Abstract
Membrane receptors are often modulated by steroids and it is necessary to distinguish the effects of steroids at these receptors from effects occurring at nuclear receptors. Additionally, it may also be mechanistically important to distinguish between direct effects caused by binding of steroids to membrane receptors and indirect effects on membrane receptor function caused by steroid perturbation of the membrane containing the receptor. In this regard, ent-steroids, the mirror images of naturally occurring steroids, are novel tools for distinguishing between these various actions of steroids. The review provides a background for understanding the different actions that can be expected of steroids and ent-steroids in biological systems, references for the preparation of ent-steroids, a short discussion about relevant forms of stereoisomerism and the requirements that need to be fulfilled for the interaction between two molecules to be enantioselective. The review then summarizes results of biophysical, biochemical and pharmacological studies published since 1992 in which ent-steroids have been used to investigate the actions of steroids in membranes and/or receptor-mediated signaling pathways.
Keywords: ent-steroid, enantioselectivity, enantiomer, diastereomer, lipid perturbation
1. Introduction
The plasma cell membrane is an extraordinarily complex platform for transmembrane cell signaling pathways. This platform is typically composed of many different types of membrane bound receptors, such as ligand-gated and voltage-gated ion channels, G-coupled protein receptors, membrane bound steroid receptors and receptor kinases, whose signaling mechanisms are the focus of study for many of the investigators at this workshop. Additionally, this platform also contains a large array of different classes of lipids and steroids (chiefly, cholesterol in mammalian cells) which determine membrane physical properties and/or become involved as second messengers in signaling pathways. Consequently, when evaluating the modulation of membrane bound receptors by steroids, it is necessary to consider both the direct actions of the steroid caused by its binding to the receptor of interest and the indirect actions of the steroid on receptor function caused by steroid alteration of the membrane environment. A priori there is no reason to disregard the possibility that both types of steroid-mediated effects may be occurring simultaneously.
Distinguishing between the direct and indirect effects of steroids on membrane receptor function can be difficult. One potential way to make the distinction relies on a stereochemical approach based on the fact that enantiomers (non-superimposible mirror images of optically active molecules –steroids and ent-steroids in this case) have mirror image shapes but identical physicochemical properties. Because enantiomers have mirror image shapes and receptors have well-defined and structurally maintained binding pockets, receptors generally can discriminate between ligands of different shapes. Hence, it is more probable than not, that binding of a ligand to its receptor will be enantioselective (i.e., one enantiomer will bind more effectively than the other enantiomer). By contrast, membrane lipids present a dynamic environment that does not maintain structurally well-defined binding sites for steroids. Hence, in the membrane, the physiochemical properties of the steroid, not it’s absolute configuration (one enantiomer or the other), will be the dominant factor that determines the degree to which the steroid affects membrane properties. Since both enantiomers have identical physicochemical properties, their effects on membrane properties will be essentially equivalent (non-enantioselective). Therefore, the direct receptor binding and indirect membrane perturbation effects of the steroid on receptor function could potentially be distinguished by differences in the magnitude of enantioselectivity observed for each mechanism of receptor modulation.
This review provides a background for understanding the difference between steroids and ent-steroids, references for the preparation of ent-steroids, a short discussion about relevant forms of stereoisomerism and the requirements that need to be fulfilled for the interaction between two molecules to be enantioselective. The review then summarizes results of biophysical, biochemical and pharmacological studies published since 1992 in which ent-steroids have been used to investigate the membrane and/or receptor-mediated actions of steroids. Additional information on the chemistry and biology of steroid enantiomers can be found in a previous review [1].
2. Occurrence of steroids and ent-steroids
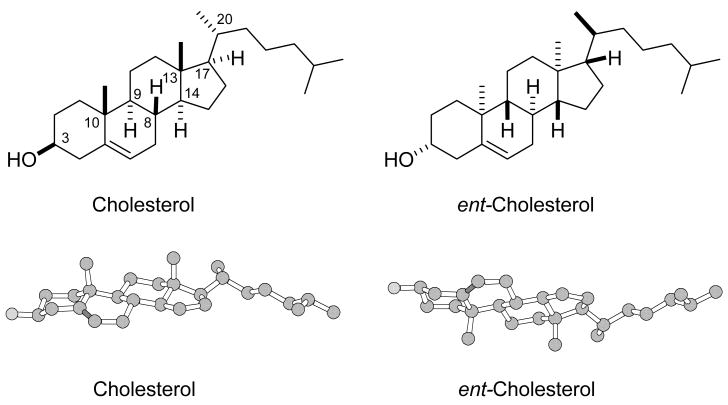
The structures of cholesterol and ent-cholesterol are shown in Figure 1. Cholesterol has eight chiral centers (C3,C8,C9,C10,C13,C14,C17,C20) in its structure and the stereochemistry at each of these chiral centers is reversed in ent-cholesterol. Since these two molecules are non-superimposable mirror images, they are enantiomers of each other. Only cholesterol is a naturally occurring steroid. The reason for this is likely related to the biosynthetic pathway for steroid formation. Steroids are formed by the enzymatic epoxidation and cyclization of squalene to form lanosterol, which is a precursor to cholesterol and other steroids [2]. Since the cyclization occurs under strict stereoelectronic control in the chiral environment of an enzyme active site, the squalene is folded in a manner that leads to lanosterol and never to its mirror image, ent-lanosterol. By analogy, this is similar to using the left hand as a mold for making left hand gloves. The left hand is never going to be a mold for making right hand gloves.
Figure 1.
The structures of cholesterol and ent-cholesterol in two dimensional (top line) and three dimensional (bottom line) drawings. Hydrogen atoms are not shown in the three dimensional drawings. The stereochemistry at all chiral centers (C3,C8,C9,C10,C13,C14,C17 and C20) is opposite in the enantiomer pair.
Since ent-steroids do not occur naturally, they must be chemically synthesized. At this time, they are not commercially available. The process we currently use for making ent-steroids [3] is an adaptation of methods developed in the pharmaceutical industry for the production of steroid hormones. Other methods for preparing ent-steroids are discussed in the earlier cited review by Biellmann. There is also a recent comprehensive review on enantioselective methods for steroid synthesis [4]. Most of these methods have not been adapted for the synthesis of ent-steroids, but there is no reason that they could not be applied for this purpose. For example, an enantioselective approach for making the secosteroid (−)-astrogorgiadiol [5] was recently adapted for the synthesis of ent-cholesterol [6].
3. Diastereomers, enantiomers and their physiochemical properties
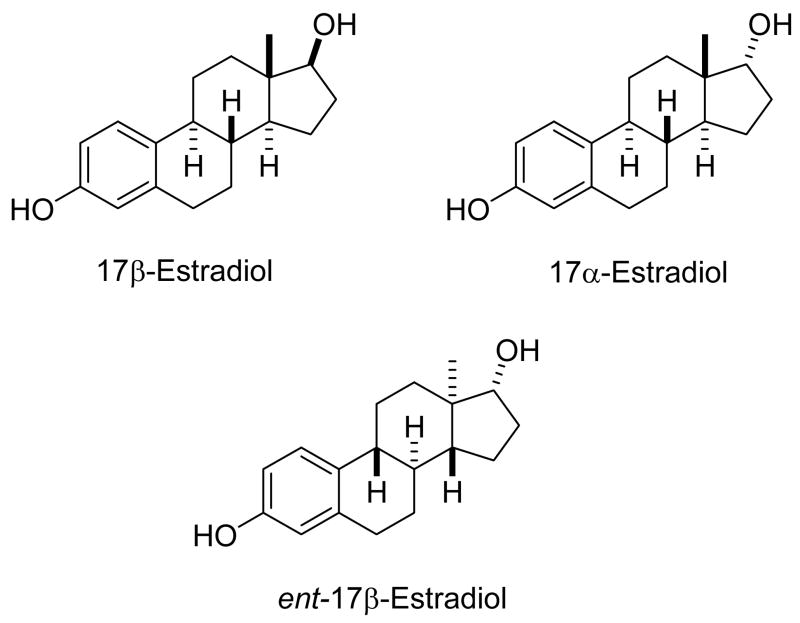
Optically active molecules that have only one chiral center can exist only as a pair of enantiomers. However, optically active molecules with two or more chiral centers have stereoisomers that are either diastereomers or enantiomers. The maximum number of stereoisomers that can exist for an optically active molecule is 2n stereoisomers, where n equals the number of chiral centers in the molecule. Figure 2 demonstrates the difference between diastereomers and enantiomers using 17β-estradiol as an example. 17α-Estradiol is the C17 diastereomer of 17β-estradiol. 17α-Estradiol is also referred to as the C17 epimer of 17β-estradiol. The stereochemical term epimer is commonly used when only one of several chiral centers in a molecule is inverted. Because 17β-estradiol has five chiral centers, and no plane or center of symmetry, it has an enantiomer and an additional thirty diastereomers. These additional diastereomers are obtained by inverting a different chiral center or any combination of up to four of the five chiral centers. Only when all five chiral centers are inverted is the enantiomer, ent-17β-estradiol, obtained. In nearly all cases in the biological literature the stereoselective actions of a steroid described are diastereoselective actions because the stereochemistry at one chiral center bearing an important substituent, as in the case of 17β- and 17α-estradiol, has been inverted. Steroid actions that are enantioselective have rarely been studied because ent-steroids have not been readily available.
Figure 2.
An example of the difference between diastereomers and enantiomers. 17β-Estradiol and 17α-estradiol are a diastereomer pair which differ only in stereochemistry at C17. 17β-Estradiol and ent-17β-estradiol are the enantiomer pair. All five chiral centers in the enantiomer pair have opposite stereochemistry.
The reason for stressing the difference between the two forms of stereoisomers is that diastereomers have different physicochemical properties and enantiomers do not (see Table 1). Thus, whereas enantiomers affect membranes in the liquid state in an identical manner (discussed in Section 4), diastereomers may not. For example, cholesterol and its C3 diastereomer, epicholesterol, orient differently in membranes to alter membrane properties in different ways [7, 8]. Likewise, anesthetic steroids and their C3 diastereomers have different mobilities in membranes [9]. Hence, in the absence of information showing that a steroid and one of its diastereomers behave in an acceptably similar manner in membranes, these stereoisomers are poorly suited for use as tools to distinguish between the direct binding and indirect membrane perturbing actions of a steroid on membrane receptor function.
Table 1.
Identical physicochemical properties of enantiomers in a non-chiral environment
| Solubility in water and other non-optically active solvents |
| Oil/water, octanol/water and similar partition coefficients |
| Melting points (provided each enantiomer is in the identical crystalline form) |
| Chromatographic mobility on non-chiral columns and adsorbents |
| Thermodynamic properties |
| Spectroscopic properties |
| Chemical reactivity with non-optically active reagents |
4. Requirements for enantioselective interactions between molecules
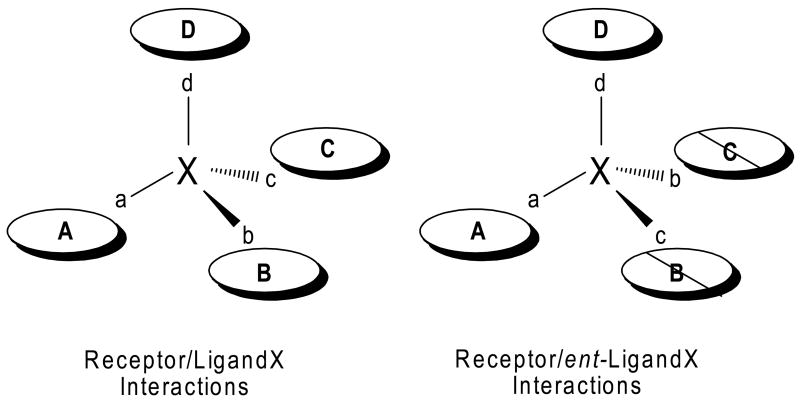
Enantiomers of a molecule can be distinguished from each other by the direction in which they rotate linearly polarized light. Additionally, they can usually be distinguished by their interactions with a different optically active molecule provided that the enantiomers cannot interact with this optically active molecule in an identical manner. For example, as shown in Figure 3 for the enantiomers of ligand X with a generic receptor ABCD, one enantiomer has four favorable interactions with the receptor, whereas the other enantiomer has only two favorable interactions with the receptor. Hence, the enantiomers are distinguishable from each other by their differential interactions with the receptor. In molecular terms, these interactions generally consist of hydrogen bonds, dipole-dipole interactions, π-bond interactions, ion-pair interactions, hydrophobic interactions and steric factors. Geometrical arguments establish that a minimum of 4 interaction constraints are needed for enantiomer discrimination by another optically active molecule [10, 11].
Figure 3.
Receptor discrimination between enantiomers. Molecules X and ent-X are an enantiomer pair containing structural elements a,b,c and d which can interact with areas A,B,C and D on receptor ABCD. The enantiomer on the left has four favorable interactions (a,A; b,B; c,C; d,D) with the receptor whereas the enantiomer on the right has two favorable interactions (a,A; d,D) and two unfavorable interactions (b,C; c,B; indicated by diagonal line through the interaction sites) thus allowing for enantiomer discrimination by the receptor.
Enantioselectivity expectations for steroid-protein and steroid-lipid interactions are summarized in Table 2. Because macromolecules like enzymes, receptors, specific transporters and antibodies that interact with steroids have architecturally well-defined binding sites that are sterically and electronically complimentary to those of the steroids they bind, it is generally expected that their interactions with steroids will be enantioselective. By contrast, lipid membrane bilayers in the liquid state, although they are composed of optically active lipids, do not maintain a defined architecture because of rapid movement of the lipids. Hence, enantioselective sterol-lipid interactions for a membrane bilayer in the liquid state would not be expected.
Table 2.
Expected outcomes for steroid enantioselectivity studies
| None to Low | Moderate to High | Unknown |
|---|---|---|
| Lipid packing in monolayer and bilayer membranes | Binding to nuclear receptors | Membrane Proteins |
| Membrane perturbation effects on receptor function | Binding to specific transporters | |
| Binding to enzymes |
In Table 2, membrane proteins are placed in a column where an expectation for enantioselectivity is not given since either (or both) outcome(s), depending on whether steroid modulation is caused by direct binding to the protein and/or by membrane perturbation, can be expected. A finding of steroid enantioselectivity strongly suggests that a steroid binding site exists on the membrane protein. The failure to observe steroid enantioselectivity either means that steroid effects are mediated by membrane perturbation or that the receptor cannot discriminate between steroid enantiomers. In such a case, additional information from other types of studies (e.g., site-directed mutagenesis, protein structural data) would be needed to distinguish between the two types of steroid modulation.
5. Biophysical studies of steroid enantioselectivity in lipid monolayers, bilayers and micelles
The issue of enantioselective interactions between cholesterol and phospholipids was first addressed using phospholipid enantiomers (reviewed in [12]). No enantioselective effects were observed. Because our rationale for using steroid enantiomers to discriminate between the direct and indirect effects of steroids on membrane receptor function is critically dependent on the hypothesis that steroid enantiomers modulate membrane properties in a non-enantioselective manner, the issue has been addressed again using cholesterol enantiomers in a variety of biophysical experiments. No enantioselectivity was observed for cholesterol effects on egg sphingomyelin bilayer properties by differential scanning calorimetry, x-ray diffraction and neutral density measurments [13]. Furthermore, the phase-transition properties of a mixture of 1-stearoyl-2-oleoylphosphatidylcholine and phosphatidyl inositol 4,5 diphosphate are not altered in an enantioselective manner by cholesterol [14].
Cholesterol enantioselectivity effects on the packing of different lipid monolayers on the surface of water have also been examined. No reproducible enantioselective effects were observed. Our group initially reported enantioselectivity for the interactions of cholesterol and ent-cholesterol with egg sphingomyelin [15, 16]. However, later results from biophysical studies with our collaborators [13] raised questions about the validity of our intial report. Subsequently, when these monolayer studies were repeated in another laboratory as part of a study of cholesterol effects on epidermal growth factor signaling, our initial results were not reproduced as no enantioselectivity was found for cholesterol-sphingomyelin interactions [17]. Most recently, the interactions of cholesterol enantiomers with dipalmitolylphosphatidylcholine enantiomers in monolayers on the surface of water were examined. Again, no enantioselectivity was observed [18].
The interactions of anesthetic steroid enantiomers with phospholipid monolayers have also been explored. No enantioselectivity was observed for the actions of the enantiomers of either pregnanolone (3α5βP) or allopregnanolone (3α5αP) on the biophysical properties of structurally diverse lipids [19]. We also measured the critical micelle concentration (cmc) of three different pairs of enantiomers of bile steroids (lithocholic acid, chenodeoxycholic acid and deoxycholic acid) and no differences in the cmc for any of the enantiomer pairs were observed [20, 21]. Most recently, we found that enantiomers of 25-hydroxycholesterol, an oxysterol that influences cellular cholesterol trafficking, affected the packing of phospholipid monolayers in an identical manner [22]. Lastly, the enantioselective effect of pregnenolone sulfate (PS) on membrane capacitance has been examined and found to be non-enantioselective [23]. Overall, these results extend the enantioselectivity studies beyond studies of cholesterol-lipid interactions and support the general conclusion that steroid enantiomers perturb membranes in an essentially equivalent manner.
6. Biological studies of cholesterol, desmosterol and U18666A enantioselectivity
Table 3 lists the outcomes of enantioselectivity studies obtained with enantiomers of cholesterol, desmosterol and U18666A. Cholesterol enantioselectivity results that were published prior to 2004 are discussed in greater detail in an earlier review [12]. As is apparent from Table 3, some membrane proteins and small molecules that localize to membranes interact with cholesterol in an enantioselective manner while others do not. Clearly, these results suggest that both indirect membrane effects and direct binding effects of cholesterol are important interactions that modulate the activity of membrane proteins and other optically active biomolecules. It can be argued that those membrane bound proteins that do not discriminate between cholesterol enantiomers have non-enantioselective binding sites for cholesterol. However, the strength of this argument seems weakened when considering the observed frequency of non-enantioselective cholesterol action.
Table 3.
Biological studies involving cholesterol, desmosterol and U18666A enantiomers.
| Biological focus | Enantioselectivity | Reference |
|---|---|---|
| Cholesterol | ||
| Amphotericin B Channel Behavior | ent gives different response | [78]
[79] |
| Crystalline Cholesterol Antibody | Not enantioselective | [80] |
| Cholesterol Oxidase Activitya | ent is very poor substrate | [17, 81] |
| SERCA2b Activity | Not enantioselective | [82] |
| ACAT1 Activity | ent is a very poor substrate and is not an allosteric activator | [25] |
| Cholesterol transporters ABCG5 and ABCG8 | ent is not efficiently transported | [83] |
| Lipid domain inducing peptides | D- and L-peptides affect domain formation differently with cholesterol enantiomers | [14] |
| Vibrio Cholera Cytolysin Pore Formation | ent essentially inactive | [84] |
| Streptolysin O Pore Formation | ent less effective | [84] |
| BAX Pore Activation | Not enantioselective | [85] |
| Multidrug Resistance P-glycoprotein | Enantioselective for some transported drugs | [81]
[24]b |
| Nicotinic Acetycholine Receptor | Not enantioselective | [86] |
| Epidermal Growth Factor Receptor | Not enantioselective | [17] |
| Caenorhabditis elegans Health & Reproduction | ent lethal for progeny development | [28] |
| Desmosterol | ||
| LXR activation | ent is very poor activator | [26] |
| U18666A | ||
| Bovine lens epitheal cells | Drug induced apoptosis and inhibition of sterol biosynthesis are not enantioselective | [33] |
This enzyme from Rhodococcus erythropolis has been shown to oxidize the hydroxyl groups in androsta-5,9(11)-diene-3β,17β-diol and its enantiomer with similar kinetics [87].
This reference addresses enantioselective actions of DHEAS on cholesterol trafficking and esterification mediated by cholesterol transport through MDR1.
Cholesterol oxidase and ACAT1 (acyl-CoA:cholesterol acyltransferase1), enzymes that use cholesterol as a substrate are highly enantioselective [17, 24, 25]. Likewise the interactions of desmosterol with the liver X receptor (LXR) is highly enantioselective [26] as are the interactions of cholesterol with the cholesterol transporters ABCG5 and ABCG8 [27]. It would be surprising if enantioselectivity were not observed in these studies, since these proteins are clearly involved in direct interactions with their steroid ligands.
The nematode Caenorhabditis elegans cannot maintain proper growth or produce subsequent generations of viable progeny when cholesterol is replaced in their diet by ent-cholesterol [28]. Since cholesterol plays only a minor structural role in this nematode [29], the result may indicate that steroid hormone(s) derived from cholesterol are involved in maintaining the growth and reproduction of this nematode and that no substitutes for them can be produced from ent-cholesterol. In a cultured mammalian cell line where bulk membrane effects of cholesterol can be satisfied with phytosteroids, those processes which require small amount of cholesterol are not completely enantioselective [30].
The enantioselectivity of oral cholesterol absorption in hamsters was explored utilizing tracer amounts of deuterated forms of cholesterol enantiomers. Uptake of the cholesterol and ent-cholesterol tracers into the intestinal mucosa at 30 min was similar, but the cholesterol tracer was retained there while the ent-cholesterol tracer rapidly entered the systemic circulation and was returned to the gut lumen. All of the ent-cholesterol tracer was excreted in the stool in 3 days, whereas ~50% of the cholesterol tracer was retained in the hamsters after this time. The mechanism(s) that explain these results remains to be delineated [31].
The amphipathic steroid U18666A inhibits multiple enzymes involved in sterol biosynthesis and causes numerous pathologies. Because of the complex actions of this compound, it was proposed that apoptosis of cultured bovine lens epithelial cells was due to membrane effects of U18666A [32]. To gain further support for this hypothesis, the enantioselectivity of U18666A action was investigated. The apoptotic actions of U18666A and ent-U1866A were found to be equal [33]. The enantioselectivity of other actions of U18666A remain to be investigated.
The ability of peptide enantiomers that induce cholesterol-rich domain formation has also been found to be influenced in a differential manner by cholesterol enantiomers. In one case, a peptide formed cholesterol-rich domains with cholesterol, but not with ent-cholesterol [14].
7. Biological studies of bile steroid enantiomers
Bile steroids are endogenous steroid detergents and they have both nuclear and membrane receptors that mediate their actions. As mentioned above in Section 5, the cmc values for the enantiomers of lithocholic acid (LCA), chenodeoxycholic acid (CDCA) and deoxycholic acid (DCA) were found to be identical to those of their natural counterparts. These results suggest that the detergent effects of the enantiomer pairs on lipid membranes will be non-enantioselective. By contrast, when the actions of the LCA and CDCA enantiomers were examined at the human farnesoid X receptor (hFXR), the heterodimeric receptor formed by this receptor and the human retinoid X receptor (hFXR/hRXR), human vitamin D receptor (hVDR), human pregnane X receptor (hPXR) and the G-protein coupled receptor TGR5, their actions were, nearly exclusively, enantioselective [20].
As summarized in Table 4, enantioselectivity for receptor activation was highest for the preferred ligand at its cognate receptor (CDCA at hFXR, hFXR/hRXR; LDA at hVDR) with ent-steroids not causing significant receptor activation. Additional studies show binding of ent-CDCA and ent-LCA to hFXR does occur as both ent-steroids are able to displace a high affinity ligand at this receptor. Enantioselectivity for activation of hPXR, a receptor known to be activated by structurally diverse ligands [34], was not significant for LCA and in the case of CDCA activation, ent-CDCA was the more potent activator. Significant enantioselectivity for the activation of TGR5 was observed for both LCA and CDCA, with both the ent-steroids being the weaker activators. This last result may be the first report of enantioselective actions of a steroid at a G-protein coupled receptor.
Table 4.
Enantioselectivity for bile steroid-receptor activationa
The overall results from these studies conform to the expectation that receptors will generally discriminate between steroid enantiomers. The enantioselectivity appears to be greatest for the highest affinity ligands of the receptor. This is in agreement with Pfeiffer’s rule which states that the degree of ligand enantioselectivity observed is directly correlated with receptor binding affinity of the natural enantiomer [35]. The finding that hPXR activation was greater for ent-CDCA than for CDCA is somewhat surprising, but as discussed in Section 8, it has also been observed in enantioselectivity studies of steroid binding to γ-aminobutyric acid type A receptors (GABAA receptors). This outcome appears to be more likely when either the receptor binds structurally diverse ligands or the ligand/receptor interactions are less than optimal.
The first use of an ent-bile steroid to answer a biological question has also been reported [21]. Bile steroids are known to decrease proliferation and induce apoptosis in colon cancer cell lines. DCA and ent-DCA were used to study the enantioselectivity of these actions. In two human colon cancer cell lines, the effects of the enantiomers on cell proliferation were very similar. By contrast, ent-DCA showed a markedly decreased ability to induce apoptosis in comparison to DCA. Studies are currently in progress to extend these enantioselectivity studies to other bile steroids and to further elaborate the mechanism of bile steroid-induced apoptosis. Because of the enantioselectivity observed thus far, it seems unlikely that detergent effects are the exclusive explanation for the apoptotic effects of bile steroids.
8. Enantioselectivity of steroid modulation of ligand-gated and voltage-gated ion channels
The reason that we first undertook the preparation of ent-steroids was to provide evidence for the existence of anesthetic steroid binding sites on GABAA receptors. In 1996, we reported that the actions of 3α5αP were enantioselective [36]. Whereas 3α5αP is a potent positive allosteroic modulator of GABAA receptors, ent-3α5αP is not (see Table 5). Until 2006, when site-directed mutagenesis studies provided direct support for the existence of these sites [37], the enantioselective actions of 3α5αP and other steroid analogues [38–41] were the strongest evidence that these sites existed.
Table 5.
Steroid enantioselectivity for modulation of ligand- and voltage-gated channels.
| Channel | Compound | Enantioselectivity of Modulation | Reference |
|---|---|---|---|
| Rat α1β2γ2 GABAA | 3 α5αP 9 (↑)a | steroid ≫ ent-steroid | [36, 38–40]b |
| 3 α5βP (↑) | steroid > ent-steroid | [41] | |
| Androsterone (↑) | ent-steroid > steroid | [42] | |
| Etiocholanolone (↑) | ent-steroid ≫ steroid | [42] | |
| PS (↓) | Not enantioselective | [44] | |
| DHEAS (↓) | steroid > ent-steroid | [44] | |
| Human ρ1 GABA-C | 3 α5αP | steroid (↑) ent-steroid (no effect) | [47] |
| 3α5βP | steroid (↓) ent-steroid (↑) | [47] | |
| C. elegans GABAA (UNC-49B-PS7) | PS (↓) | steroid > ent-steroid | [45] |
| DHEAS (↓) | Not enantioselective | [45] | |
| hα4β2 nAchR | 17β-Estradiol | No modulation by ent-steroid | [50] |
| rα4β2 nAchR | ACNc (↓) | steroid > ent-steroid | [49] |
| ECNd (↓) | Not enantioselective | [49] | |
| Rat NMDA | PS (↑) | Not enantiselective | [51] |
| Rat HVA ICa++ | ACN (↓) | steroid > ent-steroid | [56] |
| Rat LVA ICa++ | 3α5αP (↓) | steroid > ent-steroid | [55] |
| Alphaxalonee (↓) | steroid > ent-steroid | [55] | |
| ACN (↓) | steroid > ent-steroid | [54, 55] | |
| ECN (↓) | steroid > ent-steroid | [54, 55] |
Upward pointing arrow indicates the compound potentiates the channel and downward pointing arrow indicates the compound inhibits the channel.
References [38–40] also contain information on the enantioselectivity of tricyclic benz[e]indene analogues.
ACN, (3α,5α,17β)-3-hydroxyandrostane-17-carbonitrile.
ECN, (3β,5α,17β)-17-hydroxyestrane-3-carbonitrile.
Alphaxalone, (3α,5α)-3-hydroxypregnane-11,20-dione.
More recently, the enantioselectivity of androgen action at GABAA receptors has been examined. The androgens, androsterone and etiocholanolone, are weak positive allosteric modulators of GABAA receptors. Interestingly, their enantiomers are more potent and in the case of etiocholanolone, cleary more efficacious [42]. Further modifications of the unnatural enantiomers of these two ent-steroids led to compounds which have modulatory actions comparable to those of the most active steroid modulators [42]. Since the structure of the group at steroid postion C17 is the only structural feature that distinguishes these enantiomer pairs from those showing the expected enantioselectivity (steroid > ent-steroid), the result indicates that the C17 functional group is the major factor responsible for enantioselectivity of anesthetic steroid modulation of GABAA receptors. Surprisingly, site-directed mutagenesis studies suggest that etiocholanolone enantiomers bind to different sites than their steroid counterparts [43]. The reason why these receptors would have different binding sites for ent-steroids is not obvious and further studies are needed to identify these putative sites and determine if there are endogenous ligands for them.
The enantioselective actions of dehydroepiandrosterone sulfate (DHEAS), pregnenolone sulfate (PS) and (3α,5β)-3-hydroxypregnan-20-one sulfate, compounds that are negative allosteric modulators of GABAA receptors, have also been investigated using rat α 1β2γ2L GABAA receptors. Only the actions of DHEAS were found to be enantioselective [44]. By contrast, a form of the Caenorhabditis elegans GABAA receptor displayed enantioselectivity for PS, but not DHEAS [45]. These opposite enantioselectivity results in the two different systems suggest that the selectivity of GABAA receptors for steroid enantiomers is likely a function of the specific way that a particular steroid contacts its binding site on the receptor.
The human ρ1 GABA-C receptor is another type of GABA receptor that is modulated by 3α5αP, 3α5βP and other steroids. Mutations of a single key residue in a transmembrane domain (Ile307 in domain TM2) of the receptor can have major effects on steroid modulation [46]. For example, depending on the mutation, 3α5βP inhibits channel function, potentiates channel function, or has both actions depending on 3α5βP concentration. These different actions of the steroid were not attributed to different modes of steroid binding to the receptor, but were attributed instead to steroid effects on membrane lateral pressure [46].
Steroid enantioselectivity studies are not in agreement with the conclusion that effects on membrane lateral pressure account for ρ1 GABA-C receptor modulation by steroids [47, 48]. As noted in Section 5, there is no enantioselectivity for the actions of either 3α5αP or 3α5βP on membrane properties [19] so the effects of each enantiomer pair on membrane lateral pressure should be equivalent. Moreover, when the actions of the enantiomer pairs were examined at ρ1 GABA-C receptors, enantioselective steroid actions were observed. For example, enantiomers of 3α5βP had opposite actions on receptor function [47]. These enantioselectivity results suggest that steroid action in the membrane to change a membrane property, such as lateral pressure, is less likely than a direct interaction of the steroids with binding sites on ρ1 GABA-C receptors.
Other ligand-gated ion channels for which enantioselectivity of steroid allosteric modulation has been examined are the rα4β2nAchR (rat α4β2 neuronal nicotinic acetylcholine receptor) (enantioselectivity observed for one of two steroid modulators) [49], the hα4β2nAchR (human α4β2 neuronal nicotinic acetylcholine receptor) (enantioselectivity observed) [50] and the rat NMDA-type N-methyl-D-aspartate-type) glutamate receptor (enantioselectivity not observed) [51]. Because no enantioselectivity for PS potentiation of NMDA receptors was observed, and because it is known that PS potentiation of this receptor improves learning and memory [52], ent-PS was tested in vivo for its ability to facilitate learning and memory. In two different experimental paradigms this was found to be the case [51, 53].
Enantioselectivity for steroid inhibition of LVA (low voltage-gated) and HVA (high voltage-gated) calcium channels (ICa++) has been observed with the steroids being more potent blockers of calcium currents than the ent-steroids [54–56]. It should also be noted that ligand-gated glycine and 5-hydroxytryptamine3 (5-HT3) channels are also modulated by steroids [57, 58], but the enantioselectivity of steroid modulation has not been examined in these cases.
Finally, although not a direct effect on an ion-channel, enantioselectivity for a steroid effect on transmitter release has been examined. The σ1-like receptor has a presynaptic effect on glutamate release and whereas PS acting through this receptor enhanced glutamate release to augment NMDA receptor mediated currents, ent-PS did not [59].
The results summarized for steroid enantioselectivity at ion-channels cover all possible outcomes for steroid modulation: steroid > ent-steroid, steroid = ent-steroid, steroid < ent-steroid and opposite actions of steroid and ent-steroid. While lipid perturbation could explain some results in which enantioselectivity was not apparent, it is difficult to understand how the complexity of other results can be explained solely by lipid perturbation of ion channel function.
9. Steroid neuroprotection and enantioselectivity
Both 17β-estradiol and progesterone have neuroprotective actions and studies to establish the molecular basis for neuroprotection are currently of widespread interest [60–62]. Interestingly, for both steroids, neuroprotection correlates with the abilities of these steroids to reduce oxidative damage to neurons. Exactly how this occurs remains to be determined. This is an especially interesting issue for progesterone, which unlike the free radical scavenger 17β-estradiol, is not a molecule that can directly scavenge free radicals [63].
17β-Estradiol, because it is a phenolic compound, is a chemical antioxidant and this physicochemical property can contribute to its neuroprotective effects. Since 17β-estradiol and ent-17β-estradiol have identical physiochemical properties, the ent-steroid was also evaluated as a neuroprotective agent. In cell culture models of oxidative damage and in an in vivo stroke model, ent-17β-estradiol and 17β-estradiol were equally effective neuroprotectants [64, 65]. Enantiomers of other compounds structurally-related to ent-17β-estradiol also have neuroprotective actions [66–68].
The exact mechanism(s) whereby ent-17β-estradiol exerts it neuroprotective actions is still being determined. However, since this ent-steroid antagonizes uterine growth stimulated by 17β-estradiol, and has only poor affinity for nuclear estrogen receptors, it seems unlikely that its actions at estrogen receptors adequately account for its neuroprotective actions [64]. Recent studies suggest that 17β-estradiol modulation of the ERK signaling pathway is involved in the neuroprotective actions of this steroid. Both enantiomers of 17β-estradiol were found to preserve phosphatase activity in cultured neuronal cells subjected to oxidative insults thereby attenuating the persistent phosphorylation of ERK associated with neuronal death [69]. Exactly how phosphatase activity is maintained and why these actions of 17β-estradiol are non-enantioselective needs to be addressed by future studies.
Progesterone and its metabolite 3α5αP both have neuroprotective actions in a rodent model of traumatic brain injury (TBI) [70]. Mechanistically, both progesterone signaling through nuclear progesterone receptors and 3α5αP signaling through potentiation of GABAA receptor function have been evaluated by a progesterone enantioselectivity study [71]. Like progesterone, ent-progesterone was found to be neuroprotective against TBI. Unlike progesterone, the ent-steroid binds only weakly to the human nuclear progesterone receptor and it does not activate the receptor. Moreover, as mentioned in Section 8, ent-3α5αP is a very weak modulator of GABAA receptors, so if the conversion of ent-progesterone to ent-3α5αP were occurring in vivo, it would still not be expected that ent-progesterone would be as effective as progesterone in this TBI model if modulation of GABAA receptors was the key signaling pathway. Moreover, the conversion of ent-progesterone into ent-androgens which are somewhat effective positive allosteric modulators of GABAA receptors (see Section 8) seems unlikely since ent-progesterone has been shown to be an inhibitor of the human form of the lyase enzyme responsible for this transformation [72]. Overall, the results suggest that other non-enantioselective signaling pathways remain to be elaborated.
3α5αP was also recently found to prolong the lifespan of mice in a mouse model of human neurodegenerative Niemann Pick Type C disease. In an initial study, these actions of 3α5αP appeared to correlate with 3α5αP potention of GABAA receptor function [73]. However, when ent-3α5αP was subsequently evaluated in this mouse model, this ent-steroid prolonged lifespan as effectively as 3α5αP [74]. Since ent-3α5αP is not an effective potentiator of GABAA receptors, this result indicates that pathways other than GABAA receptor signaling pathways are operative. Using fibroblasts from patients with NPC disease it was recently shown that these cells were oxidatively stressed and that 3α5αP and ent-3α5αP equally attenuated oxidative stress in these cells [63]. It is hoped that future studies will reveal the mechanism that explains this interesting result as such studies should provide new insights into the mechanism(s) of neuroprotection by steroids.
10. Summary on steroid enantioselectivity
Our group initially became interested in steroid enantioselectivity as a way to provide evidence for the existence of anesthetic steroid binding sites on GABAA receptors. Subsequently, our collaborators have provided us with opportunities to expand our steroid enantioselectivity studies far beyond this initial question. To state the obvious, these studies of steroid enantioselectivity have proven to be very fruitful. Thus far, the expectation that steroid effects on membrane physical properties will be non-enantioselective has been fulfilled. Hence, enantioselective modulation of membrane protein function by steroids provides strong evidence for the existence of steroid binding sites on membrane proteins that respond to steroids in this manner. Surprisingly, not all ent-steroids are less effective modulators of protein function than their natural counterparts. In cases where ent-steroids have actions equal to or greater than their natural counterparts, there may be opportunities to develop ent-steroids as drugs. Possible advantages of ent-steroid drugs would include a potential lack of agonist activity at nuclear receptors and reduced interference with the modification of endogenous steroids by steroid transforming enzymes. However, even if ent-steroid drugs are not forthcoming, the utility of ent-steroids as tools to address the direct and indirect effects of steroids on membrane protein function now seems established.
This review reflects my perspective as a medical chemist/pharmacologist on the use of ent-steroids to study steroid modulation of particular membrane protein targets. However, Section 9 of the review indicates that ent-steroids can be useful tools for identifying steroid effects that are likely not mediated by the classical steroid receptors. There is already a well-established literature on the rapid non-genomic actions of steroids and the Mannheim Classification has been proposed as a way to classify mechanisms for these types of steroid effects [75–77]. It is hoped that future studies with ent-steroids will allow their mechanisms of action to be determined and categorized according to the Mannheim Classification.
Acknowledgments
The author wishes to thank the many collaborators who have participated in ent-steroid studies with my research group. You are too numerous to individually acknowledge by name. The following investigators in my research group have made the ent-steroids described in our publications: Zu Yun Cai, Yuefei Hu, Bryson Katona, Shirisha Komarapuri, Kathiresan Krishnan, Sampath Kumar, Kent Nilsson, Jiang Xin and Emily Westover. The author gratefully acknowledges funding support from NIH grants GM47969, AG10485 and HL67773.
References
- 1.Biellmann J-F. Enantiomeric steroids: synthesis, physical, and biological properties. Chem Rev. 2003;103:2019–33. doi: 10.1021/cr020071b. [DOI] [PubMed] [Google Scholar]
- 2.Abe I. Enzymatic synthesis of cyclic triterpenes. Nat Prod Rep. 2007;24:1311–31. doi: 10.1039/b616857b. [DOI] [PubMed] [Google Scholar]
- 3.Covey DF. Ent-steroids chemistry and biology. Polish J Chem. 2006;80:511–22. [Google Scholar]
- 4.Chapelon A-S, Moraleda D, Rodriguez R, Olliver C, Santelli M. Enantioselective synthesis of steroids. Tetrahedron. 2007;63:11511–616. [Google Scholar]
- 5.Taber DF, Malcolm SC. Synthesis of (−)-astrogorgiadiol. J Org Chem. 2001;66:944–53. doi: 10.1021/jo001519g. [DOI] [PubMed] [Google Scholar]
- 6.Belani JD, Rychnovsky SD. A concise synthesis of ent-cholesterol. J Org Chem. 2008;73:2768–73. doi: 10.1021/jo702694g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadenhead DA, Mueller-Landau F. Molecular packing in steroid-lecithin monolayers, part III: mixed films of 3-doxy cholestane and 3-doxyl-17-hydroxylandrostane with dipalmitoylphosphatidycholine. Chem Phys Lipids. 1979;25:329–43. [Google Scholar]
- 8.Murari R, Murari MP, Baumann WJ. Sterol orientations in phosphatidylcholine liposomes as determined by deuterium NMR. Biochemistry. 1986;25:1062–7. doi: 10.1021/bi00353a017. [DOI] [PubMed] [Google Scholar]
- 9.Makriyannis A, DiMeglio CM, Fesik SW. Anesthetic steroid mobility in model membrane preparations as examined by high-resolution 1H and 2H NMR spectroscopy. J Med Chem. 1991;34:1700–3. doi: 10.1021/jm00109a024. [DOI] [PubMed] [Google Scholar]
- 10.Crossley R. Chirality and the Biological Activity of Drugs. Boca Raton: CRC Press; 1995. [Google Scholar]
- 11.Mesecar AD, Koshland DE., Jr A new model for protein stereospecificity. Nature. 2000;403:614–5. doi: 10.1038/35001144. [DOI] [PubMed] [Google Scholar]
- 12.Westover EJ, Covey DF. The enantiomer of cholesterol. J Membr Biol. 2004;202:61–72. doi: 10.1007/s00232-004-0714-7. [DOI] [PubMed] [Google Scholar]
- 13.Mannock DA, McIntosh TJ, Jiang X, Covey DF, McElhaney RN. Effects of natural and enantiomeric cholesterol on the thermotropic phase behavior and structure of egg sphingomyelin bilayer membranes. Biophys J. 2003;84:1038–46. doi: 10.1016/S0006-3495(03)74920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epand RM, Rychnovsky SD, Belani JD, Epand RF. Role of chirality in peptide-induced formation of cholesterol-rich domains. Biochem J. 2005;390:541–8. doi: 10.1042/BJ20050649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalitha S, Kumar AS, Stine KJ, Covey DF. Enantiospecificity of sterol-lipid interactions: first evidence that the absolute configuration of cholesterol affects the physical properties of cholesterol-sphingomyelin membranes. Chem Commun (Camb) 2001:1192–3. [Google Scholar]
- 16.Lalitha S, Kumar AS, Stine KJ, Covey DF. Chirality in membranes: first evidence that enantioselective interactions between cholesterol and cell membranes lipids can be a determinant of membrane physical properties. J Supramol Chem. 2001;1:53–61. [Google Scholar]
- 17.Westover EJ, Covey DF, Brockman HL, Brown RE, Pike LJ. Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phosphorylation due to membrane level effects. Studies with cholesterol enantiomers. J Biol Chem. 2003;278:51125–33. doi: 10.1074/jbc.M304332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alakoskela JM, Sabatini K, Jiang X, Laitala V, Covey DF, Kinnunen PK. Enantiospecific interactions between cholesterol and phospholipids. Langmuir. 2008;24:830–6. doi: 10.1021/la702909q. [DOI] [PubMed] [Google Scholar]
- 19.Alakoskela JM, Covey DF, Kinnunen PK. Lack of enantiomeric specificity in the effects of anesthetic steroids on lipid bilayers. Biochim Biophys Acta. 2007;1768:131–45. doi: 10.1016/j.bbamem.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Katona BW, Cummins CL, Ferguson AD, Li T, Schmidt DR, Mangelsdorf DJ, et al. Synthesis, characterization, and receptor interaction profiles of enantiomeric bile acids. J Med Chem. 2007;50:6048–58. doi: 10.1021/jm0707931. [DOI] [PubMed] [Google Scholar]
- 21.Katona BW, Rath NP, Anant S, Stenson WF, Covey DF. Enantiomeric deoxycholic acid: total synthesis, characterization, and preliminary toxicity toward colon cancer cell lines. J Org Chem. 2007;72:9298–307. doi: 10.1021/jo701559q. [DOI] [PubMed] [Google Scholar]
- 22.Gale SE, Westover EJ, Dudley N, Krishnan K, Merlin S, Scherrer DE, et al. Side-chain oxygenated cholesterol regulates cellular cholesterol homeostasis through direct sterol-membrane interactions. J Biol Chem. 2008 doi: 10.1074/jbc.M807210200. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mennerick S, Lamberta M, Shu HJ, Hogins J, Wang C, Covey DF, et al. Effects on membrane capacitance of steroids with antagonist properties at GABAA receptors. Biophys J. 2008;95:176–85. doi: 10.1529/biophysj.107.124768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luker GD, Nilsson KR, Covey DF, Piwnica-Worms D. Multidrug resistance (MDR1) P-glycoprotein enhances esterification of plasma membrane cholesterol. J Biol Chem. 1999;274:6979–91. doi: 10.1074/jbc.274.11.6979. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Chang CC, Westover EJ, Covey DF, Chang TY. Investigating the allosterism of acyl-CoA:cholesterol acyltransferase (ACAT) by using various sterols: in vitro and intact cell studies. Biochem J. 2005;391:389–97. doi: 10.1042/BJ20050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang C, McDonald JG, Patel A, Zhang Y, Umetani M, Xu F, et al. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J Biol Chem. 2006;281:27816–26. doi: 10.1074/jbc.M603781200. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Sun F, Zhang DW, Ma Y, Xu F, Belani JD, et al. Sterol transfer by ABCG5 and ABCG8: in vitro assay and reconstitution. J Biol Chem. 2006;281:27894–904. doi: 10.1074/jbc.M605603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowder CM, Westover EJ, Kumar AS, Ostlund RE, Jr, Covey DF. Enantiospecificity of cholesterol function in vivo. J Biol Chem. 2001;276:44369–72. doi: 10.1074/jbc.C100535200. [DOI] [PubMed] [Google Scholar]
- 29.Entchev EV, Kurzchalia TV. Requirement of sterols in the life cycle of the nematode Caenorhabditis elegans. Semin Cell Dev Biol. 2005;16:175–82. doi: 10.1016/j.semcdb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Xu F, Rychnovsky SD, Belani JD, Hobbs HH, Cohen JC, Rawson RB. Dual roles for cholesterol in mammalian cells. Proc Natl Acad Sci U S A. 2005;102:14551–6. doi: 10.1073/pnas.0503590102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westover EJ, Lin X, Riehl TE, Ma L, Stenson WF, Covey DF, et al. Rapid transient absorption and biliary secretion of enantiomeric cholesterol in hamsters. J Lipid Res. 2006;47:2374–81. doi: 10.1194/jlr.M600165-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Cenedella RJ, Jacob R, Borchman D, Tang D, Neely AR, Samadi A, et al. Direct perturbation of lens membrane structure may contribute to cataracts caused by U18666A, an oxidosqualene cyclase inhibitor. J Lipid Res. 2004;45:1232–41. doi: 10.1194/jlr.M300469-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Cenedella RJ, Sexton PS, Krishnan K, Covey DF. Comparison of effects of U18666A and enantiomeric U18666A on sterol synthesis and induction of apoptosis. Lipids. 2005;40:635–40. doi: 10.1007/s11745-005-1426-9. [DOI] [PubMed] [Google Scholar]
- 34.Carnahan VE, Redinbo MR. Structure and function of the human nuclear xenobiotic receptor PXR. Curr Drug Metab. 2005;6:357–67. doi: 10.2174/1389200054633844. [DOI] [PubMed] [Google Scholar]
- 35.Barlow R. Enantiomers: how valid is Pfeiffer's rule? Trends Pharmacol Sci. 1990;11:148–50. doi: 10.1016/0165-6147(90)90065-G. [DOI] [PubMed] [Google Scholar]
- 36.Wittmer LL, Hu Y, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF. Enantioselectivity of steroid-induced γ-aminobutyric acidA receptor modulation and anesthesia. Mol Pharmacol. 1996;50:1581–6. [PubMed] [Google Scholar]
- 37.Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 38.Zorumski CF, Wittmer LL, Isenberg KE, Hu Y, Covey DF. Effects of neurosteroid and benz[e]indene enantiomers on GABAA receptors in cultured hippocampal neurons and transfected HEK-293 cells. Neuropharmacology. 1996;35:1161–8. doi: 10.1016/s0028-3908(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 39.Hu YF, Wittmer LL, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF. Neurosteroid analogues. Part 5. Enantiomers of neuroactive steroids and benz[e]indenes: total synthesis, electrophysiological effects on GABAA receptor function and anesthetic actions in tadpoles. J Chem Soc Perkin Trans 1. 1997:3665–71. [Google Scholar]
- 40.Zorumski CF, Mennerick SJ, Covey DF. Enantioselective modulation of GABAergic synaptic transmission by steroids and benz[e]indenes in hippocampal microcultures. Synapse. 1998;29:162–71. doi: 10.1002/(SICI)1098-2396(199806)29:2<162::AID-SYN7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Covey DF, Nathan D, Kalkbrenner M, Nilsson KR, Hu Y, Zorumski CF, et al. Enantioselectivity of pregnanolone-induced γ-aminobutyric acidA receptor modulation and anesthesia. J Pharmacol Exp Ther. 2000;293:1009–16. [PubMed] [Google Scholar]
- 42.Katona BW, Krishnan K, Cai ZY, Manion BD, Benz A, Taylor A, et al. Neurosteroid analogues. 12. Potent enhancement of GABA-mediated chloride currents at GABAA receptors by ent-androgens. Eur J Med Chem. 2008;43:107–13. doi: 10.1016/j.ejmech.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Li P, Bracamontes J, Katona BW, Covey DF, Steinbach JH, Akk G. Natural and enantiomeric etiocholanolone interact with distinct sites on the rat a1b2g2L GABAA receptor. Mol Pharmacol. 2007;71:1582–90. doi: 10.1124/mol.106.033407. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson KR, Zorumski CF, Covey DF. Neurosteroid analogues. 6. The synthesis and GABAA receptor pharmacology of enantiomers of dehydroepiandrosterone sulfate, pregnenolone sulfate, and (3α,5β)-3-hydroxypregnan-20-one sulfate. J Med Chem. 1998;41:2604–13. doi: 10.1021/jm980148h. [DOI] [PubMed] [Google Scholar]
- 45.Twede V, Tartaglia AL, Covey DF, Bamber BA. The neurosteroids dehydroepiandrosterone sulfate and pregnenolone sulfate inhibit the UNC-49 GABA receptor through a common set of residues. Mol Pharmacol. 2007;72:1322–9. doi: 10.1124/mol.107.034058. [DOI] [PubMed] [Google Scholar]
- 46.Morris KD, Amin J. Insight into the mechanism of action of neuroactive steroids. Mol Pharmacol. 2004;66:56–69. doi: 10.1124/mol.66.1.56. [DOI] [PubMed] [Google Scholar]
- 47.Li W, Covey DF, Alakoskela JM, Kinnunen PK, Steinbach JH. Enantiomers of neuroactive steroids support a specific interaction with the GABA-C receptor as the mechanism of steroid action. Mol Pharmacol. 2006;69:1779–82. doi: 10.1124/mol.106.022863. [DOI] [PubMed] [Google Scholar]
- 48.Li W, Jin X, Covey DF, Steinbach JH. Neuroactive steroids and human recombinant ρ1 GABA-C receptors. J Pharmacol Exp Ther. 2007;323:236–47. doi: 10.1124/jpet.107.127365. [DOI] [PubMed] [Google Scholar]
- 49.Paradiso K, Sabey K, Evers AS, Zorumski CF, Covey DF, Steinbach JH. Steroid inhibition of rat neuronal nicotinic α4β2 receptors expressed in HEK 293 cells. Mol Pharmacol. 2000;58:341–51. doi: 10.1124/mol.58.2.341. [DOI] [PubMed] [Google Scholar]
- 50.Paradiso K, Zhang J, Steinbach JH. The C terminus of the human nicotinic α4β2 receptor forms a binding site required for potentiation by an estrogenic steroid. J Neurosci. 2001;21:6561–8. doi: 10.1523/JNEUROSCI.21-17-06561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vallee M, Shen W, Heinrichs SC, Zorumski CF, Covey DF, Koob GF, et al. Steroid structure and pharmacological properties determine the anti-amnesic effects of pregnenolone sulphate in the passive avoidance task in rats. Eur J Neurosci. 2001;14:2003–10. doi: 10.1046/j.0953-816x.2001.01817.x. [DOI] [PubMed] [Google Scholar]
- 52.Vallee M, Mayo W, Darnaudery M, Corpechot C, Young J, Koehl M, et al. Neurosteroids: deficient cognitive performance in aged rats depends on low pregnenolone sulfate levels in the hippocampus. Proc Natl Acad Sci U S A. 1997;94:14865–70. doi: 10.1073/pnas.94.26.14865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akwa Y, Ladurelle N, Covey DF, Baulieu EE. The synthetic enantiomer of pregnenolone sulfate is very active on memory in rats and mice, even more so than its physiological neurosteroid counterpart: distinct mechanisms? Proc Natl Acad Sci U S A. 2001;98:14033–7. doi: 10.1073/pnas.241503698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Todorovic SM, Prakriya M, Nakashima YM, Nilsson KR, Han M, Zorumski CF, et al. Enantioselective blockade of T-type Ca2+ current in adult rat sensory neurons by a steroid that lacks γ-aminobutyric acid-modulatory activity. Mol Pharmacol. 1998;54:918–27. doi: 10.1124/mol.54.5.918. [DOI] [PubMed] [Google Scholar]
- 55.Pathirathna S, Brimelow BC, Jagodic MM, Krishnan K, Jiang X, Zorumski CF, et al. New evidence that both T-type calcium channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5α-reduced neuroactive steroids. Pain. 2005;114:429–43. doi: 10.1016/j.pain.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Nakashima YM, Todorovic SM, Covey DF, Lingle CJ. The anesthetic steroid (+)-3α-hydroxy-5α-androstane-17β-carbonitrile blocks N-, Q-, and R-type, but not L- and P-type, high voltage-activated Ca2+ current in hippocampal and dorsal root ganglion neurons of the rat. Mol Pharmacol. 1998;54:559–68. doi: 10.1124/mol.54.3.559. [DOI] [PubMed] [Google Scholar]
- 57.Lambert JJ, Harney SC, Belelli D, Peters JA. Neurosteroid modulation of recombinant and synaptic GABAA receptors. Int Rev Neurobiol. 2001;46:177–205. doi: 10.1016/s0074-7742(01)46063-6. [DOI] [PubMed] [Google Scholar]
- 58.Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. Mechanisms of neurosteroid interactions with GABAA receptors. Pharmacol Ther. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyer DA, Carta M, Partridge LD, Covey DF, Valenzuela CF. Neurosteroids enhance spontaneous glutamate release in hippocampal neurons. Possible role of metabotropic σ1-like receptors. J Biol Chem. 2002;277:28725–32. doi: 10.1074/jbc.M202592200. [DOI] [PubMed] [Google Scholar]
- 60.Prokai L, Simpkins JW. Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol Ther. 2007;114:1–12. doi: 10.1016/j.pharmthera.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–15. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- 62.Singh M. Progesterone-induced neuroprotection. Endocrine. 2006;29:271–4. doi: 10.1385/ENDO:29:2:271. [DOI] [PubMed] [Google Scholar]
- 63.Zampieri S, Mellon SH, Butters TD, Nevyjel M, Covey DF, Bembi B, et al. Oxidative stress in NPC1 deficient cells: Protective effect of allopregnanolone. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Green PS, Yang SH, Nilsson KR, Kumar AS, Covey DF, Simpkins JW. The nonfeminizing enantiomer of 17β-estradiol exerts protective effects in neuronal cultures and a rat model of cerebral ischemia. Endocrinology. 2001;142:400–6. doi: 10.1210/endo.142.1.7888. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, Dykens JA, Perez E, Liu R, Yang S, Covey DF, et al. Neuroprotective effects of 17β-estradiol and nonfeminizing estrogens against H2O2 toxicity in human neuroblastoma SK-N-SH cells. Mol Pharmacol. 2006;70:395–404. doi: 10.1124/mol.106.022384. [DOI] [PubMed] [Google Scholar]
- 66.Simpkins JW, Yang SH, Liu R, Perez E, Cai ZY, Covey DF, et al. Estrogen-like compounds for ischemic neuroprotection. Stroke. 2004;35:2648–51. doi: 10.1161/01.STR.0000143734.59507.88. [DOI] [PubMed] [Google Scholar]
- 67.Perez E, Cai ZY, Covey DF, Simpkins JW. Neuroprotective effects of estratriene analogs: structure-activity relationships and molecular optimization. Drug Develop Res. 2006;66:78–92. [Google Scholar]
- 68.Kumar DM, Perez E, Cai ZY, Aoun P, Brun-Zinkernagel AM, Covey DF, et al. Role of nonfeminizing estrogen analogues in neuroprotection of rat retinal ganglion cells against glutamate-induced cytotoxicity. Free Radic Biol Med. 2005;38:1152–63. doi: 10.1016/j.freeradbiomed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Yi KD, Cai ZY, Covey DF, Simpkins JW. Estrogen receptor-independent neuroprotection via protein phosphatase preservation and attenuation of persistent extracellular signal-regulated kinase 1/2 activation. J Pharmacol Exp Ther. 2008;324:1188–95. doi: 10.1124/jpet.107.132308. [DOI] [PubMed] [Google Scholar]
- 70.Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat prefrontal cortex. Neuroscience. 2004;123:349–59. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 71.Vanlandingham JW, Cutler SM, Virmani S, Hoffman SW, Covey DF, Krishnan K, et al. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology. 2006;51:1078–85. doi: 10.1016/j.neuropharm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 72.Auchus RJ, Sampath Kumar A, Andrew Boswell C, Gupta MK, Bruce K, Rath NP, et al. The enantiomer of progesterone (ent-progesterone) is a competitive inhibitor of human cytochromes P450c17 and P450c21. Arch Biochem Biophys. 2003;409:134–44. doi: 10.1016/s0003-9861(02)00491-5. [DOI] [PubMed] [Google Scholar]
- 73.Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–11. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- 74.Langmade SJ, Gale SE, Frolov A, Mohri I, Suzuki K, Mellon SH, et al. Pregnane X receptor (PXR) activation: A mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc Natl Acad Sci U S A. 2006;103:13807–12. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Falkenstein E, Norman AW, Wehling M. Mannheim classification of nongenomically initiated (rapid) steroid action(s) J Clin Endocrinol Metab. 2000;85:2072–5. doi: 10.1210/jcem.85.5.6516. [DOI] [PubMed] [Google Scholar]
- 76.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 77.Wehling M, Losel R. Non-genomic steroid hormone effects: membrane or intracellular receptors? J Steroid Biochem Mol Biol. 2006;102:180–3. doi: 10.1016/j.jsbmb.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 78.Mickus DE, Levitt DG, Rychnovsky SD. Enantiomeric cholesterol as a probe of ion-channel structure. J Am Chem Soc. 1992;114:359–60. [Google Scholar]
- 79.Richter RK, Mickus DE, Rychnovsky SD, Molinski TF. Differential modulation of the antifungal activity of amphotericin B by natural and ent-cholesterol. Bioorg Med Chem Lett. 2004;14:115–8. doi: 10.1016/j.bmcl.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 80.Geva M, Izhaky D, Mickus DE, Rychnovsky SD, Addadi L. Stereoselective recognition of monolayers of cholesterol, ent-cholesterol, and epicholesterol by an antibody. Chembiochem. 2001;2:265–71. doi: 10.1002/1439-7633(20010401)2:4<265::AID-CBIC265>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 81.Luker GD, Pica CM, Kumar AS, Covey DF, Piwnica-Worms D. Effects of cholesterol and enantiomeric cholesterol on P-glycoprotein localization and function in low-density membrane domains. Biochemistry. 2000;39:7651–61. doi: 10.1021/bi9928593. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Ge M, Ciani L, Kuriakose G, Westover EJ, Dura M, et al. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J Biol Chem. 2004;279:37030–9. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- 83.Wang J, Sun F, Zhang DW, Ma Y, Xu F, Belani JD, et al. Sterol transfer by ABCG5 and ABCG8: in vitro assay and reconstitution. J Biol Chem. 2006;281:27894–904. doi: 10.1074/jbc.M605603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zitzer A, Westover EJ, Covey DF, Palmer M. Differential interaction of the two cholesterol-dependent, membrane-damaging toxins, streptolysin O and Vibrio cholerae cytolysin, with enantiomeric cholesterol. FEBS Lett. 2003;553:229–31. doi: 10.1016/s0014-5793(03)01023-8. [DOI] [PubMed] [Google Scholar]
- 85.Christenson E, Merlin S, Saito M, Schlesinger P. Cholesterol effects on BAX pore activation. J Mol Biol. 2008;381:1168–83. doi: 10.1016/j.jmb.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Addona GH, Sandermann H, Jr, Kloczewiak MA, Miller KW. Low chemical specificity of the nicotinic acetylcholine receptor sterol activation site. Biochim Biophys Acta. 2003;1609:177–82. doi: 10.1016/s0005-2736(02)00685-5. [DOI] [PubMed] [Google Scholar]
- 87.Kitamoto D, Dieth S, Burger A, Tritsch D, Biellmann JF. Hydroxyl groups at C-3 and at C-17 of the unnatural enantiomer, ent-androsta-5,9(11)-diene-3β,17β-diol are oxidized by cholesterol oxidase from Rhodococcus erythropolis. Tetrahedron Lett. 2001;42:505–07. [Google Scholar]
- 88.Achmatowicz B, Gorobets E, Marczak S, Przezdziecka A, Steinmeyer A, Wicha J, et al. The first synthesis and biological testing of the enantiomer of 1α,25-dihydroxyvitamin D3. Tetrahedron Lett. 2001;42:2891–95. [Google Scholar]