Abstract
Pelvic organ prolapse (POP) and preterm premature rupture of the membranes (PPROM), two conditions which have in common weakening of the tensile strength of tissues, are thought to be caused, in part, by abnormal extracellular matrix synthesis and/or catabolism. We identified a new single nucleotide polymorphism (SNP) (NT_010194(LOXL1):g.45008784A>C) in the promoter of the LOXL1 gene, which is essential for elastin synthesis. Promoter studies showed that the minor “C” allele had significantly greater activity than the major “A” allele. Case-control studies examined the association of the alleles of this SNP with POP and PPROM. When comparing allele frequencies and genotypes in POP cases versus controls, no significant associations were found. A case-control study conducted in African-American neonates also found no significant associations between the promoter alleles and PPROM. We conclude that a functional SNP exists in the promoter region of LOXL1. Association studies suggest that the promoter SNP does not contribute significantly to risk of POP or PPROM.
Keywords: Lysyl oxidase-like 1, pelvic organ prolapse, preterm premature rupture of membranes, elastin
Introduction
Pelvic organ prolapse (POP) and preterm premature rupture of the membranes (PPROM) are two conditions that are thought to be caused, in part, by abnormal extracellular matrix synthesis and/or catabolism. Because the prevalence of pelvic organ prolapse (POP) increases with age, the changing demographics of the world’s population will result in a growing number of affected women. While pelvic floor disorders are not life-threatening medical conditions, they can have a significant negative impact on quality of life (Barber and Jelovsek, 2006; Fitzgerald et al. 2007).1,2
The pathophysiology of POP (Rahn et al., 2008a,b) is multifactorial with risk factors that may be categorized as predisposing (Chiaffarino et al. 1999), inciting (Mant et al. 1997), promoting (Weber & Richter 2005), or decompensating (Bump & Norton 1998 & Nygaard et al. 2004).3-9 Depending on the combination of these risk factors in an individual, prolapse may or may not develop over her lifetime. At present, the potential impact of genetic factors has not been clearly elucidated. Multiple studies have identified parturition and advanced age to be risk factors associated with development of pelvic floor disorders, but identification of positive family history provides evidence that a genetic component exists. (Jack et al. 2006; Twiss et al., 2007).10,11 Observations of lower rates of prolapse in African-American women (Rortveit et al. 2007) has also raised the question of genetic susceptibility/protection, with inherited abnormalities in collagen biosynthesis a potential target for research.12 However, only one genetic variant that confers risk for POP has been reported (Nikolova et al., 2007).13
Abnormalities in extracellular matrix metabolism and repair presents a possible genetic explanation for the development of POP. Female pelvic tissues are rich in elastic fibers that turn over slowly in most adult tissues but undergo massive remodeling in the reproductive organs through pregnancy and birth. In the generation of a Loxl1-deficient mouse model, approximately one-third of the female mutant mice developed severe POP after the first litter, and all of the remaining two-thirds developed prolapse after the second litter highlighting the essential role for elastic fiber homeostasis in pelvic floor remodeling postpartum (Liu 2006).14,15 A recent study of women with Stage III or greater prolapse showed suppression of the lysyl oxidase like-1 protein in uterosacral ligaments. (Klutke et al. 2008).16 This enzyme provides a potential source of genetic variation that may predispose women to the development of pelvic organ prolapse.
Fetal membrane rupture, occurring before 37 weeks of completed gestation, is referred to as preterm premature rupture of membranes (PPROM). PPROM complicates 1-4% of all pregnancies, and is the leading identifiable cause of preterm birth, which is the major cause of neonatal mortality and morbidity (Parry & Strauss, 1998).17 PPROM and preterm birth are more common in African-Americans.
The tensile strength of the fetal membranes, which are composed of the amnion and chorion, is provided predominantly by the extracellular matrix of the amnion (Moore et al., 2006; El Khawad et al., 2005, 2006).18-20 Fetal membranes that rupture prematurely have reduced collagen content, which could be the result of diminished synthesis or increased catabolism (Skinner et al., 1981; Hampson et al., 1997; Vadillo-Ortega et al., 1990; Wang H et al., 2004; Fujimoto et al., 2002; Ferrand et al., 2002; Devlieger et al, 2006).21-27 Reduced extracellular matrix production is related to risk of PPROM (Hieber et al., 1997; Wang et al., 2006).28,29 Fetuses affected with Ehler-Danlos syndrome are more likely to be born prematurely as a result of PPROM (Parry & Strauss, 1998).17 Moreover, a fetal polymorphism in the SERPINH1 gene, which encodes heat-shock protein 47 (Hsp47), an intracellular chaperone for collagen proteins which governs collagen elaboration, is associated with risk of PPROM in African-Americans (Wang et al., 2006).29
Lysyl oxidases are copper-dependent amine oxidases that are key enzymes involved in collagen and elastin synthesis and repair. Lysyl oxidases are responsible for the formation of cross-linking between the collagen and elastin fibrils, without which it is prone to degradation leading to weakened connective tissues. Distinct from the prototypic lysyl oxidase (LOX), the lysyl oxidase like-1 (LOXL1) protein localizes specifically to sites of elastogenesis and is an essential enzyme in producing the scaffold that ensures the spatially defined deposition of elastin 15,30
The goals of this study were to: 1) determine if functional single nucleotide polymorphisms (SNPs) exist in the promoter region of the LOXL1 gene; 2) determine if women with and without POP demonstrate a significant difference in the carriage of the SNP alleles; and 3) determine if the SNP alleles are associated with risk of PPROM.
Materials and Methods
Identification of SNPs
To search for SNPs in the LOXL1 promoter region, an 1120 base pair (bp) sequence was amplified in 10 DNA samples of unknown ethnicity and this DNA was then directly sequenced. To amplify the LOXL1 promoter region, AmpliTaq Gold with Gene Amplification from Applied BioSystems, manufactured by Roche in Branchburg, NJ was used. PCR primers used in the amplification of the LOXL1 gene were forward: 5′-AGA GCAGTATTTGGAGTGTG-3′ and reverse: 5′-CCCACTCTGAATGAATAAGC-3′. The conditions for amplification of LOXL1 are initialization at 94 degrees for 3 minutes, denaturation was begun at 94 C for 30 sec, annealing at 57 C for 45 sec, extension/elongation at 72 C for 45 sec for a total of 35 cycles, followed by elongation at 72 C for 7 min. We then acquired DNA samples from 10 Western Europeans from the CEPH DNA collection for analysis under the same conditions stated above.
Construction of Promoter-Reporter Plasmids
To determine whether the -659 A>C SNP in the LOXL1 promoter region influences transcription of the LOXL1 gene, we cloned the 1120 bp fragment of the LOXL1 promoter into the pGL3 vector, which contains the firefly luciferase gene as a reporter. The promoter fragment extended from -1431 bp to -310 bp from the ATG codon. Based on cDNA sequences deposited in GenBank (Acession Numbers: NM005576 and BC068542), the promoter construct extends into the transcribed region of the LOXL1 gene by at least 17 bp. A mutagenesis kit was used to create the targeted alleles with a uniform backbone sequence (Stratagene, QuikChange Site-Directed Mutagenesis Kit). The DNA sequences of the promoter constructs were confirmed before use, and three different plasmid preparations were prepared for each construct.
Cell Culture and Transfection
Human dermal fibroblasts were cultured in DMEM. The media was supplemented with 10 % fetal bovine serum and antibiotics (100 units/ml penicillin G, 100 units/ml streptomycin sulfate). Cells were maintained at 37 °C in a water saturated atmosphere under 5 % CO2 in air. For transfection, dermal fibroblasts were grown in a 12 well culture plate. Cells were transfected using FuGene 6 transfection reagent (Roche Diagnostics, Indianapolis, IN) with 6 micrograms of the pGL3 vector containing the LOXL1 promoter fragments. In each transfection well, 25 ng of pRL-TK (Promega), a control plasmid expressing Renilla reniformis luciferase, was used to correct for transfection efficiency. The transfected cells were cultured for 48 h in medium before collecting the cells for the luciferase assays.
Luciferase Assays
After 48 h of culture, the transfected cells were lysed in lysis buffer and 20 ml aliquots of supernatant were assayed for luciferase activity using the Dual-Luciferase Reporter Assay system (Promega). Promoter activities were expressed as the ratio between Photinus luciferase and Renilla luciferase activities.
Study Populations
After obtaining institutional review board approval, a control population of women with < Stage II POP according to the Pelvic-Organ-Prolapse Quantification System (POP-Q) was prospectively recruited from the Urogynecology and benign gynecology clinics of a study author (CAM). Control subjects were recruited to match cases on the basis of age, race, menopausal status, smoking history, body-mass-index, and parity. Exclusion criteria for all study subjects included a personal history of a systemic collagen disorder such as Ehlers-Danlos or Marfan’s syndrome. Cases were defined as women with ≥ Stage II POP and were collected via two different methods in Caucasian women in an attempt to expedite study completion. Prospective recruitment of Caucasian and African-American women with ≥ Stage II POP who presented to the Urogynecology clinic at VCU Medical center was conducted in the same manner in which control women were enrolled. In addition, Caucasian cases were retrospectively collected by accessing tissue samples of women who underwent reconstructive surgery for ≥ Stage II POP between January 2004 and January 2006 at the VCU Medical Center. Demographic data for these women was extracted from the Urogyecology case log that is maintained by our division at VCU Medical Center. Informed consent was obtained for all subjects who were prospectively enrolled. For this group, demographic data was obtained at the time of consent, including age, race, parity, menopausal status, smoking status, and BMI. Samples for genomic DNA isolation were collected by buccal smears using Simhelix Buccal Swabs, the extraction of which was performed using Simhelix Buccal Swab DNA Isolation Kit from Boca Scientific.
Subjects in the PPROM case-control study were African-American women and their neonates receiving obstetrical care at the Hospital of the University of Pennsylvania or Hutzel Hospital. Written informed consent was obtained from mothers before collection of the samples. This study was approved by the respective institutional review boards. Control samples (N=142) were obtained from neonates of singleton pregnancies delivered at term of mothers with no prior history of PPROM or preterm labor. Case samples (N=249) were collected from neonates from pregnancies complicated by rupture of membranes before 37 weeks of gestation. The diagnosis of membrane rupture was based on pooling of amniotic fluid in the vagina, amniotic fluid ferning patterns, and a positive nitrazine test. Women with multiple gestations, fetal anomalies, trauma, connective tissue diseases, and medical complications of pregnancy requiring induction of labor were excluded.
Because the African-Americans are not the isolated populations, there is a chance that any observed association could be a result of admixture stratification. To evaluate the possible effects of population stratification, we evaluated 29 ancestry-informative markers to calculate the individual biogeographical ancestry levels of the persons in the study in the context of the two primary parental populations (West African and Western European) as previously reported (Wang et al, 2006).29 This assessment of the population structure showed no significant difference in the genetic profiles between PPROM cases and the controls (p>0.05).
Genotyping
PCR was performed on the samples using AmpliTaq Gold with Gene Amplification from Applied BioSystems, manufactured by Roche in Branchburg, NJ. PCR primers used in the amplification of the LOXL1 gene include forward: 5′ GAACATGCAAAGAGGGTGTGTC -3′ and reverse: 5′ CAGACCTGCCTCTGAGGAAGG -3′. The conditions for amplification of LOXL1 are initialization at 94 degrees for 3 min, denaturation at 94 C for 30 sec, annealing at 57 C for 45 se, extension/elongation at 72 C for 45 sec for a total of 35 cycles, followed by elongation at 72 C for 7 min. Allele discrimination was performed on PCR products from cases and controls using the 5′ nuclease assay, Applied Biosystems TaqMan SNP Genotyping Assay. Conditions for this reaction: initialization at 50 C for 2 min, 95 C for 10 min, denaturation at 95 C for 15 sec, and annealing/extension at 60 C for 1 min for a total of 40 cycles.
Electrophoretic Mobility Shift Assays
Nuclear proteins were extracted using Novagen NucBuster Protein Extraction Kit (EMD Biosciences, Darmstadt, Germany). Double stranded oligonucleotide probes were constructed: -659 A sense: 5′-CATTCCAGCCCAAACGAGAAGCCAG-3′; -659 A antisense: 5′-CTGGCTTCTCGTTTGGGCTGGAATG-3′; -659 C sense: 5′-CATTCCAGCCCACACGAGAAGCCAG-3′; -659 C antisense: 5′-CTGGCTTCTCGTGTGGGCTGGAATG-3′. The double stranded oligonucleotides were labeled with T4 polynucleotide kinase and [γ32P]-ATP. The EMSA binding reaction was mixed in 4X EMSA buffer (Novagen) with 10 μg of nuclear protein, 1×105 c.p.m. of 32P-labeled double stranded oligonucleotide probe (1 ng) with or without unlabeled competitor probe in a total volume of 10 μl. The reaction incubated at room temperature for 30 min, and then was subjected to 8% PAGE at 250 V for 3 hours. The dried gels were next exposed to X-ray film.
Analysis of LOXL-1 mRNA in cultured dermal fibroblasts
We obtained 5 dermal fibroblasts lines obtained from the American Type Culture Collection (Rockville, MD) and genotyped them for the LOXL1 promoter SNP. We identified 5 cell lines homozygous for the major “A” allele and two that were heterozygous for the SNP. Cells were cultured as described by Wang et al. (2006) and RNA was extracted for quantitative real-time PCR assay of the LOXL-1 message using primer sets for actin mRNA for normalization.29
Statistical Analysis
Student’s t-test and Fisher’s Exact test were used to assess significant differences in demographic characteristics between subjects in the case-control groups. Significant differences in activities among the different promoter constructs were evaluated using Anova and Duncan’s multiple range test with P< 0.05 considered significant. The chi square tests, odds ratios and 95% confidence intervals were used to determine the significance of association between LOXL1 genotypes and POP.
Results
A SNP in the promoter region of the LOXL1 gene
In searching for a polymorphism that could affect the production of lysyl oxidase like-1 protein, we identified a new single nucleotide polymorphism in the promoter region of LOXL1 gene (NT_010194(LOXL1):g.45008784A>C), lying at -659 from the ATG codon, that has not previously been described. The TFSEARCH (http://www.cbrc.jp/research/db/TFSEAR) and ConSite (http:/www.phylofoot.org/consite) programs, which identify putative response elements, did not reveal a known response element around the (NT_010194(LOXL1):g.45008784A>C) SNP.
Distribution of the LOXL1 -659 A/C promoter SNP across populations
We analyzed the allele frequencies of the -659 A>C SNP in DNA samples from various populations (Table 1). We found that the -659 “A” allele was present in the highest frequencies among most populations. The minor “C” allele frequency was found to be highest in the Caucasian, North African and African-American population.
Table I.
Ethnic distribution of the LOXL1 promoter SNP Alleles
| Ethnic Group | No. of samples | -659 “A” allele frequencies |
|---|---|---|
| Caucasian | 9 | 0.65 |
| African (south) | 9 | 0.94 |
| African (north) | 6 | 0.63 |
| Chinese | 10 | 0.95 |
| Japanese | 10 | 0.90 |
| African American | 16 | 0.69 |
Allele frequencies of the -659 A>C LOXL1 promoter SNP among different ethnic groups
Functional Significance of the -659 A/C LOXL1 Promoter SNP
To determine whether the (NT_010194(LOXL1):g.45008784A>C) SNP was functional, we performed promoter studies in the context of dermal fibroblasts. In these studies, the minor “C” allele displayed significantly greater (1.5-2.3-fold greater, p<0.001) promoter function in dermal fibroblasts as compared to the major “A” allele (Figure1). From these data, we can speculate that the “C” allele is associated with increased production of LOXL1 protein compared with the “A” allele, which might result in greater elastin production and increased tensile strength of tissues.
Figure 1.
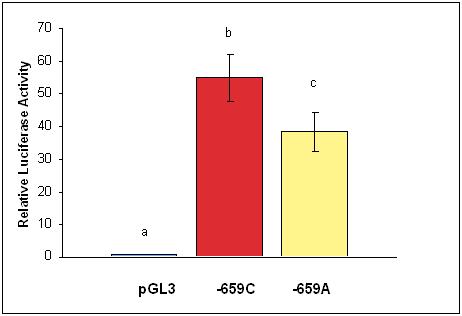
Effect of the -659 LOXL1 SNP on promoter activity in four separate experiments. LOXL1 promoter fragments representing the -659 C and A alleles were cloned into the pGL3 basic vector. Relative luciferase activities are reported as the mean +/- standard error for four separate experiments with triplicate wells in each group. The mean and standard error values are presented with statistical analysis using Anova and Duncan’s multiple range tests. Values with different letters are significantly different (p<0.001).
We measured LOXL-1 mRNA in cultured dermal fibroblasts and found that the mean abundance of these transcripts relative to actin was 1.33-fold higher in cells heterozygous for the SNP compared to cells homozygous for the major “A” allele: (A/A genotype: 0.89 ± 0.11; A/C genotype: 1.18 (range 1.08-1.28)), consistent with an enhanced rate of LOXL1 gene transcription in the presence of the -659 “C’ allele.
Proteins in nuclear extracts from dermal fibroblasts bound specifically to labeled double strand oligonucleotide probes containing the LOXL1 promoter SNP sequences (Fig. 2). However, there did not appear to be a significant difference in the affinity for binding to the two different SNP allele sequences based on competition studies with the double strand oligonucleotides representing the two promoter alleles.
Figure 2.
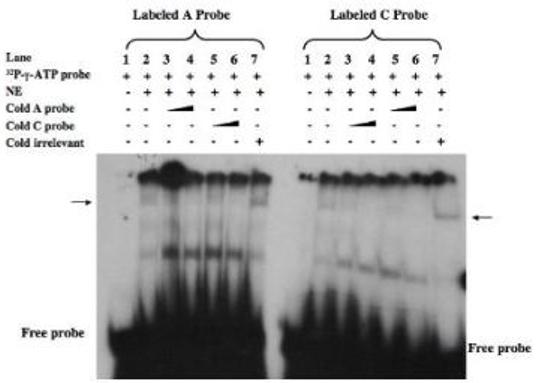
Electropohoretic mobility shift assay with nuclear extracts from dermal fibroblasts and labeled double-strand oigonucleotide probes representing the two -659 LOXL1 promoter SNP alleles. The indicate components were added (+) to the reaction mixtures prior to electrophoretic separation. The additions included unlabeled doube-strand oligonucleotides representing the two SNP alleles or an irrelevant (non-specific) unlabeled double-strand oligonucleotide. A probe = -659 “A” allele; C probe= -659 “C” allele; NE=dermal fibroblast nuclear extract. The position of the specific DNA-protein complex in the gel is indicated by an arrow.
Case-Control Study of POP and the -659 LOXL1 Promoter SNP
In a study to test the association of the (NT_010194(LOXL1):g.45008784A>C) SNP with POP, we genotyped a total of 278 women in a case-control study design. The demographic characteristics of the cases (n=137) and controls (n=141) are presented in Table II. Two hundred and twelve subjects were Caucasian, and 66 were African-American. No significant differences existed in age, parity BMI, smoking, or menopausal status. The distribution of cases by stage of prolapse was 25% stage II, 65% stage III, and 10% stage IV.
Table II.
Demographic Characteristics of Cases and Controls
| Controls (n=141) | Cases (n=137) | P value | |
|---|---|---|---|
| Age* | 60.8 +/- 12.0 | 60.7 +/- 13.4 | 0.97 |
| BMI* | 28.2 +/- 7.4 | 27.9 +/- 6.4 | 0.52 |
| Parity* | 2.3 +/- 1.22 | 2.5 +/- 1.51 | 0.42 |
| Smoking | 12.8 % | 14.6 % | 0.66 |
| Menopausal | 81.6 % post | 77.4 % post | 0.67 |
values are presented as mean ± S.D.
The genotypes for the -659 SNP in the LOXL1 promoter were analyzed in POP cases and controls. Genotyping was successful in 137 controls and 130 cases. The major “A” allele had a frequency of 0.58 in controls and 0.65 in cases. Although there was a trend for the frequency of the “A” allele, which has lower promoter activity than the minor “C” allele, to be present at lower frequency in the controls, this was not a statistically significant difference (Odds Ratio = 1.323, 95% CI: 0.918,1.908). Comparing genotypes, carriage of the minor “C” allele (i.e., comparing “A/A” individuals with “A/C” and “C/C” genotypes) revealed no association with POP (Odds Ratio = 1.147, 95% CI: 0.562, 2.341). A sub-analysis of allele frequencies by racial/ethnic group also revealed no significant associations for Caucasians (Controls=106; Cases-97; Odds Ratio = 1.243 (95% CI 0.824, 1.876)) or African-Americans (Controls=31, Cases=33; Odds Ratio = 1.6803 (95% CI: 0.602, 4.849)). Nor did an analysis comparing controls with cases having POP ≥ stage III, when stage II subjects were not considered (Odds Ratio=1.454; 95% CI: 0.577, 3.507) or when Stage II POP subjects were added to the controls (Odds Ratio=1.459; 95% CI: 0.976, 2.188).
A case-control study of PPROM in African-American neonates
To determine if the -659 LOXL1 promoter polymorphism is associated with the risk of PPROM, we performed a case-control study in African-Americans, focusing on the genotype of the offspring based on the assumption that the genotype of the extraembryonic tissues (fetal membranes) represents the primary determinant of risk of PPROM.
Analysis of the demographic characteristics of the control population, neonates born at term from normal pregnancies, and the cases, neonates from pregnancies complicated by PPROM revealed no significant differences in maternal age, gravidity and parity as previously described (Wang et al., 2006).31 However, the length of gestation (controls: 39.3 ± 1.2 (S.D.) weeks; cases: 31.8 ± 2.9, p<0.0001) and birth weight (controls: 3,308 ± 485 gm; cases: 1,934 ± 500, p<0.0001) were significantly lower in the PPROM group, as expected. There was no significant association between the -659 LOXL1 promoter SNP alleles and PPROM based on allele frequencies (Minor “C” allele frequency in 142 controls: 0.291; Minor “C” allele frequency in 249 cases: 0.306) (p=0.655, Odds Ratio=1.075, (95% C.I. 0.771, 1.494).
Since the urban African-American population in the United States from which our subjects were drawn is heterogeneous, we previously performed analyses using 29 ancestry informative markers to determine if population stratification could have affected our findings. There was no significant difference in ancestry among cases and controls analyzed in this study using a dihybrid model (Wang, H. et al., 2006).29
Discussion
We have described a new LOXL1 promoter SNP that is functional in plasmid reporter assays and associated with differences in LOXL1 mRNA abundance. The SNP is embedded in a DNA sequence, which is not identified in public databases as a known transcription binding motif, but the DNA sequence surrounding the SNP is specifically recognized by fibroblast nuclear proteins, consistent with a functional role for this element. The nature of the protein binding to the SNP element remains to be determined.
The minor “C” allele, which had greater promoter activity might be expected to increase elastin production and therefore protect against both POP and PPROM. Since there were not significant differences in the frequency of the “C” allele between Caucasians and African-Americans, a priori it is unlikely that this LOXL1 promoter SNP contributes to the differences in incidence of POP and PPROM among these two ethnic/racial groups.
Although the in vitro studies indicate that the SNP is functional, it was not found to have a major influence on risk of POP or PPROM in case-control studies. The limited impact may be explained by the relatively modest influence on promoter activity, which is consistent with the inability to detect qualitative differences in the affinity of fibroblast nuclear extract protein binding to the oligonucleotide sequence representing the two alleles. However, we cannot rule out the possibility that larger sample sizes would have revealed small but statistically significant associations. A sample size calculation indicated that with the number of total cases and controls in our study it would be possible to detect relative risk for POP of 2.5-fold with 80% power and a two-sided α of 0.05. Our findings do not exclude the possibility that other LOXL1 variants contribute to risk of POP or PPROM. Moreover, other mechanisms influencing LOXL-1 expression, including epigenetic control and post-transcriptional and post-translational processes could play roles in determining elastin content and therefore risk of POP and PPROM.
Polymorphisms in the LOXL1 gene are not only of interest relative to POP and PPROM, but to glaucoma as well. Common sequence variants have been identified in the LOXL1 gene that appear to confer susceptibility to development of exfoliation glaucoma (Thorleifsson et al. 2007).32 Whether the LOXL1 promoter polymorphism described here has an impact on the risk for developing exfoliative glaucoma is not known.
In conclusion, we have identified a new SNP in the promoter region of the LOXL1 gene that has functional significance as determined by promoter activity and examined its relationship to two conditions thought to be associated with altered matrix metabolism, POP and PPROM. There was no significant association between the SNP alleles and a diagnosis of POP. There was also no evidence for an association of the LOXL1 promoter SNP in the fetus and PPROM. Collectively, these observations suggest that the -659 LOXL1 promoter polymorphism is not a significant contributor to the two women’s health problems studied.
Acknowledgments
This research was supported by National Institutes of Health grants R01 HD034612 and P60 MD002256.
References
- 1.Fitzgerald MP, Janz NK, Wren PA, Wei JT, Weber AM, Ghetti C, Cundiff GW. Prolapse severity, symptoms and impact on quality of life among women planning sacrocolpopexy. Int J Gynaecol. Obstet. 2007;98:24–88. doi: 10.1016/j.ijgo.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Jelovsek JE, Barber MD. Women seeking treatment for advanced pelvic organ have decreased body image and quality of life. Am J Obstet Gynecol. 2006;194:1455–61. doi: 10.1016/j.ajog.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 3.Rahn DD, Acevedo JF, Word RA. Effect of vaginal distention on elastic fiber synthesis and matrix degradation in the vaginal wall: potential role in the pathogenesis of pelvic organ prolapse. Am J Physiol Regul Integr Comp Physiol. 2008 Jul 16; doi: 10.1152/ajpregu.90447.2008. [Epub ahead of print] PMID: 18635445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahn DD, Ruff MD, Brown SA, Tibbals HF, Word RA. Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Am J Obstet Gynecol. 2008;198:590, e1–6. doi: 10.1016/j.ajog.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiaffarino F, Chatenoud L, Dindelli M, Meschia M, Buonaguidi A, Amicarelli F, Surace M, Bertola E, Di Cintio E, Parazzini F. Reproductive factors, family history, occupation and risk of urogenital prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999;82:63–7. doi: 10.1016/s0301-2115(98)00175-4. [DOI] [PubMed] [Google Scholar]
- 6.Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford Family Planning Association Study. Br J Obstet Gynaecol. 1997;104:579–85. doi: 10.1111/j.1471-0528.1997.tb11536.x. [DOI] [PubMed] [Google Scholar]
- 7.Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol. 2005;10:615–34. doi: 10.1097/01.AOG.0000175832.13266.bb. [DOI] [PubMed] [Google Scholar]
- 8.Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am. 1998;25:723–46. doi: 10.1016/s0889-8545(05)70039-5. [DOI] [PubMed] [Google Scholar]
- 9.Nygaard I, Bradley C, Brandt D. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol. 2004;104:489–97. doi: 10.1097/01.AOG.0000136100.10818.d8. [DOI] [PubMed] [Google Scholar]
- 10.Jack GS, Nikolova G, Vilain E, Raz S, Rodriguez LV. Familial transmission of genitovaginal prolapse. Int. Urogynecol. J Pelvic Floor Dysfunct. 2006;17:498–501. doi: 10.1007/s00192-005-0054-x. [DOI] [PubMed] [Google Scholar]
- 11.Twiss C, Triaca V, Rodríguez LV. Familial transmission of urogenital prolapse and incontinence. Curr Opin Obstet Gynecol. 2007;19:464–8. doi: 10.1097/GCO.0b013e3282efdc21. [DOI] [PubMed] [Google Scholar]
- 12.Rortveit G, Brown JS, Thom DH, Van Den Eeden SK, Creasman JM, Subak LL. Symptomatic pelvic organ prolapse: prevalence and risk factors in a population-based, racially diverse cohort. Obstet Gynecol. 2007;109:1396–403. doi: 10.1097/01.AOG.0000263469.68106.90. [DOI] [PubMed] [Google Scholar]
- 13.Nikolova G, Lee H, Berkovitz S, Nelson S, Sinsheimer J, Vilain E, Rodríguez LV. Sequence variant in the laminin gamma1 (LAMC1) gene associated with familial pelvic organ prolapse. Hum Genet. 2007;120:847–56. doi: 10.1007/s00439-006-0267-1. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Zhao Y, Pawlyk B, Damaser M, Li T. Failure of elastic fiber homeostasis leads to pelvic floor disorders. Am J Pathol. 2006;168:519–28. doi: 10.2353/ajpath.2006.050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–82. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 16.Klutke J, Ji Q, Campeau J, Starcher B, Felix JC, Stanczyk FZ, Klutke C. Decreased endopelvic fascia elastin content in uterine prolapse. Acta Obstet. Gynecol. Scand. 2008;87:111–5. doi: 10.1080/00016340701819247. [DOI] [PubMed] [Google Scholar]
- 17.Parry S, Strauss JF., III Premature rupture of the fetal membranes. N Engl J Med. 1998;33:663–70. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 18.El Khwad M, Pandey V, Stetzer B, Mercer BM, Kumar D, Moore RM, Fox J, Redline RW, Mansour JM, Moore JJ. Fetal membranes from term vaginal deliveries have a zone of weakness exhibiting characteristics of apoptosis and remodeling. J Soc Gynecol Investig. 2006;13:191–5. doi: 10.1016/j.jsgi.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 19.El Khwad MV, Stetzer B, Moore RM, Kumar D, Mercer B, Arikat S, Redline RW, Mansour JM, Moore JJ. Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol Reprod. 2005;72:720–6. doi: 10.1095/biolreprod.104.033647. [DOI] [PubMed] [Google Scholar]
- 20.Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta. 2006;27:1037–51. doi: 10.1016/j.placenta.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Skinner SJ, Campos GA, Liggins GC. Collagen content of human amniotic membranes: effect of gestation length and premature rupture. Obstet Gynaecol. 1981;57:487–489. [PubMed] [Google Scholar]
- 22.Hampson V, Liu D, Billett E, Kirk S. Amniotic membrane collagen content and type distribution in women with preterm premature rupture of the membranes in pregnancy. Br J Obstet Gynaecol. 1997;104:1087–1091. doi: 10.1111/j.1471-0528.1997.tb12073.x. [DOI] [PubMed] [Google Scholar]
- 23.Vadillo-Ortega F, Gonzalez-Avila G, Karchmer S, Cruz NM, Ayala-Ruiz A, Lama MS. Collagen metabolism in premature rupture of membranes. Obstet Gynecol. 1990;75:84–8. [PubMed] [Google Scholar]
- 24.Wang H, Parry S, Macones G, Sammel MD, Ferrand PE, Kuivaniemi H, Tromp G, Halde I, Shriver MD, Romero R, Strauss JF., III Functionally significant SNP MMP8 promoter haplotypes and preterm premature rupture of membranes. Hum Mol Genet. 2004;13:2659–2669. doi: 10.1093/hmg/ddh287. 2004. [DOI] [PubMed] [Google Scholar]
- 25.Ferrand PE, Parry S, Sammel M, Macones GA, Kuivaniemi H, Romero R, Strauss JF., III A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol. Hum Reprod. 2002;8:494–501. doi: 10.1093/molehr/8.5.494. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto T, Parry S, Urbanek M, Sammel M, Macones G, Kuivaniemi H, Romero R, Strauss JF., III A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem. 2002;277:6296–6302. doi: 10.1074/jbc.M107865200. [DOI] [PubMed] [Google Scholar]
- 27.Devlieger R, Millar LK, Bryant-Greenwood G, Lewi L, Deprest JA. Fetal membrane healing after spontaneous and iatrogenic membrane rupture: A review of current evidence. Am J Obstet Gynecol. 2006;195:1512–20. doi: 10.1016/j.ajog.2006.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hieber AD, Corcino D, Motosue J, Sandberg LB, Roos PJ, Yu SY, Csiszar K, Kagan HM, Boyd CD, Bryant-Greenwood GD. Detection of elastin in the human fetal membranes: proposed molecular basis for elasticity. Placenta. 1997;18:301–12. doi: 10.1016/s0143-4004(97)80065-3. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Parry S, Macones G, Sammel M, Kuivaniemi H, Tromp G, Halder I, Shriver M, Romero R, Strauss JF., III A functional SNP in the promoter of the SERPINH1 gene encoding Hsp47 increases risk of preterm premature rupture of membranes and preterm birth in African-Americans. Proc. Natl. Acad. Sci. U.S.A. 2006;103:13463–13467. doi: 10.1073/pnas.0603676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hein S, Yamamoto SY, Okazaki K, Jourdan-LeSaux C, Csiszar K, Bryant-Greenwood GD. Lysyl oxidases: expression in the fetal membranes and placenta. Placenta. 2001;22:49–57. doi: 10.1053/plac.2000.0580. 2001. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Sammel MD, Tromp G, Gotsch F, Halder I, Shriver MD, Romero R, Strauss JF., 3rd A 12-bp deletion in the 5′-flanking region of the SERPINH1 gene affects promoter activity and protects against preterm premature rupture of membranes in African Americans. Hum Mutat. 2008;29:332. doi: 10.1002/humu.9522. [DOI] [PubMed] [Google Scholar]
- 32.Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson HT, Jonasdottir Adalbjorg, Jonasdottir Aslaug, Stefansdottir G, Masson G, Hardarson G, Petursson H, Arnarsson A, Motallebipour M, Wallerman O, Wadelius C, Gulcher JR, Thorsteinsdottir U, Kong A, Jonasson F, Stefansson K. Common Sequence Variants in the LOXL1 Gene Confer Susceptibility to Exfoliation Glaucoma. Science. 2007;317:1397–1400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]


