Abstract
The perioculomotor urocortin-containing population of neurons (pIIIu: otherwise known as the non-preganglionic Edinger-Westphal nucleus) is sensitive to alcohol and is involved in the regulation of alcohol intake. A recent study indicated that this brain region is also sensitive to psychostimulants. Since pIIIu has been shown to respond to stress, we investigated how psychostimulant-induced pIIIu activation compares to stress- and ethanol-induced activation, and whether it is independent from a generalized stress response. Several experiments were performed to test how the pIIIu responds to psychostimulants by quantifying the number of Fos immunoreactive nuclei after acute intraperitoneal injections of saline, 10-30 mg/kg cocaine, 5 mg/kg methamphetamine, 5 mg/kg amphetamine, 2.5 g/kg ethanol, 2 hours of restraint stress, 10 minutes of swim stress, or six applications of mild footshock in male C57BL/6J mice. We also compared Fos immunoreactivity in pIIIu after acute (20 mg/kg cocaine) and repeated cocaine exposure (7 days of 20 mg/kg cocaine) injections in male C57BL/6J mice in order to investigate the potential habituation of this response. Finally, we quantified the number of Fos immunoreactive nuclei in pIIIu after administration of saline, 2.5 g/kg ethanol, 20 mg/kg cocaine, or 2 hours of restraint stress in male Sprague-Dawley rats. We found that exposure to psychostimulants and ethanol induced significantly higher Fos levels in pIIIu compared to stress in mice. Furthermore, repeated cocaine injections did not decrease Fos immunoreactivity as would be expected if this response was due to stress. In rats, exposure to ethanol, psychostimulant and restraint stress all induced pIIIu Fos immunoreactivity compared to saline-injected controls. In both mice and rats, ethanol- and cocaine-induced Fos immunoreactivity occured exclusively in urocortin 1-, but not in tyrosine hydroxylase-, positive cells. These results provide evidence that the pIIIu Fos-response to psychostimulants is independent of a generalized stress in mice, but not rats. They additionally show that the pIIIu response to stress differs significantly between species.
Drug addiction is a significant health, social and economic problem. It is therefore important to understand the neural pathways that may be involved in the intoxicating and addictive effects of these drugs. Several neurocircuits, especially the pathways from the ventral tegmental area to striatum, extended amygdala and prefrontal cortical areas, have emerged as important components in these effects (for reviews see Koob & LeMoal, 2006; Feltenstein & See, 2008). A novel area that has recently been considered as a participant in the regulation of drug self-administration is the perioculomotor-urocortin containing population of neurons (pIIIu: also known as the non preganglionic-Edinger Westphal nucleus) (Weitemier et al., 2005; Ryabinin & Weitemier, 2006; Horn et al., 2008; May et al., 2008).
The pIIIu is the main source of neuropeptide urocortin 1 (Ucn 1) in the brain (Vaughan et al., 1995; Kozicz et al., 1998; Bittencourt et al., 1999; Morin et al., 1999; Weitemier et al., 2005, Ryabinin et al, 2005; May et al., 2008). Ucn 1 is a member of the corticotropin releasing factor (CRF) family of peptides that binds with great affinity to both the CRF receptor 1 and 2 (Vaughan et al., 1995). This commonality with CRF intrinsically links Ucn 1 with the stress response and anxiety. Indeed, an increase in immunoreactivity (ir) of inducible transcription factors Fos and Egr1 in has been observed in pIIIu following exposure to environmental challenges in rats and birds (Kozicz, 2003; Gaszner et al., 2004; Cunha et al., 2007). However, this sensitivity to stress is a matter of controversy, as exposing mice to several environmental stressors did not result in increased Fos-ir in pIIIu (Turek & Ryabinin, 2005).
Importantly for drug addiction research, several lines of evidence accumulated that the pIIIu is involved in the neural response to and regulation of alcohol consumption. First, a robust increase in Fos-ir in this nucleus was observed after experimental administration of alcohol in rats and mice (Chang et al., 1995; Ryabinin et al., 1997; Bachtell et al. 2002a). Moreover, increased Fos-ir following self-administration of alcohol in rodents occurred preferentially in pIIIu (Topple et al., 1998; Bachtell et al., 1999; Weitemier et al., 2001; Ryabinin et al., 2003; Sharpe et al., 2005). Second, a positive correlation between Ucn 1-ir and the genetic predisposition to drink alcohol was observed in mice (Bachtell et al., 2002b; Bachtell et al., 2003; Weitemier et al., 2005) and rats (Turek & Ryabinin, 2005). Third, lesions of the pIIIu disrupted ethanol preference in C57BL/6J mice (Bachtell et al., 2004). The consistency of data showing pIIIu’s sensitivity and involvement in alcohol self-administration is in contrast to a very limited number of studies attempting to analyze the participation of this nucleus in actions of other addictive drugs. Our laboratory showed that the mouse pIIIu is also responsive to morphine, barbiturates and benzodiazepines, but not nicotine (Bachtell et al., 2001; Bachtell et al. 2002b). Additional experiments in rats showed that this nucleus responds to opioids, ether and gamma-hydroxybutyrate (Gaszner et al., 2004; Singh et al., 2004; Singh et al., 2006; van Nieuwenhuijzen et al., 2009).
Recent studies in mice showed that administration of the psychostimulant methylphenidate resulted in an increase of Fos-ir in pIIIu, an effect absent in mice with a genetic ablation of the dopamine transporter. This study also reported that administration of cocaine and amphetamine increased Fos-ir in pIIIu (Trinh et al., 2003). It appears worthwhile, therefore, to examine more closely the role of pIIIu in behavioral responses to cocaine. However, the level of the pIIIu Fos-ir to psychostimulants in the published study appeared weaker than in our previous studies with ethanol (Bachtell et al., 2001; Bachtell et al., 2002a; Bachtell et al. 2002b). Since pIIIu was reported to respond to environmental stressors in rats (Gaszner et al., 2004), one could not exclude the possibility that psychostimulant-induced Fos-ir in pIIIu was due to a non-specific stress reaction.
To address this possibility we performed a series of experiments comparing Fos-ir in mice and rats following ethanol administration, stress, and administration of psychostimulants. The present report summarizes these experiments and concludes that pIIIu’s response to psychostimulants is independent of the stress response in mice, but not rats.
Materials and methods
Subjects
The National Institute of Health Guidelines for the Care and Use of Animals were followed throughout all animal procedures. Male C57BL/6J mice purchased from Jackson Laboratory (Bar Harbor, ME) and male Sprague Dawley rats purchased from Charles River Laboratory (Wilmington, MA) were group-housed in standard plastic cages and kept on a 12-hour light/dark cycle (lights on at 06:00). All animals were allowed ad libitum access to food (mice: LabDiet 5001; rats: Rodent Chow) and water. After arrival animals were acclimatized to housing for 1 week prior to experimentation. For all experiments, animals ranged in age from 6-8 weeks.
Single drug injections
Animals were habituated to handling and intraperitoneal injections for 3 days prior to any drug treatment. On the 4th day animals were injected with saline, ethanol, cocaine, amphetamine, or methamphetamine. Drugs were administered through intraperitoneal injections of 12 ml/kg approximately 3 to 5 hours from the start of their light cycle and returned back to their home cages. All drugs were dissolved in 0.9% saline. The final doses of administered drug were as follows: 2.5 g/kg ethanol (as a 20% volume/volume solution), 10, 20, or 30 mg/kg cocaine and 5 mg/kg methamphetamine hydrochloride (National Institute on Drug Abuse drug supply program: Research Triangle Institute, Research Triangle Park, NC), or 5 mg/kg amphetamine sulfate (Sigma-Aldrich, St. Louis, MO).
Repeated drug injections
All drug preparations and administration protocol remained the same in both single and repeated drug experiments. In the saline conditions mice received 7 days of saline injections; in the ethanol and acute cocaine conditions mice received 6 days of saline and on the 7th day received either 2.5 g/kg ethanol or 20 mg/kg cocaine; and in the repeated cocaine conditions mice received 7 days of 20 mg/kg cocaine.
Stress
For restraint stress, animals were placed in a polypropylene restraint tube (mouse: 25 mm diameter × 95 mm length; rat: 50 mm diameter × 200 mm length, PLAS Labs, Lansing, MI) for 2 hours in the home cage. For swim stress, mice were placed for 10 minutes into a 2000 ml glass beaker filled with 1000 ml of 30°C water. For footshock, mice were placed into a sound-attenuated plexiglass chamber (20 × 25 × 12.5 cm) with a steel shock grid floor (San Diego Instruments, San Diego, CA), where constant-current foot shock was applied at 0.75 mA intensity 6 times with a 1-minute interval.
Tissue preparation
Two hours after injections or the start of the stressor, animals were euthanized by CO2 inhalation and brains were isolated. After removal, the brains were postfixed in 2% paraformaldehyde in phosphate buffered saline (PBS) overnight and cryoprotected in 20% and 30% sucrose in PBS until saturation. Slices were collected with a CM1850 cryostat (Leica Microsystems, Inc., Deerfield, IL). Immunohistochemistry was performed on 30 μm mouse and 40 μm rat floating slices containing the entire pIIIu or nucleus accumbens.
Immunohistochemistry
To reveal the presence of Fos-containing cells, endogenous peroxidase activity was quenched with 0.3% peroxide in PBS. Blocking was accomplished with 4.5% goat serum (Vector Laboratory Inc., Burlingame, CA) in PBS and 0.3% Triton-X 100 (Sigma-Aldrich). Slices were subsequently incubated overnight in a 1:2000 dilution of a rabbit polyclonal antibody against Fos (Santa Cruz Biotech., Santa Cruz, CA) in PBS/Triton-X and 0.1% Bovine Serum Albumin (BSA). Biotinylated anti-rabbit (raised in goat) secondary antibody (Vector Laboratory Inc., Burlingame, CA) in PBS/Triton-X was used to detect the Fos antibody signal. The immunoreaction was revealed using the Vectastian ABC kit (Vector Laboratory Inc.) in PBS/Triton-X, and the immunostaining reaction was developed using the metal enhanced DAB kit (Thermo Scientific, Rockford, IL). Slices were subsequently mounted on gelatinized slides, dehydrated, and coverslipped with Cytoseal 60 mounting medium (Richard-Allan Scientific, Kalamazoo, MI).
Immunofluorescence
The simultaneous presence of both Fos and Ucn 1 was revealed through the following procedure. Floating slices (described above) were blocked for 30 minutes with 2% BSA and heparin (740 units/L) and subsequently incubated with 1:2000 rabbit polyclonal Fos antibody and 1:5000 goat polyclonal Ucn 1 antibody (Sigma-Aldrich) overnight in PBS, 0.1% BSA and 0.3% Triton-X 100. A 1-hour incubation with 0.5% AlexaFluor 488 anti-rabbit (raised in chicken) and AlexaFluor 555 anti-goat (raised in donkey) (Sigma-Aldrich) in PBS/Triton-X was used to detect the Fos and Ucn 1 signals. Slices were washed with PBS, mounted on gelatinized slide, coverslipped with VectaShield mounting medium for fluorescence with DAPI (Vector Laboratory Inc.), and sealed with clear nail polish.
The simultaneous presence of both Fos and tyrosine hydroxylase (TH) was revealed through the following procedure. Floating slices (described above) were blocked for 1 hour with 2% BSA in PBS/Triton-X and subsequently incubated with 1:2000 polyclonal rabbit Fos antibody overnight in 0.1% BSA/PBS/Triton-X. A 2-hour incubation with AlexaFluor 488 anti-rabbit (raised in chicken) (Sigma-Aldrich) in 0.1% BSA and PBS/Triton-X was used to detect the Fos signal. Slices were extensively washed with PBS prior to a second 2-hour blocking in 2% BSA in PBS/Triton-X. Slices were then incubated for 2 hours with rabbit polyclonal TH antibody (Chemicon Inc., Temecula CA) and detected with AlexaFluor 555 anti-rabbit (raised in goat) (Sigma-Aldrich) in PBS/Triton-X. Finally, slices were washed with PBS, mounted on gelatinized slide, coverslipped with VectaShield mounting medium for fluorescence with DAPI (Vector Laboratory Inc.), and sealed with clear nail polish.
Quantification
The number of Fos–containing cells was counted manually using a Leica DM4000 microscope (Bartels & Stout Inc., Bellevue, WA) by an individual masked to the group of animals. The number of Fos-containing cells was assessed in two anterior, middle and posterior sections of the pIIIu (approximate Bregma positions: -3.10 mm to -3.30 mm, -3.35 mm to -3.60 mm, -3.65 mm to -4.00 mm respectively) and averaged to produce a single data point per animal (pilot repeated ANOVA analysis did not reveal any interactions between Bregma position and treatment), in three-four sections of the paraventriculular nucleus of hypothalamus (approximate bregma positions: -0.58 mm to -0.94 mm) averaged to produce a single data point per animal, and in two sections of the shell and core of the nucleus accumbens (approximate Bregma position: 0.90 mm) averaged to produce a single data point per animal. Images were acquired with a Q-Imaging MicroPublisher 3.3 RTV (Surrey, BC, Canada) and processed using Q-Capture and ImageJ Software.
Statistics
An ANOVA was used to analyze the number of Fos-positive cells per treatment. Fisher’s PLSD post-hoc analysis was used where appropriate. All data are presented as means and S.E.M.
Results
Exposure to psychostimulants induces Fos-ir in the pIIIu of mice
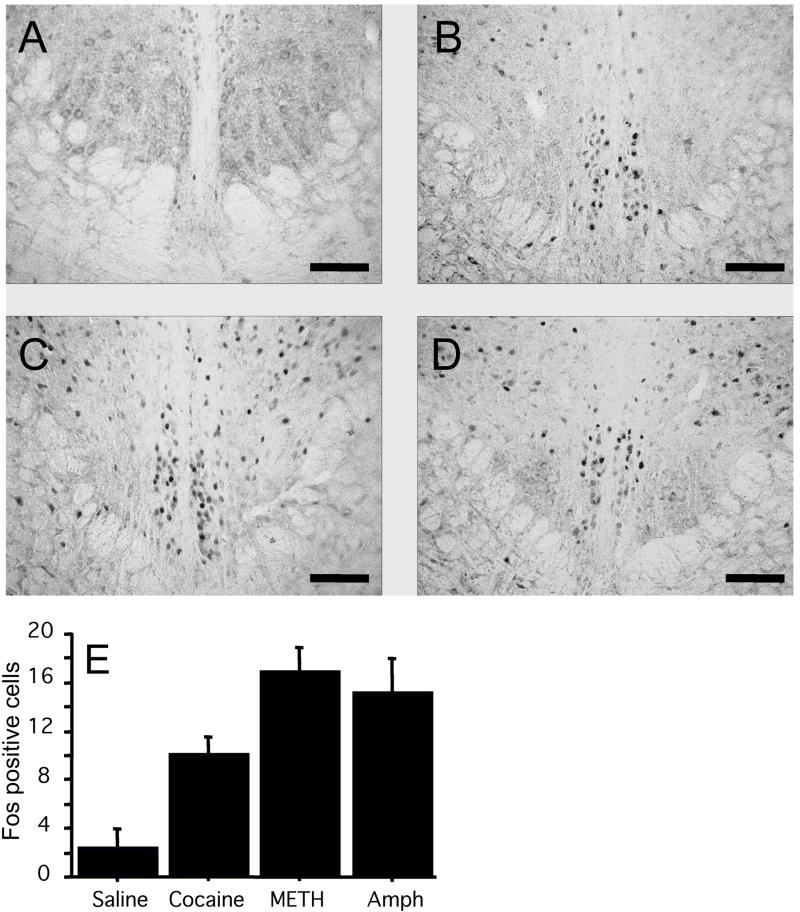
I.p. injections of the psychostimulants cocaine (30 mg/kg), methamphetamine (5 mg/kg) and amphetamine (5 mg/kg) increased the number of Fos-ir cells in the pIIIu of C57BL/6J mice (Figure 1). This was confirmed a by a significant one-way ANOVA: F (3, 16) = 10.9, p=0.0004. Post-hoc analysis revealed a significant difference between saline and cocaine conditions (p<0.05), saline and methamphetamine (p<0.0001), saline and amphetamine conditions (p<0.001), as well as cocaine and methamphetamine conditions (p<0.05).
Figure 1.
Fos-ir after administration of saline (A) 30 mg/kg cocaine (B), 5 mg/kg methamphetamine (C), or 5 mg/kg amphetamine (D) in male C57BL/6J mice (N=5 per group). DAB staining of the pIIIu nucleus revealed minimal Fos level in the saline treatment and a significant induction of Fos-ir after the cocaine, methamphetamine, and amphetamine treatments. Bar = 100μm. Quantification of induction across conditions is summarized in (E).
Since the level of the Fos response in pIIIu after psychostimulants appeared lower than in our previous experiments with ethanol, we performed an additional experiment comparing the dose response of pIIIu to cocaine (10-30 mg/kg) with its response to 2.5 g/kg of ethanol (a dose we previously determined would produce a maximal induction of Fos-ir in the mouse pIIIu – Bachtell et al, 2002b). All doses of cocaine produced a significant and dose-dependent increase in Fos-ir in pIIIu. However, even the highest dose did not reach the magnitude of the ethanol’s effect in this brain region (Figure 2). This was confirmed by a significant one-way ANOVA: F (4, 19) = 81.0. P<0.0001. Fisher PLSD post-hoc confirmed significant differences in all drug conditions versus saline (p<0.0001), and a significant difference between the ethanol and all three cocaine conditions (p<0.0001). A dose-dependence of cocaine’s effect on Fos-ir was confirmed by significant difference in Fos-ir after 10 versus 30 mg/kg of cocaine (p<0.05).
Figure 2.
Quantitative averages of Fos-positive cells found in the pIIIu after administration of saline, 2.5 g/kg ethanol, 10, 20, and 30 mg/kg cocaine in male C57BL/6J mice (N=5 per group). Induction of Fos-ir after cocaine occurs in a dose - dependent manner.
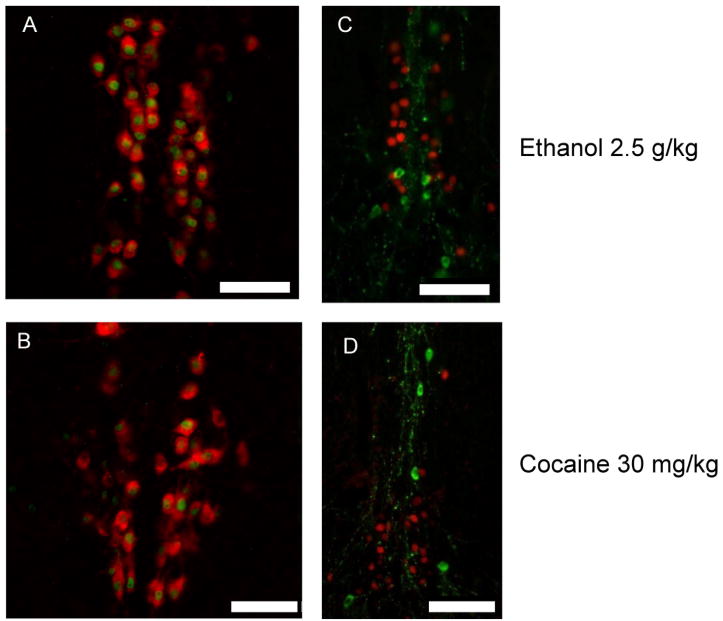
Previous studies in mice revealed that the pIIIu contains a subpopulation of dopaminergic neurons that is an extension of the rostral linear nucleus of raphe (Bachtell et al, 2003). To investigate whether both ethanol and cocaine activated the same type of neurons in pIIIu we performed double-label fluorescence immunohistochemistry experiments on brain slices from the above experiments (Figure 3). Both cocaine (30 mg/kg) and ethanol (2.5 g/kg) induced Fos-ir exclusively in Ucn 1-positive, but not in TH-positive neurons, confirming that both drugs are acting on the same type of neurons.
Figure 3.
Double label immunofluorescence staining of Ucn 1 (red) and Fos (green) confirms that both 2.5 g/kg ethanol (A) and 30 mg/kg cocaine (B) treatments induce Fos-ir exclusively in the Ucn 1-positive cells characteristic of the pIIIu nucleus in male C57BL/6J mice. Whereas, double label immunofluorescence staining of Tyrosine Hydroxylase (green) and Fos (red) reveals that Fos-ir does not occur in dopaminergic cells subsequent to injections of 2.5 g/kg ethanol (C) and 30 mg/kg cocaine treatments (D) in male C57BL/6J mice Bar = 50μm.
Fos-ir in the mouse pIIIu is increased after cocaine or ethanol, but not after restraint
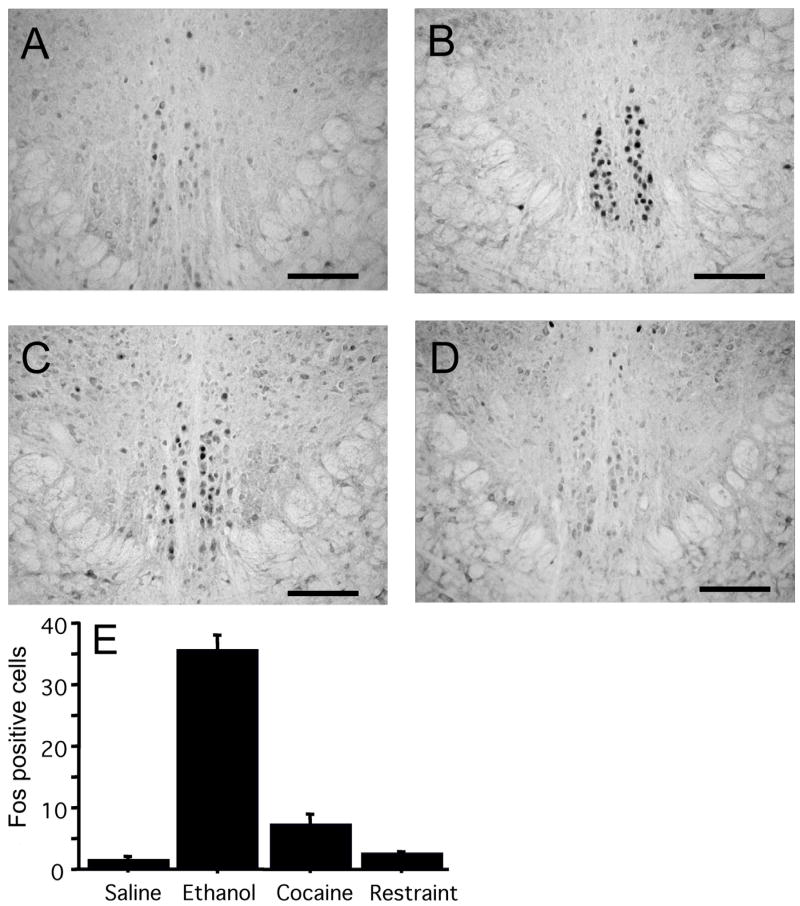
Since the magnitude of the Fos response after cocaine was substantially weaker than after ethanol, we questioned whether the effect of cocaine in pIIIu was pharmacologically-specific or, alternatively, produced by the stress of being injected with cocaine. To examine this, we tested the effect of restraint stress, a treatment known to produce a robust glucocorticoid response (Zhou et al., 1999; Lund et al., 2006), and a significant increase in Fos-ir in the rat pIIIu (Gaszner et al., 2004). We compared Fos-ir in the C57BL/6J mice following 2.5 g/kg of ethanol, 30 mg/kg of cocaine or 2 hours of restraint stress (Figure 4). Both cocaine and ethanol produced a significant induction of Fos-ir in pIIIu. In contrast, restraint stress did not induce Fos-ir. This was confirmed by a one-way ANOVA: F (3, 16) = 105.5, p<0.0001. Fisher PLSD post-hoc showed a significant difference between the ethanol treatment and all other conditions, and a significant difference between cocaine treatment versus saline injection or restraint stress (p<0.05). The lack of pIIIu response to restraint stress was in agreement with previous studies in mice (Turek & Ryabinin, 2005), and suggested that stress was unlikely to have played a significant role in cocaine-induced Fos-ir in this nucleus.
Figure 4.
Fos-ir after administration of saline (A) 2.5 g/kg ethanol (B), 30 mg/kg cocaine (C), or 2 hours of restraint stress (D) in male C57BL/6J mice (N=5 per group). DAB staining of the pIIIu nucleus revealed lack of Fos-ir after the saline and the restraint stress, but a significant increase in Fos-ir after the cocaine and ethanol treatment. Quantification of induction across conditions is summarized in (E). Bar=100 μm.
Fos-ir in mouse pIIIu is increased after cocaine and ethanol, but not after swim stress or footshock
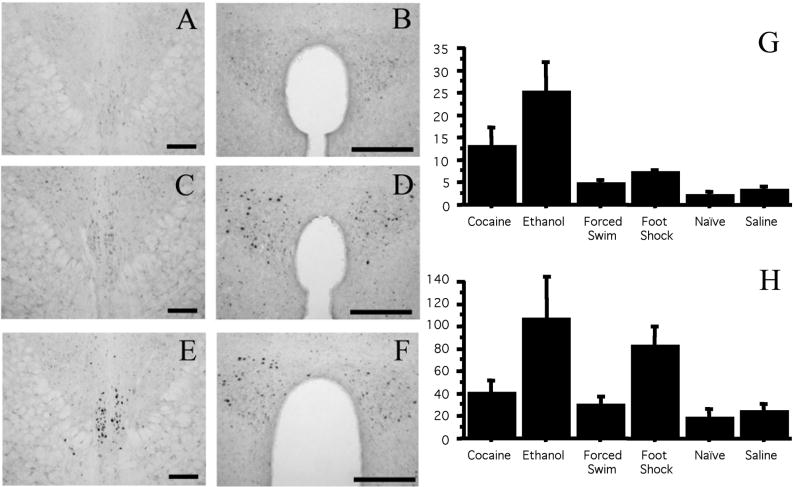
To test whether the lack of pIIIu response to restraint stress is specific to this stressor, we compared Fos-ir after 2.5 g/kg of ethanol, 30 mg/kg of cocaine, 10 minutes of swim stress or six 0.75 mA foot-shocks. In addition, a treatment-naïve control group was added, as a control for the stress treatments, and to test whether lack of difference between stressed groups and saline controls is due to potentially higher Fos-ir in animals repeatedly injected with saline. As in the previous experiments, only ethanol and cocaine administration, but not swim or footshock stress, increased Fos-ir compared to saline-injected or naïve controls (Figure 5). This was confirmed by a significant one-way ANOVA: F (5,30)=7.0, p=0.0002. Fisher PLSD post-hoc revealed significant differences between the ethanol treatment versus cocaine (p<0.05), the ethanol treatment versus all other conditions (p<0.001), and the cocaine treatment versus saline and naïve controls (p<0.05). Fos-ir in pIIIu was not different between the footshock, swim stress, saline and naïve control conditions. There was no increase in Fos-ir in pIIIu following footshock or swim stress despite a substantial increase of Fos-ir in the paraventricular nucleus of hypothalamus in footshock-stressed animals (one-way ANOVA: F (5,29)=3.1, p=0.02, Fisher PLSD post-hoc comparison: p<0.05). The lack of pIIIu response to the swim stress and footshock confirmed low sensitivity of the mouse pIIIu to various stresses, and indicated that stress was unlikely to have played a significant role in cocaine-induced Fos-ir in this nucleus.
Figure 5.
Fos-ir after administration in pIIIu and the paraventricular nucleus of hypothalamus in naïve mice or mice injected with saline, 20 mg/kg cocaine, 2.5 g/kg ethanol, or exposed to footshock or swim stress (N=6 per group). DAB staining of the pIIIu revealed lack of Fos-ir in the naïve, saline, footshock and swim stress condition, but a significant increase in Fos-ir after the cocaine and ethanol treatment. Representative staining in the pIIIu (A, C, E) or paraventricular nucleus of hypothalamus (B, D, F) in the naïve (A, B), footshock (C, D) and cocaine (E, F) groups is shown on the top. Bar=100 μm. Quantification of induction across conditions in pIIIu is summarized in (G), Quantitation across conditions in the paraventricular nucleus of hypothalamus is summarized in (H).
Repeated administration of cocaine does not desensitize the Fos response in the mouse pIIIu
To further evaluate whether cocaine-induced Fos-ir could be influenced by a non-specific stress response, we exposed C57BL/6J mice to acute or repeated administrations of cocaine (20 mg/kg) or ethanol (2.5 g/kg, as a positive control). This experiment was based on the observations that repeated exposure to a mild stressor tends to habituate the animal to this stressor resulting in decreased Fos response. A desensitized response can be observed after only a few exposures to a mild stressor (Melia et al., 1994; Watanabe et al., 1994; Ryabinin et al., 1999; Girotti et al., 1996).
Both acute and repeated cocaine produced a significant induction of Fos-ir in pIIIu (Figure 6). This was confirmed by a significant one-way ANOVA: F (3, 40) = 14.1, P<0.0001. Post-hoc Fisher PLSD analysis showed significant differences between saline and acute cocaine (p<0.05), saline and ethanol (p<0.0001), saline and chronic cocaine (p<0.01), acute cocaine and ethanol (p<0.001), ethanol and chronic cocaine (p<0.01) treatments. However, no significant difference was found between acute cocaine and chronic cocaine conditions, indicating first, that there was no desensitization of the Fos response in the pIIIu after repeated cocaine injections and second, that the cocaine-induced Fos response in the pIIIu was distinct from any generalized stress-induced Fos-ir.
Figure 6.
Quantification of Fos-ir in the pIIIu shows maintained response in male C57BL/6J mice exposed to repeated cocaine injections. C57BL/6J mice were injected with either saline for 7 days, saline for 6 days and ethanol for 1 day, saline for 6 days and 20 mg/kg cocaine for 1 day, or 20 mg/kg cocaine for 7 days (N=6 per group). DAB staining of the pIIIu revealed increased Fos-ir induction after ethanol exposure, and both acute and chronic cocaine exposure.
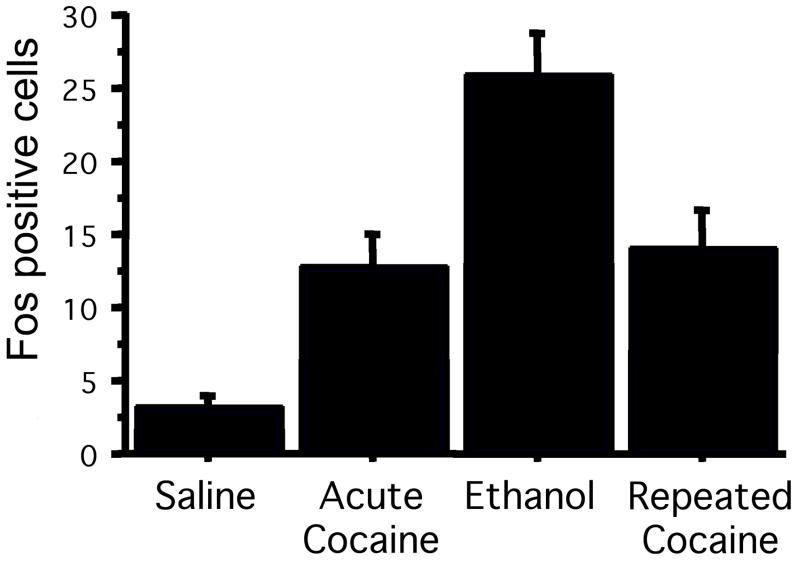
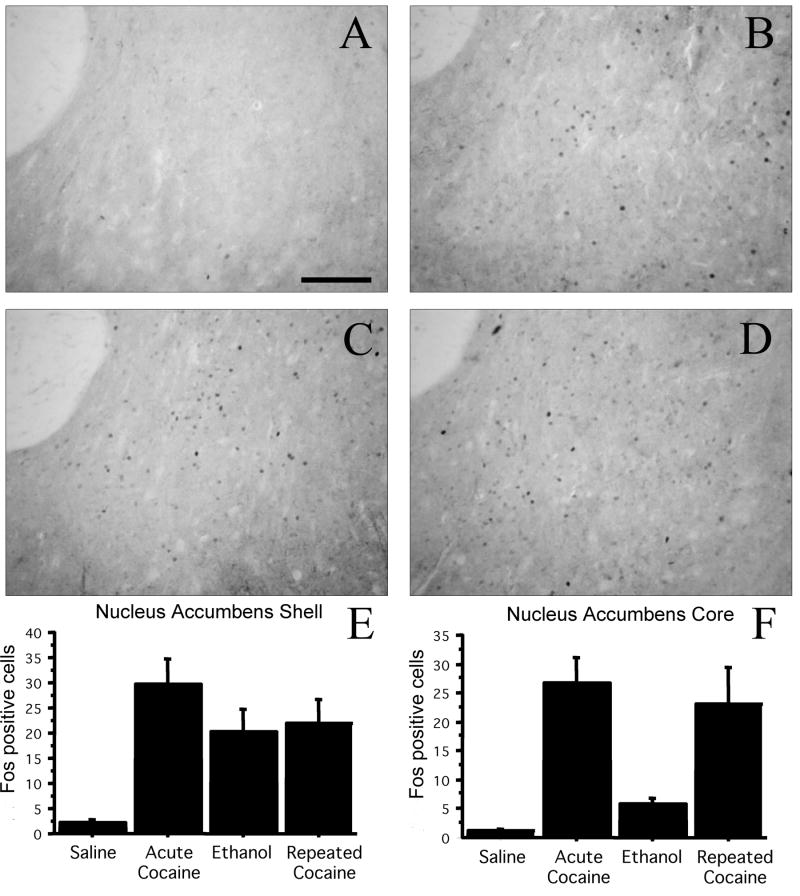
To compare the response in pIIIu to another area that is known to respond to psychostimulants, we also quantified Fos-ir in the nucleus accumbens of these animals. The Fos response in the shell and the core subregions of the nucleus accumbens was similar to what we observed in the pIIIu, except that there were weaker effects of ethanol (Figure 7). Quantification of the effect of treatment on the core of the nucleus accumbens showed a significant induction after both acute and chronic cocaine ANOVA: F (3, 19) = 9.4, p<0.0005). Post-hoc Fisher PSLD confirmed significant differences between saline and acute cocaine (p<0.0005), saline and chronic cocaine (p<0.005), acute cocaine and ethanol (p<0.005), and ethanol vs. chronic cocaine (p<0.01). Similar effects were found in the shell of the nucleus accumbens ANOVA: F (3, 10) = 6.6, p<0.005. Post-hoc analysis confirmed significant differences between saline and acute cocaine (p<0.0005), saline and ethanol (p<0.05), and saline and chronic cocaine (p<0.01).
Figure 7.
Fos-ir in the core and shell of the nucleus accumbens shows no desensitization in male C57BL/6J mice exposed to repeated cocaine injections. C57BL/6J mice were injected with either saline for 7 days (A), saline for 6 days and ethanol for 1 day (B), saline for 6 days and 20 mg/kg cocaine for 1 day (C), or 20 mg/kg cocaine for 7 days (D). Bar=100 μm. Quantitation of Fos-ir in the nucleus accumbens shell (E) and core (F) revealed increased Fos-ir after both acute and chronic cocaine exposure, and elevated Fos-ir after ethanol exposure in the shell (N=6 per group).
The increased Fos-ir in the rat pIIIu occurs after ethanol, cocaine and restraint
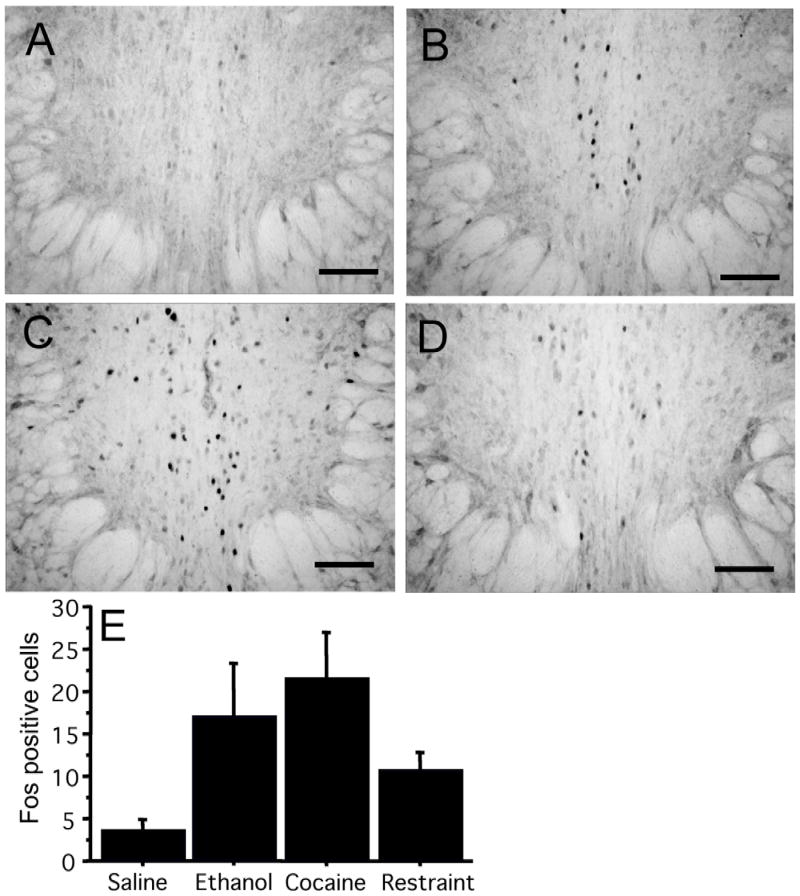
The lack of the mouse pIIIu response to restraint stress contrasts with studies in other species showing that pIIIu is stress-sensitive (Gaszner et al., 2004; Cunha et al., 2007; Kozicz et al., 2007). Therefore, we hypothesized that the magnitude of the response of pIIIu to cocaine, ethanol and stress may be different in other species. To address this hypothesis we compared induction of Fos-ir in pIIIu after cocaine (20 mg/kg), ethanol (2.5 g/kg) or 2 hours of restraint stress in male Sprague-Dawley rats. All three types of stimulation resulted in an increased Fos-ir in the rat pIIIu compared to saline-injected rats (Figure 8). This was confirmed by a significant one-way ANOVA: F (3, 28) = 7.0, p=0.001. Post-hoc Fisher PLSD analysis confirmed significant differences between all three treatments and the saline group (p<0.05). As in mice, the ethanol group showed higher Fos-ir than all other groups (p<0.05). However, unlike the experiments in mice, Fos-ir in the restraint group was not different from the cocaine group.
Figure 8.
Fos-ir in saline-injected (A), 2.5 g/kg ethanol-injected (B), 20 mg/kg cocaine-injected (C), or 2 hour restraint-stressed (D) male Sprague Dawley rats (N=8 per group). DAB staining of the pIIIu nucleus revealed minimal Fos immunoreactivity after the saline treatment, and significant elevation after restraint stress, ethanol and cocaine treatments. Quantification of induction across conditions is summarized in (E). Bar = 100μm.
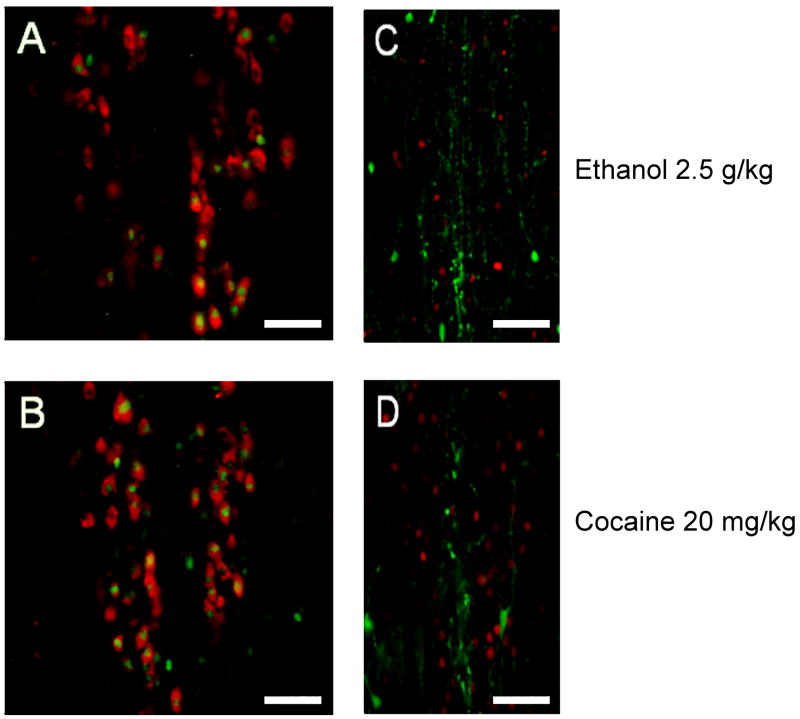
To test whether cocaine and ethanol recruited similar populations of neurons in the rat pIIIu, we analyzed whether Fos-ir in pIIIu after cocaine occurred strictly in Ucn 1-positive neurons, or whether it occurred also in TH-positive subpopulation of cells using double-label fluorescence immunohistochemistry (Figure 9). Similarly to mice, all Fos-ir colocalized exclusively with Ucn 1-ir, but not with TH-ir, indicating the both cocaine and ethanol regulated activity of the same type of neurons in pIIIu.
Figure 9.
Double label immunofluorescence staining of Ucn 1 (red) and Fos (green) confirms both 2.5 g/kg ethanol (A) and 20 mg/kg cocaine (B) treatments alter Fos-ir in Ucn 1-positive cells characteristic of the pIIIu nucleus in male Sprague Dawley rats. However, double label immunofluorescence staining of TH (green) and Fos (red) reveals that Fos-ir does not occur in dopaminergic cells subsequent to injections of 2.5 g/kg ethanol (C) and 20 mg/kg cocaine treatments (D) in male Sprague Dawley rats. Bar = 50μm.
Discussion
The results of the present study demonstrate that the pIIIu responds to psychostimulants in both rats and mice, and that and this response is separable from a stress response in mice, but not rats. The issue of sensitivity of the pIIIu to stress has been debated. On the one hand, it has been shown that exposure of rats to restraint, lipopolysaccharide (LPS) challenge, and to a lesser degree, to ether, hyperosmotive and hyposmotic shock, results in induction of Fos-ir (Gaszner et al., 2004; Korosi et al., 2005; Kozicz et al., 2004). Similarly, restraint stress in birds increased ir of Egr1, another inducible transcription factor (Cunha et al., 2007). On the other hand, our previous studies in mice did not find any induction of Fos or Egr1-ir following restraint stress, LPS, administration of yohimbine, food deprivation or exposure to a novel environment (Bachtell et al., 2002a; Bachtell & Ryabinin, 2005; Turek & Ryabinin, 2005; Spangler & Ryabinin, unpublished observations). Lack of significant change in gene expression could be interpreted as a lack of induction, a lack of sensitivity of method or the wrong time point for measuring the effect. However, Turek and Ryabinin (2005) analyzed Fos-ir in pIIIu at several time points and demonstrated a lack of Fos response in pIIIu occurs despite increased Fos–ir in other areas. The present study also indicates that swim and footshock stress does not result in increased Fos-ir in pIIIu. We conclude therefore that, at least in mice, ethanol and cocaine are substantially more potent activators of Fos-ir in pIIIu than stress.
Repeated exposure to a homotypic stressor results in habituation of the global Fos response in the brain (Melia et al., 1994; Watanabe et al., 1996; Ryabinin et al., 1999; Girotti et al., 2006). Since our study did not demonstrate a habituation of Fos-ir in pIIIu to repeated cocaine, there is another argument for the independence of this effect from stress and for a pharmacological specificity of pIIIu response to psychostimulants. This result, however, should be considered alongside studies that showed lack of attenuation of the Fos-ir in the mouse pIIIu following repeated exposure to an ether challenge (Korosi et al., 2005). It seems likely that ether induced Fos-ir in pIIIu via mechanisms similar to the actions of cocaine, and not via mechanisms of a general stressor.
It is important to note that the lack of Fos in pIIIu after exposure to stressors does not mean that this nucleus is completely unresponsive to these treatments. Increased Fos-ir reflects activation of several specific signal transduction cascades (Herdegen & Leah, 1998). It is possible that stress engages other mechanisms that do not increase Fos-ir. Studies in mice showed that restraint stress and ether exposure, as well as a genetic deletion of CRF, can increase levels of Ucn 1 mRNA in pIIIu, while transgenic overexpression of CRF leads to a decrease in Ucn 1 mRNA in pIIIu (Weninger et al., 2000; Kozicz et al, 2004). Recent studies also found that chronic social stress decreases Ucn 1-ir in the tree shrew pIIIu (Kozicz et al., 2008a) and that starvation decreases Ucn 1-ir in the pIIIu of Xenopus (Calle et al., 2006). Importantly, differences in Ucn 1 and brain-derived neurotrophic factor levels in pIIIu have been observed in human suicide victims (Kozicz et al., 2008b), a finding suggesting that stimuli leading to depression can change activity of this nucleus in humans. Thus, our findings of activation of inducible transcription factors by ethanol and cocaine, but not by stress, do not indicate that this nucleus is completely unresponsive to stress, but that stress and addictive drugs employ different mechanisms in this nucleus.
Since the observed effect of psychostimulants in pIIIu is induced by pharmacologically-specific mechanisms it is important to consider the neurotransmitter mechanisms of this induction. The three psychostimulants used in this study all act on the dopamine transporter. It is logical to assume that they induce Fos-ir via the dopamine system. Interestingly, studies in rats showed that 6-hydroxy-dopamine lesion of dopaminergic innervation of pIIIu did not block stress-induced Fos-ir in this brain region (Gaszner & Kozicz, 2003). It would be important to investigate what such lesions do to psychostimulant-induced Fos-ir. In agreement with the involvement of dopaminergic mechanisms, mice lacking the dopamine transporter did not show induction of Fos-ir in pIIIu following administration of a psychostimulant (Trinh et al., 2003). Another study suggested the involvement of dopamine in pIIIu activity by showing that haloperidol blocks ethanol-induced increase of Fos-ir in this brain region (Bachtell et al., 2002). Interestingly, this study also showed that ethanol and morphine induce Fos-ir in this brain region via different mechanisms because morphine-, but not ethanol-, induced Fos-ir in pIIIu was blocked by the opioid antagonist naltrexone. Therefore, dopamine is most likely not the only neurotransmitter involved in activation of pIIIu. Since the Fos response of pIIIu to ethanol is reliably higher than the response to psychostimulants, we hypothesize that additional mechanisms, independent of dopamine, play a role in ethanol’s effects in this nucleus. GABAergic and noradrenergic mechanisms have also been implicated in the pIIIu Fos response (Bachtell et al, 2002). Effects of some of these mechanisms could be additive with the effects of dopamine.
Studies discussed so far show that pIIIu is sensitive to several addictive drugs. A more important consideration is whether this nucleus is also involved in mediating effects of these drugs, especially their addictive potential. This possibility has been confirmed in studies with alcohol. Thus, Ucn 1-ir in pIIIu is significantly higher in inbred alcohol-preferring C57BL/6J mice than alcohol avoiding DBA/2J mice (Bachtell et al., 2002b; Bachtell et al., 2003; Weitemier et al., 2005). Similarly, Ucn 1-ir in pIIIu is significantly higher in two replicate lines of selectively bred high alcohol preferring mice compared to corresponding lines of low alcohol preferring mice (Bachtell et al., 2003). Ucn 1-ir is also significantly higher in mice selectively bred for high alcohol-induced place preference compared to mice selectively bred for low alcohol-induced place preference (Kiianmaa et al., 2003). Moreover, electrolytic lesions of pIIIu block alcohol preference in C57BL/6J mice (Bachtell et al., 2004). Taken together this evidence indicates that pIIIu is not only sensitive to ethanol, but also is involved regulation of alcohol consumption. It is possible therefore, that pIIIu could be involved in regulation of self-administration of other drugs of abuse, such as psychostimulants. In fact, data collected in this study are in agreement with this idea. Thus, we show that administration of drugs producing hedonic effects consistently leads to increased Ucn 1-ir in pIIIu across species. In contrast, stress, which is not a hedonic factor, does not result in a consistent increase in Fos-ir in pIIIu across species. We hypothesize therefore, that activation of pIIIu is related to positive reinforcing properties of drugs of abuse. On the other hand, since Ucn 1 is known to act on both CRF receptor 1 and CRF receptor 2, it is possible that the role of pIIIu could be biphasic: enhancing reinforcing effects of drugs via one type of receptors at low levels of activity, and blocking these effects via another receptor at higher levels of activity. Interestingly, biphasic effects of CRF-related peptides have been recently demonstrated in the dorsal raphe, a brain region innervated by pIIIu (Kirby et al., 1999; Pernar et al., 2004). Addressing this hypothesis will be an important direction for further research on the mechanisms of psychostimulant addiction.
Acknowledgments
We would like to thank Ronjon Datta, Charlene Voorhees, Jason Jaworski, and Simranjit Kaur for technical help. This work was supported by NIH Grants AA013738, AA016647 and DA018165.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999;847:157–165. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Ryabinin AE. Interactive effects of nicotine and alcohol co-administration on expression of inducible transcription factors in mouse brain. Neurosci. 2001;103:941–954. doi: 10.1016/s0306-4522(01)00042-2. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther. 2002a;302:512–524. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Strain differences in urocortin expression in the Edinger-Westphal nucleus and its relation to alcohol-induced hypothermia. Neurosci. 2002b;113:421–434. doi: 10.1016/s0306-4522(02)00174-4. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Galvan-Rosas A, Tsivkovskaia NO, Risinger FO, Phillips TJ, Grahame NJ, Ryabinin AE. The Edinger-Westphal-lateral septum urocortin pathway and its relationship to alcohol consumption. J Neurosci. 2003;23:2477–2487. doi: 10.1523/JNEUROSCI.23-06-02477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Ryabinin AE. Lesion of the Edinger-Westphal nucleus in C57BL/6J mice disrupts ethanol-induced hypothermia and ethanol consumption. Eur J Neurosci. 2004;20:1613–1623. doi: 10.1111/j.1460-9568.2004.03594.x. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against persuasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- Calle M, Kozicz T, van der Linden E, Desfeux A, Veening JG, Barendregt HP, Roubos EW. Effects of starvation on Fos and neuropeptide immunoreactivities in the brain and pituitary gland of Xenopus laevis. Gen Comp Endocrinol. 2006;147:237–246. doi: 10.1016/j.ygcen.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Chang SL, Patel NA, Romero AA. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679:89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- Cunha RP, Reiner A, Toledo CA. Involvement of urocortinergic neurons below the midbrain central gray in the physiological response to restraint stress in pigeons. Brain Res. 2007;1177:175–83. doi: 10.1016/j.brainres.2007.01.122. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Brit J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaszner B, Kozicz T. Interaction between catecholaminergic terminals and urocortinergic neurons in the Edinger-Westphal nucleus in the rat. Brain Res. 2003;989:117–121. doi: 10.1016/s0006-8993(03)03367-5. [DOI] [PubMed] [Google Scholar]
- Gaszner B, Csernus V, Kozicz T. Urocortinergic neurons respond in a differentiated manner to various acute stressors in the Edinger-Westphal nucleus in the rat. J Comp Neurol. 2004;480:170–179. doi: 10.1002/cne.20343. [DOI] [PubMed] [Google Scholar]
- Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neurosci. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Horn AK, Eberhorn A, Hartig W, Ardeleanu P, Messoudi A, Buttner-Ennever JA. Perioculomotor cell groups in monkey and man defined by their histochemical and functional properties: reappraisal of the Edinger-Westphal nucleus. J Comp Neurol. 2008;507:1317–1335. doi: 10.1002/cne.21598. [DOI] [PubMed] [Google Scholar]
- Kiianmaa K, Hyytiä P, Samson HH, Engel JA, Svensson L, Söderpalm B, Larsson A, Colombo G, Vacca G, Finn DA, Bachtell RK, Ryabinin AE. New neuronal networks involved in ethanol reinforcement. Alcohol: Clin Exp Res. 2003;27:209–219. doi: 10.1097/01.ALC.0000051020.55829.41. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacol. 1999;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of Addiction. San Diego: Elsevier; 2006. [Google Scholar]
- Korosi A, Schotanus S, Olivier B, Roubos EW, Kozicz T. Chronic ether stress-induced response of urocortin 1 neurons in the Edinger-Westphal nucleus in the mouse. Brain Res. 2005;1046:172–179. doi: 10.1016/j.brainres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Kozicz T. Neurons colocalizing Urocortin and cocaine and amphetamine-regulated transcript immunoreactivities are induced by acute lipopolysaccharide stress in the Edinger-Westphal nucleus in the rat. Neurosci. 2003;116:315–320. doi: 10.1016/s0306-4522(02)00772-8. [DOI] [PubMed] [Google Scholar]
- Kozicz T. CRF and CRF-related peptides in stress adaptation: from invertebrates to man. Gen Comp Neurol. 2007;153:198–199. doi: 10.1016/j.ygcen.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Korosi A, Korsman C, Tilburg-Ouwens D, Groenink L, Veening J, van Der Gugten J, Roubos E, Olivier B. Urocortin expression in the Edinger-Westphal nucleus is down-regulated in transgenic mice over-expressing neuronal corticotropin-releasing factor. Neurosci. 2004;123:589–594. doi: 10.1016/j.neuroscience.2003.10.042. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Bordewin LAP, Czeh B, Fuchs E, Roubos EW. Chronic psychosocial stress affects corticotrophin-releasing factor in the paraventricular nucleus and central extended amygdala as well as Urocortin 1 in the non-preganglionic Edinger-Westphal nucleus of the tree shrew. Psychoneuroendocrinol. 2008a;33:741–54. doi: 10.1016/j.psyneuen.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Tillburg-Ouwens D, Faludi G, Palkovits M, Roubos E. Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neurosci. 2008b;152:1015–1023. doi: 10.1016/j.neuroscience.2007.12.050. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Yanaihara H, Arimura A. Distribution of Urocortin-like immunoreactivity in the central nervous system of the rat. J Comp Neurol. 1998;391:1–10. doi: 10.1002/(sici)1096-9861(19980202)391:1<1::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5apha-dehydrostrerone and its metabolite 5alpha-androstan-3beta,17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, Reiner AJ, Ryabinin AE. Comparison of the distributions of urocortin-containing and cholinergic neurons in the perioculomotor midbrain of the cat and macaque. J Comp Neurol. 2008;507:1300–1316. doi: 10.1002/cne.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restrain stress. J Neurosci. 1994;14:5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin SM, Ling N, Liu X-J, Kahl SD, Gehlert DR. Differential distribution of urocortin- and corticotropin-releasing factor-like immunoreactivities in the rat brain. Neurosci. 1999;92:281–291. doi: 10.1016/s0306-4522(98)00732-5. [DOI] [PubMed] [Google Scholar]
- Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2:32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Galvan-Rosas A, Bachtell RK, Risinger FO. High alcohol/sucrose consumption during dark circadian phase in C57BL/6J mice: involved of hippocampus, lateral septum and urocortin-positive cells of the Edinger-Westphal nucleus. Psychopharmacol. 2003;165:296–305. doi: 10.1007/s00213-002-1284-y. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Tsivkovskaia NO, Ryabinin SA. Urocortin 1-containing neurons in the human Edinger-Westphal nucleus. Neurosci. 2005;134:1317–1323. doi: 10.1016/j.neuroscience.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Wang Y-M, Finn DA. Different levels of Fos immunoreactivity after repeated handing and injection stress in two inbred strains of mice. Pharm Biochem Behav. 1999;63:143–151. doi: 10.1016/s0091-3057(98)00239-1. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Weitemier AZ. The urocortin 1 neurocircuit: ethanol sensitivity and potential involvement in alcohol consumption. Brain Res Rev. 2006;52:368–380. doi: 10.1016/j.brainresrev.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Tsivkivskaia NO, Ryabinin AE. Ataxia and c-fos expression in mice drinking in a limited access session. Alcohol: Clin Exp Res. 2005;29:1419–1426. doi: 10.1097/01.alc.0000174746.64499.83. [DOI] [PubMed] [Google Scholar]
- Singh ME, Verty AN, Price I, McGregor IS, Mallet PE. Modulation of morphine-induced Fos-immunoreactivity by the cannabinoid receptor antagonist SR 141716. Neuropharmacol. 2004;47:1157–1169. doi: 10.1016/j.neuropharm.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Singh ME, McGregor IS, Mallet PE. Perinatal exposure to delta(9)-tetrahydrocannabinol alters heroin-induced place conditioning and fos-immunoreactivity. Neuropsychopharmacol. 2006;31:58–69. doi: 10.1038/sj.npp.1300770. [DOI] [PubMed] [Google Scholar]
- Topple AN, Hunt GE, McGregor IS. Possible neurosubstrates of beer-craving in rats. Neurosci Lett. 1998;252:99–102. doi: 10.1016/s0304-3940(98)00574-6. [DOI] [PubMed] [Google Scholar]
- Turek VF, Ryabinin AE. Expression of c-Fos in the mouse Edinger-Westphal nucleus following ethanol administration is not secondary to hypothermia or stress. Brain Res. 2005;1063:132–139. doi: 10.1016/j.brainres.2005.09.056. [DOI] [PubMed] [Google Scholar]
- Trinh JV, Nehrenberg DL, Jacobsen JP, Caron MG, Wetsel WC. Differential psychostimulant-induced activation of neural circuits in dopamine transporter knockout and wild type mice. Neurosci. 2003;118:297–310. doi: 10.1016/s0306-4522(03)00165-9. [DOI] [PubMed] [Google Scholar]
- van Nieuwenhuijzen PS, McGregor IS, Hunt GE. The distribution of gamma-hydroxybutyrate-induced Fos expression in rat brain: Comparison with baclofen. Neurosci. 2009;158:441–455. doi: 10.1016/j.neuroscience.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, et al. Urocortin, a mammalian neuropeptide related to fish urotensin 1 and to corticotropin-release factor. Nature. 1995;378:287–92. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Stone E, McEwen BS. Induction and habituation of c-fos and zif/268 by acute and repeated stressors. Neuroreport. 1994;5:1321–1324. [PubMed] [Google Scholar]
- Weitemier AZ, Woerner A, Bäckström P, Hyytiä P, Ryabinin AE. Expression of c-Fos in Alko alcohol rats responding for ethanol in an operant paradigm. Alcohol: Clin Exp Res. 2001;25:704–710. [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Brain region-specific regulation of Urocortin 1 innervation and corticotrophin-releasing factor receptor type 2 binding by ethanol exposure. Alcohol: Clin Exp Res. 2005;29:1610–1620. doi: 10.1097/01.alc.0000179363.44542.05. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Tsivkovskaia NO, Ryabinin AE. Urocortin 1 distribution in mouse brain is strain-dependent. Neurosci. 2005;132:729–740. doi: 10.1016/j.neuroscience.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Weninger SC, Peters LL, Majzoub JA. Urocortin expression in the Edinger-Westphal nucleus is up-regulated by stress and corticotropin-releasing hormone deficiency. Endocrinol. 2000;141:253–263. doi: 10.1210/endo.141.1.7277. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cheshire A, Howell LA, Ryan DH, Harris RBS. Neuroautoantibody immunoreactivity in relation to aging and stress in aoplipoprotein E-deficient mice. Brain Res Bull. 1999;49:173–179. doi: 10.1016/s0361-9230(99)00052-0. [DOI] [PubMed] [Google Scholar]