Abstract
The vascular system – through its development, response to injury, and remodeling during disease – constitutes one of the key organ systems sustaining normal human physiology; conversely, its dysregulation also underlies multiple pathophysiologic processes. Regulation of vascular endothelial cell function requires the integration of complex signals via multiple cell types, including arterial smooth muscle, capillary and post-capillary pericytes, and other perivascular cells such as glial and immune cells. Here, we focus on the pericyte and its roles in microvascular remodeling, reviewing current concepts in microvascular pathophysiology and offering new insights into the specific roles that pericyte-dependent signaling pathways may play in modulating endothelial growth and microvascular tone during pathologic angiogenesis and essential hypertension.
Overview
A complete understanding of the complex regulation of the vascular system requires both a systemic, structural understanding of vascular function, as well as the focused dissection of multiply-intertwined signaling pathways in both vascular and non-vascular cell types.1 While tumor growth is known to depend on concomitant angiogenesis,2 it has been further suggested that angiogenesis may, in fact, be a common template underlying numerous other disparate phenomena including wound healing, diabetic retinopathy, age-related macular degeneration, chronic inflammatory states, capillary permeability, and microvascular tone regulation.3 A complete understanding of the physiological cross-talk between endothelial cells and the cellular regulators that modulate their (dys)function holds promise for understanding the underpinnings of key physiologic and patholologic processes, while offering opportunities for innovation in the treatment of life threatening and chronic illness.
Interactions between endothelial cells and surrounding perivascular cells have long been thought to mediate control of the vascular system on a local level. In addition to the endothelial cell, several perivascular cell types play key roles in dynamic regulation of the vascular system, including arterial smooth muscle4 and capillary and venular pericytes.5, 6 Regulation of the capillary microenvironment by these perivascular cell types occurs via three principal mechanisms: (1) communication with the underlying endothelium by soluble mediators and cell-cell contact, (2) synthesis, remodeling, and maintenance of the basement membrane, and (3) regulation of microvascular tone. All of these mechanisms involve an overlapping array of biochemical and biomechanical signaling pathways,7–9 with considerable gaps in knowledge prompting extensive current interest and investigation. A complete understanding of the cellular physiology underpinning vascular development, blood-brain barrier function, capillary permeability, and microvascular tone regulation, therefore, may be expected to illuminate the corresponding pathophysiology of tumor angiogenesis,10 age-related macular degeneration, 11 and diabetic retinopathy,12 as well as both pulmonary and systemic hypertension.13, 14
Physiological and Pathological Angiogenesis: Current Concepts and Challenges
Pericyte control of microvascular remodeling and proliferative status
The pericyte in particular has drawn increased attention as an emerging key mediator in multiple microvascular processes, including: (i) endothelial cell proliferation and differentiation,15, 16 (ii) contractility and tone,17, 18 (iii) stabilization and permeability,19 and (iv) morphogenesis during disease onset.20 First described in early studies of vascular development by Rouget in 1873,21 pericytes have subsequently been shown to regulate multiple stages of vascular development and differentiation.6–8 During angiogenesis, nascent microvessels are heralded by an actively motile and proliferative endothelium with an immature basement membrane. This migratory and proliferative phase yields a primitive capillary tube, followed by a microvascular maturation phase marked by an endothelial FGF-2- and PDGF-dependent recruitment of presumptive pericytes, occurring concomitantly with basement membrane remodeling. Triggered by endothelial cell contact, the presumptive pericyte then assumes a mature contractile status by initiating expression of its smooth muscle contractile protein repertoire.22
Pericytes have been postulated to govern the phenotypic change from a proliferative angiogenic sprout to a mature microvascular conduit possessing a quiescent capillary endothelium.15, 23–26 Through both pericyte/endothelial cell contact-dependent as well as endothelial-independent mechanisms, pericytes suppress endothelial growth27 and migration.28 Additionally, in situ, there is striking coincidence of pericyte investment and microvessel stabilization,19, 23 and densely pericyte-invested capillary beds demonstrate reduced rates of endothelial turnover.29–31 Interestingly, pericyte investment has also been directly implicated in conferring capillary resistance to regression in vivo.32, 33 As well, knockout studies using pericyte-deficient PDGF-receptor-beta mice demonstrate endothelial hyperplasia in the usually densely-pericyte associated capillary beds of the brain.34, 35 This work suggests that in order for pathological neovascularization to occur, the quiescent endothelium must escape from its growth-arrested phenotype, perhaps actively destabilizing and disengaging from its association with pericytes as it re-enters the cell cycle. Outcomes of this would be both consistent with the observable ‘pericyte dropout’ seen histologically in proliferative diabetic retinopathy36–38 as well as the notion that pericyte apoptosis need not be required for initiation of pathologic angiogenesis.
Pericyte dysregulation in pathological angiogenesis
There is growing interest in the regulatory roles for pericytes in modulating endothelial phenotype during pathologic angiogenesis, such as tumor-induced angiogenesis, age related macular degeneration and proliferative diabetic retinopathy. Based on early histological observations, frank pericyte loss (‘pericyte dropout’) via apoptosis or de-differentiation was initially proposed to underlie the observed endothelial dysfunction and hyperplasia in these states.39 Recent models of anti-angiogenic chemotherapy confirm that small molecule- and antibody-mediated disruption of endothelial survival signaling show specific destruction of non- or loosely pericyte-associated vessels, while mature vessels with secure pericyte association may be more refractory to therapy.19, 32, 40 Other studies predicted that alterations in basement membrane composition could modulate pericyte-endothelial interactions,41, 42 and that matrix-associated molecular signaling via endothelial-derived FGF-2 could control pericyte recruitment,43 suggesting possible mechanisms for dysfunction of pericyte-endothelial growth regulation without actual pericyte loss. As well, El-Bizri et al. have shown that pericyte-targeted deletion of bone morphogenetic protein receptor 1A (BMPR1A) is associated with reduced matrix metalloproteinase activity and pericyte resistance to apoptosis in vitro, associated with failure of appropriate vascular pruning and impaired organogenesis in vivo.33 Also consistent with a prominent role for matrix as an arbiter of endothelial-pericyte interaction, rapid regrowth of neovascular sprouts into empty basement membrane sleeves was also observed following regression of immature tumor vasculature less than 24 hours following discontinuation of VEGF-targeted monotherapy.44 As the importance of the platelet-derived growth factor (PDGF) system to the recruitment and differentiation of pericytes at the level of the nascent capillary has also been strongly supported by multiple developmental34, 35, 45–47 and pharmacological48 studies, PDGF has become a promising additional target for anti-angiogenic chemotherapy. Several groups have shown augmented efficacy and greater durability of anti-angiogenic therapy by combining small molecule inhibition of both VEGF and PDGF receptor signaling in tumors.48–55 A similar mechanism has also been shown in models of ocular neovascularization,56 where microvascular regression is coupled to the ‘drop-out’ of vessel-associated pericytes, derived either by apoptosis or microenvironmentally-driven ‘de-differentiation’ without frank loss. Recent reports of pericyte drop-out in mouse models of diabetic retinopathy suggest that one of the local mediators controlling this phenomenon in the diabetic eye may be the hyperglycemia-induced production of Ang-2, leading to a Tie2-mediated increases in pericyte apoptosis and migration in vitro that mirrors the dissociation of pericytes from retinal capillaries observed in vivo.57, 58 The identification of such molecular cues for pericyte de-differentiation and dissociation is a promising source of both new drug targets for diabetic retinopathy, as well as a potential source of insight into the pathophysiology of hyperglycemic damage in other organ beds.
Consistent with the theory that dysfunctional pericyte stabilization of tumor endothelium may prove to be a potent additional target for tumor microvascular instability and microvascular regression, one group’s careful analysis of baseline tumor vasculature by thick-section confocal microscopy revealed pericyte association with greater than 97% of tumor vessels, but with electron-microscopically evident abnormal associations between endothelial cells, pericytes, and matrix.59, 60 Interestingly, intravital microscopy performed on glioma vasculature growing in a dorsal skinfold chamber during treatment with a small molecule inhibitor of both VEGF receptor-2 and PDGF receptor-beta revealed that vascular regression occurred despite a surprisingly high index of continued endothelial investment by pericytes.51 These data imply that pericyte-endothelial destabilization without frank pericyte drop-out or apoptosis may be sufficient to produce marked vascular regression. Interestingly, recent work has revealed that the ability of pericytes to growth-arrest adjacent endothelial cells in vitro can be attenuated by manipulation of pericyte Rho GTP status. These results demonstrate that alterations in the mechanochemical coupling of endothelial cells and pericytes may be sufficient to initiate pathological angiogenesis, without the requirement for pericyte drop-out or apoptosis.61
Soluble and trans-matrix extracellular signaling via TGF-beta
The above-described bridging between intracellular signaling and force transduction pathways in pericytes parallels the emerging role for the transforming growth factor-beta (TGF-beta) family of ligands, receptors, and signal-transducing effectors (recently reviewed by Bertolino62). In vascular endothelial cells, human analogues of the Drosophila mothers against decapentaplegic (MAD) family of proteins have recently been characterized as primary transducers of TGF-beta signaling through multiple pathways. These include the mitogen-activated protein kinase, MAPK,63 which may function through the cyclic AMP-responsive element binding protein CREB.64 There is also evidence to indicate that a subset of MAD family proteins (Smads 6 and 7) are shear stress-inducible, and therein constitute a mechanism of fluid flow-sensitive mechanochemical regulation of endothelial gene expression.65 Smad-mediated signaling also plays a role in the regulation of alpha-smooth muscle actin expression and proliferative state in pericytes, which are downstream of TGF-beta and FGF-210, 22 signal transduction. Given that both TGF-beta66 and FGF-241 are known to be associated with sub-endothelial matrix (reviewed by Dinbergs et al.67), these data together raise the possibility that a mechanochemical link exists between pericytes and endothelial cells: in particular, TGF-beta- and SMAD-dependent signaling may mediate a continuous signal originating from the luminal endothelial surface, spanning sub-endothelial matrix, and arriving at a neighboring pericyte. Further, it is likely that this heterocellular mechanochemical coupling is mediated at the pericyte level by a Rho GTP-dependent network of cytoskeletal effectors.
Interestingly, recent evidence from of acute lung injury models also implicate Rho and Rho kinase-dependent signaling pathways in the transduction of TGF-beta signaling in endothelial cells. Increased levels of circulating TGF-beta have been noted in trauma patients and are associated with the development of the acute respiratory distress syndrome (ARDS) and sepsis.68 In two animal models of ARDS, adenovirus-mediated overexpression of TGF-beta induces69 and delivery of soluble TGF-beta receptor inhibits70 the acute increase in vascular permeability as well as the later development of pulmonary fibrosis in animal models of septic lung injury modeling human ARDS patients. One group has recently demonstrated that treatment with TGF-beta induces myosin light chain phosphorylation, stress fiber formation, and endothelial permeability, and that these alterations in cytoskeletal structure are partially dependent on downstream signaling through Rho GTPase and its principle effector, Rho kinase.71, 72
The in vivo finding that circulating systemic TGF-beta has potent effects on the microvasculature of the lung is consistent with the known role of local TGF-beta signaling in pericyte-mediated endothelial maturation and survival. Recent in vivo experiments showing that pericyte-derived TGF-beta enhances retinal endothelial cell survival through upregulation of endothelial VEGF receptor-173 complements extensive evidence in vitro implicating TGF-beta as a key mediator of pericyte control of endothelial growth state and phenotype.27, 74–79 Importantly, the biochemical activity of TGF-beta is dependent upon activation requiring the involvement of both alpha-V integrins (such as beta-6 and beta-8)80 as well as latent TGF-beta binding protein (recently reviewed by Sheppard et al.81), allowing specific local control of TGF-beta activation even in the face of its systemic presence.
Although TGF-beta is currently the best understood soluble mediator of pericyte-endothelial cell interactions, recent evidence indicates that other as yet unknown molecules may also play a parallel role. Kondo et al. recently noted that treatment of cultured retinal endothelial cells by retinal pericyte-conditioned media suppressed endothelial cell proliferation, and that this effect was only partially reversible by a TGF-beta-1 blocking antibody, in contrast to the complete reversibility observed following heat-treatment.82 As well, our lab has recently demonstrated that signaling through the Rho GTPase and its downstream effector Rho kinase underlies both pericyte contractility61, 83 as well as pericyte regulation of endothelial growth, which appears to be TGF-beta-independent.61 Thus, while matrix-associated TGF-beta and FGF243 play roles in modulating pericyte recruitment, pericyte-endothelial interactions, and microvascular junctional integrity, there are also likely roles for as yet undescribed soluble mediators, which function to regulate endothelial growth in both contact-dependent and -independent manners.61, 84, 85
Intracellular signaling via Rho GTPase – Smooth muscle cell pathways
Evidence is accumulating that vascular morphogenesis may be regulated by members of the Rho family of small GTPases (as recently reviewed by Bryan and D’Amore,86 as well as Mammoto et al.87). Rho GTPase-mediated cytoskeletal adaptations modulate normal maintenance of the arterial vasculature, as well as mediate the tone dysregulation and hypertrophic remodeling observed during essential hypertension.88–91 Here, we will briefly review the Rho signaling in vascular smooth muscle as a template for understanding its role in pericytes.
Biochemical tools
Multiple experimental tools are available for both the in vitro and in vivo perturbation of signaling through Rho GTPase and its downstream effector, Rho kinase. The first such signaling studies were conducted using GTP-gamma-S-bound Rho;92 shortly thereafter, an array of Rho mutants were established by site-directed mutagenesis, including two dominant-active (Rho-V14 and RhoL-63) and one dominant-negative (Rho-N19) mutant.93, 94 As well, microinjection or transfection with either Clostridium botulinum exoenzyme C3 or Clostridium difficile toxin B produces inactivation of endogenous Rho via ADP-ribosylation and glucosylation, respectively.92, 95
Recent focus has also been placed on the development of small molecular pharmacologic inhibitors of Rho kinase (also referred to as ROCK1), one of the principle downstream effectors of Rho GTPase signaling in multiple cell types (recently reviewed by Liao et al.96). The first of these specific Rho kinase inhibitors was Y-27632, a pyridine derivative which binds to and inhibits the catalytic site of Rho kinase in an ATP-competitive fashion, and is efficiently taken up via carrier-mediated facilitated diffusion.97 The second major well-characterized inhibitor was fasudil (also referred to as HA1077 and AT877), initially explored for use as a calcium antagonist in models of subarachnoid hemorrhage and smooth muscle-mediated cerebral vasospasm.98 Unlike other previously described calcium channel blockers investigated for their protective effect in vasospasm, fasudil’s mechanism of action was independent of upstream calcium channel blockade.99 Subsequently, this mechanism was elucidated as dependent on the selective inhibition of Rho kinase in smooth muscle.100 Of the described Rho kinase inhibitors thus far, fasudil has demonstrated safety and efficacy in multiple clinical trials of cardiovascular disease treatment, beginning with the prevention of subarachnoid hemorrhage-induced smooth muscle-mediated vasospasm in the early 1990’s.101, 102 Since its clinical introduction, it has been explored for further use in cerebral infarction,103 coronary vasospastic angina and ischemia,104, 105 ventricular remodeling and myocardial fibrosis following myocardial infarction,106, 107 intimal hyperplasia,108 pulmonary and essential hypertension (reviewed by Fukumoto et al.109 and Shimokawa and Takeshita,110 respectively), pulmonary interstitial fibrosis,111 and peripheral nerve injury.112 The growing clinical applications of fasudil and other small-molecule inhibitors of Rho kinase signaling in human disease are beyond the scope of this review, but have been recently and thoroughly reviewed by Olson113 and others.
Rho GTPase and Rho kinase signaling in vascular smooth muscle
Through application of the above biochemical tools, the importance of Rho GTPase signaling through Rho kinase has been explored in some detail in smooth muscle cells, and has been extensively reviewed elsewhere.114, 115 While considerable overlap between Rho signaling in smooth muscle cells and pericytes exists, clear evidence for pericyte-specific aspects of the Rho cascade is emerging as well.61, 116 In order to highlight both parallel and divergent pathways of Rho GTP-dependent signaling in pericytes, we will briefly outline the Rho pathway in vascular smooth muscle here.
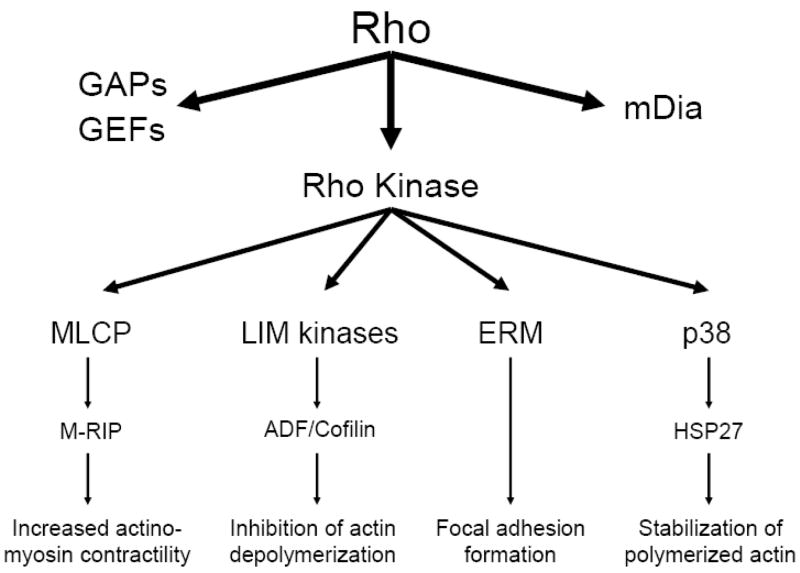
Downstream signaling via activation of the Rho GTPase involves three principle pathways: dynamic interactions with Rho GAPs (G-protein activating proteins) and GEFs (guanine nucleotide-binding factors), activation of mDia, and activation of Rho kinase (see Figure 1). Regulators of GAPs and GEFs include such kinases as Src and FAK, with principle downstream regulation of focal adhesion formation. mDia leads to activation of profilin, an actin monomer-binding protein which orchestrates actin filament and stress fiber assembly. Downstream of Rho kinase there appear to be multiple targets, ultimately signaling through focal adhesion kinase and alpha-smooth muscle actin to regulate stress fiber formation.117 Four Rho kinase-mediated pathways in particular deserve detailed attention as promising candidates for bridging the activation of Rho kinase seen in smooth muscle with direct functional relationships likely to be central in remodeling pericyte cytoskeletal dynamics during contractility: myosin light chain kinase, ADF/cofilin, ERM family proteins, and p38 MAP kinase.
Figure 1. The Rho GTPase – Rho kinase signaling pathway in vascular smooth muscle.
The Rho GTPase mechanochemical signaling apparatus links transmembrane and intracellular signaling-associated Rho GTPase with functional alterations in the actin cytoskeleton. Rho kinase, a principal downstream effector of Rho GTPase, mediates linkage to the actin cytoskeleton via four principle pathways discussed in the text: myosin light chain phosphatase (MLCP), ADF/cofilin, ERM proteins, and p38 MAP kinase.
Myosin light chain kinase and myosin phosphatase
In smooth muscle cells, contractility is controlled by the myosin light chain; the phosphorylation state of its myosin-binding subunit is mediated by the dynamic balance between calcium/calmodulin-dependent myosin light chain kinase [MLCK; reviewed by Kamm and Stull118) and myosin phosphatase activity (reviewed by Ito et al.119). While MLCK activity is regulated by intracellular calcium and calmodulin-based signaling, myosin phosphatase activity is chiefly governed by signaling through Rho kinase.100, 120, 121 Recent work also describes linkage of myosin light chain phosphatase to the cytoskeleton via a Rho GTPase-interacting protein, linking Rho both structurally and functionally to myosin phosphatase regulation.122–124 The possibility of a similar biomechanical link in pericytes is currently under investigation (Surks and Herman, unpublished data).
ADF/Cofilin
An additional force transduction system known to operate downstream of Rho kinase hinges upon phosphorylation-inhibition of members of the actin depolymerizing factor (ADF)/cofilin family, and their control of actin filament disassembly (reviewed by Bamburg125). Phosphorylation of ADF/cofilin family members is controlled by the LIM kinases, LIMK1 and LIMK2, of which LIMK1 operates under the control of Rac126, 127 while LIMK2 is the principal ADF/cofilin effector downstream of Rho and Cdc42.128–130 Pericyte-specific aspects of the ADF/cofilin cascade remain to be thoroughly characterized.
ERM family proteins
A third mechanism of force transduction known to interact with the Rho GTPase pathway is the ezrin/radixin/moesin (ERM) family of proteins, which governs crosslinking of actin filaments to the plasma membrane (reviewed by Louvet-Vallee131). ERM proteins are activated via both Rho kinase-dependent132 as well as -independent mechanisms.133, 134 Recent work in our laboratory further describes the actin-specific regulator betacap-73 as a critical bridge between ezrin and the actin network via its association with Rho GTPase effectors (Durham et al., 2008; manuscript in preparation).
p38 MAP Kinase
A fourth downstream effector of Rho GTPase via Rho kinase reported in both smooth muscle and endothelial cells is p38 MAP kinase135, 136, via the stabilization of polymerized actin.137, 138 p38 MAP kinase has also been shown to contribute to the production of inflammatory cytokines in response to endotoxin exposure in both the myocardium139 and pulmonary arteries,140. In the pulmonary vasculature, this is particularly of interest, as inflammatory cytokines such as TNF-alpha, IL-1, and IL-6 are known to contribute to hypoxic pulmonary vasoconstriction, possibly through induction of pro-contractile signaling at the smooth muscle, and perhaps pericyte, level.141–144
Intracellular signaling via Rho GTPase – Pericyte-specific pathways
The Rho family of small GTPases has long been known to play a role in control of the actin cytoskeleton in many cell types other than vascular smooth muscle.145 With reference to the above-described pathways, recent work has also demonstrated that similar mechanisms are operative in pericytes. These Rho GTP-dependent (as well as other Rho GTP-independent) pathways function to coordinate pericyte growth and contractile phenotype while simultaneously modulating capillary endothelial function and microvascular tone.
Pericyte-specific assays of Rho activity
Previous work in our lab had indicated that Rho (but not Rac or Cdc42) GTPase signaling modulates pericyte morphology and contractile phenotype in an isoactin-dependent manner.83 Using a transient transfection system in which cultured pericyte cytoskeletal elements were analyzed differentially based on Rho phenotype, it was shown that expression of dominant-positive Rho fostered specific remodeling of alpha-smooth muscle actin- and myosin- containing stress fiber arrays, whereas nonmuscle actin-containing stress fibers were sustained. These results suggest that, in pericytes, alterations in Rho GTPase control the isoactin-specific regulation of pericyte shape and resultant contractile phenotype. Using adenoviral-mediated delivery of dominant-active and -negative Rho mutants,61, 146, 147 we have also described two novel assays to quantify both pericyte contractile phenotype as well as pericyte-mediated juxtacrine modulation of endothelial growth state. Results of these experiments not only reveal central parallels between Rho GTP-dependent signaling in vascular smooth muscle and microvacular pericytes; at the same time, there appears to be distinct divergence in Rho GTP-mediated alterations in pericytes as compared to smooth muscle cells. Clearly, additional work will be needed to continue to define the distinct Rho-mediated pathways existing in vascular smooth muscle cells and pericytes and their differential contributions to vascular remodeling during development and disease.
Rho GTPases in pericyte contractility
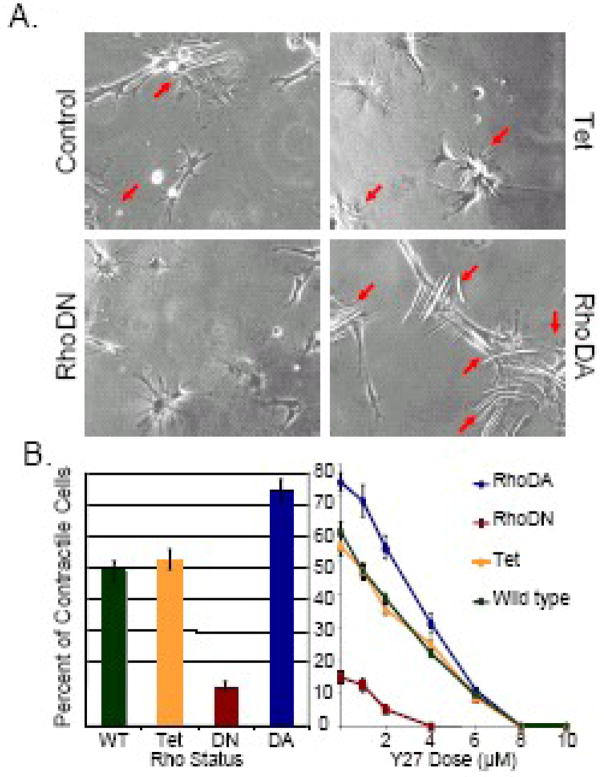
Based on morphological differences observed in dominant-active RhoA-transfected pericytes compared to control- and dominant-negative RhoA-transfected pericytes previously described,83 we hypothesized that this phenotypic change reflected a mechanochemical coupling mechanism both in the pericyte cytoplasmic space as well as between pericytes and endothelial cells in the regulation of microvascular tone. In order to dissect the functional aspects of this pathway, we re-implemented methods pioneered by Harris and others148 to quantify cellular force transduction as a function of Rho activation. Real-time imaging reveals that pericyte deformation of extracellular matrix-treated silicone substrates can be altered by expression of mutant Rho GTPases and treatment with Rho GTP-specific pharmacologic inhibitors (see Figure 3).61 These results indicate that pericyte contractility is dependent upon and proportional to signaling through Rho and its downstream effectors, in parallel to the known dependence of smooth muscle contraction on Rho GTP-dependent signaling.149, 150 These findings raise the possibility that the control of microvascular flow, regulation of endothelial cell migration and growth, and the maintenance of capillary permeability are each orchestrated via mechanochemical signal transduction cascades involving Rho GTPase. Thus, whereas the mechanical functionality of Rho signaling has been explored thoroughly in vascular smooth muscle, the importance of Rho GTP-dependent signaling in pericytes is only now emerging.
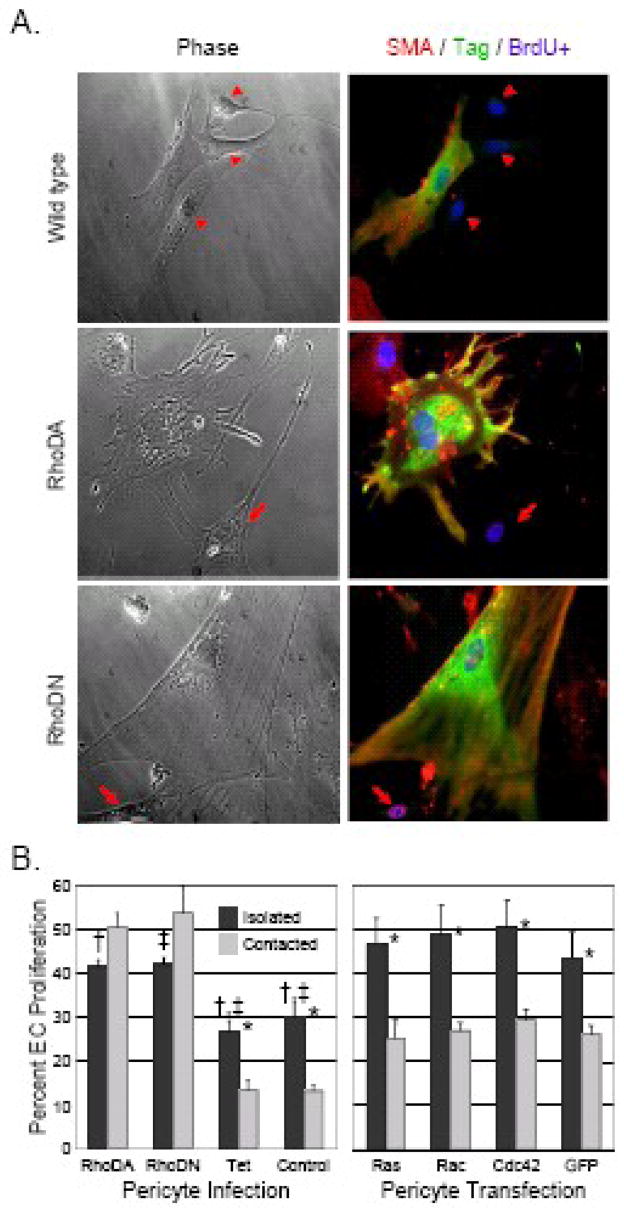
Figure 3. Alteration of pericyte Rho (but not Ras, Rac1, or Cdc42) GTPase signaling impedes pericyte-mediated endothelial cell growth arrest.
Bovine retinal pericytes were either transfected with plasmids (A) or infected with adenoviruses (B) containing dominant-active Rho GTPase (RhoDA) or dominant-negative Rho GTPase (RhoDN). Mock-transfected (Wild type) as well as tetracycline transactivator-infected (Tet) and mock-infected (Control) pericytes were used as controls as labeled. Rho-altered pericytes were then co-cultured with bovine retinal endothelial cells for 24 hours. BrdU was incorporated into the co-culture medium for the last 4 hours. A: Altered Rho-transfected pericyte co-cultures were then fixed and stained for the pericyte marker cytoplasmic alpha-smooth muscle actin and proliferative endothelial cell nuclear BrdU incorporation (αSMA + BrdU: red), the Myc epitope tag as a marker of transfection (Myc: green), and nuclei by Hoechst (Hoechst: blue). Parallel phase image are provided. Images are 200x. Arrows: BrdU-positive, pericyte-contacting proliferating endothelial cell. Arrowheads: BrdU-negative, pericyte-contacting quiescent endothelial cell. B: Adenoviral Rho GTPase-altered pericytes as well as dominant-active Ras, Rac1, Cdc42, and GTP control-transfected pericytes, were co-cultured for 24 hours with bovine retinal endothelial cells as above. Co-cultures were then scored for nuclear BrdU-positive endothelial proliferation as a function of pericyte contact and GTPase status. Results are expressed as mean percentages ± standard error (n > 400 cells/condition, experiments in triplicate). *: p < 0.05 for differences between lone and pericyte-contacting endothelial cells with same GTPase status; †, ‡: p < 0.05 for differences between lone endothelial cell populations with different Rho GTPase status.
Rho GTPases in pericyte-mediated endothelial growth arrest
One of the hallmarks of pericyte function in the microvascular milieu is the ability to induce the growth arrest of nearby endothelial cells via both contact dependent- and independent pathways. 6, 15 Contact-dependent pericyte-mediated arrest of endothelial growth was originally described by comparison of side-by-side and porous membrane-separated models of endothelial cell co-culture, in which pericytes and smooth muscle cells (but not fibroblasts or epithelial cells) were noted to inhibit endothelial proliferation.27 Subsequent work also identified TGF-beta as a soluble, pericyte-derived regulator of endothelial cell migration and proliferation,28 requiring activation from its latent form for pro-proliferative activity.78 In addition to endothelial growth arrest, pro-survival signaling via pericyte-derived juxtacrine vascular endothelial growth factor (VEGF) was demonstrated.76 Taken together, these studies solidify a model in which pericyte investment constitutes a critical stage in angiogenesis, at which migratory, proliferative endothelium transitions into stable, quiescent endothelium.151
Building on these initial co-culture models, we developed an assay in which the ability of pericytes to arrest the growth of nearby endothelial cells could be quantified based on pericyte Rho signaling status. In side-by-side pericyte/endothelial co-cultures in which pericyte Rho GTP signaling was modified using mutant Rho GTPase constructs, both over- and under-expression of Rho were associated with distinct morphological changes but uniform inhibition of pericyte-mediated endothelial growth arrest (see Figure 4).61 These results indicate that pericyte contact-mediated endothelial growth arrest is sensitively dependent on Rho signaling, and is disrupted by altering cellular steady state levels of Rho GTP, since both over- as well as under-expression of Rho GTP could be shown to modulate pericyte-driven control of endothelial cell proliferation.
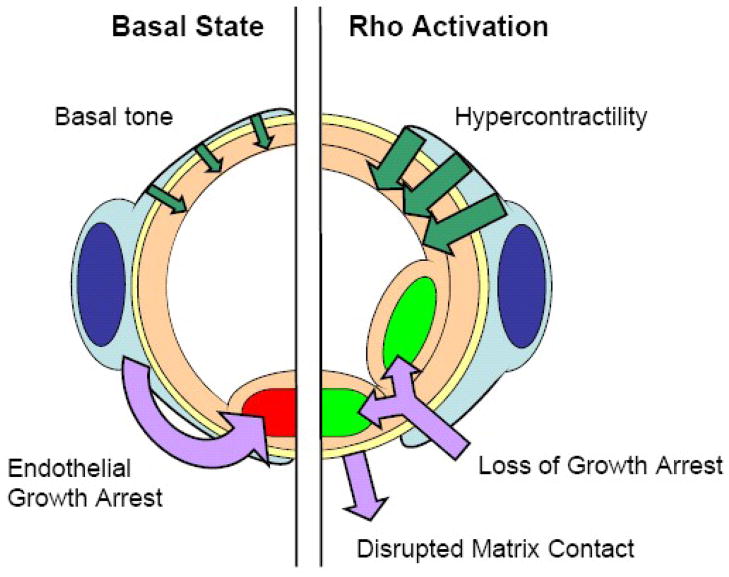
Figure 4. Diagrammatic representation of downstream changes of Rho activation in microvascular pericytes.
Rho activation causes increased pericyte contractility, loss of endothelial contact-mediated growth arrest, and disruption of cell-matrix interactions. On the level of the microvascular bed, pathological activation of Rho may lead to microvascular hypertension, pathological angiogenesis, and increased capillary permeability.
Indeed, endothelial cell proliferation rate was noted to be more than 20% higher in endothelial cells co-cultured with either dominant-active or -negative Rho-expressing pericytes compared with controls, raising the possibility of an additional contact-independent soluble mediator that is also Rho GTP-regulated. TGF-beta was initially investigated as a likely candidate, but preliminary experiments indicate that protein levels of both latent and active TGF-beta as well as soluble TGF-beta receptor II are identical in both experimentally manipulated and control cultures (M.E.K., J.T. Durham, and I.M.H., unpublished observations). In fact, a recent study by another group82 has demonstrated a similar phenomenon in a contact-independent system examining the effects of pericyte-conditioned media on endothelial growth. Here, a TGF-beta-1-specific function-blocking antibody only partially reverses the endothelial growth suppression caused by exposure to pericyte-conditioned media, while heat-treatment of the media completely abrogates the effect. This suggests that additional, currently uncharacterized soluble mediators are likely to exist downstream of pericyte Rho signaling. Intriguingly, the above findings suggest that the Rho GTPase signaling pathway in pericytes involve both an acute, juxtacrine, contact-mediated phase of immediate alteration in contractile tone, as well as a slower-acting, structural phase via regional regulation of angiogenesis by soluble mediators.
Rho signaling in microvascular tone dysregulation
In addition to the role of disrupted endothelial control in vasoproliferative disease, microvascular signaling dysregulation is emerging as a causative factor in the pathogenesis of several non-proliferative vascular pathologies as well. Based on the initial understanding of Rho family GTPase signaling in the regulation of smooth muscle contractility,92 a key role for Rho signaling through Rho kinase has recently been elucidated in both physiological maintenance89 and pathological dysregulation88, 90 of the cardiovascular system. In particular, dysregulated Rho kinase signaling in vascular smooth muscle is being demonstrated and actively explored in animal models of cerebral vasospasm,152, 153 stroke,103 coronary vasospasm,154, 155 post-myocardial infarction ventricular remodeling156 and heart failure,157 atherosclerosis,158, 159 and oxidative stress. 160, 161 Investigation of Rho kinase signaling in endothelial cells is also producing insight into the processes of atherogenesis,162 cardiovascular protection,163 and endothelial damage in diabetes.164, 165 In the following paragraphs, we offer evidence in support of the notion that, in addition to the tonal dysregulation present at the smooth muscle/arterial level, parallel but distinct regulatory pathways exist at the level of the microvascular pericyte, which are relevant to the pathophysiology underlying hypertension.
Pulmonary hypertension
The pulmonary vasculature represents one of the most dynamically flow-responsive vascular beds known. Physiologically, the lung must accommodate the sudden, wide fluctuations in cardiac output required to balance varying oxygen demand and systemic vascular resistance, while simultaneously protecting alveoli from unpredictable changes in arterial pressure. Intricate mechanisms to control local vasomotor tone and capillary recruitment have evolved in order to translate fluctuating pulmonary arterial pressure into constant, predictable alveolar capillary pressure. Both pulmonary artery vasoconstriction and vascular remodeling are key components of the pathology underlying failure of these mechanisms and resultant pulmonary arterial hypertension; see recent extensive reviews by Mandegar,166 Morrell,167 and Pak.168 Reduced endothelial-derived nitric oxide production has been implicated in this vasomotor dysfunction, and in vitro work in human pulmonary artery endothelial cells suggests that the hypoxia-induced downregulation of endothelial nitric oxide synthase is mediated by Rho signaling through Rho kinase. 169 Furthermore, several studies have shown that both the acute vasoconstriction and the chronic vascular remodeling associated with hypoxia are attenuated by Rho kinase inhibition.170–173 These recent studies inform and reinforce the importance of original ultrastructural studies in human and animal lung, which revealed both increased medial thickness and extent of the muscular wall in the pulmonary arterial tree during chronic hypoxia, involving both smooth muscle cells and pericytes.174, 175 In fact, recent in vitro evidence suggests that part of the morphological vascular change observed during chronic hypoxia may be due to transdifferentiation of endothelial cells into a smooth muscle-like phenotype.176 Indeed, the role of Rho signaling in the pathology of pulmonary arterial hypertension currently represents an exciting and active research arena, with prospects for the elucidation of pericyte- versus smooth muscle-dependent mechanisms.
Essential hypertension
Rho kinase plays a key role in the pathogenesis of essential hypertension and its attendant microvascular remodeling (recently reviewed by Lee et al.91 and Loirand et al.90) The calcium-dependent dynamic balance between ligand-activated contractility177 and nitric oxide-mediated relaxation178 in the determination of vascular tone appears to be mediated by Rho kinase at the level of smooth muscle-invested arteries.179–182 In parallel, emerging data from human patients indicates that inhibition of the Rho kinase pathway can correct the peripheral vascular tone dysregulation present in heart failure,183 coronary artery disease,184 and even in cigarette smoking,160 while having minimal effects on tone in normal controls, pointing to the Rho kinase pathway as a promising therapeutic target in vascular disease. Many of these therapeutic uses of Rho antagonists have been shown using in vitro assays of smooth muscle function or in whole-animal and human studies. However, while in vitro assays specifically demonstrate the role of smooth muscle in arterial and arteriolar tension, whole-animal models (for example, mouse models of hypertension) may incorporate additional microvascular- and, notably, pericyte-specific effects of Rho inhibition at the capillary and post-capillary levels that have so far been attributed solely to smooth muscle. It will be important to further clarify the role of pericytes in the global pathophysiology of tone dysregulation in order to develop potential new avenues for pericyte-specific therapeutic intervention in systemic hypertension.
Specific roles for pericytes in microvascular tone regulation
Although systemic arterial pressure is the principal parameter used to monitor and study essential hypertension, cellular-level metabolic exchange principally occurs under conditions of microvascular capillary flow. In addition to protective mechanisms at the arterial and pre-capillary arteriolar level shielding the capillary bed from both extreme fluctuations in pressure as well as consistent hypertension, the microvasculature appears to have additional complementary regulatory mechanisms to control tone and flow on a local level. Important components of the real-time, dynamic regulation of such vascular beds as the central nervous system and retinal vasculature, for example, may rest with Rho GTP-dependent signaling in microvascular pericytes. In the central nervous system, control of vascular tone is exquisitely sensitive to metabolic demands.185–187 Indeed, within the cerebellum, functional imaging studies have shown that in vivo moment-to-moment local control of demand-induced functional hyperemia is mediated by neuronal nitric oxide synthase.188–193 Inhibition of Rho kinase signaling by nitric oxide has been demonstrated in the regulation of extracranial arterial tone as well,181 and in situ hybridization and immunohistochemical evidence indicate the extensive presence of Rho and Rho kinase in cerebellar tissue.194–196 Altogether, these findings suggest that Rho kinase may regulate cerebellar functional hyperemia at some level. Additionally, a role for Rho kinase has been demonstrated in the hypertensive brainstem, including likely novel vasodilation-independent effects of Rho kinase activation in the regulation of the sympathetic nervous system,197–199 suggesting that Rho kinase may underlie both vasogenic as well as neurogenic mechanisms of hypertension. Interestingly, early comparative studies on the cerebral microvasculature in spontaneously hypertensive rats versus normotensive Wistar-Kyoto rats revealed two- to five-fold increases in pericyte investment of endothelial cells as well as loss of normal stress fiber distribution in pericytes associated with the hypertensive microvasculature both in situ and in cell culture.200, 201 This work suggests that Rho kinase-mediated pericyte contractility may be a novel means of local tone regulation, particularly in the pericyte-rich cerebral microvasculature.
Parallel evidence from the retinal microvasculature supports and extends the hypothesis that Rho kinase signaling in pericytes may play a principle role in tone regulation in other capillary beds as well. Calcium-dependent chloride channel activation downstream of multiple vasoactive ligands mediates pericyte contractility in the retina,202–206 in which nitric oxide has again been shown to counterbalance ligand-mediated contraction by promoting pericyte relaxation.207, 208 The retina is a particularly elegant microvascular bed for the investigation of pericyte-autonomous control of local microvascular tone, as it is one of the most densely pericyte-invested vascular beds in the human body209 and lacks the smooth muscle precapillary sphincters that play a regulatory role in many other vascular beds.210 Careful dissection of smooth muscle signaling pathways underlying tone dysregulation in the arterial system of both spontaneously hypertensive as well as angiotensin II-treated rodent models of hypertension implicate activation of Rho kinase downstream of angiotensin receptor-1211–214 in smooth muscle contraction; this contractile impulse appears to be dynamically balanced by the countervailing Rho kinase-mediated release of nitric oxide from adjacent endothelial cells,215–217 suggesting cell-type specificity in the downstream signaling of Rho kinase. Recent work from our laboratory highlights the role of signaling via Rho kinase in the regulation of pericyte contractility as well as both pericyte contact-dependent and -independent control of endothelial growth state in vitro.61 Specifically, activation of Rho signaling in pericytes was seen to cause a hypercontractile phenotype concomitant with a failure to growth arrest nearby endothelial cells, demonstrating failure of the mechanochemical feedback which normally regulates microvascular tone (see Figure 3 and 4). Indeed, Rho and the Rho kinase pathway may play a parallel role to that of smooth muscle regulation at the arteriolar level by controlling pericyte contractility at the level of the capillary bed in vivo.
Interestingly, recent results indicate that the pericyte may in fact be of previously unrecognized importance not only at the local capillary bed level, but as a mediator of the systemic effects of microvascular tone dysregulation. In both cerebellar cortex and retina, as well as in contracting striated muscle, increased oxygen demand initiates a vasodilatory response in nearby capillary segments that propagates through a gap junctional network of capillary endothelial cell-associated pericytes, extending far enough to dilate proximally-located precapillary arterioles.218–222 This work implies that regulation of vascular tone in some capillary beds may in fact originate locally at the level of the capillary-associated pericyte, with subsequent feedback conducted upstream to proximal arteriole-associated smooth muscle cells. This intriguing possibility suggests that some elements of vascular tone regulation previously attributed to smooth muscle alone may, in fact, be pericyte-mediated. If this is so, further investigation into the regulation of microvascular tone and endothelial cell function may elucidate novel capillary-level signaling mechanisms, orchestrated by the activity of multiple perivascular cell types.
Conclusions and future directions
Recent work indicates that Rho GTPase and its downstream signaling cascade, previously thought to be confined to vascular smooth muscle cells, has been functionally extended to include the microvascular pericyte. Although the bulk of current literature has investigated the specific nuances of vascular smooth muscle cell-associated and Rho GTPase-dependent signaling, interest is currently developing in the exploration of parallel but distinct roles for Rho signaling in the microvascular pericyte. This body of work proceeds from the first suggestion of independent mechanisms of regulation between arterial and capillary tone, framed in August Krogh’s 1920 Nobel Prize acceptance speech, initially implicating ‘Rouget cells’ (now known as pericytes) as specific regulators of capillary contractility.223 Subsequent work has described critical components of microvascular cross-talk between pericytes and endothelial cells involving soluble, matrix-mediated, and direct contact-mediated pathways. These interactions have been further shown to dynamically regulate the physiology of vascular development, capillary permeability, and microvascular tone regulation as well as the pathophysiology accompanying tumor angiogenesis, macular degeneration, and diabetic neuropathy. A fruitful area of recent inquiry has emerged in elucidating the role of Rho signaling in pulmonary and systemic hypertension, with potential therapeutic uses in multiple human pulmonary and cardiovascular disease states. While much of this work has focused on Rho signaling in smooth muscle cells, we postulate that Rho signaling in pericytes plays parallel but unique roles in the regulation of microvascular tone – particularly within such pericyte-rich vascular beds as the central nervous system, the retinal microcirculation, and striated muscle – via capillary-autonomous dynamic fluctuations in vascular tone above and beyond precapillary arteriolar contractility. Indeed, we predict that a deepened understanding of the detailed mechanisms of dynamic capillary-level regulation of tone will likely complement and significantly extend current models of end-organ damage in systemic hypertension. In turn, this would present a new avenue for novel drug discovery and the hopeful creation of innovative therapeutic modalities.
In the pursuit of understanding the dynamics of Rho signaling in pericytes, several challenges exist. Signaling mediators specifically linking Rho and Rho kinase with the pericyte actin cytoskeleton must be carefully elucidated. And, while several in vitro models exist for the fine dissection of these pathways in pericyte contractility, cell-to-cell signaling, and pericyte-endothelial interactions, the nature of biochemical cross-talk between the various microvascular cell types involved must be carefully dissected. To this end, the mechanochemical signaling that links contractile force production with respect to matrix and neighboring cells must also be examined; this will likely require innovative three-dimensional systems and interdisciplinary investigation of the biophysics principles involved governing this dynamic reciprocity. As well, in vivo systems for the examination and elucidation of pericyte-specific, Rho GTP-dependent signaling are also needed. In this way, the dynamic and specific contributions of pericytes and pericyte-dependent signal transduction can be dissected and characterized in situ. We look forward to the next decades of microvascular research, which should help to unveil the regulatory roles that pericytes play in the global control of vascular dynamics.
Figure 2. Altered Rho GTPase signaling modulates pericyte contractile phenotype.
Bovine retinal pericytes were transduced with dominant-active (RhoDA) or dominant-negative (RhoDN) Rho GTPase-expressing adenovirus, tetracycline-repressible transactivator (Tet)-expressing adenovirus, or were mock-infected (Control). Pericytes were then re-plated onto plasma glow discharge-prepared, Type I collagen-coated silicon substrates containing 0–10 μM of the small molecule Rho kinase inhibitor Y-27632, and monitored by real-time phase contrast imaging. A: Representative images are provided in as labeled, where arrows indicate substrate-wrinkling, actively contractile pericytes; images are 400x. B: After 24 hours, force transduction was quantified as the percentage of cells capable of producing substrate-deforming contractile force sufficient to produce substrate wrinkling visible by phase-contrast microscopy.
Acknowledgments
We wish to recognize the technical assistance of Lindsey Wolf and Katy Riley, and the parallel experiments of Jennifer Durham. Adenoviral construct assistance and reagents provided by Dr. Howard Surks. Support for this work is partially provided by a Williams Fellowship (MEK) and NIH EY 15125 (IMH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 4.Hungerford JE, Little CD. Developmental biology of the vascular smooth muscle cell: building a multilayered vessel wall. J Vasc Res. 1999;36:2–27. doi: 10.1159/000025622. [DOI] [PubMed] [Google Scholar]
- 5.D’Amore PA, Orlidge A, Herman IM. Growth control in the retinal microvasculature. Progress in retinal research. 1998;7:233–258. [Google Scholar]
- 6.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–98. [PubMed] [Google Scholar]
- 7.Beck L, Jr, D’Amore PA. Vascular development: cellular and molecular regulation. Faseb J. 1997;11:365–73. [PubMed] [Google Scholar]
- 8.Darland DC, D’Amore PA. Cell-cell interactions in vascular development. Curr Top Dev Biol. 2001;52:107–49. doi: 10.1016/s0070-2153(01)52010-4. [DOI] [PubMed] [Google Scholar]
- 9.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–87. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 10.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–70. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 11.Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7:860–72. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 12.Fong DS, Aiello LP, Ferris FL, 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 2004;27:2540–53. doi: 10.2337/diacare.27.10.2540. [DOI] [PubMed] [Google Scholar]
- 13.Cogolludo A, Moreno L, Villamor E. Mechanisms controlling vascular tone in pulmonary arterial hypertension: implications for vasodilator therapy. Pharmacology. 2007;79:65–75. doi: 10.1159/000097754. [DOI] [PubMed] [Google Scholar]
- 14.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259:381–92. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 15.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 16.Hirschi KK, D’Amore PA. Control of angiogenesis by the pericyte: molecular mechanisms and significance. Exs. 1997;79:419–28. doi: 10.1007/978-3-0348-9006-9_18. [DOI] [PubMed] [Google Scholar]
- 17.Herman IM, D’Amore PA. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol. 1985;101:43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rucker M, Strobel O, Vollmar B, Roesken F, Menger MD. Vasomotion in critically perfused muscle protects adjacent tissues from capillary perfusion failure. Am J Physiol Heart Circ Physiol. 2000;279:H550–8. doi: 10.1152/ajpheart.2000.279.2.H550. [DOI] [PubMed] [Google Scholar]
- 19.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–9. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Yamagishi S, Imaizumi T. Pericyte biology and diseases. Int J Tissue React. 2005;27:125–35. [PubMed] [Google Scholar]
- 21.Rouget C. Mémoire sur le développement, la structure et les proprietés physiologiques des capillaires sanguins et lymphatiques. Arch Physiol Norm et Path. 1873:603–663. [Google Scholar]
- 22.Papetti M, Shujath J, Riley KN, Herman IM. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: a role for myf-5 and Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci. 2003;44:4994–5005. doi: 10.1167/iovs.03-0291. [DOI] [PubMed] [Google Scholar]
- 23.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–64. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. Exs. 2005:115–25. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- 25.Darland DC, D’Amore PA. Blood vessel maturation: vascular development comes of age. J Clin Invest. 1999;103:157–8. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirschi KK, Rohovsky SA, D’Amore PA. Cell-cell interactions in vessel assembly: a model for the fundamentals of vascular remodelling. Transpl Immunol. 1997;5:177–8. doi: 10.1016/s0966-3274(97)80034-2. [DOI] [PubMed] [Google Scholar]
- 27.Orlidge A, D’Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987;105:1455–62. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–15. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denekamp J, Hobson B. Endothelial-cell proliferation in experimental tumours. Br J Cancer. 1982;46:711–20. doi: 10.1038/bjc.1982.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engerman RL, Pfaffenbach D, Davis MD. Cell turnover of capillaries. Lab Invest. 1967;17:738–43. [PubMed] [Google Scholar]
- 31.Hirst LW, Smiddy WE, Kays DL, Hoffman R, Stark WJ. Transmitted light microscopy of the unstained endothelial cell layer of excised human corneal tissue. Invest Ophthalmol Vis Sci. 1982;22:811–8. [PubMed] [Google Scholar]
- 32.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–8. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 33.El-Bizri N, Guignabert C, Wang L, et al. SM22alpha-targeted deletion of bone morphogenetic protein receptor 1A in mice impairs cardiac and vascular development, and influences organogenesis. Development. 2008;135:2981–91. doi: 10.1242/dev.017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellstrom M, Gerhardt H, Kalen M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–53. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–5. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 36.Crocker DJ, Murad TM, Geer JC. Role of the pericyte in wound healing. An ultrastructural study. Exp Mol Pathol. 1970;13:51–65. doi: 10.1016/0014-4800(70)90084-5. [DOI] [PubMed] [Google Scholar]
- 37.Hammes HP. Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res. 2005;37(Suppl 1):39–43. doi: 10.1055/s-2005-861361. [DOI] [PubMed] [Google Scholar]
- 38.Kuwabara T, Cogan DG. Retinal vascular patterns. VI. Mural cells of the retinal capillaries. Arch Ophthalmol. 1963;69:492–502. doi: 10.1001/archopht.1963.00960040498013. [DOI] [PubMed] [Google Scholar]
- 39.Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV Diabetic retinopathy. Arch Ophthalmol. 1961;66:366–78. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- 40.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–65. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Healy AM, Herman IM. Preparation of fluorescent basic fibroblast growth factor: localization in living retinal microvascular endothelial cells. Exp Eye Res. 1992;55:663–9. doi: 10.1016/0014-4835(92)90171-n. [DOI] [PubMed] [Google Scholar]
- 42.Newcomb PM, Herman IM. Pericyte growth and contractile phenotype: modulation by endothelial-synthesized matrix and comparison with aortic smooth muscle. J Cell Physiol. 1993;155:385–93. doi: 10.1002/jcp.1041550220. [DOI] [PubMed] [Google Scholar]
- 43.Healy AM, Herman IM. Density-dependent accumulation of basic fibroblast growth factor in the subendothelial matrix. Eur J Cell Biol. 1992;59:56–67. [PubMed] [Google Scholar]
- 44.Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–21. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindblom P, Gerhardt H, Liebner S, et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–40. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–55. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 47.Bjarnegard M, Enge M, Norlin J, et al. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–57. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- 48.Wilkinson-Berka JL, Babic S, De Gooyer T, et al. Inhibition of platelet-derived growth factor promotes pericyte loss and angiogenesis in ischemic retinopathy. Am J Pathol. 2004;164:1263–73. doi: 10.1016/s0002-9440(10)63214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bagley RG, Rouleau C, Morgenbesser SD, et al. Pericytes from human non-small cell lung carcinomas: an attractive target for anti-angiogenic therapy. Microvasc Res. 2006;71:163–74. doi: 10.1016/j.mvr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. Faseb J. 2004;18:338–40. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 52.Farhadi MR, Capelle HH, Erber R, Ullrich A, Vajkoczy P. Combined inhibition of vascular endothelial growth factor and platelet-derived growth factor signaling: effects on the angiogenesis, microcirculation, and growth of orthotopic malignant gliomas. J Neurosurg. 2005;102:363–70. doi: 10.3171/jns.2005.102.2.0363. [DOI] [PubMed] [Google Scholar]
- 53.Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23:939–52. doi: 10.1200/JCO.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 54.Shaheen RM, Tseng WW, Davis DW, et al. Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms. Cancer Res. 2001;61:1464–8. [PubMed] [Google Scholar]
- 55.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–9. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jo N, Mailhos C, Ju M, et al. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 2006;168:2036–53. doi: 10.2353/ajpath.2006.050588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai J, Kehoe O, Smith GM, Hykin P, Boulton ME. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:2163–71. doi: 10.1167/iovs.07-1206. [DOI] [PubMed] [Google Scholar]
- 58.Pfister F, Feng Y, Vom Hagen F, et al. Pericyte migration: A novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes. 2008 doi: 10.2337/db08-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163:1801–15. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kutcher ME, Kolyada AY, Surks HK, Herman IM. Pericyte Rho GTPase mediates both pericyte contractile phenotype and capillary endothelial growth state. Am J Pathol. 2007;171:693–701. doi: 10.2353/ajpath.2007.070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bertolino P, Deckers M, Lebrin F, ten Dijke P. Transforming growth factor-beta signal transduction in angiogenesis and vascular disorders. Chest. 2005;128:585S–590S. doi: 10.1378/chest.128.6_suppl.585S. [DOI] [PubMed] [Google Scholar]
- 63.Brown JD, DiChiara MR, Anderson KR, Gimbrone MA, Jr, Topper JN. MEKK-1, a component of the stress (stress-activated protein kinase/c-Jun N-terminal kinase) pathway, can selectively activate Smad2-mediated transcriptional activation in endothelial cells. J Biol Chem. 1999;274:8797–805. doi: 10.1074/jbc.274.13.8797. [DOI] [PubMed] [Google Scholar]
- 64.Topper JN, DiChiara MR, Brown JD, et al. CREB binding protein is a required coactivator for Smad-dependent, transforming growth factor beta transcriptional responses in endothelial cells. Proc Natl Acad Sci U S A. 1998;95:9506–11. doi: 10.1073/pnas.95.16.9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Topper JN, Cai J, Qiu Y, et al. Vascular MADs: two novel MAD-related genes selectively inducible by flow in human vascular endothelium. Proc Natl Acad Sci U S A. 1997;94:9314–9. doi: 10.1073/pnas.94.17.9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flanders KC, Thompson NL, Cissel DS, et al. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989;108:653–60. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dinbergs ID, Brown L, Edelman ER. Cellular response to transforming growth factor-beta1 and basic fibroblast growth factor depends on release kinetics and extracellular matrix interactions. J Biol Chem. 1996;271:29822–9. doi: 10.1074/jbc.271.47.29822. [DOI] [PubMed] [Google Scholar]
- 68.Laun RA, Schroder O, Schoppnies M, Roher HD, Ekkernkamp A, Schulte KM. Transforming growth factor-beta1 and major trauma: time-dependent association with hepatic and renal insufficiency. Shock. 2003;19:16–23. doi: 10.1097/00024382-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–76. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pittet JF, Griffiths MJ, Geiser T, et al. TGF-beta is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–44. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Birukova AA, Birukov KG, Adyshev D, et al. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol. 2005;204:934–47. doi: 10.1002/jcp.20359. [DOI] [PubMed] [Google Scholar]
- 72.Clements RT, Minnear FL, Singer HA, Keller RS, Vincent PA. RhoA and Rho-kinase dependent and independent signals mediate TGF-beta-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol. 2005;288:L294–306. doi: 10.1152/ajplung.00213.2004. [DOI] [PubMed] [Google Scholar]
- 73.Shih SC, Ju M, Liu N, Mo JR, Ney JJ, Smith LE. Transforming growth factor beta1 induction of vascular endothelial growth factor receptor 1: mechanism of pericyte-induced vascular survival in vivo. Proc Natl Acad Sci U S A. 2003;100:15859–64. doi: 10.1073/pnas.2136855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antonelli-Orlidge A, Saunders KB, Smith SR, D’Amore PA. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989;86:4544–8. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Darland DC, D’Amore PA. TGF beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis. 2001;4:11–20. doi: 10.1023/a:1016611824696. [DOI] [PubMed] [Google Scholar]
- 76.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–88. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 77.Kocher O, Madri JA. Modulation of actin mRNAs in cultured vascular cells by matrix components and TGF-beta 1. In Vitro Cell Dev Biol. 1989;25:424–34. doi: 10.1007/BF02624627. [DOI] [PubMed] [Google Scholar]
- 78.Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin DB. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol. 1990;111:757–63. doi: 10.1083/jcb.111.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vinals F, Pouyssegur J. Transforming growth factor beta1 (TGF-beta1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-alpha signaling. Mol Cell Biol. 2001;21:7218–30. doi: 10.1128/MCB.21.21.7218-7230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–34. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sheppard D. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev. 2005;24:395–402. doi: 10.1007/s10555-005-5131-6. [DOI] [PubMed] [Google Scholar]
- 82.Kondo T, Hosoya K, Hori S, Tomi M, Ohtsuki S, Terasaki T. PKC/MAPK signaling suppression by retinal pericyte conditioned medium prevents retinal endothelial cell proliferation. J Cell Physiol. 2005;203:378–86. doi: 10.1002/jcp.20237. [DOI] [PubMed] [Google Scholar]
- 83.Kolyada AY, Riley KN, Herman IM. Rho GTPase signaling modulates cell shape and contractile phenotype in an isoactin-specific manner. Am J Physiol Cell Physiol. 2003;285:C1116–21. doi: 10.1152/ajpcell.00177.2003. [DOI] [PubMed] [Google Scholar]
- 84.Hirschi KK, Rohovsky SA, D’Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–14. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ding R, Darland DC, Parmacek MS, D’Amore PA. Endothelial-mesenchymal interactions in vitro reveal molecular mechanisms of smooth muscle/pericyte differentiation. Stem Cells Dev. 2004;13:509–20. doi: 10.1089/scd.2004.13.509. [DOI] [PubMed] [Google Scholar]
- 86.Bryan BA, D’Amore PA. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol Life Sci. 2007;64:2053–65. doi: 10.1007/s00018-007-7008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mammoto A, Mammoto T, Ingber DE. Rho signaling and mechanical control of vascular development. Curr Opin Hematol. 2008;15:228–34. doi: 10.1097/MOH.0b013e3282fa7445. [DOI] [PubMed] [Google Scholar]
- 88.Pacaud P, Sauzeau V, Loirand G. Rho proteins and vascular diseases. Arch Mal Coeur Vaiss. 2005;98:249–54. [PubMed] [Google Scholar]
- 89.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290:C661–8. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–34. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 91.Lee DL, Webb RC, Jin L. Hypertension and RhoA/Rho-kinase signaling in the vasculature: highlights from the recent literature. Hypertension. 2004;44:796–9. doi: 10.1161/01.HYP.0000148303.98066.ab. [DOI] [PubMed] [Google Scholar]
- 92.Hirata K, Kikuchi A, Sasaki T, et al. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. J Biol Chem. 1992;267:8719–22. [PubMed] [Google Scholar]
- 93.Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–53. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qiu RG, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci U S A. 1995;92:11781–5. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Just I, Fritz G, Aktories K, et al. Clostridium difficile toxin B acts on the GTP-binding protein Rho. J Biol Chem. 1994;269:10706–12. [PubMed] [Google Scholar]
- 96.Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol. 2007;50:17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ishizaki T, Uehata M, Tamechika I, et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57:976–83. [PubMed] [Google Scholar]
- 98.Takayasu M, Suzuki Y, Shibuya M, et al. The effects of HA compound calcium antagonists on delayed cerebral vasospasm in dogs. J Neurosurg. 1986;65:80–5. doi: 10.3171/jns.1986.65.1.0080. [DOI] [PubMed] [Google Scholar]
- 99.Asano T, Ikegaki I, Satoh S, et al. Mechanism of action of a novel antivasospasm drug, HA1077. J Pharmacol Exp Ther. 1987;241:1033–40. [PubMed] [Google Scholar]
- 100.Uehata M, Ishizaki T, Satoh H, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–4. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 101.Shibuya M, Suzuki Y, Sugita K, et al. Dose escalation trial of a novel calcium antagonist, AT877, in patients with aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 1990;107:11–5. doi: 10.1007/BF01402606. [DOI] [PubMed] [Google Scholar]
- 102.Shibuya M, Suzuki Y, Sugita K, et al. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg. 1992;76:571–7. doi: 10.3171/jns.1992.76.4.0571. [DOI] [PubMed] [Google Scholar]
- 103.Rikitake Y, Kim HH, Huang Z, et al. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–7. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003;41:15–9. doi: 10.1016/s0735-1097(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 105.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–7. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 106.Hattori T, Shimokawa H, Higashi M, et al. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234–9. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 107.Ishimaru K, Ueno H, Kagitani S, Takabayashi D, Takata M, Inoue H. Fasudil attenuates myocardial fibrosis in association with inhibition of monocyte/macrophage infiltration in the heart of DOCA/salt hypertensive rats. J Cardiovasc Pharmacol. 2007;50:187–94. doi: 10.1097/FJC.0b013e318064f150. [DOI] [PubMed] [Google Scholar]
- 108.Negoro N, Hoshiga M, Seto M, et al. The kinase inhibitor fasudil (HA-1077) reduces intimal hyperplasia through inhibiting migration and enhancing cell loss of vascular smooth muscle cells. Biochem Biophys Res Commun. 1999;262:211–5. doi: 10.1006/bbrc.1999.1129. [DOI] [PubMed] [Google Scholar]
- 109.Fukumoto Y, Tawara S, Shimokawa H. Recent progress in the treatment of pulmonary arterial hypertension: expectation for rho-kinase inhibitors. Tohoku J Exp Med. 2007;211:309–20. doi: 10.1620/tjem.211.309. [DOI] [PubMed] [Google Scholar]
- 110.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25:1767–75. doi: 10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- 111.Moriyama T, Nagatoya K. The Rho-ROCK system as a new therapeutic target for preventing interstitial fibrosis. Drug News Perspect. 2004;17:29–34. doi: 10.1358/dnp.2004.17.1.829023. [DOI] [PubMed] [Google Scholar]
- 112.Hiraga A, Kuwabara S, Doya H, et al. Rho-kinase inhibition enhances axonal regeneration after peripheral nerve injury. J Peripher Nerv Syst. 2006;11:217–24. doi: 10.1111/j.1529-8027.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 113.Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20:242–8. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hilgers RH, Webb RC. Molecular aspects of arterial smooth muscle contraction: focus on Rho. Exp Biol Med (Maywood) 2005;230:829–35. doi: 10.1177/153537020523001107. [DOI] [PubMed] [Google Scholar]
- 115.Gerthoffer WT. Actin cytoskeletal dynamics in smooth muscle contraction. Can J Physiol Pharmacol. 2005;83:851–6. doi: 10.1139/y05-088. [DOI] [PubMed] [Google Scholar]
- 116.Lee JS, Kang Decker N, Chatterjee S, Yao J, Friedman S, Shah V. Mechanisms of nitric oxide interplay with Rho GTPase family members in modulation of actin membrane dynamics in pericytes and fibroblasts. Am J Pathol. 2005;166:1861–70. doi: 10.1016/S0002-9440(10)62495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–9. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- 118.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- 119.Ito M, Nakano T, Erdodi F, Hartshorne DJ. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- 120.Seko T, Ito M, Kureishi Y, et al. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res. 2003;92:411–8. doi: 10.1161/01.RES.0000059987.90200.44. [DOI] [PubMed] [Google Scholar]
- 121.Kawano Y, Fukata Y, Oshiro N, et al. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023–38. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Surks HK, Richards CT, Mendelsohn ME. Myosin phosphatase-Rho interacting protein. A new member of the myosin phosphatase complex that directly binds RhoA. J Biol Chem. 2003;278:51484–93. doi: 10.1074/jbc.M305622200. [DOI] [PubMed] [Google Scholar]
- 123.Surks HK, Riddick N, Ohtani K. M-RIP targets myosin phosphatase to stress fibers to regulate myosin light chain phosphorylation in vascular smooth muscle cells. J Biol Chem. 2005;280:42543–51. doi: 10.1074/jbc.M506863200. [DOI] [PubMed] [Google Scholar]
- 124.Riddick N, Ohtani K, Surks HK. Targeting by myosin phosphatase-RhoA interacting protein mediates RhoA/ROCK regulation of myosin phosphatase. J Cell Biochem. 2008;103:1158–70. doi: 10.1002/jcb.21488. [DOI] [PubMed] [Google Scholar]
- 125.Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 126.Arber S, Barbayannis FA, Hanser H, et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–9. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 127.Yang N, Higuchi O, Ohashi K, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–12. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 128.Amano T, Tanabe K, Eto T, Narumiya S, Mizuno K. LIM-kinase 2 induces formation of stress fibres, focal adhesions and membrane blebs, dependent on its activation by Rho-associated kinase-catalysed phosphorylation at threonine-505. Biochem J. 2001;354:149–59. doi: 10.1042/0264-6021:3540149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sumi T, Matsumoto K, Takai Y, Nakamura T. Cofilin phosphorylation and actin cytoskeletal dynamics regulated by rho- and Cdc42-activated LIM-kinase 2. J Cell Biol. 1999;147:1519–32. doi: 10.1083/jcb.147.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem. 2001;276:670–6. doi: 10.1074/jbc.M007074200. [DOI] [PubMed] [Google Scholar]
- 131.Louvet-Vallee S. ERM proteins: from cellular architecture to cell signaling. Biol Cell. 2000;92:305–16. doi: 10.1016/s0248-4900(00)01078-9. [DOI] [PubMed] [Google Scholar]
- 132.Matsui T, Maeda M, Doi Y, et al. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–57. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Matsui T, Yonemura S, Tsukita S. Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr Biol. 1999;9:1259–62. doi: 10.1016/s0960-9822(99)80508-9. [DOI] [PubMed] [Google Scholar]
- 134.Yonemura S, Matsui T, Tsukita S. Rho-dependent and -independent activation mechanisms of ezrin/radixin/moesin proteins: an essential role for polyphosphoinositides in vivo. J Cell Sci. 2002;115:2569–80. doi: 10.1242/jcs.115.12.2569. [DOI] [PubMed] [Google Scholar]
- 135.Nwariaku FE, Rothenbach P, Liu Z, Zhu X, Turnage RH, Terada LS. Rho inhibition decreases TNF-induced endothelial MAPK activation and monolayer permeability. J Appl Physiol. 2003;95:1889–95. doi: 10.1152/japplphysiol.00225.2003. [DOI] [PubMed] [Google Scholar]
- 136.Galaria II, Fegley AJ, Nicholl SM, Roztocil E, Davies MG. Differential regulation of ERK1/2 and p38(MAPK) by components of the Rho signaling pathway during sphingosine-1-phosphate-induced smooth muscle cell migration. J Surg Res. 2004;122:173–9. doi: 10.1016/j.jss.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 137.Guay J, Lambert H, Gingras Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110( Pt 3):357–68. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- 138.Larsen JK, Yamboliev IA, Weber LA, Gerthoffer WT. Phosphorylation of the 27-kDa heat shock protein via p38 MAP kinase and MAPKAP kinase in smooth muscle. Am J Physiol. 1997;273:L930–40. doi: 10.1152/ajplung.1997.273.5.L930. [DOI] [PubMed] [Google Scholar]
- 139.Wang M, Sankula R, Tsai BM, et al. P38 MAPK mediates myocardial proinflammatory cytokine production and endotoxin-induced contractile suppression. Shock. 2004;21:170–4. doi: 10.1097/01.shk.0000110623.20647.aa. [DOI] [PubMed] [Google Scholar]
- 140.Nick JA, Young SK, Brown KK, et al. Role of p38 mitogen-activated protein kinase in a murine model of pulmonary inflammation. J Immunol. 2000;164:2151–9. doi: 10.4049/jimmunol.164.4.2151. [DOI] [PubMed] [Google Scholar]
- 141.Johnson A, Ferro TJ. TNF-alpha augments pulmonary vasoconstriction via the inhibition of nitrovasodilator activity. J Appl Physiol. 1992;73:2483–92. doi: 10.1152/jappl.1992.73.6.2483. [DOI] [PubMed] [Google Scholar]
- 142.Liu SF, Crawley DE, Rohde JA, Evans TW, Barnes PJ. Role of nitric oxide and guanosine 3′,5′-cyclic monophosphate in mediating nonadrenergic, noncholinergic relaxation in guinea-pig pulmonary arteries. Br J Pharmacol. 1992;107:861–6. doi: 10.1111/j.1476-5381.1992.tb14538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ertel W, Morrison MH, Ayala A, Chaudry IH. Hypoxemia in the absence of blood loss or significant hypotension causes inflammatory cytokine release. Am J Physiol. 1995;269:R160–6. doi: 10.1152/ajpregu.1995.269.1.R160. [DOI] [PubMed] [Google Scholar]
- 144.Castaneda J, Isusi A, Tamayo L. Hypoxic pulmonary vasoconstriction increases during endotoxemia in the perfused rat lung. J Trauma. 2001;50:882–6. doi: 10.1097/00005373-200105000-00018. [DOI] [PubMed] [Google Scholar]
- 145.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 146.Hoshijima M, Sah VP, Wang Y, Chien KR, Brown JH. The low molecular weight GTPase Rho regulates myofibril formation and organization in neonatal rat ventricular myocytes. Involvement of Rho kinase. J Biol Chem. 1998;273:7725–30. doi: 10.1074/jbc.273.13.7725. [DOI] [PubMed] [Google Scholar]
- 147.Kalman D, Gomperts SN, Hardy S, Kitamura M, Bishop JM. Ras family GTPases control growth of astrocyte processes. Mol Biol Cell. 1999;10:1665–83. doi: 10.1091/mbc.10.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208:177–9. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 149.Tsai MH, Jiang MJ. Rho-kinase-mediated regulation of receptor-agonist-stimulated smooth muscle contraction. Pflugers Arch. 2006;453:223–32. doi: 10.1007/s00424-006-0133-y. [DOI] [PubMed] [Google Scholar]
- 150.Woodsome TP, Polzin A, Kitazawa K, Eto M, Kitazawa T. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J Cell Sci. 2006;119:1769–80. doi: 10.1242/jcs.02805. [DOI] [PubMed] [Google Scholar]
- 151.Klagsbrun M, Moses MA. Molecular angiogenesis. Chem Biol. 1999;6:R217–24. doi: 10.1016/S1074-5521(99)80081-7. [DOI] [PubMed] [Google Scholar]
- 152.Sato M, Tani E, Fujikawa H, Kaibuchi K. Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ Res. 2000;87:195–200. doi: 10.1161/01.res.87.3.195. [DOI] [PubMed] [Google Scholar]
- 153.Watanabe Y, Faraci FM, Heistad DD. Activation of Rho-associated kinase during augmented contraction of the basilar artery to serotonin after subarachnoid hemorrhage. Am J Physiol Heart Circ Physiol. 2005;288:H2653–8. doi: 10.1152/ajpheart.00923.2004. [DOI] [PubMed] [Google Scholar]
- 154.Kandabashi T, Shimokawa H, Miyata K, et al. Inhibition of myosin phosphatase by upregulated rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1beta. Circulation. 2000;101:1319–23. doi: 10.1161/01.cir.101.11.1319. [DOI] [PubMed] [Google Scholar]
- 155.Kandabashi T, Shimokawa H, Miyata K, et al. Evidence for protein kinase C-mediated activation of Rho-kinase in a porcine model of coronary artery spasm. Arterioscler Thromb Vasc Biol. 2003;23:2209–14. doi: 10.1161/01.ATV.0000104010.87348.26. [DOI] [PubMed] [Google Scholar]
- 156.Hattori T, Shimokawa H, Higashi M, et al. Long-term treatment with a specific Rho-kinase inhibitor suppresses cardiac allograft vasculopathy in mice. Circ Res. 2004;94:46–52. doi: 10.1161/01.RES.0000107196.21335.2B. [DOI] [PubMed] [Google Scholar]
- 157.Qvigstad E, Sjaastad I, Brattelid T, et al. Dual serotonergic regulation of ventricular contractile force through 5-HT2A and 5-HT4 receptors induced in the acute failing heart. Circ Res. 2005;97:268–76. doi: 10.1161/01.RES.0000176970.22603.8d. [DOI] [PubMed] [Google Scholar]
- 158.Morishige K, Shimokawa H, Eto Y, et al. Adenovirus-mediated transfer of dominant-negative rho-kinase induces a regression of coronary arteriosclerosis in pigs in vivo. Arterioscler Thromb Vasc Biol. 2001;21:548–54. doi: 10.1161/01.atv.21.4.548. [DOI] [PubMed] [Google Scholar]
- 159.Mallat Z, Gojova A, Sauzeau V, et al. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res. 2003;93:884–8. doi: 10.1161/01.RES.0000099062.55042.9A. [DOI] [PubMed] [Google Scholar]
- 160.Noma K, Higashi Y, Jitsuiki D, et al. Smoking activates rho-kinase in smooth muscle cells of forearm vasculature in humans. Hypertension. 2003;41:1102–5. doi: 10.1161/01.HYP.0000067062.92836.9E. [DOI] [PubMed] [Google Scholar]
- 161.Higashi M, Shimokawa H, Hattori T, et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–75. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 162.Ming XF, Barandier C, Viswambharan H, et al. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation. 2004;110:3708–14. doi: 10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- 163.Wolfrum S, Dendorfer A, Rikitake Y, et al. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–7. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rikitake Y, Liao JK. Rho-kinase mediates hyperglycemia-induced plasminogen activator inhibitor-1 expression in vascular endothelial cells. Circulation. 2005;111:3261–8. doi: 10.1161/CIRCULATIONAHA.105.534024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Didion SP, Lynch CM, Baumbach GL, Faraci FM. Impaired endothelium-dependent responses and enhanced influence of Rho-kinase in cerebral arterioles in type II diabetes. Stroke. 2005;36:342–7. doi: 10.1161/01.STR.0000152952.42730.92. [DOI] [PubMed] [Google Scholar]
- 166.Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX. Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc Res. 2004;68:75–103. doi: 10.1016/j.mvr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 167.Morrell ED, Tsai BM, Crisostomo PR, et al. Therapeutic concepts for hypoxic pulmonary vasoconstriction involving ion regulation and the smooth muscle contractile apparatus. J Mol Cell Cardiol. 2006;40:751–60. doi: 10.1016/j.yjmcc.2006.03.431. [DOI] [PubMed] [Google Scholar]
- 168.Pak O, Aldashev A, Welsh D, Peacock A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J. 2007;30:364–72. doi: 10.1183/09031936.00128706. [DOI] [PubMed] [Google Scholar]
- 169.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 170.Nagaoka T, Fagan KA, Gebb SA, et al. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med. 2005;171:494–9. doi: 10.1164/rccm.200405-637OC. [DOI] [PubMed] [Google Scholar]
- 171.Guilluy C, Sauzeau V, Rolli-Derkinderen M, et al. Inhibition of RhoA/Rho kinase pathway is involved in the beneficial effect of sildenafil on pulmonary hypertension. Br J Pharmacol. 2005;146:1010–8. doi: 10.1038/sj.bjp.0706408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fagan KA, Oka M, Bauer NR, et al. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L656–64. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- 173.Abe K, Shimokawa H, Morikawa K, et al. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94:385–93. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- 174.Meyrick B, Reid L. Ultrastructural findings in lung biopsy material from children with congenital heart defects. Am J Pathol. 1980;101:527–42. [PMC free article] [PubMed] [Google Scholar]
- 175.Meyrick B, Fujiwara K, Reid L. Smooth muscle myosin in precursor and mature smooth muscle cells in normal pulmonary arteries and the effect of hypoxia. Exp Lung Res. 1981;2:303–13. doi: 10.3109/01902148109052325. [DOI] [PubMed] [Google Scholar]
- 176.Zhu P, Huang L, Ge X, Yan F, Wu R, Ao Q. Transdifferentiation of pulmonary arteriolar endothelial cells into smooth muscle-like cells regulated by myocardin involved in hypoxia-induced pulmonary vascular remodelling. Int J Exp Pathol. 2006;87:463–74. doi: 10.1111/j.1365-2613.2006.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 178.Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000;184:409–20. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 179.Ming XF, Viswambharan H, Barandier C, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–77. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sauzeau V, Rolli-Derkinderen M, Lehoux S, Loirand G, Pacaud P. Sildenafil prevents change in RhoA expression induced by chronic hypoxia in rat pulmonary artery. Circ Res. 2003;93:630–7. doi: 10.1161/01.RES.0000093220.90027.D9. [DOI] [PubMed] [Google Scholar]
- 181.Chitaley K, Webb RC. Nitric oxide induces dilation of rat aorta via inhibition of rho-kinase signaling. Hypertension. 2002;39:438–42. doi: 10.1161/hy02t2.102960. [DOI] [PubMed] [Google Scholar]
- 182.Carter RW, Begaye M, Kanagy NL. Acute and chronic NOS inhibition enhances alpha(2)- adrenoreceptor-stimulated RhoA and Rho kinase in rat aorta. Am J Physiol Heart Circ Physiol. 2002;283:H1361–9. doi: 10.1152/ajpheart.01101.2001. [DOI] [PubMed] [Google Scholar]
- 183.Kishi T, Hirooka Y, Masumoto A, et al. Rho-kinase inhibitor improves increased vascular resistance and impaired vasodilation of the forearm in patients with heart failure. Circulation. 2005;111:2741–7. doi: 10.1161/CIRCULATIONAHA.104.510248. [DOI] [PubMed] [Google Scholar]
- 184.Nohria A, Grunert ME, Rikitake Y, et al. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res. 2006;99:1426–32. doi: 10.1161/01.RES.0000251668.39526.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 186.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–65. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 187.Lauritzen M. Relationship of spikes, synaptic activity, and local changes of cerebral blood flow. J Cereb Blood Flow Metab. 2001;21:1367–83. doi: 10.1097/00004647-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 188.Akgoren N, Fabricius M, Lauritzen M. Importance of nitric oxide for local increases of blood flow in rat cerebellar cortex during electrical stimulation. Proc Natl Acad Sci U S A. 1994;91:5903–7. doi: 10.1073/pnas.91.13.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hayashi T, Katsumi Y, Mukai T, et al. Neuronal nitric oxide has a role as a perfusion regulator and a synaptic modulator in cerebellum but not in neocortex during somatosensory stimulation--an animal PET study. Neurosci Res. 2002;44:155–65. doi: 10.1016/s0168-0102(02)00122-0. [DOI] [PubMed] [Google Scholar]
- 190.Iadecola C, Li J, Ebner TJ, Xu X. Nitric oxide contributes to functional hyperemia in cerebellar cortex. Am J Physiol. 1995;268:R1153–62. doi: 10.1152/ajpregu.1995.268.5.R1153. [DOI] [PubMed] [Google Scholar]
- 191.Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:9157–64. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang G, Chen G, Ebner TJ, Iadecola C. Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. Am J Physiol. 1999;277:R1760–70. doi: 10.1152/ajpregu.1999.277.6.R1760. [DOI] [PubMed] [Google Scholar]
- 193.Yang G, Zhang Y, Ross ME, Iadecola C. Attenuation of activity-induced increases in cerebellar blood flow in mice lacking neuronal nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2003;285:H298–304. doi: 10.1152/ajpheart.00043.2003. [DOI] [PubMed] [Google Scholar]
- 194.Hashimoto R, Nakamura Y, Kosako H, et al. Distribution of Rho-kinase in the bovine brain. Biochem Biophys Res Commun. 1999;263:575–9. doi: 10.1006/bbrc.1999.1409. [DOI] [PubMed] [Google Scholar]
- 195.O’Kane EM, Stone TW, Morris BJ. Distribution of Rho family GTPases in the adult rat hippocampus and cerebellum. Brain Res Mol Brain Res. 2003;114:1–8. doi: 10.1016/s0169-328x(03)00121-9. [DOI] [PubMed] [Google Scholar]
- 196.Olenik C, Barth H, Just I, Aktories K, Meyer DK. Gene expression of the small GTP-binding proteins RhoA, RhoB, Rac1, and Cdc42 in adult rat brain. Brain Res Mol Brain Res. 1997;52:263–9. doi: 10.1016/s0169-328x(97)00270-2. [DOI] [PubMed] [Google Scholar]
- 197.Ito K, Hirooka Y, Sakai K, et al. Rho/Rho-kinase pathway in brain stem contributes to blood pressure regulation via sympathetic nervous system: possible involvement in neural mechanisms of hypertension. Circ Res. 2003;92:1337–43. doi: 10.1161/01.RES.0000079941.59846.D4. [DOI] [PubMed] [Google Scholar]
- 198.Ito K, Hirooka Y, Kishi T, et al. Rho/Rho-kinase pathway in the brainstem contributes to hypertension caused by chronic nitric oxide synthase inhibition. Hypertension. 2004;43:156–62. doi: 10.1161/01.HYP.0000114602.82140.a4. [DOI] [PubMed] [Google Scholar]
- 199.Ito K, Hirooka Y, Kimura Y, Shimokawa H, Takeshita A. Effects of hydroxyfasudil administered to the nucleus tractus solitarii on blood pressure and heart rate in spontaneously hypertensive rats. Clin Exp Hypertens. 2005;27:269–77. [PubMed] [Google Scholar]
- 200.Herman IM, Jacobson S. In situ analysis of microvascular pericytes in hypertensive rat brains. Tissue Cell. 1988;20:1–12. doi: 10.1016/0040-8166(88)90002-x. [DOI] [PubMed] [Google Scholar]
- 201.Herman IM, Newcomb PM, Coughlin JE, Jacobson S. Characterization of microvascular cell cultures from normotensive and hypertensive rat brains: pericyte-endothelial cell interactions in vitro. Tissue Cell. 1987;19:197–206. doi: 10.1016/0040-8166(87)90005-x. [DOI] [PubMed] [Google Scholar]
- 202.Kawamura H, Oku H, Li Q, Sakagami K, Puro DG. Endothelin-induced changes in the physiology of retinal pericytes. Invest Ophthalmol Vis Sci. 2002;43:882–8. [PubMed] [Google Scholar]
- 203.Kawamura H, Sugiyama T, Wu DM, et al. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2003;551:787–99. doi: 10.1113/jphysiol.2003.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kawamura H, Kobayashi M, Li Q, et al. Effects of angiotensin II on the pericyte-containing microvasculature of the rat retina. J Physiol. 2004;561:671–83. doi: 10.1113/jphysiol.2004.073098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wu DM, Kawamura H, Li Q, Puro DG. Dopamine activates ATP-sensitive K+ currents in rat retinal pericytes. Vis Neurosci. 2001;18:935–40. [PubMed] [Google Scholar]
- 206.Wu DM, Kawamura H, Sakagami K, Kobayashi M, Puro DG. Cholinergic regulation of pericyte-containing retinal microvessels. Am J Physiol Heart Circ Physiol. 2003;284:H2083–90. doi: 10.1152/ajpheart.01007.2002. [DOI] [PubMed] [Google Scholar]
- 207.Haefliger IO, Zschauer A, Anderson DR. Relaxation of retinal pericyte contractile tone through the nitric oxide-cyclic guanosine monophosphate pathway. Invest Ophthalmol Vis Sci. 1994;35:991–7. [PubMed] [Google Scholar]
- 208.Sakagami K, Kawamura H, Wu DM, Puro DG. Nitric oxide/cGMP-induced inhibition of calcium and chloride currents in retinal pericytes. Microvasc Res. 2001;62:196–203. doi: 10.1006/mvre.2001.2343. [DOI] [PubMed] [Google Scholar]
- 209.Shepro D, Morel NM. Pericyte physiology. Faseb J. 1993;7:1031–8. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 210.Pannarale L, Onori P, Ripani M, Gaudio E. Precapillary patterns and perivascular cells in the retinal microvasculature. A scanning electron microscope study. J Anat. 1996;188( Pt 3):693–703. [PMC free article] [PubMed] [Google Scholar]
- 211.Touyz RM, He G, El Mabrouk M, Diep Q, Mardigyan V, Schiffrin EL. Differential activation of extracellular signal-regulated protein kinase 1/2 and p38 mitogen activated-protein kinase by AT1 receptors in vascular smooth muscle cells from Wistar-Kyoto rats and spontaneously hypertensive rats. J Hypertens. 2001;19:553–9. doi: 10.1097/00004872-200103001-00006. [DOI] [PubMed] [Google Scholar]
- 212.Sagara Y, Hirooka Y, Nozoe M, Ito K, Kimura Y, Sunagawa K. Pressor response induced by central angiotensin II is mediated by activation of Rho/Rho-kinase pathway via AT1 receptors. J Hypertens. 2007;25:399–406. doi: 10.1097/HJH.0b013e328010b87f. [DOI] [PubMed] [Google Scholar]
- 213.Moriki N, Ito M, Seko T, et al. RhoA activation in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Hypertens Res. 2004;27:263–70. doi: 10.1291/hypres.27.263. [DOI] [PubMed] [Google Scholar]
- 214.Faraci FM, Lamping KG, Modrick ML, Ryan MJ, Sigmund CD, Didion SP. Cerebral vascular effects of angiotensin II: new insights from genetic models. J Cereb Blood Flow Metab. 2006;26:449–55. doi: 10.1038/sj.jcbfm.9600204. [DOI] [PubMed] [Google Scholar]
- 215.Budzyn K, Marley PD, Sobey CG. Opposing roles of endothelial and smooth muscle phosphatidylinositol 3-kinase in vasoconstriction: effects of rho-kinase and hypertension. J Pharmacol Exp Ther. 2005;313:1248–53. doi: 10.1124/jpet.104.082784. [DOI] [PubMed] [Google Scholar]
- 216.Lohn M, Steioff K, Bleich M, Busch AE, Ivashchenko Y. Inhibition of Rho-kinase stimulates nitric oxide-independent vasorelaxation. Eur J Pharmacol. 2005;507:179–86. doi: 10.1016/j.ejphar.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 217.Savoia C, Ebrahimian T, He Y, Gratton JP, Schiffrin EL, Touyz RM. Angiotensin II/AT2 receptor-induced vasodilation in stroke-prone spontaneously hypertensive rats involves nitric oxide and cGMP-dependent protein kinase. J Hypertens. 2006;24:2417–22. doi: 10.1097/01.hjh.0000251902.85675.7e. [DOI] [PubMed] [Google Scholar]
- 218.Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol. 1997;78:651–9. doi: 10.1152/jn.1997.78.2.651. [DOI] [PubMed] [Google Scholar]
- 219.Berg BR, Cohen KD, Sarelius IH. Direct coupling between blood flow and metabolism at the capillary level in striated muscle. Am J Physiol. 1997;272:H2693–700. doi: 10.1152/ajpheart.1997.272.6.H2693. [DOI] [PubMed] [Google Scholar]
- 220.Cohen KD, Berg BR, Sarelius IH. Remote arteriolar dilations in response to muscle contraction under capillaries. Am J Physiol Heart Circ Physiol. 2000;278:H1916–23. doi: 10.1152/ajpheart.2000.278.6.H1916. [DOI] [PubMed] [Google Scholar]
- 221.Cohen KD, Sarelius IH. Muscle contraction under capillaries in hamster muscle induces arteriolar dilatation via K(ATP) channels and nitric oxide. J Physiol. 2002;539:547–55. doi: 10.1113/jphysiol.2001.013388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–4. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Krogh A. A Contribution to the Physiology of the Capillaries. 1920 [Google Scholar]