received the recommended pegylated IFN dosage.
Adherence and early virologic response (EVR)
A total of 134 patients (71%) achieved EVR. EVR was more common among patients with ⩾85% adherence than it was among patients with <85% adherence to pegylated IFN treatment (73% vs. 29%; P = 02) and ribavirin treatment (73% vs. 55%; P = 08) (figure 4). Among the 123 patients with ⩾100% adherence to both pegylated IFN and ribavirin treatment, 35 (28%) did not achieve EVR.
Figure 4.
Early virologic response among 188 patients, by level of adherence to pegylated IFN (A) and ribavirin (B) therapy. The bars on each data point represent 95% CIs.
Adherence among subgroups
HIV-infected patients had a higher median adherence to pegylated IFN (109.8% vs. 102.8%; P = 01) and ribavirin treatment (110.0% vs. 102.7%; P = 3), compared with HIV-uninfected individuals. Patients with a history of depression, posttraumatic stress disorder, or alcohol abuse did not have poorer adherence, compared with those without a history of these conditions.
DISCUSSION
To our knowledge, this study is the first, outside of clinical trials, to evaluate the relationship between adherence to combination HCV therapy during the initial 12 weeks of treatment and virologic outcomes. Adherence of ⩾85% to pegylated IFN and ribavirin treatment, measured by pharmacy refill data, was associated with increased HCV suppression and EVR. Decreases in HCV load became even greater when patients had ⩾85% adherence to their regimen and were prescribed the recommended weight-based ribavirin dosage throughout the initial 12 weeks of treatment. Adherence to pegylated IFN treatment was statistically greater than adherence to ribavirin treatment, but the magnitude of this difference was small and unlikely to be clinically significant. Moreover, adherence levels to the 2 anti-HCV medications were comparable for individuals. Finally, patients with a history of HIV infection, depression, posttraumatic stress disorder, or alcohol abuse did not have poorer adherence, compared with those without a history of these conditions.
This study demonstrates that maintaining high levels of adherence to pegylated IFN and ribavirin regimens throughout the initial 12 weeks of therapy is associated with better virologic outcomes, particularly when recommended weight-based ribavirin dosages are prescribed and maintained. Thus, in addition to administering optimal dosages of anti-HCV medications, providers should also encourage high levels of adherence to both anti-HCV medications before and throughout treatment to ensure maximum response to therapy. Identifying suboptimal (i.e., <85%) adherence to pegylated IFN and/or ribavirin therapy with use of pharmacy refill data could allow HCV treatment providers to help patients improve their adherence during treatment, which could help improve virologic response rates. Although interventions to increase adherence to HCV therapy have not been tested, providers could ask patients about barriers to adherence (e.g., forgetting doses) and help them identify potential solutions (e.g., using reminder alarms).
Our findings suggest that HCV suppression does not increase with adherence to pegylated IFN and ribavirin therapy >85%. In addition, the proportion of patients with an EVR was similar for all adherence levels ⩾85%. It is possible that 85% adherence might represent a threshold adherence level below which virologic suppression is reduced. We were unable to determine the presence of a threshold statistically because of the small numbers of patients with adherence <85%. Future studies will need to determine whether such a threshold exists and whether it occurs at this 85% adherence level. If confirmed, this threshold would represent a target for treatment adherence interventions.
Our study also examined whether certain subgroups of patients demonstrated lower levels of adherence. Patients with a history of depression, posttraumatic stress disorder, and alcohol abuse are considered to be at risk for nonadherence to treatment, but our results did not demonstrate poorer adherence among patients in these subgroups. These findings should not be attributed to selection bias, because the characteristics of excluded patients were similar to those of patients who were included. Because of this finding, HCV providers should not be reluctant to initiate treatment for these patients because of a perceived risk of nonadherence to treatment. Additional risk factors for nonadherence to HCV therapy should be examined to determine high-risk subgroups and whom to target with interventions to improve adherence.
Our results have several potential limitations. First, the retrospective study design did not permit HCV load testing at the same time during treatment for each patient, but we required week 12 viral loads to have been obtained in a narrow window to reduce misclassification of outcomes. Second, although it is possible that we could have misclassified adherence as good for some patients who had late first and/or second refills of anti-HCV medications but on-time third refills, we still found a strong association between our adherence measure and virologic outcomes, which suggests that the amount of misclassification of adherence was minimal. Third, by including only patients who had a follow-up viral load measurement obtained between weeks 11 and 13, we may have selected individuals who were more likely than others to be adherent to their anti-HCV regimen. However, the characteristics of patients who were excluded from the study were similar to those of patients who were included; thus, these exclusions should not have altered our conclusions. Fourth, all measures of treatment adherence are imperfect. However, the potential misclassifications of the measure that we used would tend to bias results to show no difference, and we still found significant associations. Finally, only US veterans were included in this study, which potentially limits the generalizability of our results; however, it is unlikely that the relationship between adherence and virologic response is different in other populations.
In conclusion, providers should consider poor treatment adherence as a possible cause of virologic nonresponse during HCV therapy. Future studies should evaluate risk factors for poor adherence and monitor changes in the magnitude of adherence beyond the initial 12 weeks of HCV therapy to determine the optimal timing and nature of interventions to maximize adherence.
Acknowledgments
We thank Dr. Brian Strom for his critical reviews of the manuscript.
Financial support. Agency for Healthcare Research and Quality Centers for Education and Research on Therapeutics cooperative agreement (#HS10399) and National Institute of Allergy and Infectious Diseases (K01-AI070001 to V.L.R.).
Footnotes
Presented in part: 43rd Annual Meeting of the European Association of the Liver, Milan, Italy, 23–27 April 2008 (abstract 1623) and 24th International Conference on Pharmacoepidemiology, Copenhagen, Denmark, 17–21 August 2008 (abstract 50).
Potential conflicts of interest. All authors: no conflicts
References
- 1.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa–2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 2.Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–55. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 3.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa–2b plus ribavirin compared with interferon alfa–2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: management of hepatitis C: 2002—June 10–12, 2002. Hepatology. 2002;36:S3–20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 5.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–3. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 6.Gross R, Bilker WB, Friedman HM, Strom BL. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS. 2001;15:2109–17. doi: 10.1097/00002030-200111090-00006. [DOI] [PubMed] [Google Scholar]
- 7.McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1–infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–9. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 8.Raptopoulou M, Tsantoulas D, Vafiadi I, et al. The effect of adherence to therapy on sustained response in daily or three times a week interferon alpha–2b plus ribavirin treatment of naive and nonresponder chronic hepatitis C patients. J Viral Hepat. 2005;12:91–5. doi: 10.1111/j.1365-2893.2005.00549.x. [DOI] [PubMed] [Google Scholar]
- 9.Reddy KR, Shiffman ML, Morgan TR, et al. Impact of ribavirin dose reductions in hepatitis C virus genotype 1 patients completing peginterferon alfa–2a/ribavirin treatment. Clin Gastroenterol Hepatol. 2007;5:124–9. doi: 10.1016/j.cgh.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Shiffman ML, Ghany MG, Morgan TR, et al. Impact of reducing peginterferon alfa–2a and ribavirin dose during retreatment in patients with chronic hepatitis C. Gastroenterology. 2007;132:103–12. doi: 10.1053/j.gastro.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Weiss JJ, Bhatti L, Dieterich DT, et al. Hepatitis C patients’ self-reported adherence to treatment with pegylated interferon and ribavirin. Aliment Pharmacol Ther. 2008;28:289–93. doi: 10.1111/j.1365-2036.2008.03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–71. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 13.Tien PC. Management and treatment of hepatitis C virus infection in HIV-infected adults: recommendations from the Veterans Affairs Hepatitis C Resource Center Program and National Hepatitis C Program Office. Am J Gastroenterol. 2005;100:2338–54. doi: 10.1111/j.1572-0241.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 14.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records: description and validation. Med Care. 1988;26:814–23. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 16.Grossberg R, Zhang Y, Gross R. A time-to-prescription-refill measure of antiretroviral adherence predicted changes in viral load in HIV. J Clin Epidemiol. 2004;57:1107–10. doi: 10.1016/j.jclinepi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–80. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]





