Abstract
Adolescence may be critical period for drug addiction. Young adolescent male rats have greater locomotor responses than adults after acute low dose cocaine administration. Further, repeated cocaine administration produces as much or more conditioned place preference but reduced locomotor sensitization in adolescents compared to adults. Acute activation of neurons by cocaine induces long-term changes in behavior by activating transcriptional complexes. The purpose of the present study was to correlate cocaine-induced locomotor activity with neuronal activation in subregions of the striatum and cortex by acute cocaine in young adolescent (post natal (PN) 28) and adult (PN 65) male rats by measuring the induction of the plasticity-associated immediate early genes (IEGs) c-fos and zif268 using in situ hybridization. Animals were treated with saline, low (10 mg/kg), or high (40 mg/kg) dose cocaine in locomotor activity chambers and killed 30 min later. Low dose cocaine induced more locomotor activity and striatal c-fos expression in adolescents than adults whereas high dose cocaine induced more locomotor activity, striatal c-fos, and striatal zif268 expression in adults. Locomotor activity correlated with the expression of both genes in adults but correlated with striatal c-fos only in adolescents. Finally, there was a significant correlation between the expression of c-fos and zif268 in the adult striatum but not in adolescents. Our results suggest that the coordinated expression of transcription factors by cocaine continues to develop during adolescence. The immature regulation of transcription factors by cocaine could explain why adolescents show unique sensitivity to specific long-term behavioral alterations following cocaine treatment.
Keywords: cocaine, adolescence, c-fos, zif268, locomotion, rat
Adolescence is a critical period for drug addiction in humans (reviewed in Chambers et al. 2003). Most life long addiction is initiated during adolescence (reviewed in Spear 2000). Drug use initiated during adolescence is more commonly associated with worse long-term outcomes (Reviewed in Chambers et al. 2003; Spear 2000). The progression from first use to addiction appears to be shorter during adolescence (Estroff et al. 1989; Chen and Kandel 1995; Clark et al. 1998). Early adolescence represents a time of particular risk: alcohol, marijuana, or illicit drug use early in adolescence predicts worse outcomes than use later in adolescence (Barnes and Welte 1986; Hawkins et al. 1997; Kandel and Davies 1992; Robins and Pryzbeck 1985; Yamaguchi and Kandel 1984). Understanding the neural mechanisms which mediate addiction vulnerability during this crucial developmental phase is important to developing age-appropriate prevention and treatment programs.
Forebrain dopamine systems represent a logical substrate to mediate adolescent vulnerability to drug addiction as they contribute to both the rewarding and locomotor activating effects of addictive stimulants (Kelley 1999; Kelley et al. 2004). Evidence suggests that forebrain dopamine function in young adolescents differs significantly from adults. Young adolescents may also find low, but not high doses of cocaine more rewarding than older adolescent and adult rats (Badanich et al. 2006). Locomotor responses to stimulants like cocaine also may be highest during early adolescence. We have shown that young adolescents (PN 28) have greater locomotor responses to acute low dose cocaine (Caster et al. 2005, 2007; Parylak et al. 2008) than older adolescents (PN 42) or young adults (PN 65). In contrast, less marked and inconsistent differences in locomotor responses to acute cocaine have been reported between older adolescents and adults (Frantz et al. 2007; Laviola et al. 1995; Maldonado and Kirstein 2005). These behavioral data point to dopaminergic function during early adolescence as a potential mediator of addiction risk during this developmental stage.
Both pre- and post-synaptic dopaminergic mechanisms may contribute to these enhanced behavioral responses to low dose cocaine in adolescence. Recent studies from our laboratory and elsewhere suggest that adolescents may exhibit greater percent increases in dopamine after cocaine, even if basal dopamine levels are lower (Badanich et al. 2006; Walker et al. 2008). Both D1 and D2 receptors attain maximal expression during mid-adolescence, although the time frame varies somewhat by region, and in some areas including n. accumbens, receptor density is higher in early adolescence than in adulthood (Andersen and Teicher 2000; Schambra et al. 1994; Teicher et al. 1995). Therefore, both pre- and postsynaptic mechanisms have the potential to increase behavioral responses to uptake inhibitors like cocaine in adolescence.
These behavioral and neurochemical studies suggest that low doses of cocaine may cause greater neural responses in forebrain dopamine systems early in adolescence compared to later in adolescence or adulthood. However, the development of addiction also depends upon critical events downstream from initial receptor activation including transcriptional events that mediate the long-term changes in CNS (reviewed in McClung and Nestler 2008): unique molecular responses to drugs of abuse during adolescence could underlie a biological vulnerability to addiction during adolescence. The induction of various immediate early genes (IEGs), including c-fos and zif268, widely used markers of neuronal activity (eg. Brandon and Steiner 2003; Shramm et al. 2007), represents such a process. IEG induction is required for drug-induced “learning,” processes like conditioned place preference (CPP) and locomotor sensitization (Valjent et al. 2000, 2006; Zhang et al. 2006). These are adaptations to which adolescents may to be more and less (respectively) sensitive to compared to adults (Badanich et al. 2006; Brenhouse et al. 2008; Collins and Izenwasser 2002; Frantz et al. 2007; Laviola et al. 1995; Schramm-Sapyta et al. 2004). However, only a few studies have compared the regional induction of c-fos and/or zif268 in young to mid adolescents with adults. These studies have shown that induction in twenty one day old rats after acute amphetamine (Andersen et al. 2001) or thirty five day old rats after nicotine (Shram et al. 2007) are greater than in older animals, while induction after high doses of cocaine was comparable in young adolescents (day 28) and adults. (Cao et al. 2007; Kosofsky et al. 1995). These few studies are insufficient to determine whether there is an underlying developmental difference in IEG induction that parallels the greater sensitivity to the rewarding and locomotor stimulating effects of cocaine and other psychostimulants.
The purpose of the present study was to measure the activation of forebrain dopamine systems by high and low dose cocaine in young adolescents and adults using IEG expression as a marker of neuronal activity. We used in situ hybridization to measure c-fos and zif268 mRNA levels in adolescent (PN 28) and adult (PN 65) rats treated with 0, 10, or 40 mg/kg cocaine. We measured the expression of both c-fos and zif268 since recent studies have shown that the induction of these genes by cocaine influences distinct behavioral phenotypes (Brami-Cherrier et al. 2005; Valjent et al. 2006; Zhang et al. 2006). In addition to measuring regional mRNA levels, we correlated the magnitude of locomotor activation with the expression of each gene in individual animals. We further correlated the regional expression of c-fos and zif268 in individual animals to investigate the potential development of coordinated IEG induction by cocaine during adolescence. The results of these experiments will help elucidate the relationship between acute locomotor activation and transcriptional activity during adolescence and could provide some potential mechanistic understanding of why adolescents and adults demonstrate distinct behavioral adaptations following stimulant exposures.
Experimental procedures
Animals
We used Sprague-Dawley (CD) rats PN 28 and 65 to represent early adolescence and early adulthood, respectively (Spear 2000). These ages were selected as we have reliably observed locomotor differences at these ages following low cocaine doses (Caster et al. 2005, 2007; Parylak et al. 2008). Most studies of dopamine neuron ontogeny have shown that dopaminergic neurotransmission is fully mature by PN 65 (Andersen and Teicher 2000; Galineau et al. 2004; Giorgi et al. 1987; Trauth et al. 2001). Male Sprague-Dawley rats were obtained from Charles River Laboratories (Raleigh, NC) one week before experimentation (+/- one day). Rats were group housed (2 adults or 4 adolescents/cage) in suspended, self-ventilated cages (Techniplast) on laboratory bedding and provided with unrestricted access to laboratory rat chow and water. In both facilities, all animals were under a 12 hr light/dark cycle (lights on at 700 h and off at 1900 h). All drug treatments were given during the lights on cycle between 900 and 1300 h. All animal experiments were approved by the Institutional Animal Care and Use Committee and meet guidelines set forth by the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Drugs
Cocaine HCl (courtesy of NIDA) was diluted in saline (final concentration of 10 or 40 mg/ml) just prior to experimentation. All injections were given intraperitoneally to ensure rapid absorption.
Drug treatments
A total of 87 rats were used for the in situ hybridization experiments. To measure cocaine-induced IEG expression, animals were placed in the locomotor chambers and allowed to habituate for 1 hr. Animals were then injected with saline, 10, or 40 mg/kg cocaine and locomotor activity was recorded for 30 min. We selected 10 mg/kg cocaine as a low dose of cocaine as it is near the threshold for locomotor activation in male rats. We selected 40 mg/kg cocaine as a high dose of cocaine as we have previously shown that this dose induces locomotor and stereotypies in animals of all ages and is sufficient to induce single dose sensitization in animals of all ages (Caster et al. 2007). To measure basal IEG expression levels, rats were killed by decapitation immediately following removal from the home cage. All animals were immediately killed by decapitation at the end of the 30 min session. Brains were removed and snap frozen in mounting media and stored at -80°C until processing.
A separate cohort of 47 rats were used for the RT-PCR experiments. Animals were habituated to the locomotor chambers for 1 hr and then injected with saline or 40 mg/kg cocaine. Animals were then killed 15, 30, or 60 min post-injection. Several animals were also killed immediately following saline injection as a measure of baseline (time 0).
Locomotor activity
Locomotor activity was assessed using photobeam recording software (Kinder Scientific, Poway, CA). Behavioral activity was measured in Plexiglas open-field locomoter boxes measuring 40 × 40 cm. Each side of the box has sixteen photobeams spaced 2.5 cm apart. Animals of all ages were tested in the chambers under identical conditions. The box assignments were counterbalanced daily to randomize location.
Tissue preparation and in situ hybridization
Coronal sections corresponding to +1.60, +0.40, and -0.80 mm relative to bregma were cryostat cut to a thickness of 12 microns at -20°C. Sections were thaw-mounted onto glass micro-slides (SuperFrost Plus microslides, VWR International, Westchester, PA) and fixed in 4% paraformaldehyde solution (in 0.1 M phosphate-buffered saline (PBS)) for 5 min and then rinsed 3 × 30 sec in 0.1 M PBS. In preparation for in situ hybridization, slides were acetylated in a fresh solution of 0.25% acetic anhydride in 0.1M triethanolamine/0.2M SSC for 10 min, rinsed 3 × 30 sec in 0.2 M SSC, and air dried.
Oligonucleotide RNA probes (48mers) were in vitro transcribed from 70mer DNA oligonucleotide sequences containing T7 or SP6 RNA polymerase promoters and bases 207-254 of the c-fos mRNA (GenBank accession number X06769) or bases 352-399 of zif268 mRNA (GenBank accession number M18416). DNA oligonucleotides were generated by Molecula Research Laboratories LLC (Columbia, MD). Probes complimentary to these specific 48mer sequences have been used in previous studies (Brandon and Steiner 2003; Willuhn et al. 2003). Sense-strand probes were also generated and used as a negative control for both genes. [35]S-UTP was incorporated into probes during transcription using an in vitro transcription kit (Promega, Madison, WI). Labeled probe (1 × 106 cpm) in hybridization buffer was added to each slide. The sections were then coverslipped and incubated in an oil bath at 65°C overnight. After incubation, slides were washed 2 × 30 sec in chloroform, 2 × 30 sec in 2M SSC containing 0.1% 2-mercaptoethanol, decoverslipped, and soaked for 1 hr in 2M SSC/0.1% 2-mercaptoethanol at room temperature. Slides were then washed for 30 min in a 50% formamide 2M SSC/0.1% 2-mercaptoethanol solution at 65°C and then for 30 min in a 0.02M SSC solution at 65°C. After washing, slides were dried in a graded ethanol rinse and then air dried and apposed to X-Ray film (BioMax MR film, Kodak Co, USA) for 10-14 days. Exposure times were optimized to remain below the limits of saturation. Values approaching the saturation limits were excluded from our analyses.
Sampling areas
We selected four rostral to caudal coronal sections from which to sample c-fos and zif268 mRNA levels. Figure 1 shows a schematic representation of regions quantified by in situ hybridization. We analyzed 12 striatal regions from 3 rostral to caudal sections. Multiple striatal regions were analyzed because they have different functional roles mediating different aspects of addiction (reviewed in Kalivas and Volkow 2005, Volkow et al. 2006) and specific subregions within the dorsal striatum (caudate-putamen) and nucleus accumbens mediate specific psychomotor behaviors (Delfs and Kelley 1990; Kelly and Iverson 1976). The selected subregions also receive glutamate input from distinct cortical areas (below indicated in parentheses). In the most rostral striatal section (+1.60 mm) we measured c-fos and zif268 mRNA levels in the dorsal caudate (dorsal agranular, sensorimotor cortex), medial caudate (dorsal and ventral anterior cingulate), lateral caudate (sensorimotor cortex), nucleus accumbens core (prelimbic, agranular insular cortex), medial nucleus accumbens shell (prelimbic, infralimbic cortex), and the ventral nucleus accumbens shell (agranular insular cortex). In the medial section (+0.40 mm) we measured c-fos and zif268 mRNA levels in the dorsal caudate (dorsal agranular, sensorimotor cortex), lateral caudate (sensorimotor cortex), ventromedial caudate (sensorimotor cortex), and the ventrolateral caudate (sensorimotor cortex). From the most caudal section (-0.80 mm) we measured c-fos and zif268 mRNA levels in the dorsal caudate (dorsal agranular, sensorimotor cortex) and the ventrolateral caudate (sensorimotor cortex). We also measured c-fos and zif268 expression in the above listed cortical regions. From the most rostral section (+3.20 mm), we quantitated mRNA levels in the orbital and cingulate cortex. We measured c-fos and zif268 mRNA levels in five cortical regions in all of the remaining three sections: the dorsal agranular (premotor) cortex, dorsal anterior cingulate, ventral anterior cingulate, sensorimotor, and agranular insular cortex. Previous studies have shown that the general pattern of psychostimulant-induced c-fos induction on PN28 resembles that observed in adults (Brandon and Steiner 2003, Kosofsky et al 1995). Since the basic organization of both basal ganglia and cortex are mature by PN 28 in rats (Fentress et al 1981, Uyling et al. 1990), we were confident that we were sampling analogous areas in PN28 and PN 65 animals.
Figure 1. Regions analyzed.
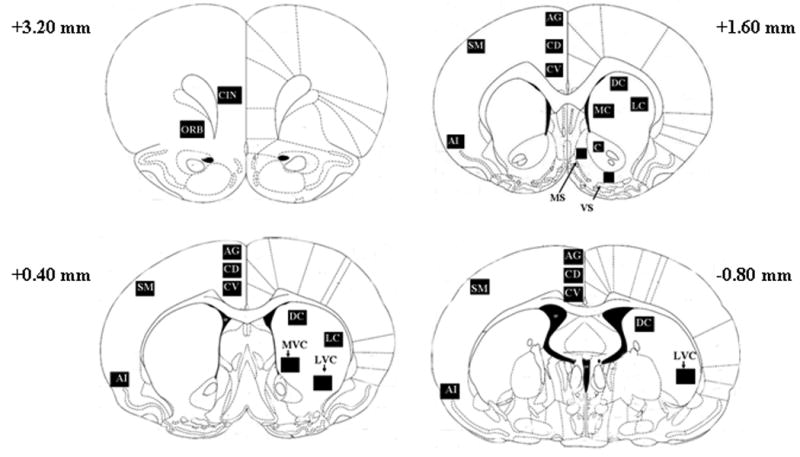
Schematic diagrams of the 29 subregions measured in four rostral-caudal coronal sections. In the most rostral section (+3.20 mm) we measured mRNA levels in the cingulate (CIN) and orbital cortex (ORB). We measured c-fos and zif268 mRNA levels from the same five cortical regions in each of the three remaining sections: the dorsal agranular cortex (AG), dorsal anterior cingulate (CD), ventral anterior cingulate (CV), sensorimotor cortex (SM), and the agranular insular cortex (AI). Cortical regions are indicated in the left hemispheres. We measured mRNA levels in 12 subregions of the striatum indicated in the right hemispheres. In the most rostral section corresponding to +1.60 mm, we measured mRNA levels in the dorsal caudate (DC), medial caudate (MC), lateral caudate (LC), nucleus accumbens core (C), medial nucleus accumbens shell (MS), and ventral nucleus accumbens shell (VS). In the middle section (+0.40 mm) we measured the dorsal caudate (DC), lateral caudate (LC), ventromedial caudate (MVC), and the ventrolateral caudate (LVC). In the caudal section (-0.80, bottom image) we measured in the dorsal caudate (DC) and the ventrolateral caudate (LVC).
Analysis of autoradiograms
C-fos and zif268 mRNA levels were quantitated using a PC based imaging analysis system (Scion Image, Scion Corp., Frederick, MD). Radiographic brain images were converted to digital images (JPEGs) using a digital scanner (Seiko Epson Corp., Long Beach, CA). Digital images were not altered or adjusted in any way prior to or during densitometric analysis. All experimenters were blinded to the age and treatment condition of animals and their corresponding radiographic brain images. Mean density values for each region were recorded from both hemispheres and an average value was obtained. We normalized average mean density values by subtracting background values measured over corpus callosum white matter. Normalized average values are presented as corrected c-fos and zif268 values. For publication, representative brain images were placed in a single file and the contrast was equalized.
Real-time PCR
We analyzed the time course of cocaine-induced striatal c-fos and zif268 induction by determining the relative expression levels (compared to GAPDH) of c-fos and zif268 15, 30 and 60 min after an injection of saline or 40 mg/kg cocaine in young adolescent and adult rats. GAPDH expression levels did not differ as a function of age or time after cocaine injection. Relative c-fos and zif268 expression levels were obtained by comparison with animals treated with saline and killed at the corresponding post-injection time. Time zero levels were obtained by killing animals immediately following and injection of saline. Animals were killed by dissection and the dorsal striatum was rapidly dissected and snap-frozen with dry ice. Total RNA was isolated using the Trizol method (Invitrogen, Carelsbad, CA) and converted to cDNA using Bio-Rad iScript Select cDNA synthesis kits (BioRad Inc., Hurcules, CA). cDNA was stored at -20°C until utilized for RT-PCR. Real-time PCR was performed using a Roche LightCycler® RT-PCR instrument (F. Hoffman-La Roche Ltd, Switzerland) and reaction mixtures were generated using Platinum® SYBR® Green qPCR SuperMix-UDG kits (Invitrogen, Carlesbad, CA). Reaction cycles proceeded as follows: 50°C for 2 min hold, 95°C for 2 min hold, followed by 45 cycles of 94°C for 5 sec, 55°C for 10 sec, 72°C for 10 sec. Melting curve analysis (to verify single product reactions) of all samples was performed following each reaction by ramping the temperature to 95°C and reducing the temperature at a rate of 0.5°C per sec to 40°C.
GAPDH, zif268, and c-fos primers for RT-PCR were generated using PrimerQuest© software available from Integrated DNA Technology (Coralville, IA). C-fos primers were determined using the published rat c-fos mRNA (Genebank accession number X06769). The forward primer sequence (5′-3′) was AATGCCGCACTAAAGCGGATGAAC and the reverse primer sequence was TTTGCCAGACAGAGGACAGCGTAT. Zif268 primers were determined using the published rat zif268 mRNA (Genbank accession number M18416). The forward primer sequence (5′-3′) was TCTGAATAACGAGAAGGCCGTGGT and the reverse sequence was (5′-3′) ACAAGGCCACTGACTAGGCTGAAA. GAPDH primers were determined using the published rat GAPDH sequence (Genebank accession number NM 017008). The forward primer sequence was ACAAGATGGTGAAGGTCGGTGTGA and the reverse primer sequence was AGCTTCCCATTCTCAGCCTTGACT. All primers were synthesized by Integrated DNA Technologies, received as lyophilized powders, reconstituted to 100 μM in sterile RNAse/DNAse free water, and stored in aliquots at -20°C. The size of products from all primer pairs was validated by running standard PCR reactions followed by agarose gel electrophoresis. Melting curve analysis following each RT-PCR reaction further confirmed that all primer pairs produced detectable levels of only one significant product. We also validated the quantitative accuracy of our primer pairs by performing RT-PCR with 1:1, 1:2, 1:4, 1:8, 1:16, and 1:32 template dilutions.
Statistical analyses
The effects of age, dose, and region on striatal c-fos and zif268 mRNA levels were initially examined using a four-factor repeated measures ANOVA (NCSS 2000, NCSS, Kaysville, UT) with between subject variables of age and dose and repeated measures of region and gene. In this and all analyses, main effects and interactions were considered significant at P<0.05. Newman-Keuls post-hoc analyses were used to investigate significant main effects. Subsequent ANOVAs filtered by significant terms were employed to further investigate significant interactions.
Cortical c-fos and zif268 mRNA levels were measured using two ANOVAs for specific cortical regions. Five cortical regions were repeatedly analyzed at three rostral-caudal coordinates. We employed a four-factor repeated measures ANOVA with subject variables of age and dose and repeated measures of region and gene to initially examine gene expression in these regions. C-fos and zif268 expression in two other cortical areas from the most rostral section (+3.20 mm) were analyzed separately using the same four-factor design. The same ANOVAs were used to calculate relative increases (as a percent of saline) in cocaine stimulated c-fos and zif268 expression.
We used linear regression analyses to correlate locomotor activity with c-fos and zif268 expression in specific striatal and cortical subregions within individual animals. We performed both parametric (Pearson) and non-parametric (Spearman) correlations and observed nearly identical significant correlations (subsequently, only parametric results are presented). The effects of age and region on these correlations were analyzed using a four-factor ANCOVA (JMP statistical software, SAS, Cary, NC) with age, dose, gene, and region as variables. Correlations for striatal and cortical subregions were analyzed using separate ANCOVAs. The effects of age and dose on the correlation between c-fos and zif268 expression within individual animals were analyzed using three-factor ANCOVAs with age, dose, and region as variables. Correlations in striatal and cortical subregions were analyzed using separate ANCOVAs.
Results
Locomotor activity
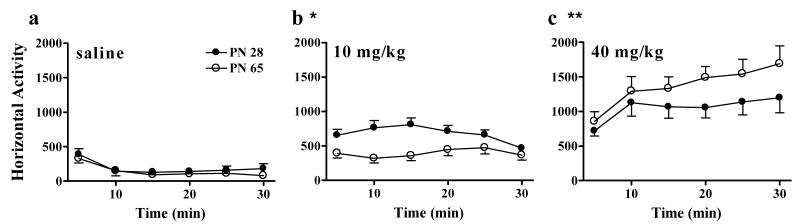
Following 1 hr of habituation, animals were treated with saline, 10, or 40 mg/kg cocaine and locomotor activity was recorded for 30 min (Figure 2). Cocaine dose-dependently increased locomotor activity in animals of both ages but the relative magnitude of locomotor activity in young adolescents and adults differed by dose. ANOVA indicated a main effect of dose (F(2,82)=62.7, P<0.001) and interactions of time × dose (F(10,410)=7.4, P<0.001) and age × dose (F(2,82)=7.6, P<0.001). Post-hoc analysis demonstrated that locomotor activity was similar in young adolescents and adults following saline, young adolescents had greater locomotor responses than adults to 10 mg/kg cocaine and adults had greater locomotor responses than young adolescents to 40 mg/kg cocaine.
Figure 2. Locomotor responses to cocaine.
Locomotor responses in adolescents (filled symbols) and adults (open symbols) following saline (a), 10 mg/kg cocaine (b), or 40 mg/kg cocaine (c) are plotted against time. Symbols represent mean +/- SEM. * indicates P<0.05 greater than PN 65. ** indicates P<0.05 greater than PN 28. N=6-15 for each age × treatment group. Cocaine dose-dependently increased locomotor activity in adolescents and adults. Locomotion increased more in adolescents after 10 mg/kg and more in adults after 40 mg/kg.
Striatal c-fos and zif268 expression
Cocaine dose-dependently increased c-fos and zif268 expression in the striatum in a regionally specific manner. Table 1 shows the mean +/- S.E.M. for background corrected c-fos (top) and zif268 (bottom) values in each brain region. ANOVA indicated a main effect of dose [F(2,66)=126.0, P<0.001] and region [F(11,706)=64.5, P<0.001] and interactions of age × dose [F(2,66)=8.2, P<0.001], age × region [F(11,706)=2.2, P<0.05], dose × region [F(22,706)=18.9, P<0.001], and age × dose × region [F(22,706)=3.7, P<0.001]. Further, these effects were gene specific as ANOVA indicated interactions of dose × gene [F(2,37)=21.6, P<0.001], age × dose × gene [F(2,37)=3.9, P<0.05], region × gene [F(11,375)=25.6, P<0.001], dose × region × gene [F(22,375)=11.0, P<0.001] and age × dose × region × gene [F(22,375)=2.7, P<0.001]. To investigate these interactions, we subsequently measured the effects of age, dose, and region in each gene separately.
Table 1. Mean density values for striatal c-fos and zif268 mRNA levels.
Background corrected values for c-fos (top) and zif268 (bottom) are shown for adolescents (left) and adults (right). Italics indicate greater than age-matched saline treated animals. + indicates significantly greater than age-matched 10 mg/kg. *indicates greater than PN 65. **indicates greater than PN 28. Cocaine increased c-fos
| PN 28 | PN 65 | |||||
|---|---|---|---|---|---|---|
| Striatal Region | saline | 10 mg/kg | 40 mg/kg | saline | 10 mg/kg | 40 mg/kg |
| Rostral (+1.6) | ||||||
| Dorsal Caudate | 2.9 +/- 0.2 | 67.3 +/- 10.5* | 78.8 +/- 7.9 | 1.6 +/- 0.6 | 21.3 +/- 4.2 | 107.6 +/- 9.5**+ |
| Medial Caudate | 2.7 +/- 0.8 | 93.3 +/- 14.5* | 99.8 +/- 11.0 | 1.6 +/- 0.5 | 25.0 +/- 4.3 | 120.3 +/- 12.1+ |
| Lateral Caudate | 1.5 +/- 0.8 | 59.4 +/- 10.1* | 71.2 +/- 10.5 | 1.4 +/- 1.0 | 15.3 +/- 3.6 | 118.8 +/- 7.0**+ |
| Nuc Acc Core | 3.3 +/- 1.3 | 20.7 +/- 3.4* | 25.3 +/- 4.7 | 2.5 +/- 1.0 | 7.6 +/- 1.2 | 33.2 +/- 7.6+ |
| M Nuc Acc shell | 8.0 +/- 1.6 | 51.2 +/- 7.0* | 75.3 +/- 9.7 | 4.7 +/- 1.5 | 21.8 +/- 2.6 | 83.0 +/- 7.8+ |
| V Nuc Acc shell | 4.3 +/- 1.2 | 12.8 +/- 2.8 | 17.9 +/- 2.7 | 3.2 +/- 1.2 | 4.3 +/- 1.2 | 13.2 +/- 3.8 |
| Medial (+0.40) | ||||||
| Dorsal Caudate | 3.0 +/- 0.7 | 61.8 +/- 8.7* | 108.3 +/-9.6+ | 2.9 +/- 2.5 | 28.6 +/- 7.0 | 123.7 +/- 9.5+ |
| Lateral Caudate | 3.0 +/- 0.9 | 74.1 +/- 10.7* | 132.7 +/- 14.5+ | 3.1 +/- 0.7 | 27.8 +/- 7.5 | 136.4 +/- 10.6+ |
| M Vent Caudate | 4.1 +/- 0.9 | 73.0 +/- 10.7* | 119.1 +/- 13.3+ | 1.7 +/- 1.8 | 30.1 +/- 7.5 | 104.4 +/- 9.4+ |
| Lat Vent Caudate | 3.6 +/- 0.6 | 13.7 +/- 4.2 | 57.1 +/- 17.0+ | 1.8 +/- 1.9 | 4.8 +/- 1.9 | 73.0 +/- 3.9+ |
| Caudal (-0.80) | ||||||
| Dorsal Caudate | 4.2 +/- 1.5 | 86.0 +/- 11.1* | 117.8 +/- 11.9+ | 5.9 +/- 0.9 | 38.5 +/- 6.75 | 124.7 +/- 8.7+ |
| Lat Vent Caudate | 1.3 +/- 0.9 | 4.8 +/- 1.7 | 58.7 +/- 19.0+ | 1.9 +/- 1.5 | 4.4 +/- 2.5 | 69.5 +/- 6.1+ |
| zif288 | ||||||
| Rostral (+1.6) | ||||||
| Dorsal Caudate | 17.8 +/- 2.5* | 45.4 +/- 6.5* | 52.3 +/- 5.2 | 9.3 +/- 1.3 | 27.4 +/- 3.5 | 64.4 +/- 5.3+ |
| Medial Caudate | 21.8 +/- 3.5* | 57.1 +/- 7.6* | 67.8 +/- 4.9 | 8.8 +/- 1.2 | 34.0 +/- 4.0 | 68.6 +/- 4.7+ |
| Lateral Caudate | 18.2 +/- 2.8* | 40.5 +/- 6.6* | 47.7 +/- 5.4 | 9.3 +/- 1.2 | 25.4 +/- 3.3 | 61.7 +/- 4.3+ |
| Nuc Acc Core | 11.1 +/- 1.9 | 25.8 +/- 6.5 | 26.7 +/- 3.3 | 5.5 +/- 1.4 | 11.6 +/- 2.2 | 38 +/- 8.2 |
| M Nuc Acc shell | 16.2 +/- 3.5* | 41.5 +/- 7.2 | 57.9 +/- 7.8 | 7.8 +/- 1.4 | 28.5 +/- 4.5 | 77.4 +/- 6.5+ |
| V Nuc Acc shell | 11.5 +/- 2.0* | 19.6 +/- 3.0 | 22.3 +/- 3.5 | 7.8 +/- 2.1 | 14.2 +/- 2.9 | 19.3 +/- 2.8 |
| Medial (+0.40) | ||||||
| Dorsal Caudate | 15.5 +/- 2.5* | 38.9 +/- 4.2 | 62.9 +/- 5.1+ | 9.3 +/- 1.3 | 28.1 +/- 5.2 | 57.1 +/- 5.8+ |
| Lateral Caudate | 16.8 +/- 2.4* | 41.2 +/- 4.1 | 59.1 +/- 5.5+ | 9.1 +/- 1.4 | 28.7 +/- 4.7 | 57.1 +/- 4.9+ |
| M Vent Caudate | 10.3 +/- 3.4 | 32.2 +/- 4.0 | 47.8 +/- 6.3 | 7.3 +/- 1.9 | 30.1 +/- 6.3 | 39.4 +/- 3.9 |
| Lat Vent Caudate | 11.9 +/- 1.6 | 29.2 +/- 3.7 | 57.8 +/- 8.4+ | 6.5 +/- 1.2 | 21.7 +/- 3.9 | 53.4 +/- 5.0+ |
| Caudal (-0.80) | ||||||
| Dorsal Caudate | 12.8 +/- 1.8 | 44.8 +/- 7.3 | 68.5 +/- 5.9+ | 8.3 +/- 2.3 | 39.3 +/- 0.9 | 63.2 +/- 6.5+ |
| Lat Vent Caudate | 10.4 +/- 2.5 | 22.9 +/- 2.7 | 45.3 +/- 6.6+ | 8.5 +/- 2.9 | 20.6 +/- 3.8 | 50.4 +/- 2.8+ |
Cocaine induced similar anatomical patterns of striatal c-fos expression in young adolescents and adults (Table 1, top) although the relative effects of dose in some regions differed by age. ANOVA indicated a main effect of dose [F(2,62)=104.2, P<0.001] and region [F(11,642)=66.8, P<0.001] as well as interactions of age × dose [F(2,62)=10.2, P<0.001], age × region [F(11,642)=2.7, P<0.001], dose × region [F(22,642)=21.5, P<0.001], and age × dose × region [F(22,642)=4.5, P<0.001] for corrected c-fos mRNA levels. Striatal c-fos expression was minimal in all regions of the striatum following an injection of saline and did not differ significantly by age. Drug effects were robust throughout most of the caudate. Low dose (10 mg/kg) cocaine significantly increased c-fos expression in all regions of the caudate in young adolescents except the ventrolateral caudate. Drug effects in the nucleus accumbens were modest and regionally selective: cocaine significantly increased c-fos expression in the medial nucleus accumbens shell, but had minimal effects in the ventral shell and core. A similar but less robust trend was observed in adults. High dose (40 mg/kg) cocaine substantially increased c-fos expression in all regions of the caudate in animals of both ages and some of these increases were higher in adults. These analyses demonstrate that cocaine can induce c-fos expression in all areas of the dorsal striatum (measured in this study) in both young adolescents and adults and that young adolescents may be more sensitive to striatal c-fos induction by lower doses of cocaine than adults.
Cocaine also induced similar anatomical patterns of zif268 expression in the striatum in young adolescents and adults (Table 1, bottom). ANOVA indicated a main effect of dose [F(2,41)=57.5, P<0.001], region [F(11,439)=28.4, P<0.001] and interactions of dose × region [F(22,439)=6.9, P<0.001] and age × dose × region [F(22,439)=2.2, P<0.01] for corrected zif268 mRNA levels. Post-hoc analyses indicated that basal zif268 levels (saline animals) were higher in young adolescents than adults. Similar to c-fos, cocaine caused greater increases in zif268 expression in the caudate compared to the nucleus accumbens. Significant increases in zif268 expression were seen following cocaine in most regions of the caudate after both doses in both age groups.
We next calculated our c-fos and zif268 mRNA levels as a percentage of baseline (saline) to determine how much the different doses of cocaine increased the relative expression of each gene. The effects of age and dose on % increases in striatal IEG expression were gene specific. ANOVA indicated a main effect of dose [F(1,46)=56.3, P<0.001], region [F11,486)=49.6, P<0.001], gene [F(1,30)=725.4, P<0.001], and interactions of age × dose [F(1,46)=12.8, P<0.001], age × region [F(11,486)=6.0, P<0.001], dose × region [F(11,486)=11.2, P<0.001], age × dose × region [F(11,486)=10.3, P<0.001], dose × gene [F(1,30)=187.7, P<0.001] age × dose × gene [F(1,30)=41.1, P<0.001], region × gene [F(11,308)=97.8, P<0.001], age × region × gene [F(11,308)=11.2, P<0.001], dose × region × gene [F(11,308)=21.6, P<0.001], and age × dose × region × gene [F(11,308)=19.7, P<0.001]. Therefore, we analyzed the effects of age and dose on regional cocaine-induced c-fos and zif268 expression separately.
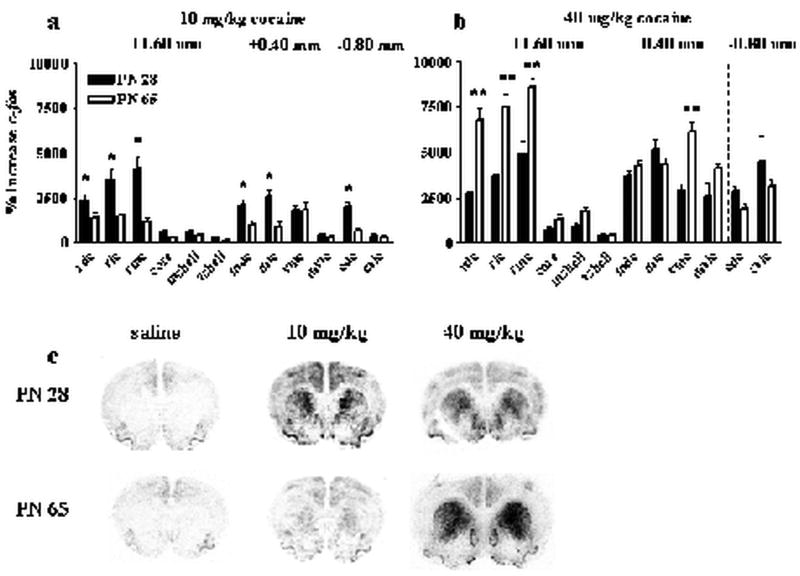
While cocaine induced similar anatomical patterns of striatal c-fos and zif268 in young adolescents and adults, the relative magnitude of cocaine-stimulated increases in c-fos and zif268 mRNAs differed by dose. When expressed as a percent of saline, high and low dose cocaine induced opposite age-effects on c-fos induction in the striatum (Figure 3). ANOVA indicated a main effect of dose [F(1,45)=93.6, P<0.001], region [F(11,463)=82.7, P<0.001], and interactions of age × dose [F(1,45)=20.9, P<0.001], age × region [F(11,463)=9.5, P<0.001], dose × region [F(11,463)=18.5, P<0.001], and age × dose × region [F(11,463)=17.0, P<0.001]. Post-hoc analyses demonstrated that cocaine dose-dependently increased c-fos expression in both young adolescents and adults. These analyses also showed that 10 mg/kg cocaine induced more c-fos expression in young adolescents than adults whereas 40 mg/kg cocaine induced more c-fos expression in adults than young adolescents. Significant effects of age in specific regions are indicated in figure 3 a-b.
Figure 3. Cocaine-induced increases in striatal c-fos expression.
Top panels show the percent increase in c-fos expression (compared to saline) in adolescents (filled bars) and adults (open bars) after 10 mg/kg cocaine (a) or 40 mg/kg cocaine (b). The x axes indicate specific striatal regions abbreviated RDC (rostral dorsal caudate), RMC (rostral medial caudate), RLC (rostral lateral caudate), core (nucleus accumbens core), mshell (medial nucleus accumbens shell), vshell (ventral nucleus accumbens shell), MDC (medial dorsal caudate), MLC (medial lateral caudate), VMC (ventromedial caudate), and MVLC (medial ventrolateral caudate), CDC (caudal dorsal caudate), and CVLC (caudal ventrolateral caudate). Dashed lines separate regions measured in different rostral to caudal sections. *indicates PN 28 significantly greater than PN 65 in designated regions. **indicates PN 65 significantly greater than PN 28 in designated regions. Representative images from the most rostral section (+1.60 mm) from adolescents (top) and adults (bottom) are shown in (c). N=6-15 for each age × treatment group. Cocaine increased c-fos more in adolescents than adults in dorsal striatum after 10 mg/kg, and increased c-fos more in dorsal striatum after 40 mg/kg in adults than adolescents.
Cocaine also dose-dependently produced age-specific effects on the relative induction of striatal zif268 (Figure 4). ANOVA indicated a main effect of age [F(1,31)=29.2, P<0.001], dose [F(1,31)=44.4, P<0.001], region [F(11,331)=17.2, P<0.001], and interactions of age × dose [F(2,31)=11.6, P<0.001], age × region [F(11,331)=5.4, P<0.001], dose × region [F(11,331)=4.7, P<0.001], and age × dose × region [F(11,331)=4.2, P<0.001]. Post-hoc analysis showed that 10 mg/kg cocaine stimulated a comparable induction of zif268 in young adolescents and adults but high dose cocaine induced more zif268 expression in adults than young adolescents. Significant effects of age at each region are indicated in figure 4.
Figure 4. Cocaine-induced increases in striatal zif268 expression.
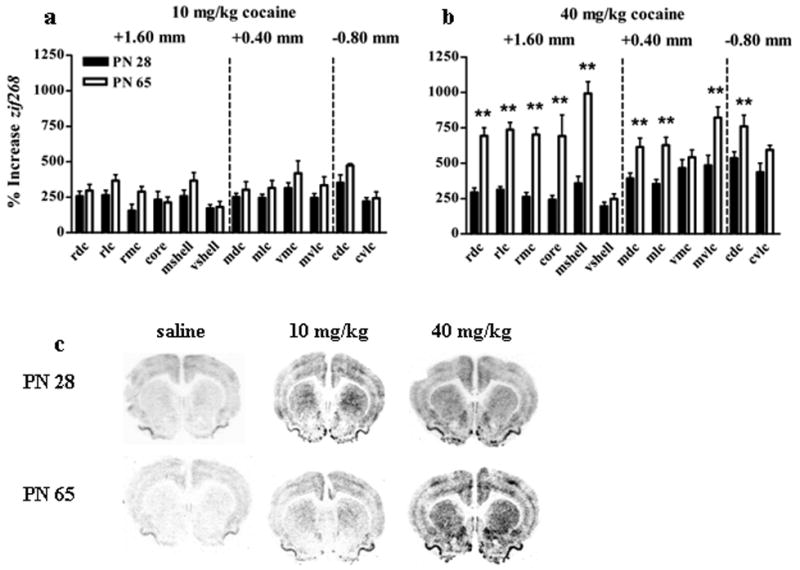
Top panels show the percent increase in zif268 expression (compared to saline) in adolescents (filled bars) and adults (open bars) after 10 mg/kg cocaine (a) or 40 mg/kg cocaine (b). The x axes indicate specific striatal regions abbreviated RDC (rostral dorsal caudate), RMC (rostral medial caudate), RLC (rostral lateral caudate), core (nucleus accumbens core), mshell (medial nucleus accumbens shell), vshell (ventral nucleus accumbens shell), MDC (medial dorsal caudate), MLC (medial lateral caudate), VMC (ventromedial caudate), and MVLC (medial ventrolateral caudate), CDC (caudal dorsal caudate), and CVLC (caudal ventrolateral caudate). Dashed lines separate regions measured in different rostral to caudal sections. **indicates significantly greater than PN 28 in designated regions. Representative images from the most rostral section (+1.60 mm) from adolescents (top) and adults (bottom) are shown in (c). N=6-15 for each age × treatment group. Cocaine increased zif268 comparably in adolescents and adults in all areas after 10 mg/kg, and increased zif268 more in adults than adolescents in all areas after 40 mg/kg.
Cortical c-fos and zif268 expression
Compared to the striatum, cocaine only induced modest changes in the expression of c-fos and zif268 in the cortical areas we measured. Table 2 shows the mean +/- S.E.M. for background corrected c-fos (top) and zif268 (bottom) values in each cortical brain region. For the five cortical areas we measured in three rostral-caudal slices, ANOVA indicated a main effect of age [F(1,66)=27.6, P<0.001], dose [F(2,66)=30.2, P<0.001], region [F(4,264)=127.4, P<0.001], and interactions of age × region [F(4,264)=3.0, P<0.05], dose × region [F(8,264)=5.4, P<0.001], region × gene [F(4,148)=10.9, P<0.001], age × dose × gene [F(2,37)=4.1, P<0.05], and dose × region × gene [F(8,148)=2.3, P<0.05]. Two-factor ANOVAs were then employed to investigate the significant three-way interactions.
Table 2. Mean density values for cortical c-fos and zif268 mRNA levels.
Background corrected values for c-fos (top) and zif268 (bottom) are shown for adolescents (left) and adults (right). Italics indicate greater than age-matched saline treated animals. + indicates significantly greater than age-matched 10 mg/kg.
| c-fos | PN 28 | PN 65 | ||||
|---|---|---|---|---|---|---|
| Region | saline | 10 mg/kg | 40 mg/kg | saline | 10 mg/kg | 40 mg/kg |
| +3.2 mm | ||||||
| Cingulate | 36.4 +/- 5.1 | 73.9 +/- 5.9 | 66.2 +/- 4.9 | 28.2 +/- 7.0 | 57.2 +/- 4.9 | 48.7 +/- 7.6 |
| Orbital Cortex | 47.7 +/- 6.7 | 89.9 +/- 6.9 | 77.3 +/- 6.9 | 29.4 +/- 3.6 | 54.3 +/- 5.0 | 49.8 +/- 7.5 |
| +1.6 mm | ||||||
| Dorsal Agranular | 19.7 +/- 3.1 | 58.6 +/- 6.8 | 49.5 +/- 5.6 | 9.9 +/- 2.5 | 26.0 +/- 2.9 | 28.8 +/- 9.4 |
| Dorsal Cingulate | 29.4 +/- 3.5 | 61.7 +/- 5.8 | 50.3 +/- 6.8 | 16.8 +/- 1.8 | 34.8 +/- 4.2 | 32.2 +/- 4.0 |
| Ventral Cingulate | 29.3 +/- 3.0 | 62.8 +/- 6.9 | 53.7 +/- 6.4 | 17.2 +/- 3.1 | 37.1 +/- 3.7 | 34.8 +/- 3.8 |
| Sensorimotor | 9.1 +/- 1.4 | 22.9 +/- 2.1 | 19.7 +/- 3.6 | 2.6 +/- 1.0 | 10.9 +/- 2.3 | 8.8 +/- 4.1 |
| Agranular Insular | 18.7 +/- 2.7 | 34 +/- 3.7 | 28.7 +/- 2.3 | 10.9 +/- 2.2 | 21.6 +/- 2.9 | 16.4 +/- 2.2 |
| +0.40 mm | ||||||
| Dorsal Agranular | 18.0 +/- 2.0 | 48.3 +/- 5.5 | 44.2 +/- 5.9 | 17.4 +/- 4.1 | 32.4 +/- 5.6 | 34.9 +/- 3.6 |
| Dorsal Cingulate | 23.0 +/- 1.8 | 43.6 +/- 5.1 | 41.1 +/- 6.3 | 17.2 +/- 3.9 | 33.9 +/- 3.8 | 33.3 +/- 4.1 |
| Ventral Cingulate | 23.8 +/- 2.4 | 36.9 +/- 4.3 | 37.3 +/- 7.6 | 17.1 +/- 3.8 | 33.1 +/- 3.9 | 29.1 +/- 3.3 |
| Sensorimotor | 11.2 +/- 1.5 | 22.8 +/- 3.0 | 34.9 +/- 10.9 | 6.8 +/- 1.8 | 23.0 +/- 4.3 | 24.5 +/- 4.5 |
| Agranular Insular | 15.9 +/- 1.8 | 25.6 +/- 3.6 | 23.3 +/- 5.9 | 15.4 +/- 4.8 | 19.3 +/- 2.6 | 14.8 +/- 4.2 |
| -0.80 mm | ||||||
| Dorsal Agranular | 12.0 +/- 1.9 | 44.3 +/- 6.1 | 34.7 +/- 6.0 | 14.2 +/- 1.9 | 18.5 +/- 3.1 | 31.9 +/- 3.9 |
| Dorsal Cingulate | 16.6 +/- 7.2 | 42.3 +/- 6.3 | 39.9 +/- 5.1 | 13.3 +/- 1.4 | 21.8 +/- 3.0 | 27.0 +/- 3.3 |
| Ventral Cingulate | 24.7 +/- 3.2 | 52.2 +/- 5.4 | 50.0 +/- 5.3 | 16.5 +/- 0.9 | 34.3 +/- 5.0 | 28.9 +/- 3.3 |
| Sensorimotor | 11.6 +/- 3.0 | 29.7 +/- 5.2 | 34.7 +/- 8.6 | 11.3 +/- 2.8 | 18.4 +/- 3.9 | 25.1 +/- 4.6 |
| Agranular Insular | 9.4 +/- 1.7 | 19.8 +/- 3.3 | 14.1 +/- 4.3 | 6.4 +/- 2.7 | 6.3 +/- 2.0 | 9.3 +/- 3.6 |
| zif288 | ||||||
| +3.2 mm | ||||||
| Cingulate | 31.7 +/- 4.3 | 45.6 +/- 6.4 | 63.3 +/- 8.6 | 23.5 +/- 5.0 | 49.2 +/- 9.6 | 51.2 +/- 5.0 |
| Orbital Cortex | 42.2+/- 6.6 | 63.8 +/- 8.9 | 94.0 +/- 6.2+ | 26.1 +/- 4.9 | 57.1 +/- 6.6 | 65.0 +/- 7.7 |
| +1.6 mm | ||||||
| Dorsal Agranular | 45.3 +/- 9.1 | 57.2 +/- 8.1 | 57.8 +/- 4.5 | 23.2 +/- 3.2 | 43.1 +/- 5.8 | 48.1 +/- 11.8 |
| Dorsal Cingulate | 46.2 +/- 8.1 | 63.2 +/- 8.5 | 63.6 +/- 5.4 | 20.1 +/- 2.8 | 44.2 +/- 4.5 | 54.9 +/- 4.3 |
| Ventral Cingulate | 39.1 +/- 6.3 | 55.5 +/- 7.6 | 59.4 +/- 5.6 | 20.8 +/- 3.3 | 50.2 +/- 7.7 | 55.1 +/- 3.8 |
| Sensorimotor | 24.2 +/- 5.0 | 35.8 +/- 6.1 | 34.1 +/- 4.7 | 9.9 +/- 1.6 | 24.9 +/- 3.0 | 26.7 +/- 4.4 |
| Agranular Insular | 23.6 +/- 6.2 | 36.3 +/- 4.9 | 40.1 +/- 3.9 | 14.3 +/- 3.0 | 30.9 +/- 2.9 | 31.3 +/- 2.2 |
| +0.40 mm | ||||||
| Dorsal Agranular | 36.0 +/- 5.8 | 47.8 +/- 4.4 | 55.9 +/- 4.6 | 22.3 +/- 4.0 | 43.5 +/- 7.4 | 39.4 +/- 4.7 |
| Dorsal Cingulate | 34.1 +/- 4.8 | 41.6 +/- 3.9 | 53.9 +/- 5.3 | 18.5 +/- 2.9 | 40.2 +/- 5.7 | 41.7 +/- 5.1 |
| Ventral Cingulate | 36.1 +/- 5.8 | 39.8 +/- 3.5 | 52.0 +/- 7.8 | 19.8 +/- 5.4 | 34.6 +/- 5.2 | 37.4 +/- 5.1 |
| Sensorimotor | 29.9 +/- 4.7 | 39.5 +/- 3.8 | 48.0 +/- 6.8 | 16.8 +/- 2.0 | 28.1 +/- 5.4 | 31.1 +/- 3.2 |
| Agranular Insular | 16.7 +/- 2.7 | 21.8 +/- 2.3 | 27.4 +/- 4.4 | 9.9 +/- 1.9 | 25.6 +/- 3.9 | 18.5 +/- 2.7 |
| -0.80 mm | ||||||
| Dorsal Agranular | 30.8 +/- 4.5 | 42.9 +/- 5.8 | 58.5 +/- 5.7 | 15.8 +/- 2.0 | 41.6 +/- 0.7 | 46.4 +/- 2.9 |
| Dorsal Cingulate | 27.3 +/- 3.9 | 37.9 +/- 5.1 | 54.2 +/- 4.6 | 18.2 +/- 1.6 | 42.7 +/- 2.6 | 42.8 +/- 5.2 |
| Ventral Cingulate | 31.0 +/- 4.0 | 47.0 +/- 6.8 | 72.3 +/- 7.5 | 19.4 +/- 2.9 | 53.6 +/- 4.6 | 51.9 +/- 5.7 |
| Sensorimotor | 29.1 +/- 4.7 | 39.8 +/- 4.5 | 54.1 +/- 6.7 | 17.4 +/- 3.3 | 49.4 +/- 2.5 | 43.9 +/- 4.4 |
| Agranular Insular | 13.4 +/- 1.8 | 18.6 +/- 2.4 | 25.5 +/- 4.1 | 6.3 +/- 3.4 | 19.2 +/- 6.5 | 15.3 +/- 1.3 |
We then examined the effects of dose on regional c-fos and zif268 expression separately. For c-fos, ANOVA indicated a main effect of dose [F(2,65)=15.2, P<0.001], region [F(4,260)=71.5, P<0.001] and a dose × region interaction [F(8,260)=5.4, P<0.001]. Post-hoc analysis showed that c-fos induction was not dose dependent. C-fos mRNA levels were higher than saline after both doses of cocaine but 40 mg/kg did not induce higher expression levels than 10 mg/kg. C-fos mRNA levels were higher after cocaine treatment (either dose) than saline in all regions except the agranular insular cortex. Cocaine had very similar dose effects on regional zif268 expression. ANOVA indicated a main effect of dose [F(2,44)=14.3, P<0.001], region [F(4,176)=104.3, P<0.001], and a dose × region interaction [F(8,176)=3.5, P<0.001]. Similar to c-fos, zif268 expression was not dose-dependent in animals of either age. However, zif268 mRNA levels were higher after both doses of cocaine (compared to saline) in all five cortical regions including the agranular insular cortex.
We then investigated the effects of age and dose on cortical c-fos and zif268 expression separately. ANOVA indicate a main effect of age and dose for both genes (P<0.001 for all). There was no significant interaction between age and dose for either gene. Post-hoc analyses demonstrated that neither c-fos nor zif268 expression were dose-responsive in animals of either age. Further, post-hoc analysis demonstrated that young adolescents had higher c-fos and zif268 expression than adults after both saline and cocaine.
Finally, we analyzed c-fos and zif268 expression levels in the orbital cortex and rostral cingulate from the +3.20 mm section (using a 4 factor repeated measures ANOVA as before). ANOVA indicated a main effect of a dose [F(2,63)=15.6, P<0.001] and an age × region interaction [F(1,63)=6.4, P<0.05]. Consistent with the other cortical regions, neither c-fos nor zif268 expression was dose-dependent. Young adolescents had higher c-fos and zif268 mRNA levels than adults in the orbital cortex. This age effect was drug independent as it was observed after saline and cocaine. These analyses demonstrate that, in general, cocaine increases c-fos and zif268 expression in the cortical regions we measured in both young adolescents and adults. Further, these effects are independent of cocaine dose in all of the cortical regions we analyzed.
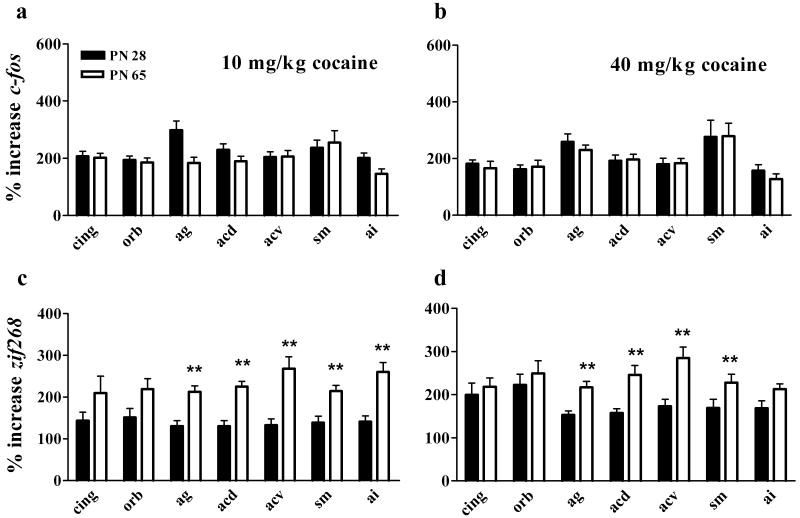
We also expressed our cortical data as a percent of saline (Figure 5). In the orbital and rostral cingulate cortex (+3.20 mm), ANOVA indicated a region × gene interaction [F(1,28)=19.9, P<0.001] and post-hoc analysis showed that cocaine caused bigger increases in zif268 than c-fos expression in the orbital cortex. ANOVA indicated a main effect of region [F(4,184)=8.1, P<0.001], gene [F(1,30)=4.9, P<0.05], and interactions of age × gene [F(1,30)=12.0, P<0.001], and region × gene [F(4,120)=11.8, P<0.001] for the five regions measured repeatedly in three sections. Cocaine caused similar increases in c-fos in young adolescents and adults. At the same time, cocaine caused bigger increases in the expression of zif268 in adults compared to young adolescents. These differences reflect lower basal zif268 expression levels in adults but also suggest that cocaine may induce greater neural responses in the adult cortex.
Figure 5. Effects of cocaine on cortical c-fos and zif268 expression.
Top panels show the percent increase in c-fos expression (compared to saline) in adolescents (filled bars) and adults (open bars) following 10 mg/kg cocaine (a) or 40 mg/kg cocaine (b). Bottom panels show the percent increase in zif268 expression following 10 mg/kg cocaine (c) or 40 mg/kg cocaine (d). The x axes indicate specific cortical regions abbreviated CING (cingulate), ORB, (orbital cortex), AG (dorsal agranular cortex), ACD (dorsal anterior cingulate), ACV (ventral anterior cingulate), SM (sensorimotor cortex), and AI (agranular insular cortex). **indicates significantly greater than PN 28 in designated regions. N=6-15 for each age × treatment group. Cocaine increased c-fos comparably in adolescents and adults in all cortical areas, and increased zif268 more in ag, acd, acv, sm and ai in adults than in adolescents after 40 mg/kg.
Basal zif268 expression
We demonstrated above that young adolescents had higher levels of striatal and cortical zif268 expression than adults after an injection of saline in a novel environment. To determine if this represents higher basal zif268 expression or greater zif268 responses to stressors (injection or novelty) in young adolescents, we treated adolescents and adults with saline in the locomotor chambers or removed them from their home cages and immediately collected brains for in situ hybridization. We determined that young adolescents have higher basal expression of zif268 than adults in the striatum and the cortex (Table 3). In the striatum, ANOVA indicated a main effect of age (F(1,20)=30.1, P<0.001). In the cortex, ANOVA indicated a main effect of age (F(1,20)=33.8, P<0.001), region (F(4,80)=46.1, P<0.001), and interactions of age × region (F(4,80)=10.1, P<0.001), treatment × region (F(4,80)=3.9, P<0.01) and age × treatment × region (F(4,80)=8.3, P<0.001). Young adolescents had higher average zif268 levels than adults in both tissues. Further, significant differences between saline-treated and home cage animals were only observed in a limited number of cortical areas in adults.
Table 3. Effect of saline injection in a novel environment on basal zif268 expression.
Background corrected zif268 mRNA levels are shown for adolescents (left) and adults (right) treated with saline in a novel environment or killed upon removal from the home cage. Mean values from striatal subregions are shown on top and mean values from cortical regions are shown on the bottom. + indicates significantly less than saline treated animals. *indicates greater than PN 65.
| PN 28 | PN 65 | |||
|---|---|---|---|---|
| Striatum | saline | home cage | saline | home cage |
| Rostral (+1.6) | ||||
| Dorsal Caudate | 37.6 +/- 5.7* | 38.7 +/- 12.3 | 18.9 +/- 2.0 | 19.4 +/- 2.5 |
| Medial Caudate | 38.2 +/- 6.3* | 41.3 +/- 6.7* | 18.2 +/- 1.8 | 18.7 +/- 3.5 |
| Lateral Caudate | 48.0 +/- 8.5* | 36.0 +/- 5.0* | 19.3 +/- 2.4 | 20.8 +/- 3.0 |
| Nuc Acc Core | 38.2 +/- 6.4* | 27.0 +/- 3.0 | 15.6 +/- 2.1 | 16.1 +/- 4.2 |
| Medial Nuc Acc shell | 44.7 +/- 10.2* | 43.1 +/- 1.3* | 15.2 +/- 2.5 | 23.9 +/- 5.7 |
| Ventral Nuc Acc shell | 39.1 +/- 8.9* | 25.7 +/- 2.0 | 13.5 +/- 1.7 | 21.6 +/- 5.6 |
| Medial (+0.40) | ||||
| Dorsal Caudate | 35.9 +/- 6.0* | 36.7 +/- 10.1 | 16.2 +/- 2.1 | 14.4 +/- 2.3 |
| Lateral Caudate | 36.3 +/- 7.0* | 35.6 +/- 8.1* | 17.6 +/- 2.2 | 14.0 +/- 2.0 |
| Med Ventral Caudate | 39.8 +/- 4.8* | 39.0 +/- 12.4* | 19.7 +/- 2.4 | 10.8 +/- 2.3 |
| Lat Ventral Caudate | 36.9 +/- 6.5* | 40.2 +/- 9.4* | 15.8 +/- 1.7 | 16.6 +/- 2.4 |
| Caudal (-0.80) | ||||
| Dorsal Caudate | 33.7 +/- 6.5 | 30.5 +/- 2.5* | 20.8 +/- 4.5 | 19.2 +/- 3.5 |
| Lat Ventral Caudate | 33.0 +/- 7.4 | 30.8 +/- 0.2* | 19.5 +/- 4.5 | 19.8 +/- 2.9 |
| Cortex | ||||
| Rostral (+1.6) | ||||
| Dorsal Agranular | 70.5 +/- 9.8* | 69.5 +/- 5.6* | 43.7 +/- 2.6 | 25.5 +/- 3.4 |
| Dorsal Cingulate | 71.0 +/- 9.8* | 68.2 +/- 11.2* | 39.7 +/- 2.6 | 29.5 +/- 2.4 |
| Ventral Cingulate | 66.9 +/- 15.8* | 54.8 +/- 9.8 | 39.1 +/- 3.1 | 43.4 +/- 4.2 |
| Sensorimotor | 60.1 +/- 12.8* | 86.8 +/- 10.7* | 28.4 +/- 2.8 | 22.9 +/- 4.3 |
| Agranular Insular | 48.4 +/- 9.4 | 36.2 +/- 3.9* | 27.5 +/- 2.7 | 18.6 +/- 2.4 |
| Medial (+0.40) | ||||
| Dorsal Agranular | 64.8 +/- 12.6* | 69.5 +/- 6.7* | 39.3 +/- 7.0 | 21.0 +/- 2.3 |
| Dorsal Cingulate | 70.7 +/- 10.0* | 62.0 +/- 8.1* | 35.2 +/- 5.0 | 24.3 +/- 5.1 |
| Ventral Cingulate | 75.4 +/- 13.0* | 58.7 +/- 11.2* | 32.9 +/- 4.0 | 34.1 +/- 2.9 |
| Sensorimotor | 62.1 +/- 12.5 | 78.3 +/- 13.1* | 40.1 +/- 4.1 | 22.1 +/- 4.1 |
| Agranular Insular | 36.9 +/- 6.8 | 28.5 +/- 6.0 | 24.2 +/- 4.5 | 12.9 +/- 3.3 |
| Caudal (-0.80) | ||||
| Dorsal Agranular | 53.1 +/- 11.2 | 58.5 +/- 6.8* | 49.8 +/- 8.9 | 28.3 +/- 5.0 |
| Dorsal Cingulate | 47.6 +/- 9.2 | 46.7 +/- 4.2* | 47.0 +/- 7.6 | 32.2 +/- 4.0 |
| Ventral Cingulate | 59.4 +/- 9.0 | 51.8 +/- 9.1 | 59.4 +/- 10.4 | 36.9 +/- 3.8 |
| Sensorimotor | 61.3 +/- 8.4 | 72.3 +/- 9.7* | 46.4 +/- 7.6 | 37.2 +/- 5.9 |
| Agranular Insular | 36.3 +/- 11.2 | 29.3 +/- 3.6* | 23.3 +/- 5.6 | 18.7 +/- 2.4 |
Locomotor activity and c-fos or zif268 expression in individual animals
We used linear regression analysis to determine if striatal c-fos and/or zif268 expression correlated with locomotor activity in young adolescents and adults. Locomotor activity during the 30 min session was summed for each animal. Our initial ANCOVA indicated a main effect of age, [F(1,31)=6.3, P<0.05], dose [F(1,31)=68.0, P<0.001], region [F(11,31)=18.1, P<0.001], and an age × gene × locomotion interaction [F(1,410)=16.3, P<0.001]. Subsequent ANCOVAs were used to compare locomotor activity and the expression of c-fos and zif268 separately.
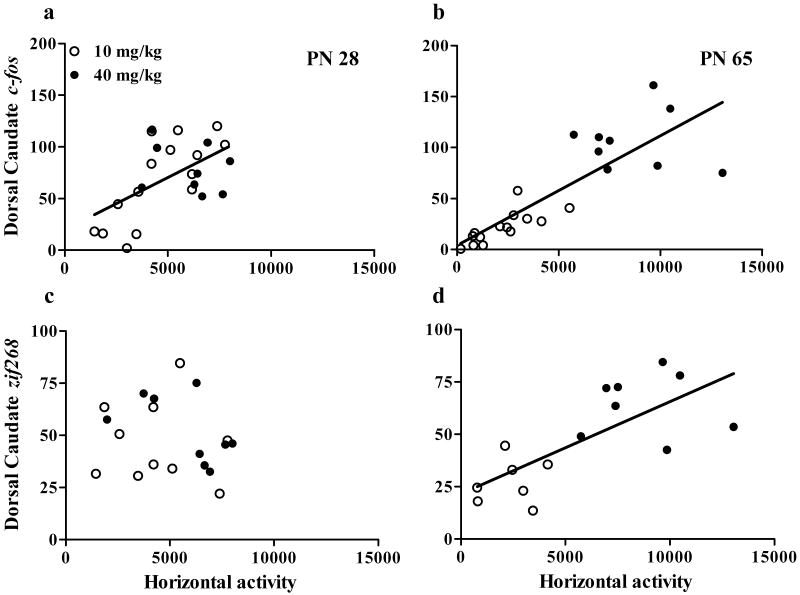
We first examined the correlation between striatal c-fos and locomotor activity. There was a significant correlation between c-fos expression and locomotor activity in most regions of the striatum in both young adolescents and adults. ANCOVA indicated a main effect of locomotion [F(1,45)=71.9, P<0.001], age [F(1,45)=19.9, P<0.001], dose [F(,45)=48.5, P<0.001] and region [F(11,45)=28.6, P<0.001] on c-fos expression. Figure 6a-b shows the correlation between c-fos expression in the dorsal caudate of the rostral section and locomotor activity as a representative image. Table 4 shows the P-value and, when statistically significant, the correlation coefficient for the correlations between locomotion and c-fos (top) of all striatal regions.
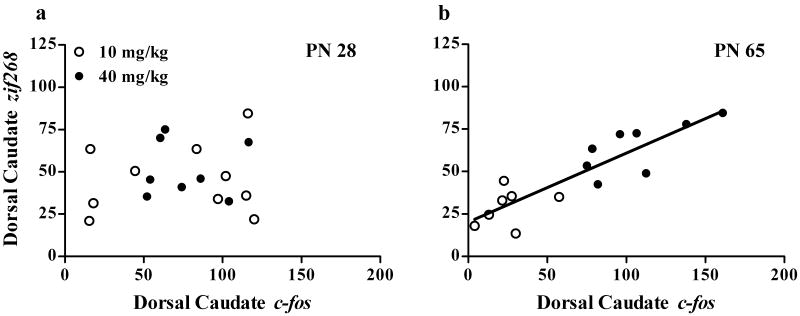
Figure 6. Correlation between striatal c-fos and zif268 expression and locomotor activity.
Correlations in adolescents are shown in (a) and adults are shown in (b). Open symbols represent animals treated with 10 mg/kg cocaine and closed symbols represent animals treated with 40 mg/kg cocaine. Regression lines are shown for significant correlations (P<0.05). N=6-15 for each age × treatment group. Cocaine-stimulated locomotion correlated with c-fos induction in both adolescents and adults but correlated with zif268 induction only in adults.
Table 4. Correlation between IEG expression and locomotor activity.
Correlations are shown between c-fos (top) or zif268 (bottom) and horizontal activity in adolescents (left) and adults (right). + indicate significant correlations. R2 values are only given for significant correlations.
| c-fos | PN 28 | PN 65 | ||
|---|---|---|---|---|
| Striatal Region | P- Value | R2 | P- Value | R2 |
| Rostral (+1.6 mm) | ||||
| Dorsal Caudate | P<0.01+ | 0.30 | P<0.001+ | 0.69 |
| Medial Caudate | P<0.001+ | 0.41 | P<0.001+ | 0.72 |
| Lateral Caudate | P<0.01+ | 0.29 | P<0.001+ | 0.76 |
| Nuc Acc Core | P>0.05 | P<0.01+ | 0.31 | |
| Medial Nuc Acc shell | P<0.01+ | 0.28 | P<0.001+ | 0.75 |
| Ventral Nuc Acc shell | P>0.05 | P>0.05 | ||
| Medial (+0.40 mm) | ||||
| Dorsal Caudate | P<0.05+ | 0.22 | P<0.001+ | 0.78 |
| Lateral Caudate | P<0.05+ | 0.24 | P<0.001+ | 0.81 |
| MV Caudate | P>0.05 | P<0.001+ | 0.59 | |
| LV Caudate | P>0.05 | P<0.001+ | 0.74 | |
| Caudal (-0.80) | ||||
| Dorsal Caudate | P<0.05+ | 0.25 | P<0.001+ | 0.70 |
| LV Caudate | P>0.05 | P<0.001+ | 0.71 | |
| zif268 | ||||
| Rostral (+1.6 mm) | ||||
| Dorsal Caudate | P>0.05 | P<0.01+ | 0.54 | |
| Medial Caudate | P>0.05 | P<0.001+ | 0.59 | |
| Lateral Caudate | P>0.05 | P<0.001+ | 0.61 | |
| Nuc Acc Core | P>0.05 | P<0.05+ | 0.32 | |
| Medial Nuc Acc shell | P>0.05 | P<0.001+ | 0.69 | |
| Ventral Nuc Acc shell | P>0.05 | P>0.05 | ||
| Medial (+0.40 mm) | ||||
| Dorsal Caudate | P>0.05 | P<0.001+ | 0.50 | |
| Lateral Caudate | P>0.05 | P<0.001+ | 0.55 | |
| MV Caudate | P>0.05 | P>0.05 | ||
| LV Caudate | P>0.05 | P<0.001+ | 0.52 | |
| Caudal (-0.80) | ||||
| Dorsal Caudate | P>0.05 | P<0.05+ | 0.31 | |
| LV Caudate | P>0.05 | P<0.001+ | 0.71 | |
In contrast to c-fos, locomotor activity only correlated with zif268 expression in individual adults. ANCOVA indicated a significant effect of age [F(1,31)=6.3, P<0.05], dose [F(1,31)=68.0, P<0.001], region [F(11,31)=18.1, P<0.001], and an age × locomotion interaction [F(1,410)=16.3, P<0.001] on zif268 mRNA levels. Figure 6c-d shows the correlation between zif268 expression in the dorsal caudate of the rostral section and locomotor activity as a representative image. Table 4 (bottom) shows the P-value and correlation coefficients between locomotion and zif268 in all measured striatal areas.
We also tried to correlate locomotor activity with the expression of c-fos and zif268 in cortical subregions. However, we did not identify any significant correlations between locomotor activity and c-fos or zif268 expression in any cortical subregion in individual animals. This is perhaps not surprising as locomotor activity was dose responsive whereas cortical c-fos and zif268 expression were not. No significant correlations were observed when doses were analyzed separately.
C-fos and zif268 expression in individual animals
Our correlational studies between locomotor activity and c-fos and zif268 suggest that the quantitative relationship between c-fos and zif268 should correlate in adults (both measures were highly correlated with locomotion in the same animals) but might not correlate in young adolescents. To address this, we plotted corrected striatal c-fos vs. striatal zif268 mRNA levels in individual animals. As predicted, c-fos correlated with zif268 only in adults. ANCOVA indicated a main effect of region [F(11,31)=3.4, P<0.001], c-fos [F(1,31)=73.4, P<0.001], and an age × c-fos interaction [F(1,386)=10.1, P<0.01] on the expression of zif268. Figure 7 shows the correlation between c-fos and zif268 in individual adolescents and adults and table 5 shows the P-value and correlation coefficients between c-fos and zif268 expression in adolescents and adults in all striatal regions. These results demonstrate that the quantitative expression of at least two immediate early genes is not correlated in individual adolescents.
Figure 7. Correlation between striatal c-fos and zif268 mRNA levels.
Correlations in adolescents are shown in (a) and adults are shown in (b). Open symbols represent animals treated with 10 mg/kg cocaine. Closed symbols represent animals treated with 40 mg/kg cocaine. Regression lines are shown for when significant correlations were observed. N=6-10 for each age × treatment group. Zif268 induction correlated with c-fos induction only in adults.
Table 5. Correlation between c-fos and zif268 expression.
Correlations between the two genes in striatal region are shown in adolescents (left) and adults (right). + indicates significant correlations. R2 values are only given for significant correlations.
| Striatal Region | PN 28 | PN 65 | ||
|---|---|---|---|---|
| P- Value | R2 | P- Value | R2 | |
| Rostral (+1.6) | ||||
| Dorsal Caudate | >0.05 | <0.001+ | 0.78 | |
| Medial Caudate | >0.05 | <0.001+ | 0.81 | |
| Lateral Caudate | >0.05 | <0.001+ | 0.82 | |
| Nuc Acc Core | >0.05 | <0.001+ | 0.89 | |
| Medial Nuc Acc shell | >0.05 | <0.001+ | 0.76 | |
| Ventral Nuc Acc shell | >0.05 | <0.05+ | 0.35 | |
| Medial (+0.40) | ||||
| Dorsal Caudate | >0.05 | <0.05+ | 0.31 | |
| Lateral Caudate | >0.05 | <0.05+ | 0.44 | |
| Medial Ventral Caudate | >0.05 | >0.05 | ||
| Lateral Ventral Caudate | <0.05+ | 0.37 | <0.05+ | 0.39 |
| Caudal (-0.80) | ||||
| Dorsal Caudate | <0.01+ | 0.36 | <0.01+ | 0.48 |
| Lateral Ventral Caudate | <0.001+ | 0.51 | <0.01+ | 0.63 |
Cocaine-induced increases in striatal c-fos and zif268 expression
We wanted to confirm that the age differences in cocaine-induced c-fos and zif268 that we observed by in situ hybridization did not represent a more rapid on/off of mRNA levels in adolescents and adults. Previous studies examining c-fos and/or zif268 expression have killed animals 30-60 min post-injection. We measured relative cocaine-stimulated c-fos and zif268 mRNA expression in adolescent and adult dorsal striata by treating animals PN 28 and 65 with 40 mg/kg cocaine or saline in locomotor test chambers and killed them 15, 30, or 60 min post injection. Relative mRNA levels were measured using RT-PCR. ANOVA indicated a main effect of age [F(1,46)=19.5, P<0.001], time [F(3,46)=32.1, P<0.001], gene [F(1,91)=142.9, P<0.001], and interactions of age × time [F(1,91)=4.15, P<0.05], and gene × time [F(3,91)=20.2, P<0.05]. We then investigated the effects of age and time in each gene separately (Figure 8).
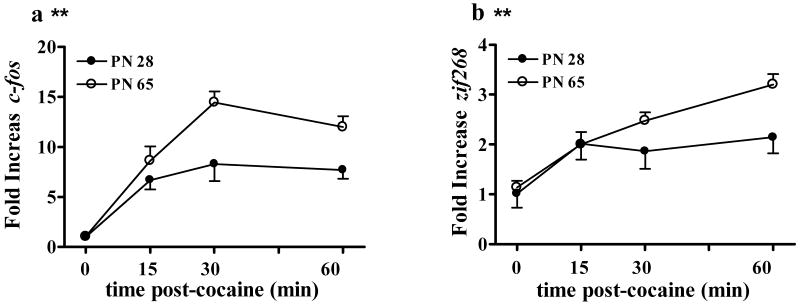
Figure 8. Time course of c-fos and zif268 induction by high dose cocaine in the adolescent and adult striatum.
Cocaine induced a similar time course of expression for both c-fos (a) and zif268 (b) in young adolescent and adult male rats. Closed symbols represent young adolescents and open symbols represent adults. **indicates greater in PN 65 than PN 28. N=5-8 for all age × time groups. Induction of both c-fos and zif268 were greater in adults than adolescents both at 30 min and 60 min.
For c-fos, ANOVA indicated a main effect of age [F(1,46)=17.6, P<0.001] and time [F(3,46)=27.8, P<0.001]. Post-hoc analysis showed that cocaine caused bigger fold increases in c-fos expression in adults than adolescents (Figure 8a) and that c-fos expression was maximal at 30 min. For zif268, ANOVA indicated a main effect of age [F(1,44)=7.9, P<0.05] and time [F(3,44)=17.2, P<0.001]. As with c-fos, post-hoc analysis showed that cocaine stimulated greater increases in zif268 expression in adults than adolescents. However, post-hoc analyses showed that zif268 expression was higher at 60 min than at earlier time points (Figure 8b). These results suggest that it is not possible to measure maximal c-fos and zif268 expression in the same animals. At the same time, they demonstrate that relative age differences are maintained at both 30 and 60 min post cocaine and show that age-differences observed at these times do not represent a more rapid induction or turnover of mRNA in adolescents or adults.
Discussion
The present study demonstrated that cocaine-induced striatal c-fos and zif268 expression varies as a function of age and dose. Low dose cocaine (10 mg/kg) induced more striatal c-fos in young adolescents than in adults whereas high dose cocaine (40 mg/kg) caused greater increases in striatal c-fos and zif268 expression in adults. Cocaine also caused greater increases in the expression of zif268 in adults in some cortical areas. These results are summarized in table 6. We further demonstrated that while locomotor activity correlated with striatal c-fos expression in individual young adolescents and adults, it correlated with striatal zif268 expression only in adults. Accordingly, striatal c-fos and zif268 expression were significantly correlated in individual adults but not young adolescents. Neither cortical c-fos nor zif268 expression correlated with locomotor activity in animals of either age. Our results demonstrate that the transcriptional regulation of specific gene targets by cocaine changes during adolescence. Developmental changes in the coordinated induction of transcription factors could result in the expression of different long-lasting cellular changes in response to cocaine in adolescents and adults. These observations could partially explain why adolescents and adults show different sensitivities to some of the lasting behavioral effects of cocaine like CPP and locomotor sensitization.
Table 6. Summary of behavioral and IEG responses to cocaine in adolescent and adult rats.
| Low Dose Cocaine | High Dose Cocaine | |
|---|---|---|
| Locomotion | Adol > Adult | Adult > Adol |
| C-fos Caudate | Adol > Adult | Adult > Adol |
| Zif 268 Caudate | Adol = Adult | Adult > Adol |
| C-fos Cortex | Adol = Adult | Adult > Adol |
| Zif 268 cortex | Adult > Adol | Adult > Adol |
Locomotor responses to acute cocaine
Consistent with our previous reports (Caster et al. 2005, 2007; Parylak et al. 2008) we demonstrated that locomotor responses to cocaine are age-specific and dose dependent. While adolescents have consistently been reported as hyporesponsive to amphetamine (Bolanos et al. 1998; Lanier and Isaacson 1977; Vasilev et al. 2003), a brief review of the literature suggests that enhanced locomotor responses to acute cocaine are observed following lower doses of cocaine during early adolescence. We have shown here and in previous studies that young adolescent male rats (PN 28) have greater locomotor responses than adults to lower (<15 mg/kg) but not higher (> 25 mg/kg) doses of cocaine (Caster et al. 2005, 2007; Parylak et al. 2008). Similarly, Badanich et al. (2008) showed that young adolescents (PN 35) have greater locomotor responses than both mid-adolescent (PN 45) and adult rats after 5 and 20 mg/kg cocaine. Previous studies also showed that locomotor responses to cocaine fall from the second or third postnatal week to around PN 30 to 40, although they did not follow the response into adulthood. (Snyder et al. 1998; Spear and Brick 1979). Laviola et al. (1995) showed that cocaine (10-20 mg/kg) induced fewer specific behaviors such as stereotyped sniffing and rearing in mid-adolescents (PN 35-39) compared to adults. Maldonado and Kirstein (2005) observed similar locomotor responses to 30 mg/kg cocaine in PN 45 and PN 60 rats.
The magnitude of difference in the locomotor response to cocaine in adolescents and adults also depends upon dose. Several previous studies, including our own, have not observed any age-differences in locomotor activity between young adolescents and adults after higher doses (25 and 40, 30 mg/kg, respectively) of cocaine (Caster et al. 2005; Collins and Izenwasser 2002; Parylak et al. 2008). In summary, age-differences in locomotor responses to acute cocaine appear to be most consistent following low, but not high dose cocaine and are restricted to early adolescence. This difference could be relevant to human addiction as the onset of drug taking early in adolescence (<14 years) is associated with higher abuse rates than onset later in adolescence or adulthood (reviewed in Chambers et al. 2003; Laviola et al. 1999).
Cocaine-induced striatal c-fos and zif268 expression
Cocaine stimulated nearly identical anatomical patterns of striatal c-fos and zif268 expression in adolescents and adults. Cocaine preferentially increased c-fos and zif268 expression in the dorsal striatum (caudate-putamen) compared to the ventral striatum, as described in previous studies (Brandon and Steiner 2003; Daunais and McGinty 1994, 1995; Graybiel et al. 1990; Kosofsky et al. 1995; Moratalla et al. 1993; Steiner and Gerfen 1993; Willuhn et al. 2003).
While the overall anatomic pattern of IEG induction was similar in adolescents and adults, we did observe robust dose-dependent age differences in the magnitude of cocaine-induced striatal gene expression. The age effects of cocaine-induced striatal IEG expression largely mirrored those of cocaine-induced locomotor activity: low dose cocaine induced more striatal c-fos expression in young adolescents than adults whereas high dose cocaine induced more c-fos and zif268 expression in adults than young adolescents. These observations suggest that low dose cocaine causes greater activation of striatal neurons in young adolescents and high dose cocaine causes greater activation of striatal neurons in adults.
These effects are not easily explained by cocaine pharmacokinetics as our laboratory and others have already demonstrated that cocaine produces similar brain cocaine concentrations in young adolescent and adult rats (Caster et al. 2005, Frantz et al., 2007). Another possible explanation could be a greater increase in extracellular dopamine after cocaine in adolescents than adults. Cocaine indirectly activates postsynaptic dopamine receptors by binding to and blocking the dopamine transporter, thereby increasing extracellular dopamine levels (Hurd and Ungerstedt 1989). Our laboratory and others have shown that low (15-20 mg/kg) dose cocaine produces similar extracellular dopamine levels in the striatum and nucleus accumbens in adolescents and adults (Frantz et al. 2007; Walker and Kuhn 2008). This is surprising, considering that most markers of presynaptic dopamine terminal density in the striatum, such as dopamine transporter binding, dopamine content, and vesicular monoamine transporter, reach maximal levels somewhere between PN 50 and PN 70 in rats (Coulter et al. 1997; Coyle and Campochiaro 1976, Galineau et al., 2004, Giorge et al. 1987; Nomura et al. 1976; Porcher and Heller 1972; Slotkin et al. 2002; Tarazi et al. 1998; Trauth et al. 2001). Modeling of dopamine uptake and release kinetics suggests that differences in transporter function (a higher affinity for dopamine) might enhance uptake in adolescents despite the lower transporter density (Walker et al. 2008).
Postsynaptic mechanisms represent another potential mediator of the enhanced response to adolescents to low dose cocaine, although data supporting this possibility are mixed. Dopamine receptor numbers in the caudate, nucleus accumbens, and PFC peak and regress during adolescence, but dopamine receptor number appears fairly comparable in all of these regions around PN 30 and PN 60 (Andersen et al., 2000; Andersen and Teicher 2000; Schambra et al., 1994, Teicher et al., 1995). Downstream signaling molecules that have been studied including adenylyl cyclase and DARP32 in whole striatum show a gradual increase across adolescence like presynaptic targets (Coyle and Campochiaro 1976, Ehrlich et al., 1990, Sakagami et al., 1995). One study showed that D1-mediated cyclase activation was enhanced in the adolescent striatum, whereas D2-mediated inhibition was reduced (Andersen 2002). Since maximal striatal IEG induction requires synergism between D1 and D2 receptor stimulation (Alonzo et al. 1999; Gerfen et al. 1995) it seems unlikely that these age differences in cyclase stimulation should result in enhanced c-fos expression following low dose cocaine in adolescents. It is possible that maturational differences in cocaine-stimulated intracellular signaling downstream of these events could underlie age-specific IEG induction by cocaine.
Enhanced c-fos induction by low dose cocaine in young adolescents could also be influenced by non-dopaminergic mechanisms. Dopamine-glutamate interactions represent one possible candidate to mediate enhanced c-fos activation by low dose cocaine in adolescents. Dopamine and glutamate fibers converge on medium spiny neurons in the striatum (Jay 2003; Sesack et al. 2003) and postsynaptic interactions between the two neurotransmitters mediate the effects of stimulants like cocaine (reviewed in Vanderschuren and Kalivas 2000). Selective antagonists of D1 dopamine (Graybiel et al. 1990; Young et al. 1991) or glutamate receptors (Konradi et al. 1996; Wang et al. 1994 a,b) can block stimulant-induced IEG expression in the striatum.
Glutamate mechanisms show a developmental time course that is consistent with a role in enhanced IEG induction by cocaine during early adolescence. Cortical NMDA receptor binding is maximal around PN 28 (Insel et al. 1990), although cortical projections to the striatum continue to develop over the next several weeks (Brenhouse et al., 2008). Further, locomotor responses to the NMDA antagonist MK-801 fall to adult levels during adolescence (Frantz and Van Hartzeveldt 1999; Vasilev et al. 2003) in parallel with rapid increase in glutamate levels and uptake sites in the striatum during the second-fourth weeks of life (Campochiaro and Coyle 1978). The role of glutamate mechanisms in neuroplastic events related to addiction vulnerability in adolescents represents an important area for future study.
Serotonin signaling could also contribute to age and dose-specific cocaine-mediated c-fos induction. Bhat and Baraban (1992, 1993) demonstrated that serotonin can potentiate dopamine-mediated striatal gene expression. Several classes of serotonin (5HT) receptors, including 5HT-2a and 5HT-1a, undergo significant reductions during adolescence (reviewed in Crews et al. 2007). In summary, the innervation and maturation of glutamate and serotonin inputs could play an important role in age-specific responses to low dose cocaine.
The greater locomotor and IEG responses to high dose cocaine in adults are more consistent with the developmental pattern in gradual increase in indices of pre and postsynaptic dopamine function that occur over this time period. As discussed above, a number of presynaptic markers demonstrate that dopamine innervation density in the striatum is immature early in adolescence compared to adulthood. It is possible that saturation of the transporter by high dose cocaine could result in greater extracellular dopamine levels in adults compared to adolescents, similar to treatment with dopamine releasing agents (Laviola et al. 2001). Greater efflux of dopamine and/or greater postsynaptic receptor number in adults following high dose cocaine could result in greater locomotor stimulation and transcriptional activation in adults compared to adolescents.
Cocaine-Induced Increases in Cortical gene expression
Basal levels of both genes were significantly higher in the cortex than the striatum and cocaine caused smaller increases in c-fos and zif268 expression. The elevated basal levels of IEGs and lesser increase after cocaine in cortex relative to striatum have been observed previously (Daunais and McGinty 1994; Steiner and Gerfen 1993; Wilhuhn et al, 2003). The more modest IEG response probably reflects the more diffuse distribution of dopamine transporters in the cortex (Leroux-Nicollet and Costentin 1988) and smaller increases in extracellular dopamine levels in the cortex than the striatum after cocaine (Ikegami and Duvauchelle 2004). Adolescents showed both higher basal levels of IEGs as well as higher levels after cocaine, but the percent increase was no different than adults. Therefore, the developmental differences in response to cocaine are restricted to the caudate.
Correlations between c-fos and cocaine-induced locomotion in individual animals
Striatal c-fos expression correlated with locomotor activity in individual adolescents and adults. One previous study (Szucs et al. 2005) showed a significant correlation between locomotor activity and the number of c-fos immunopositive cells in the nucleus accumbens core and the medial caudate, but not the nucleus accumbens shell or the lateral caudate in adults. Measuring mRNA as opposed to protein, we showed that c-fos levels in virtually all regions of the striatum correlated with locomotor activity in individual adults. A similar but less dramatic profile was observed in adolescents. We observed a significant correlation between locomotor activity and striatal zif268 expression only in adults. We had predicted that the expression of c-fos and/or zif268 in some cortical areas, including the premotor (agranular) and sensorimotor cortex, might correlate with locomotor activity in individual animals, as these centers provide glutamate input to the caudate that is important for cocaine-stimulated locomotion, but this correlation was not observed. Whether these are causally related or not, this finding suggests that individual differences in behavioral response to psychomotor stimulants are at least paralleled by exaggerated early neuroplastic changes in target neurons. Given the importance of IEG induction as an early step in the neuroplastic events leading to addiction (McClung and Nestler 2008), it supports the broad literature emphasizing the importance of individual vulnerabilities to addiction (Bardo et al., 1996; Deminiere et al., 1989).
Several methodologic issues suggest caution in implying that there are necessarily causal relationships among dopaminergic stimulation, behavior and IEG induction in this study. First, we measured mRNA not protein expression, and so obtained a sensitive indication of neural activity (the primary goal of this study) but did not obtain an accurate assessment of the neuroplastic responses. Furthermore, the experimental design required killing animals at a single time point in order to minimize number of animals and analyses. The real-time PCR study showed that the developmental relationship was maintained at all time points, but also showed that c-fos and zif268 likely did not attain maximal levels at the same time. Therefore, comparison of absolute levels of the two must be tempered by this experimental design. Finally in-situ hybridization provides a semi-quantitative signal. Despite these caveats, these data clearly indicate that cocaine induced a dose- dependent, regionally specific induction of both c-fos and zif268 that exhibited robust age differences
Correlation of c-fos with zif268 induction
The correlated expression of c-fos and zif268 in adults was expected as it has been proposed that D1 dopamine receptor stimulation causes the coordinated induction of multiple IEGs. Both genes show similar dopamine-mediated regulation in the same cell types: D1 receptor stimulation increases c-fos and zif268 (and other IEGs) expression in striatonigral (enkephalin negative) medium spiny neurons, D2 receptor stimulation decreases IEG expression in striatopallidal (enkephalin positive) medium spiny neurons, and combined D1 and D2 receptor stimulation synergistically increases IEG expression in striatonigral neurons (Berreta et al. 1992; Bertran-Gonzalez et al. 2008; Bhat et al. 1992; Gerfen et al. 1990, 1995; Moratalla et al. 1992; Robertson and Jian 1995; Robertson et al. 1990, 1992;). These and other studies have also demonstrated that c-fos and zif268 show similar anatomical patterns of induction and dose-response relationships to stimulants including methylphenidate and cocaine (Daunais and McGinty 1994; Kosofsky et al. 1995; Brandon and Steiner 2003).
Despite their similarities, the two genes do contain different combinations of enhancer sequences in their upstream promotors and there is evidence of distinct regulation. For example, zif268 has four serum response elements (SRE) binding sites in its promotor whereas c-fos only has one (Christy and Nathans 1989). Differences such as these could enable distinct intracellular signals to preferentially activate the expression of one gene or the other. Several studies have shown selective modulation of dopamine-mediated c-fos and zif268 expression in striatal neurons. In the dopamine depleted striatum, low-moderate doses of selective NMDA receptor antagonists blocked D1-stimulated c-fos expression whereas much higher doses were necessary to reduce zif268 expression (Keefe and Gerfen 1996). Mitogen and Stress-Activated Protein Kinase 1 (MSK1) knockout mice show normal cocaine-stimulated increases in zif268 but significantly reduced cocaine-induced increases in striatal c-fos expression (Brami-Cherrier et al. 2005). Mu opioid receptor blockade attenuates cocaine and methamphetamine-induced striatal zif268 expression without affecting striatal c-fos expression (Horner and Keefe 2006). One intriguing possibility raised by the NMDA antagonist study is that the balance of NMDA and D1 stimulation to IEG induction differs in adolescents and adults. Studies are underway to explore this possibility.
A number of groups, including Moratalla et al. (1992), have proposed that transcriptional cooperativity underlies the complexity of functional responses produced by dopamine modulation Dopamine agonists can stimulate an array of IEGs from at least two gene families (Cole et al. 1992; Graybiel et al. 1990; Moratalla et al. 1992, 1993; Young et al. 1991). Further, the known genes of these families have different collections of upstream elements in their promotors and their products are capable of acting in different combinatorial patterns (Janssen-Timmen et al.1989; Treisman 1995; Tsai-Morris et al. 1988). Several studies using knock outs of c-fos, zif268, and an upstream kinase (MSK-1), have demonstrated that locomotor sensitization to cocaine requires the coordinated expression of both genes whereas cocaine CPP appears to require only zif268 expression (Brami-Cherrier et al. 2005; Valjent et al. 2006; Zhang et al. 2006). An evolving combinatorial IEG response to psychostimulants with age might contribute to evolving behavior as well as evolving neuroplasticity.
Relevance of IEG induction for Addiction Vulnerability
C-fos and zif268 are transcription factors that coordinate the long-lasting effects of stimulants (Zhang et al. 2006; Valjent et al. 2006). Our results imply that young adolescents may be differentially susceptible to some of the neuroplasticities induced by repeated low dose cocaine. The prominent c-fos response to low but not high dose cocaine but absence of c-fos/zif268 coordination could partially explain why adolescents may be less sensitive to cocaine-induced locomotor sensitization (Laviola et al. 1995; Collins and Izenwasser 2002; Frantz et al. 2007) but at the same time show similar or enhanced development of CPP (Badanich et al. 2006; Brenhouse et al. 2008; Campbell et al. 2000; Schramm-Sapyta et al. 2004). However, the lack of c-fos and zif268 coordination in adolescents implies that adults may be actually more vulnerable to some of the long-lasting effects of high dose cocaine than young adolescents that depend upon a coordinated IEG induction. The present results to not provide clear evidence of enhanced transcriptional responses that simplistically predict increased addiction vulnerability in adolescents. A clear understanding of the neuroplastic events, including later-developing transcriptional responses like delta fos b (McClung and Nestler 2008) will be extremely important to understand whether there is a neurobiologic basis for the increased addiction vulnerability of adolescents.
Acknowledgments
This work was generously supported by NIDA grant #DA09079 and NIH pre-doctoral service award (F31) #DA022813-01.
List of abbreviations
- CPP
Conditioned placed preference
- IEG
Immediate early gene
- PN
Post natal
- 5HT
Serotonin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alanzo R, Gnanadicom H, Frechin N, Fournier M, Le Fur G, Soubrie P. Blockade of neurotensin receptors suppresses the dopamine D1/D2 synergism on immediate early gene expression in the rat brain. Eur J Neurosci. 1999;11:967–974. doi: 10.1046/j.1460-9568.1999.00506.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Changes in second messenger cAMP signaling during development may underlie the motoric symptoms in attention deficit/hyperactivity disorder (ADHAD) Behav Brain Res. 2002;130:197–201. doi: 10.1016/s0166-4328(01)00417-x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Sex difference in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24:137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Andersen SL, LeBlanc CJ, Lyss PJ. Maturational increases in c-fos expression in ascending dopamine systems. Synapse. 2001;41:345–350. doi: 10.1002/syn.1091. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Maldonado AM, Kirstein CL. Early adolescents show enhanced acute cocaine-induced locomotor activity in comparison to late adolescent and adult rats. Dev Psychobiol. 2008;50:127–133. doi: 10.1002/dev.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77(12):23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Welte JW. Patterns and predictors of alcohol use among 7-12th graders in New York State. J Stud Alcohol. 1986;47:53–62. doi: 10.15288/jsa.1986.47.53. [DOI] [PubMed] [Google Scholar]
- Berretta S, Robertson HA, Graybiel AM. Dopamine and glutamate agonists stimulate neuron-specific expression of fos-like protein in the striatum. J Neurophysiol. 1992;68:767–777. doi: 10.1152/jn.1992.68.3.767. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RV, Cole AJ, Baraban JM. Role of monoamine systems in activation of zif268 by cocaine. J Psychiatry Neurosci. 1992;19:94–102. [PMC free article] [PubMed] [Google Scholar]
- Bhat RV, Baraban JM. Activation of transcription factor genes in striatum by cocaine: role of both serotonin and dopamine systems. J Pharmacol Exp Ther. 1993;267:496–505. [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in the periadolescent rats: a behavioral and neurochemical analysis. Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Herve D, Darragh J, Corvol JC, Pages C, Simon AJ, Girault JA, Caboche J. Parsing molecular and behavioral effects of cocaine in mitogen and stress-activated protein kinase-1- deficient mice. J Neurosci. 2005;25:11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon CL, Steiner H. Repeated methylphenidate treatment in adolescent rats alters gene regulation in the striatum. Eur J Neurosci. 2003;18:1584–1592. doi: 10.1046/j.1460-9568.2003.02892.x. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivation salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Campochiaro P, Coyle JT. Ontogenetic development of kainite neurotoxicity: correlates with glutamate innervation. Proc Natl Acad Sci. 1978;75:2025–2029. doi: 10.1073/pnas.75.4.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Lotfipour S, Loughlin SE, Leslie FM. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology. 2007;32:2279–2289. doi: 10.1038/sj.npp.1301349. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology. 2005;183:218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. A single high dose of cocaine induces differential sensitization to specific behaviors across adolescence. Psychopharmacology. 2007;193:247–260. doi: 10.1007/s00213-007-0764-5. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Pub Health. 1995;84:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B, Nathans D. Functional serum response elements upstream of the growth factor-inducible gene zif268. Mol Cell Bio. 1989;9:4889–4895. doi: 10.1128/mcb.9.11.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Tarter RE. Adolescent versus adult onset and the development of substance use disorders. Drug Alcohol Dep. 1998;49:115–121. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- Cole AJ, Bhat RV, Patt C, Worley PF, Baraban JM. D1 dopamine receptor activation of multiple transcription factor genes in rat striatum. J Neurochem. 1992;58:1420–1426. doi: 10.1111/j.1471-4159.1992.tb11358.x. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Res Dev Brain Res. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Happe HK, Murrin LC. Dopamine transporter development in postnatal rat striatum: an autoradiographic study with [3H]WIN 35,428. Dev Brain Res. 1997;104:55–62. doi: 10.1016/s0165-3806(97)00135-1. [DOI] [PubMed] [Google Scholar]
- Coyle, Campochiaro P. Ontogenesis of dopaminergic-cholinergic interactions in the rat striatum: a neurochemical study. J Neurochem. 1976;27(3):673–8. doi: 10.1111/j.1471-4159.1976.tb10393.x. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, McGinty JF. Acute and chronic cocaine administration differentially alters striatal opioid and nuclear transcription factor mRNAs. Synapse. 1994;18:35–45. doi: 10.1002/syn.890180106. [DOI] [PubMed] [Google Scholar]
- Daunais JB, McGinty JF. Cocaine binges differentially alter striatal preprodynorphin and zif268 messenger RNAs. Mol Brain Res. 1995;29:201–210. doi: 10.1016/0169-328x(94)00246-b. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Kelley AE. The role of D1 and D2 dopamine receptors in oral stereotypy induced by dopaminergic stimulation of the ventrolateral striatum. Neuroscience. 1990;39:59–67. doi: 10.1016/0306-4522(90)90221-o. [DOI] [PubMed] [Google Scholar]
- Deminiere JM, Piazza PV, Le Moal M, Simon H. Experimental approach to individual vulnerability to psychostimulant addiction. Neurosci Biobehav Rev. 1989 Summer-Fall;13(23):141–7. doi: 10.1016/s0149-7634(89)80023-5. 1989. [DOI] [PubMed] [Google Scholar]
- Ehrlich ME, Rosen NL, Kurihara T, Shalaby IA, Greengard P. DARPP-32 development in the caudate nucleus is independent of afferent input from the substantia nigra. Brain Res Dev Brain Res. 1990;54(2):257–63. doi: 10.1016/0165-3806(90)90148-r. [DOI] [PubMed] [Google Scholar]
- Fentress JC, Stanfield BB, Cowan WM. Observations on the development of the striatum in mice and rats. Anat. Embryol. 1981;163:275–298. doi: 10.1007/BF00315705. [DOI] [PubMed] [Google Scholar]
- Estroff TW, Schwartz RH, Hoffmann NG. Adolescent cocaine abuse: addictive potential, behavioral and psychiatric effects. Clin Ped. 1989;28:550–555. doi: 10.1177/000992288902801201. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, Van Hartesveldt C. The locomotor effects of MK801 in the nucleus accumbens of developing and adult rats. Eur J Pharmacol. 1999;368:125–135. doi: 10.1016/s0014-2999(99)00009-6. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O'Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Galineau L, Kodas E, Guilloteau D, Vilar MP, Chalon S. Ontogeny of the dopamine and serotonin transporters in the rat brain: an autoradiographic study. Neurosci Lett. 2004;363(3):266–71. doi: 10.1016/j.neulet.2004.04.007. 17. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Keefe KA, Gauda EB. D1 and D2 dopamine receptor function in the striatum: coactivation of D1 and D2 dopamine receptors on separate populations of neurons results in potentiated immediate early gene response in D1-containing neurons. J Neurosci. 1995;15:8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Giorgi O, De Montis G, Porceddu ML, Mele S, Calderini G, Toffano G, Biggio G. Developmental and age-related changes in D1-dopamine receptors and dopamine content in the rat striatum. Dev Brain Res. 1987;35:283–290. doi: 10.1016/0165-3806(87)90053-8. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age and alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J Stud Alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner KA, Keefe KA. Regulation of psychostimulant-induced preprodynorphin, c-fos, and zif268 messenger RNA expression in the rat dorsal striatum by mu opioid receptor blockade. Eur J Neurosci. 2006;532:61–73. doi: 10.1016/j.ejphar.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Ungerstedt U. Cocaine: an in vivo microdialysis evaluation of its acute action on dopamine transmission in rat striatum. Synapse. 1989;3:48–54. doi: 10.1002/syn.890030107. [DOI] [PubMed] [Google Scholar]
- Ikegami A, Duvauchelle CL. Nucleus accumbens and prefrontal cortex dopaminergic responses to self-administered cocaine in naïve rats. Neurosci Lett. 2004;354:205–208. doi: 10.1016/j.neulet.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino-acid receptors in the rat forebrain: N-methyl-D-aspartate and quisquilate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Janssen-Timmen U, Lemaire P, Mattei MG, Revelant O, Charnay P. Structure, chromosome mapping, and regulation of the mouse zinc-finger gene Krox-24; evidence for a common regulatory pathway for immediate-early serum-response genes. Gene. 1989;80:325–336. doi: 10.1016/0378-1119(89)90296-5. [DOI] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psych. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Davies M. Progression to regular marijuana involvement: phenomenology and risk-factors for near daily use. In: Glantz MD, Pickens RW, editors. Vulnerability to drug abuse. Washington, DC: American Psychological Association Press; 1992. pp. 211–253. [Google Scholar]
- Keefe KA, Gerfen CR. D1 dopamine recptor-mediate induction of zif268 and c-fos in the dopamine-depleted striatum: differential regulation and independence from NMDA receptors. J Comp Neurol. 1996;367:165–176. doi: 10.1002/(SICI)1096-9861(19960401)367:2<165::AID-CNE1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann NY Acad Sci. 1999;877:71–90. doi: 10.1111/j.1749-6632.1999.tb09262.x. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part 1. Ann NY Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Iverson SD. Selective 6OHDA induced destruction of mesolimbic dopamine neurons: ablation of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends upon post-synaptic NMDA receptors and calcium. J Neurosci. 1994;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosofsky BE, Genova LM, Hyman SE. Postnatal age defines specificity of immediate early gene induction by cocaine in developing rat brain. J Comp Neurol. 1995;351:27–40. doi: 10.1002/cne.903510104. [DOI] [PubMed] [Google Scholar]
- Lanier LP, Isaacson RL. Early developmental stages in the locomotor response to amphetamine and their relation to hippocampal function. Brain Res. 1977;126:567–575. doi: 10.1016/0006-8993(77)90610-2. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Pascucci T, Peirette S. Striatal dopamine sensitization to D-amphetamine in periadolescent but not adult rats. Pharmacol Biochem Behav. 2001;68:115–124. doi: 10.1016/s0091-3057(00)00430-5. [DOI] [PubMed] [Google Scholar]
- Leroux-Nicollet I, Costentin J. In vivo and in vitro autoradiographic labeling of central dopaminergic systems with [3H] GBR12783 in rodents. Neurosci Lett. 1988;95:7–12. doi: 10.1016/0304-3940(88)90623-4. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology. 2008;33(1):3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- Maldonado AM, Kirstein CL. Handling alters cocaine-induced activity in adolescent but not adult male rats. Phys Behav. 2005;84:321–326. doi: 10.1016/j.physbeh.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R, Robertson HA, Graybiel AM. Dynamic regulation of NGF1-A (zif268, egr1) gene expression in the rat striatum. J Neurosci. 1992;12:2609–2622. doi: 10.1523/JNEUROSCI.12-07-02609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R, Vickers EA, Robertson HA, Cochran BH, Graybiel AM. Coordinate expression of c-fos and jun B is induced in the rat striatum by cocaine. J Neurosci. 1993;13:423–433. doi: 10.1523/JNEUROSCI.13-02-00423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y, Naitoh F, Segawa T. Regional changes in monoamine content and uptake of the rat brain during postnatal development. Brain Res. 1976;101:305–315. doi: 10.1016/0006-8993(76)90271-7. [DOI] [PubMed] [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav. 2008;89:314–323. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcher W, Heller A. Regional development of catecholamine biosynthesis in rat brain. J Neurochem. 1972;19:1917–1930. doi: 10.1111/j.1471-4159.1972.tb01480.x. [DOI] [PubMed] [Google Scholar]
- Roberston GS, Jian M. D1 and D2 dopamine receptors differentially increase Fos-like immunoreactivity in accumbal projections to the ventral pallidum and midbrain. Neuroscience. 1995;64:1019–1034. doi: 10.1016/0306-4522(94)00426-6. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Vincent SR, Fibiger HC. Striatonigral projection neurons contain D1 receptor-activated c-fos. Brain Res. 1990;523:288–290. doi: 10.1016/0006-8993(90)91498-6. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Vincent SR, Fibiger HC. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992;49:285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Robins LN, Pryzbeck TR. Age of onset of drug use as a factor in drug and other disorders. In: Jones CL, Battjes R, editors. Etiology of drug abuse: implications for prevention. Rockville, MD; National Institute on Drug Abuse; 1985. pp. 178–179. (Research Monograph Series, no 56). [Google Scholar]
- Sakagami H, Sawamura Y, Kondo H. Synchronous patchy pattern of gene expression for adenylyl cyclase and phosphodiesterase but discrete expression for G-protein in developing rat striatum. Brain Res Mol Brain Res. 1995;33(2):185–91. doi: 10.1016/0169-328x(95)00123-a. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Robinson TE. Why does rapid delivery of drugs to the brain promote addiction? Trends Pharm Sci. 2005;26:82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Schambra UB, Duncan GE, Breese GR, Fornaretto MG, Caron MG, Fremeau RT., Jr Ontogeny of D1A and D2 dopamine receptor subtypes in rat brain using in situ hybridization and receptor binding. Neuroscience. 1994;62(1):65–85. doi: 10.1016/0306-4522(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann NY Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Pratt AR, Winder DG. Effects of periadolescent versus adult cocaine exposure on cocaine conditioned place preference and motor sensitization in mice. Psychopharmacology. 2004;173:41–48. doi: 10.1007/s00213-003-1696-3. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li z, Le AD. Acute nicotine enhanced c-fos mRNA expressioni differentially in reward-related substrates of adolescent and adult rat brain. Neurosci Lett. 2007;418:286–291. doi: 10.1016/j.neulet.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Functional alterations in CNS catecholamine systems in adolescence and adulthood after neonatal chlorpyrifos exposure. Dev Brain Res. 2002;133:163–173. doi: 10.1016/s0165-3806(02)00284-5. [DOI] [PubMed] [Google Scholar]
- Snyder KJ, Katovic NM, Spear LP. Longevity of the expression of behavioral sensitization to cocaine in preweanling rats. Pharmacol Biochem Behav. 1998;60:909–914. doi: 10.1016/s0091-3057(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brick J. Cocaine-induced behavior in the developing rat. Behav Neural Bio. 1979;26:401–415. doi: 10.1016/s0163-1047(79)91410-9. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993;13:5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs RP, Frankel PS, McMahon LR, Cunningham CA. Relationship of cocaine-induced c-Fos expression to behaviors and the role of serotonin 5-HT2A receptors in cocaine-induced c-Fos expression. Behav Neurosci. 2005;119:1173–1183. doi: 10.1037/0735-7044.119.5.1173. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomisini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neurosci Lett. 1998;254:21–24. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89(2):167–72. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res. 2001;892:269–280. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- Triesman R. Journey to the surface of the cell: Fos regulation and the SRE. EMBO J. 1995;14:4905–4913. doi: 10.1002/j.1460-2075.1995.tb00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai-Morris CH, Cao X, Sukhatme VP. 5′ flanking sequence and genomic structure of EGR-1, a murine mitogen inducible zinc-finger encoding gene. Nucleic Acids Res. 1998;16:8835–8846. doi: 10.1093/nar/16.18.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HBM, van Eden CG, Parnavelas JG, Kalsbeek A. The prenatal and postnatal development of rat cerebral cortex. In: Kolb B, Tees RC, editors. The Cerebral Cortex of the Rat Cambridge (USA) MIT Press; 1990. pp. 35–76. [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Aubier B, Corbille AG, Brami-Cherrier K, Caboche J, Topilko P, Girault JA, Herve D. Plasticity-associate gene Krox24/Zif268 is required for the long-lasting behavioral effects of cocaine. J Neurosci. 2006;26:4956–4960. doi: 10.1523/JNEUROSCI.4601-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamaturgic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psycopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vasilev V, Veskov R, Janac B, Rakic L, Stojiljkovic M. Age-related differences in MK-801 and amphetamine-induced locomotor and stereotypic activities of rats. Neurobiol Aging. 2003;24:715–723. doi: 10.1016/s0197-4580(02)00232-4. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Folwer JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in the dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Kuhn CM. Cocaine increases stimulated dopamine release more in periadolescent than adult rats. Neurotox Terat. 2008;30(5):412–8. doi: 10.1016/j.ntt.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Daunais JB, McGinty JF. NMDA receptors mediate amphetamine-induced upregulation of zif268 and preprodynorphin mRNA expression in the rat striatum. Synapse. 1994a;18:343–353. doi: 10.1002/syn.890180410. [DOI] [PubMed] [Google Scholar]
- Wang J, Daunais JB, McGinty JF. Role of kainate/AMPA receptors in the induction of striatal zif268 and preprodynorphin mRNA expression in rat striatum. Mol Brain Res. 1994b;27:118–126. doi: 10.1016/0169-328x(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Sun W, Steiner H. Topography of cocaine-induced gene regulation in the rat striatum: relationship to cortical inputs and role of behavioral context. Eur J Neurosci. 2003;17:1053–1066. doi: 10.1046/j.1460-9568.2003.02525.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Kandel DB. Patterns of drug abuse from young adolescence to adulthood: III. Predictors of progression. Am J Pub Health. 1984;74:673–681. doi: 10.2105/ajph.74.7.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, Porrino LJ, Iadarola JM. Cocaine induces striatal c-fos immunoreactive proteins via dopaminergic D1 receptors. Proc Nat Acad Sci. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Jiao H, Zhang Q, Lou D, Katz JL, Xu M. c-Fos facilitates the acquisition and extinction of cocaine-induced persistent changes. J Neurosci. 2006;26:13287–13296. doi: 10.1523/JNEUROSCI.3795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]