Summary
Iron is essential for nearly all living organisms. Innate immunity effectively restricts iron availability to microbial invaders. Some microbes have evolved effective countermeasures that blunt the effect of iron restriction. Recent epidemiologic studies have highlighted the potentiating effect of iron on microbial infections. Laboratory studies have focused on specific immune mechanisms that mediate iron withholding from microbes constitutively and in response to infections. Specialized inflammation-regulated proteins chelate iron, trap siderophores, and transport iron or modulate its transport to alter its tissue distribution during infections.
Introduction
Iron is an essential trace element for humans and other vertebrate hosts, as well as for their microbial invaders. Although iron is abundant in the Earth’s crust, its natural forms are poorly soluble, and so its supply is often insufficient for optimal growth and function of either microbes or their hosts. This review discusses specific mechanisms of innate immunity that further restrict the availability of iron on host surfaces exposed to environmental microbes, and to an even greater extent in infected tissues. Within hours of infection in humans and other vertebrates, concentrations of iron in extracellular fluid and plasma dramatically decrease (hypoferremia). Macrophages involved in recycling iron from erythrocytes limit the release of the recycled iron, thus lowering the extracellular iron concentration. Under the influence of cytokines, macrophages infected by intracellular microbes inhibit their multiplication by moving iron from the phagosomes to cytoplasmic ferritin. As part of their host defense repertoire, neutrophils accumulating in infected tissues secrete proteins that chelate various forms of iron. Among microbes that are well adapted to their hosts, some have evolved countermeasures that allow them to obtain iron from host ferroproteins, including hemoglobin, ferritin, and transferrin, other microbes specifically interfere with iron-sequestering host mechanisms. Host genetics and nutritional/medical practices also affect tissue and extracellular iron concentrations and are thought to modulate susceptibility to infection.
Epidemiology of iron and infections
Accumulated evidence supports the concept that infections with a variety of microbes are increased in frequency and severity in humans with iron overload (due to iron supplementation, hereditary hemochromatosis, erythrocyte transfusions or unknown causes, as reviewed in [1;2] with recent new data [3–5]). Some infections (notably malaria) may be less frequent and less severe in iron-deficient patients [6;7] and this has raised questions about iron supplementation in regions endemic for malaria [8]. The potential mechanisms of the role of iron in susceptibility and resistance to infections have been recently reviewed [9–11]. Surprisingly, iron overload may exacerbate some virus-mediated diseases as well, either by facilitating viral replication or by promoting tissue injury or secondary infections [12].
Animal models of the interaction between iron and infections
The severity of infections in mice with the specialized human pathogen Neisseria meningiditis is strongly dose-dependent on the co-administration of human iron transferrin (holotransferrin) [13]. Neisseria have a specific iron acquisition receptor for human holotransferrin that does not recognize the murine version of the protein. Mice made transgenic in human transferrin developed prolonged bacteremia with N. meningiditis and but their wild-type counterparts rapidly cleared the infection [14]. In a mouse model of M. tuberculosis infection, iron supplementation resulted in about 10-fold increase in bacterial CFU compared to unsupplemented mice[15]. These models make it plausible that the hypoferremia associated with infections could significantly decrease the rate of microbial proliferation.
Specific mechanisms of iron sequestration in innate immunity
Multiple mechanisms of innate immunity restrict the iron supply to microbial invaders (Table I). Some of these mechanisms function constitutively to protect vulnerable exposed tissues but most are inducible by exposure to pathogen-associated molecules, either directly or in response to specific cytokines.
Table I.
Representative host mechanisms that sequester iron during infections
| Mechanism | Protein | Tissues | Response to infection | Effect on iron |
|---|---|---|---|---|
| High affinity binding of Fe | lactoferrin | Neutrophils, mucosal epithelia | Local degranulation or secretion | Decreased availability to most pathogens |
| High affinity binding of siderophores | lipocalin-2 | Neutrophils, epithelia, hepatocytes | Degranulation or secretion | Decreased availability to microbes using affected siderophores |
| Fe transport | Nramp1 (Slc11a1) | Macrophages, neutrophils | Activation in phagosomal membranes | Iron export from phagocytic vacuoles |
| Fe transport | ferroportin (Slc40a1) | Macrophages Enterocytes | Increased hepcidin degrades ferroportin | Decreased extracellular and plasma iron |
| Fe transport | transferrin receptor 1 (TfR1) | Many | Interferon-γ suppresses TfR1 expression in macrophages | Iron depletion of activated macrophages |
Lactoferrin
Lactoferrin is an 80kD glycoprotein structurally very similar to transferrin (reviewed in [16]). Like transferrin, it binds two ferric ions very tightly but differs from transferrin in not releasing the iron at acid pH prevalent in infected or hypoxic areas. Lactoferrin is found at high concentration in neutrophils and mucosal secretions. It has been reported to be antimicrobial through multiple mechanisms (reviewed in [17]) including its ability to chelate iron[18]. Surprisingly, mice with ablation of the lactoferrin gene had a very mild phenotype manifested by a two-fold increase in spontaneous staphylococcal infections but no difference in resistance to S. aureus and P. aeruginosa in various models of infection. These observations suggest that the function of lactoferrin is largely redundant or that lactoferrin more narrowly targets as yet unidentified specific pathogens.
Siderocalin (also called: lipocalin-2, neutrophil gelatinase-associated lipocalin or NGAL, or in mice: 24p3)
Siderocalin is a 24 kD protein that binds enterobactin of E. coli (also called enterochelin) and other catechol-related siderophores, small secreted organic iron chelators used by microbes to acquire iron from their environment (reviewed in [19]). Siderocalin is selectively bacteriostatic to bacteria that are dependent on siderophores scavenged by siderocalin. The synthesis of siderocalin is massively induced during sepsis and mice deficient in siderocalin are more susceptible to bacteremia and death from E. coli sepsis [20]. As indicated by increased mycobacterial invasion of epithelial cells in siderocalin-deficient mice, siderocalin may also be protective in the airways [21]. Recent studies have identified bacterial evasion mechanisms [19;22] that covalently modify enterobactin to weaken its interaction with siderocalin.
Natural resistance-associated macrophage protein-1 (Nramp1, also called Slc11a1)
Nramp1 is an 90–100kD integral membrane protein of macrophage and neutrophil phagosomes that functions as a divalent metal-proton symporter (reviewed in [23]). The role of the Nramp1 gene in innate immunity was originally identified because a mutation in Nramp1 occurring in common mouse strains conferred susceptibility to infection with intracellular microbes that localize in macrophages, including Mycobacteria, Salmonella typhimurium and Leishmania donovanii. Although controversy about the specific mechanism still persists, it now appears that Nramp1 acts by depleting Fe2+, Co2+ and Mn2+ from the phagosome, and thereby starving the phagosomal microbes of these essential nutrients. Moreover, a recent study suggests that Nramp1 also acts by an as yet unknown mechanism to decrease cellular iron content [24]. Both of these mechanisms should inhibit microbial proliferation within macrophages. Nramp1 expression in macrophages is transcriptionally induced by interferon-γ and lipolysaccharide through a mechanism involving the Interferon Regulatory Factor 8 [25], thus linking macrophage activation to a specific iron-dependent microbistatic mechanism.
Transferrin receptor-1 (TfR1)
Cell membrane TfR1 binds iron-transferrin (Tf-Fe) from plasma. After endocytosis, the TfR1-Tf-Fe complex releases its iron in acidified vacuoles, and TfR1-Tf returns to plasma membrane and returns (apo)Tf into plasma. TfR1 is essential for iron utilization by erythrocyte precursors but it is also present on many other cell types, including macrophages. Several studies have shown that TfR1 is reduced in activated macrophages and that interferon-γ potently suppresses TfR1 expression [26–29], and reduces iron incorporation by macrophages. Reduced availability of iron limits the multiplication of Legionella pneumophila, an intracellular bacterium that resides in macrophages. The mechanism of TfR1 suppression is not fully understood but may be in part mediated by the effects of inflammation-induced nitric oxide-nitrosonium on IRP2 [30].
Hepcidin and ferroportin
Hepcidin is a 2.7 kD peptide hormone that controls extracellular iron concentrations by regulating dietary iron absorption and the release of iron from stores in macrophages and hepatocytes. The receptor for hepcidin is the cellular iron exporter ferroportin, a 70kD integral membrane protein expressed in all iron-transporting tissues including duodenal epithelium, macrophages that recycle iron from senescent erythrocytes and iron-storing hepatocytes. When hepcidin binds to ferroportin, ferroportin is internalized and degraded, and the export of cellular iron to plasma is diminished (reviewed in [31;32]). The continued utilization of iron then depletes the relatively small pool of plasma and extracellular iron. In mice, the injection of 50 μg of hepcidin produces rapid and profound hypoferremia that lasts for 48 hours. During inflammation, the synthesis of hepcidin is transcriptionally induced by inflammatory stimuli and specifically by IL-6 [33] acting through the transcription factor STAT3 [34–36]. After an inflammatory stimulus such as IL-6 or lipopolysaccharide, the development of hypoferremia in human volunteers coincided with the rise in hepcidin[37;38]. The same response is seen in patients with malaria [39]. Thus, extracellular microbes face low iron concentrations during inflammation.
For intracellular microbes in phagosomes of macrophages, iron concentrations would be determined by the balance between mechanisms that move iron into and out of phagosomes. On one hand, hepcidin-mediated degradation of ferroportin and the resulting retention of iron in macrophage cytoplasmic ferritin could provide additional iron for intracellular pathogens, as suggested by the effects of hepcidin in macrophage-like cell lines and cultured macrophages, with or without ferroportin overexpression [40;41]. In some cells, ferroportin is found on phagosomal membranes [42] thus providing a pathway for moving hepcidin from ferritin into phagosomes. Ferroportin was not detected in inclusion bodies of Chlamydia, and it is not clear how the accumulated cellular iron reaches the bacteria. Mechanisms that favor iron movement out of phagosomes include the presence of inflammation-stimulated Nramp1 as well as Nramp2 in phagosomal membranes [23]. Additional mechanisms that may modulate the iron content of phagosomes include the autocrine production of hepcidin by macrophages [43] and the feedback effects of intracellular iron concentrations on ferroportin expression [44;45]. The ability of intracellular microbes to manipulate the composition of phagosomes, to alter the expression of iron transporters and to degrade ferritin [46] further complicates any attempts at modeling the net effect of inflammation on the subcellular iron distribution in macrophages.
What is the effect of the hepcidin-ferroportin interaction on innate immunity in vivo? Genetic deficiency of hepcidin is the fundamental cause of hereditary hemochromatosis, with additional cases due to ferroportin mutations causing resistance to hepcidin. Hepcidin deficiency may result from mutations in hepcidin regulators (hemochromatosis protein HFE, transferrin receptor 2 and hemojuvelin) or in the hepcidin gene itself. Hereditary hemochromatoses are known to be associated with a predisposition to a variety of infections [2], most characteristically with Yersinia and Vibrio spp. However, the specific associations are subject to reporting bias which may result in underreporting of common infections compared to exotic ones. More detailed studies are needed to establish whether the predisposition is due to baseline high iron saturation of plasma transferrin, or to the reduced ability of the patients with hemochromatosis or other forms of iron overload to develop inflammatory hypoferremia.
Conclusions
The evidence is strong that iron restriction is an important component of innate immunity, and a potential target for therapeutic intervention. The specific mechanisms are multiple and complex and are met with microbial countermeasures. The net effect of iron restriction on microbes in vivo is a timely subject for further study.
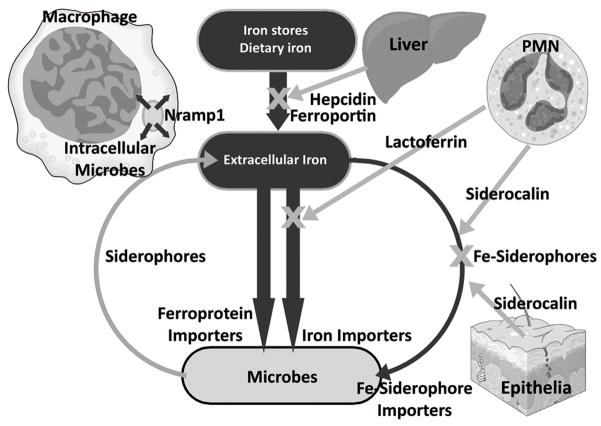
Figure 1.
Key host defense mechanisms restricting iron supply to microbes. Iron flows are shown in black.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1•.Boelaert JR, Vandecasteele SJ, Appelberg R, Gordeuk VR. The effect of the host’s iron status on tuberculosis. J Infect Dis. 2007;195:1745–1753. doi: 10.1086/518040. A comprehensive and balanced analysis of evidence for the role of iron in the pathogenesis of tuberculosis. [DOI] [PubMed] [Google Scholar]
- 2.Khan FA, Fisher MA, Khakoo RA. Association of hemochromatosis with infectious diseases: expanding spectrum. Int J Infect Dis. 2007;11:482–487. doi: 10.1016/j.ijid.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Vidal C, Upton A, Kirby KA, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041–1050. doi: 10.1086/591969. Cumulative erythrocyte transfusion history is associated with invasive mold infections in allogeneic hematopoietic stem cell transplant recipients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermid JM, Jaye A, Schim van der Loeff MF, Todd J, Bates C, Austin S, Jeffries D, Awasana AA, Whittlex AA, Prentice A. Elevated iron status strongly predicts mortality in West African adults with HIV infection. J Acquir Immune Defic Syndr. 2007;46:498–507. doi: 10.1097/qai.0b013e31815b2d4b. [DOI] [PubMed] [Google Scholar]
- 5•.Rawat R, Humphrey J, Ntozini R, Mutasa K, Iliff P, Stoltzfus R. Elevated iron stores are associated with HIV disease severity and mortality among postpartum women in Zimbabwe. Public Health Nutr. 2008:1–9. doi: 10.1017/S136898000800390X. 4 and 5. Markers of iron overload are associated with increased all-cause mortality in HIV infection but the specific mechanism responsible for increased deaths has not been pinpointed. [DOI] [PubMed] [Google Scholar]
- 6.Kabyemela ER, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Decreased susceptibility to Plasmodium falciparum infection in pregnant women with iron deficiency. J Infect Dis. 2008;198:163–166. doi: 10.1086/589512. Iron deficiency may protect against malaria through an as yet unknown mechanism. [DOI] [PubMed] [Google Scholar]
- 7•.Koka S, Foller M, Lamprecht G, Boini KM, Lang C, Huber SM, Lang F. Iron deficiency influences the course of malaria in Plasmodium berghei infected mice. Biochem Biophys Res Commun. 2007;357:608–614. doi: 10.1016/j.bbrc.2007.03.175. In a mouse model of malaria, iron deficiency increased mouse survival and decreased parasitemia, in part through increased clearance of infected iron-deficient erythrocytes compared to infected normal erythrocytes. [DOI] [PubMed] [Google Scholar]
- 8••.Prentice AM. Iron Metabolism, Malaria, and Other Infections: What Is All the Fuss About? J Nutr. 2008;138:2537–2541. doi: 10.3945/jn.108.098806. This knowledgeable review advises caution in using indiscriminate iron supplementation in areas with endemic malaria. Directing iron supplementation only to iron-deficient children, or combining therapy for malaria with iron supplementation may be safer strategies. [DOI] [PubMed] [Google Scholar]
- 9.Denic S, Agarwal MM. Nutritional iron deficiency: an evolutionary perspective. Nutrition. 2007;23:603–614. doi: 10.1016/j.nut.2007.05.002. The work questions whether iron deficiency in regions with a high burden of infections is a disease or a protective evolutionary adaptation. Although the article contains some errors, the question it raises may have important implications for the treatment of iron deficiency. [DOI] [PubMed] [Google Scholar]
- 10.Doherty CP. Host-pathogen interactions: the role of iron. J Nutr. 2007;137:1341–1344. doi: 10.1093/jn/137.5.1341. [DOI] [PubMed] [Google Scholar]
- 11.Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 12•.Drakesmith H, Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6:541–552. doi: 10.1038/nrmicro1930. This article reviews the evidence for the facilitating role of iron in the pathogenesis of viral infections. [DOI] [PubMed] [Google Scholar]
- 13.Holbein BE. Enhancement of Neisseria meningitidis infection in mice by addition of iron bound to transferrin. Infect Immun. 1981;34:120–125. doi: 10.1128/iai.34.1.120-125.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarantonelli ML, Szatanik M, Giorgini D, Hong E, Huerre M, Guillou F, Alonso JM, Taha MK. Transgenic mice expressing human transferrin as a model for meningococcal infection. Infect Immun. 2007;75:5609–5614. doi: 10.1128/IAI.00781-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaible UE, Collins HL, Priem F, Kaufmann SH. Correction of the iron overload defect in beta-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J Exp Med. 2002;196:1507–1513. doi: 10.1084/jem.20020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker EN, Baker HM. Molecular structure, binding properties and dynamics of lactoferrin. Cell Mol Life Sci. 2005;62:2531–2539. doi: 10.1007/s00018-005-5368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenssen H, Hancock RE. Antimicrobial properties of lactoferrin. Biochimie. 2008 doi: 10.1016/j.biochi.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Gallin JI, Zarember K. Lessons about the pathogenesis and management of aspergillosis from studies in chronic granulomatous disease. Trans Am Clin Climatol Assoc. 2007;118:175–185. [PMC free article] [PubMed] [Google Scholar]
- 19.Abergel RJ, Clifton MC, Pizarro JC, Warner JA, Shuh DK, Strong RK, Raymond KN. The siderocalin/enterobactin interaction: a link between mammalian immunity and bacterial iron transport. J Am Chem Soc. 2008;130:11524–11534. doi: 10.1021/ja803524w. The work provides structural analysis of the interaction of siderophores with siderocalin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 21•.Saiga H, Nishimura J, Kuwata H, Okuyama M, Matsumoto S, Sato S, Matsumoto M, Akira S, Yoshikai Y, Honda K, Yamamoto M, Takeda K. Lipocalin 2-Dependent Inhibition of Mycobacterial Growth in Alveolar Epithelium. J Immunol. 2008;181:8521–8527. doi: 10.4049/jimmunol.181.12.8521. This article analyzes the increased susceptibility of siderocalin-deficient mice to M. tuberculosis, and identifies a role for siderocalin of epithelial cells in resistance to these bacteria. [DOI] [PubMed] [Google Scholar]
- 22•.Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, Liu DR, Raymond KN, Wanner BL, Strong RK, Walsh CT, Aderem A, Smith KD. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc Natl Acad Sci USA. 2006;103:16502–16507. doi: 10.1073/pnas.0604636103. Some bacteria evade siderocalin by covalently modifying their siderophores to decrease its affinity for this host defense protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect. 2007;9:1662–1670. doi: 10.1016/j.micinf.2007.09.006. An up-to-date review of the activity and probable role of Nramp1 in phagocytes. [DOI] [PubMed] [Google Scholar]
- 24•.Fritsche G, Nairz M, Theurl I, Mair S, Bellmann-Weiler R, Barton HC, Weiss G. Modulation of macrophage iron transport by Nramp1 (Slc11a1) Immunobiology. 2007;212:751–757. doi: 10.1016/j.imbio.2007.09.014. A stimulating article that analyzed the impact of wild-type and mutated Nramp1 on iron distribution in RAW264.7 mouse macrophage-like cell lines. Functional Nramp1 reduced iron availability in macrophages by a complex mechanism. [DOI] [PubMed] [Google Scholar]
- 25•.ter-Koltunoff M, Goren S, Nousbeck J, Feng CG, Sher A, Ozato K, Azriel A, Levi BZ. Innate immunity to intraphagosomal pathogens is mediated by interferon regulatory factor 8 (IRF-8) that stimulates the expression of macrophage-specific Nramp1 through antagonizing repression by c-Myc. J Biol Chem. 2008;283:2724–2733. doi: 10.1074/jbc.M707704200. The article analyzes the mechanism of Nramp1 induction by interferon-γ. [DOI] [PubMed] [Google Scholar]
- 26.Byrd TF, Horwitz MA. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest. 1989;83:1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrd TF, Horwitz MA. Regulation of transferrin receptor expression and ferritin content in human mononuclear phagocytes. Coordinate upregulation by iron transferrin and downregulation by interferon gamma. J Clin Invest. 1993;91:969–976. doi: 10.1172/JCI116318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrd TF, Horwitz MA. Lactoferrin inhibits or promotes Legionella pneumophila intracellular multiplication in nonactivated and interferon gamma-activated human monocytes depending upon its degree of iron saturation. Iron-lactoferrin and nonphysiologic iron chelates reverse monocyte activation against Legionella pneumophila. J Clin Invest. 1991;88:1103–1112. doi: 10.1172/JCI115409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrd TF, Horwitz MA. Chloroquine inhibits the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. A potential new mechanism for the therapeutic effect of chloroquine against intracellular pathogens. J Clin Invest. 1991;88:351–357. doi: 10.1172/JCI115301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S, Ponka P. Effects of interferon-gamma and lipopolysaccharide on macrophage iron metabolism are mediated by nitric oxide-induced degradation of iron regulatory protein 2. J Biol Chem. 2000;275:6220–6226. doi: 10.1074/jbc.275.9.6220. [DOI] [PubMed] [Google Scholar]
- 31.Ganz T. Molecular control of iron transport. J Am Soc Nephrol. 2007;18:394–400. doi: 10.1681/ASN.2006070802. [DOI] [PubMed] [Google Scholar]
- 32•.Ganz T, Nemeth E. Regulation of iron acquisition and iron distribution in mammals. Biochim Biophys Acta. 2006;1763:690–699. doi: 10.1016/j.bbamcr.2006.03.014. 31–32. Recent reviews of hepcidin, ferroportin and their role in systemic iron homeostasis. [DOI] [PubMed] [Google Scholar]
- 33.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, Ernst M, Klein C, Trautwein C. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132:294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 36••.Verga Falzacappa MV, Vujic SM, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358. doi: 10.1182/blood-2006-07-033969. 34.–36. Three complementary articles describe the molecular mechanism of transcriptional induction of hepcidin by IL-6. [DOI] [PubMed] [Google Scholar]
- 37.Kemna E, Pickkers P, Nemeth E, van der HH, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 38.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.de Mast Q, Nadjm B, Reyburn H, Kemna EH, Amos B, Laarakkers CM, Silalye S, Verhoef H, Sauerwein RW, Swinkels DW, van dV. Assessment of Urinary Concentrations of Hepcidin Provides Novel Insight into Disturbances in Iron Homeostasis during Malarial Infection. J Infect Dis. 2008 doi: 10.1086/595790. 39. Hepcidin is increased in malaria, causes iron restriction, and this probably contributes to the anemia associated with this disease. [DOI] [PubMed] [Google Scholar]
- 40.Chlosta S, Fishman DS, Harrington L, Johnson EE, Knutson MD, Wessling-Resnick M, Cherayil BJ. The iron efflux protein ferroportin regulates the intracellular growth of Salmonella enterica. Infect Immun. 2006;74:3065–3067. doi: 10.1128/IAI.74.5.3065-3067.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Paradkar PN, De DI, Durchfort N, Zohn I, Kaplan J, Ward DM. Iron depletion limits intracellular bacterial growth in macrophages. Blood. 2008;112:866–874. doi: 10.1182/blood-2007-12-126854. 40–41. Two articles indicate that ferroportin overexpression suppresses and accordingly, hepcidin treatment increases the growth of intracellular microbes. These findings are somewhat counterintuitive and point to a possible dichotomy between the effect of hepcidin on extracellular vs. intracellular bacteria. The results should be interpreted cautiously as they rely on overexpressing cell lines or isolated macrophages, systems that alter the physiologic regulation of hepcidin and ferroportin synthesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Van Zandt KE, Sow FB, Florence WC, Zwilling BS, Satoskar AR, Schlesinger LS, Lafuse WP. The iron export protein ferroportin 1 is differentially expressed in mouse macrophage populations and is present in the mycobacterial-containing phagosome. J Leukoc Biol. 2008;84:689–700. doi: 10.1189/jlb.1107781. The response of ferroportin to stimulation by cytokines or mycobacteria differs between macrophages isolated from different sites. If confirmed, the observed expression of ferroportin on phagosomal membranes would further complicate any predictions about iron fluxes in and out of phagosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Theurl I, Theurl M, Seifert M, Mair S, Nairz M, Rumpold H, Zoller H, Bellmann-Weiler R, Niederegger H, Talasz H, Weiss G. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood. 2008;111:2392–2399. doi: 10.1182/blood-2007-05-090019. The production of hepcidin by monocytes and macrophages may locally regulate iron export from macrophages and lead to local (as opposed to systemic) iron sequestration. [DOI] [PubMed] [Google Scholar]
- 44.Delaby C, Pilard N, Goncalves AS, Beaumont C, Canonne-Hergaux F. Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood. 2005;106:3979–3984. doi: 10.1182/blood-2005-06-2398. [DOI] [PubMed] [Google Scholar]
- 45••.Delaby C, Pilard N, Puy H, Canonne-Hergaux F. Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: early mRNA induction by haem, followed by iron-dependent protein expression. Biochem J. 2008;411:123–131. doi: 10.1042/BJ20071474. A careful analysis of macrophage response to the heme and iron load presented by phagocytosis of erythrocytes. The cellular regulation of ferroportin is an area that needs more investigation. [DOI] [PubMed] [Google Scholar]
- 46.Larson JA, Howie HL, So M. Neisseria meningitidis accelerates ferritin degradation in host epithelial cells to yield an essential iron source. Mol Microbiol. 2004;53:807–820. doi: 10.1111/j.1365-2958.2004.04169.x. [DOI] [PubMed] [Google Scholar]