Abstract
Previous work from our laboratory has shown that the ability of estradiol to enhance object memory consolidation in young ovariectomized mice is dependent on dorsal hippocampal activation of the extracellular signal-regulated kinase/mitogen activated protein kinase (ERK/MAPK) signaling pathway (Fernandez et al., 2008). However, it is unclear if estradiol modulates memory or ERK activation similarly in the presence of progesterone. Therefore, the present study investigated effects of combined estradiol and progesterone treatment on object memory consolidation and dorsal hippocampal ERK activation in young ovariectomized C57BL/6 mice. Object memory was tested in a novel object recognition task. Immediately after training, mice received intraperitoneal (i.p.) injections of vehicle, 17β-estradiol (E2; 0.2 mg/kg), or E2 plus 5, 10, or 20 mg/kg progesterone (P). Forty-eight hours later, mice receiving E2 alone or E2 plus 10 or 20 mg/kg P exhibited significantly enhanced memory for the novel object relative to chance, whereas those receiving vehicle or E2 plus 5 mg/kg P spent no more time than chance with the novel object. Two weeks later, ERK phosphorylation was measured in the dorsal hippocampus 1 hour after i.p. injection of vehicle, E2, or E2 plus P. Consistent with our previous work (Fernandez et al., 2008), E2 alone significantly increased phospho-p42 ERK protein levels in the dorsal hippocampus relative to vehicle controls. In contrast, no combination of E2 and P affected dorsal hippocampal phosphoERK levels. These data indicate that, unlike E2 alone, the beneficial effects of combined E2 plus P treatment on memory are not associated with ERK activation in the dorsal hippocampus 1 hour after treatment, and suggest that E2 alone and combined E2 plus P may influence ERK activation in different timeframes or enhance memory through different mechanisms.
Keywords: Object recognition, Non-spatial memory, Mouse, Estrogen, Progestin, MAPK
Introduction
Understanding the effects of combined estrogen and progestin treatment on the brain and behavior has become critically important as questions have been raised about the effectiveness of hormone therapy to reduce cognitive decline in menopausal women (Rapp et al., 2003, Shumaker et al., 2003, Espeland et al., 2004, Shumaker et al., 2004). Numerous studies in rodents have demonstrated beneficial effects of the most potent estrogen, 17β-estradiol, on memory and hippocampal function (for recent reviews see (Daniel, 2006, Woolley, 2007, Frick, 2009)). In contrast to estradiol, far less is known about the effects of progestins, such as progesterone, on hippocampal memory and physiology. Some studies suggest that progesterone can reduce the beneficial effects of estradiol in the hippocampus; for example, progesterone blocks estradiol’s neuroprotective effects in the hippocampus (Rosario et al., 2006) and on brain-derived neurotrophic factor (BDNF) levels in the entorhinal cortex (Bimonte-Nelson et al., 2004). Also in the hippocampus, two estradiol injections followed 48 hrs later by a progesterone injection initially increases CA1 dendritic spine density in young ovariectomized rats, but then decreases spine density more than if estradiol was administered alone (Woolley and McEwen, 1993). Consistent with this biphasic effect on spines, when this same hormone regimen is administered to rats prior to Morris water maze training, spatial memory is improved 90 min, but not 24 hrs, after the progesterone injection (Sandstrom and Williams, 2001). Similarly, chronic estradiol plus progesterone treatment given prior to training impairs spatial memory in the Morris water maze in rats (Bimonte-Nelson et al., 2006) and footshock avoidance learning in mice (Farr et al., 1995) relative to estradiol treatment alone.
Interestingly, estradiol plus progesterone treatment administered immediately after training (i.e. post-training) reportedly enhances spatial and non-spatial object memory in young ovariectomized rats (Walf et al., 2006, Frye et al., 2007). In a post-training paradigm, rodents are trained in a single day and then are given hormone treatment immediately after training. Retention is then tested after several hours or days. Although this treatment does not simulate the chronic treatment given to menopausal women, it permits the direct observation of mnemonic effects of hormones in the absence of non-mnemonic performance confounds. Many such studies use water-soluble cyclodextrin encapsulated versions of estradiol and progesterone that can be metabolized within 24 hrs (Pitha et al., 1986). Beacause these hormones are not in the circulation during training or testing, hormones can only affect the consolidation phase of memory. Eliminating performance confounds is particularly important for progesterone, which has been shown to influence anxiety (Bitran et al., 1991).
We have previously demonstrated that post-training intraperitoneal (i.p.) injections or intrahippocampal infusions of cyclodextrin encapsulated estradiol (Gresack and Frick, 2004, Gresack and Frick, 2006, Fernandez et al., 2008) or progesterone (Harburger et al., 2008, Orr et al., submitted) enhance hippocampal-dependent object memory consolidation in young ovariectomized mice. However, it is unclear whether combined estradiol plus progesterone treatment would also enhance hippocampal object memory in young ovariectomized mice. In young ovariectomized rats, post-training subcutaneous (s.c.) injections of estradiol plus progesterone improved spatial and non-spatial object memory (Walf et al., 2006, Frye et al., 2007). However, these studies injected hormones dissolved in oil and tested memory 4 hrs later, when hormones were likely not fully metabolized. Therefore, potential effects of each hormone on non-mnemonic performance factors may have influenced behavior during testing. In addition, these prior studies examined only a single hormone combination (0.9 mg/kg estradiol plus 4 mg/kg progesterone) (Walf et al., 2006, Frye et al., 2007), leaving open the question of whether other dose combinations would have affected memory differently. Indeed, we have shown that the mnemonic effects of estradiol and progesterone alone are dose-dependent (Gresack and Frick, 2006, Harburger et al., 2008). Further, we recently showed in aged ovariectomized mice that 20 mg/kg, but not 5 or 10 mg/kg, progesterone completely blocked estradiol’s beneficial effects on spatial memory (Harburger et al., 2007), suggesting that different dose combinations of estradiol plus progesterone may have discrepant effects on memory. Thus, determining an effective range of hormone treatments is important to establishing cognitively effective doses for clinical hormone therapies.
Identifying molecular mechanisms underlying the mnemonic benefits of effective estradiol plus progesterone treatments is also critical to the development of safe and effective hormone therapies (Frick, 2009). One important signaling pathway linked to learning and memory is the ERK/MAPK pathway (for review see (Sweatt, 2001)). ERK is activated (i.e., phosphorylated) in the hippocampus after training in hippocampal-dependent tasks (Atkins et al., 1998, Blum et al., 1999, Cammarota et al., 2000) and is necessary for long-term memory formation (Walz et al., 1999, Schafe et al., 2000, Kelly et al., 2003). Estradiol enhances ERK activation in hippocampal neurons (Kuroki et al., 2000, Nilsen and Brinton, 2002, Wade and Dorsa, 2003), and our laboratory has shown that dorsal hippocampal ERK activation is necessary for estradiol to enhance object memory consolidation in young ovariectomized mice (Fernandez et al., 2008). Although, progesterone, with and without estradiol, has been shown to activate hippocampal ERK in rats in vitro (Nilsen and Brinton, 2002, Nilsen and Brinton, 2003), and progesterone alone also increases hippocampal ERK in rats in vivo (Guerra-Araiza et al., 2008), no study has examined the effects of combined estradiol plus progesterone treatment on hippocampal ERK activation in vivo.
Therefore, the present study investigated the effects of post-training i.p. injections of cyclodextrin encapsulated estradiol and progesterone on non-spatial object memory consolidation in ovariectomized mice. Following the completion of behavioral testing, dorsal hippocampal ERK activation was examined 1 hr after i.p. hormone injections. Object memory was assessed using a version of the object recognition task in which total exploration time is fixed, and in which hippocampal involvement has been demonstrated (Clark et al., 2000, Baker and Kim, 2002, Fernandez et al., 2008). We have previously shown in young ovariectomized mice that 0.2 mg/kg estradiol, 10 or 20 mg/kg progesterone, but not 5 mg/kg progesterone, enhance object recognition in this task (Gresack and Frick, 2004, Gresack and Frick, 2006, Fernandez et al., 2008, Harburger et al., 2008). Therefore, mice in the present study were treated with an effective dose of estradiol (0.2 mg/kg) combined with progesterone doses of varying effectiveness (5, 10, or 20 mg/kg). Although they effectively enhance memory on their own, it was hypothesized that the 10 or 20 mg/kg doses of progesterone might reduce the beneficial effects of estradiol on memory, given that 20 mg/kg progesterone previously reduced the beneficial effects of 0.2 mg/kg estradiol on spatial memory consolidation in aged ovariectomized mice (Harburger et al., 2007).
Materials and Methods
Subjects
Subjects were 67 C57BL/6 female mice ovariectomized by Taconic (Germantown, NY) at 8 weeks and shipped to Yale at 9 weeks of age. Mice were housed up to 5 per shoebox cage in a room with a 12:12 light/dark cycle (lights on at 07:00). All behavioral testing took place during the light phase. Mice had ad libitum access to food and water in their cages. Upon arrival, mice were handled 5 times for 5 min to habituate them to being picked up by the experimenter. All procedures were approved by the Institutional Animal Care and Use Committee of Yale University, and conformed to the guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Hormone treatment
Mice were randomly assigned to treatment groups that would receive vehicle, 0.2 mg/kg 17β-estradiol (E2), or 0.2 mg/kg E2 combined with 5, 10, or 20 mg/kg progesterone (P). These treatments will hereafter be referred to as follows: vehicle (n = 15); 0.2 E2 (n = 13), 0.2 E2 + 5 P (n = 13), 0.2 E2 + 10 P (n = 14), and 0.2 E2 + 20 P (n = 12). Vehicle-treated mice received acute intraperitoneal (i.p.) injections of 2-hydroxypropyl-β-cyclodextrin (HBC) dissolved in physiological saline (Sigma, St. Louis, MO). Hormone-treated mice received injections of an estradiol-HBC inclusion complex and a progesterone-HBC inclusion complex (Sigma, St. Louis, MO), both dissolved in physiological saline. HBC enhances the solubility for hydrophobic steroid hormones and does not alter the bioefficacy of the hormones (Pitha, 1985).
Object recognition task
The object recognition task tested non-spatial object memory and was conducted as previously described (Frick and Gresack, 2003, Harburger et al., 2008). Mice were habituated in an empty white open field box for 5 min during which no data were collected. Twenty-four hours later, mice were rehabituated in the empty open field for 1 min. They were then placed in a holding cage while two identical objects were placed in the northeast and northwest corners of the box, approximately 5 cm from the wall. Mice were then returned to the box and allowed to freely explore the two identical objects until they accumulated 30 s of time exploring the objects, after which they were removed from the box, immediately injected, and then returned to their homecage.
Retention was tested 48 hrs later. Mice were placed in the open field with one of the same objects presented on the first day (familiar object) and a new (novel) object and were allowed to accumulate 30 s exploring the objects. Object exploration was recorded when the front paws or nose were in direct contact with the object. Because mice have a natural affinity for novelty, an increase in the time spent exploring the novel object relative to chance (15 s) demonstrates memory for the familiar object (Frick and Gresack, 2003). The location of the novel object was counterbalanced across mice in each group. Time spent exploring the objects (s) and elapsed time to accumulate 30 sec of exploration were recorded during testing using a custom-written computer program.
Western blotting
Two weeks after the completion of behavioral testing, mice were injected with hormone or vehicle and decapitated 1 hr later. ERK activation was examined 1 hr after injection because we previously reported that i.p. injections of 0.2 mg/kg estradiol increase phosphorylation of the p42, but not the p44, isoform of ERK in the dorsal hippocampus 1 hr after injection (Fernandez et al., 2008), and because the effects of estradiol and progesterone on ERK in vitro have also been demonstrated at the same time point (Nilsen and Brinton, 2002, Nilsen and Brinton, 2003). Immediately after decapitation, the dorsal hippocampus was dissected bilaterally on ice. Tissue samples were homogenized with a probe sonicator in 1:50 w/v lysis buffer. Western blotting was conducted as previously reported (Fernandez et al., 2008, Lewis et al., 2008a). Because all samples could not be run on the same blot, vehicle control samples were run twice: once with the 0.2 mg/kg E2 group and again with the 0.2 E2 + 5 P, 0.2 E2 + 10 P, and 0.2 E2 + 20 P groups. Hormone samples were statistically compared only to vehicle samples run on their blot. Blots were incubated overnight with either anti-phospho-p44/42 MAPK antibody (Thr202/Tyr204) (1:1000; Cell Signaling Technology; Danvers, MA, USA) or anti-total p44/42 MAPK antibody (1:2000; Cell Signaling Technology). A Kodak Image Station 440CF was used to detect the signal and Kodak 1D 3.6 software was used to conduct densitometry.
Data analysis
For the object recognition task, separate one-sample t-tests were performed to determine if the time spent with each object differed from chance (15 s) (SPSS, SPSS Inc., Chicago, IL). These analyses were used because the time spent with each object to accumulate 30 s is not independent (time exploring one object necessarily reduces exploration time of the other) (Frick and Gresack, 2003). Elapsed time to accumulate 30 s was analyzed using one-way ANOVAs with Treatment as the independent variable and Elapsed Time as the dependent variable. Fisher’s Protected Least Significant Difference (PLSD) post-hocs were performed on all significant main effects of Treatment. An alpha level of 0.05 was used to reject the null hypothesis.
Phosphorylated p42 and p44 ERK levels were normalized to total p42 and p44 ERK levels and then the percent change from vehicle controls was calculated. These values were analyzed using separate independent samples t-tests to compare each treatment group to the vehicle controls run on their blot. As an additional control, independent samples t-tests were also used to compare total p42/p44 ERK levels of each hormone treatment group to that of vehicle controls run on their blot. An alpha level of 0.05 was used to reject the null hypothesis.
Results
Object recognition
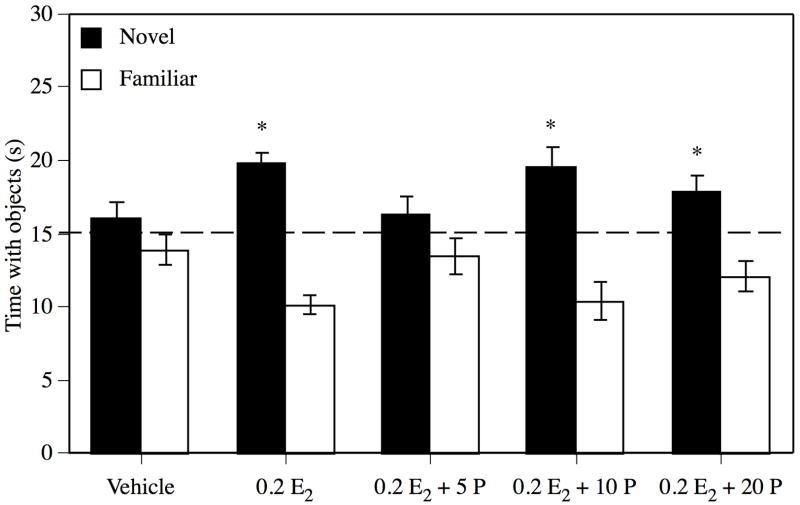
Mice in the 0.2 E2, 0.2 E2 + 10 P, or 0.2 E2 + 20 P groups demonstrated intact memory for the familiar object 48 hrs after training (see Figure 1). These groups spent significantly more time than the chance value of 15 s with the novel object (0.2 E2, t(12) = 7.30, P = 0.0001, 0.2 E2 + 10 P, t(13) = 3.47, P = 0.004, 0.2 E2 + 20 P, t(11) = 2.81, P = 0.02). However, groups treated with vehicle or 0.2 E2 + 5 P spent no more time than chance with the novel object (vehicle, t(14) = 1.10, P = 0.29; 0.2 E2 + 5 P, t(12) = 1.18, P= 0.26), indicating that these groups did not remember the familiar object. The main effect of Treatment for elapsed time was not significant (F(4, 62) = 1.28, P = 0.29). Mean (± standard error of the mean; S.E.M.) elapsed times (s) for each group were as follows: vehicle = 291.6 ± 42.9, 0.2 E2 = 353.1 ± 92.3, 0.2 E2 + 5 P = 260.9 ± 35.8, 0.2 E2 + 10 P = 231.5 ± 22.2, and 0.2 E2 + 20 P = 205.3 ± 18.3.
Figure 1.
Forty-eight hrs after training, mice treated with 0.2 E2, 0.2 E2 + 10 P, and 0.2 E2 + 20 P demonstrated memory for the familiar object by spending significantly more time with the novel object relative to chance (dashed line at 15 s; * p < 0.05), whereas vehicle-and 0.2 E2 + 5 P-treated mice did not. Each bar represents the mean (± S.E.M.) time spent with the novel or familiar object 48 hrs after training.
Western blotting
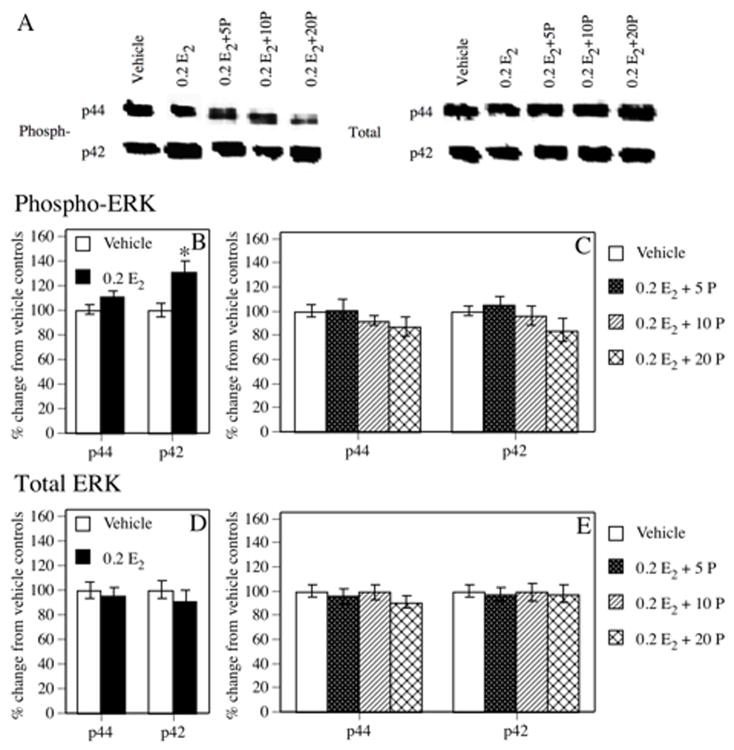
As expected, a single i.p. injection of 0.2 mg/kg E2 significantly increased phospho-p42 ERK protein levels in the dorsal hippocampus relative to vehicle controls, see Figure 2 (t(22) = −3.43, P = 0.002). Although 0.2 E2 also increased dorsal hippocampal phospho-p44 ERK levels, this effect was not significant (t(22) = −1.87, P = 0.07). In contrast, no dose of progesterone combined with 0.2 E2 significantly increased phospho-p42 ERK levels (0.2 E2 + 5 P mg/kg, t(23) = −0.71, P = 0.49; 0.2 E2 + 10 P, t(24) = 0.42, P = 0.68; 0.2 E2 + 20 P, t(22) = 1.46, P = 0.16), or phospho-p44 ERK levels (0.2 E2 + 5 P mg/kg, t(23) = −0.09, P = 0.93; 0.2 E2 + 10 P, t(24) = 1.16, P = 0.26; 0.2E2 + 20P, t(22) = 1.29, P = 0.21) in the dorsal hippocampus relative to vehicle controls. Hormone injections had no significant effect on total p42 ERK (0.2 E2, t(22) = 0.77, P = 0.45; 0.2 E2 + 5 P mg/kg, t(23) = 0.33, P = 0.75; 0.2 E2 + 10 P, t(24) = 0.06, P = 0.95; 0.2 E2 + 20 P, t(22) = 0.27, P = 0.79) or total p44 ERK (0.2 E2, t(22) = 0.46, P = 0.65; 0.2 E2 + 5 P mg/kg, t(23) = 0.47, P = 0.64; 0.2 E2 + 10 P, t(24) = 0.12, P = 0.91; 0.2 E2 + 20 P, t(22) = 1.23, P = 0.23) protein levels relative to vehicle controls.
Figure 2.
(A) Representative Western blot images illustrating phosphorylated and total p42 and p44 ERK protein levels in each group. (B) Densitometric analyses indicated that phospho-p42 ERK levels in the dorsal hippocampus were significantly increased 1 hr after a single i.p. injection of 0.2 E2 relative to vehicle controls (* P < 0.05). (C) In contrast, none of the groups treated with 0.2 E2 + progesterone exhibited increased phospho-p42 ERK levels. Phospho-p44 ERK protein levels were not significantly elevated by 0.2 E2 alone or any combination of 0.2 E2 + progesterone. (D and E) No treatment affected total p42 or p44 ERK protein immunoreactivity. Each bar in B–E represents the mean percent change from vehicle controls (± S.E.M.).
Discussion
Results from the present study demonstrate that the beneficial effects of estradiol and progesterone on memory consolidation are dependent on the dose of combined hormone treatment. Previous studies from our laboratory have reported that when estradiol or progesterone are administered alone, 0.2 mg/kg E2, 10 mg/kg P, and 20 mg/kg P enhance object recognition, whereas 5 mg/kg P has no effect on this type of memory (Gresack and Frick, 2004, Gresack and Frick, 2006, Gresack et al., 2007b, Gresack et al., 2007a, Fernandez et al., 2008, Harburger et al., 2008). In the present study, post-training treatment with either effective progesterone dose (10 or 20 mg/kg P) combined with an effective estradiol dose (0.2 mg/kg E2) significantly enhanced object recognition after a 48-hr delay. On the other hand, treatment with a sub-effective progesterone dose (5 mg/kg P) combined with an effective estradiol dose (0.2 mg/kg E2) had no effect on memory tested 48 hrs after training.
Together, the behavioral results from the present study and our recently published report illustrating that 10 or 20 mg/kg P alone enhance object recognition (Harburger et al., 2008) suggest that a progesterone dose that is beneficial on its own is necessary for estradiol plus progesterone treatment to enhance memory consolidation in young mice. This finding is consistent with prior studies in ovariectomized rats that reported beneficial effects on spatial and non-spatial object memory of post-training treatment with an effective estradiol dose combined with an effective progesterone dose (Walf et al., 2006, Frye et al., 2007). Interestingly, 5 mg/kg P did not improve memory when administered with estradiol, despite the fact that estradiol alone improves memory. Thus, 5 mg/kg P may not only be ineffective in enhancing memory (Harburger et al., 2008), but also may be detrimental to memory consolidation. Because this dose has no effect on object memory on its own (Harburger et al., 2008), the detrimental effects may only be observed when administered in combination with an effective dose of estradiol.
One possible confound of the design of the present study is that the injections themselves may have influenced performance due to factors such as increased circulating stress hormones (Belz et al., 2003). These effects could have been altered by estradiol or progesterone administration. However, we find it unlikely that the beneficial effects of estradiol or progesterone are due to an interaction with stress homornes. Previous work from our laboratory has demonstrated that estradiol administered to middle-aged female mice in the drinking water also enhances object recognition (Fernandez and Frick, 2004). In this study, all three doses of estradiol enhanced object recognition using the same protocol and retention delay used in the present study. Because these improvements were observed in the absence of injections, these data suggest that the beneficial effects of estradiol and progesterone in the present study were not related to effects of the injection procedure.
Interestingly, the present finding that the combination of beneficial estradiol and progesterone doses improves object memory in young ovariectomized mice differs from our recently published data in aged ovariectomized mice. We have previously found in aged ovariectomized mice that 5 and 10 mg/kg P, but not 20 mg/kg P, enhanced 48-hr object recognition (Lewis et al., 2008b). However, when the 10 and 20 mg/kg P doses were combined in aged females with 0.2 mg/kg estradiol and administered post-training after spatial Morris water maze training, both doses reduced the beneficial effects of estradiol, particularly 20 mg/kg P, which completely reversed the beneficial effects of estradiol (Harburger et al., 2007). Nevertheless, a similar experiment has not yet been conducted in aged females tested in object recognition, so mnemonic and non-mnemonic differences between the spatial Morris water maze task and the nonspatial object recognition task may account for our differing results. Alternatively, the fact that these dose combinations were not beneficial to one type of hippocampal-dependent memory in aged females suggests that the interactions between estradiol and progesterone in the hippocampus may be considerably different in young and aging females, perhaps due to the fact that estrogen receptor density decreases with age in the female hippocampus (Mehra et al., 2005, Yamaguchi-Shima and Yuri, 2007). It is unknown if hippocampal progesterone receptors are also decreased with age. In contrast to the effects of simultaneous post-training estradiol plus progesterone treatment on spatial memory in aged mice, a previous study demonstrated that long-term cyclic estradiol and progesterone treatment improves spatial memory in a delayed matching-to-position task in aged ovariectomized rats (Gibbs, 2000b). Therefore, estradiol and progesterone treatment may be more beneficial in young or aged females when administered cyclically rather than simultaneously. Indeed, the beneficial effects of estradiol and progesterone on certain measures of basal forebrain cholinergic function were greater when these hormones were administered using a cyclic regimen compared to a simultaneous silastic capsule regimen (Gibbs, 2000a). Future studies in aging females should examine multiple estradiol and progesterone dose combinations in different treatment regimens and on multiple memory tests to determine if any combination can reduce age-related memory decline.
Treatment with 0.2 mg/kg E2 alone significantly increased memory and phospho-p42 ERK levels in the dorsal hippocampus relative to vehicle controls. This finding is consistent with previous reports from other laboratories indicating that estradiol increases ERK activation in rat hippocampal neurons in vitro (Nilsen and Brinton, 2002, Nilsen and Brinton, 2003) and replicates previous work from our laboratory reporting that 0.2 mg/kg E2 increases phospho-p42 ERK levels in young ovariectomized mice (Fernandez et al., 2008, Lewis et al., 2008a). One prior study from our laboratory also showed that dorsal hippocampal infusions of the MEK inhibitor U0126 block the beneficial effects of estradiol on object recognition, demonstrating that ERK activation is necessary for estradiol to enhance object memory (Fernandez et al., 2008). Therefore, it is interestesting that estradiol plus progesterone treatments in the present study that enhanced object memory did not increase dorsal hippocampal ERK activation 1 hr after injection. Indeed, all doses of progesterone, whether behaviorally effective or not, appeared to block estradiol’s effects on ERK activation. One possible explanation for these results is that progesterone (more specifically, the progesterone metabolite 3α-5α-THP) activates GABA neurotransmission by binding to GABA-A receptors (Wilson, 1996). Because GABA decreases ERK phosphorylation in the hippocampus (Zheng et al., 2007), progesterone may decrease estradiol-enhanced ERK by acting as a GABA agonist. Progesterone may also reduce estradiol-enhanced ERK activation by binding to membrane-bound estrogen receptors (ERs) in addition to the intracellular progesterone receptors (PRs). Toran-Allerand and colleagues found that progesterone is capable of binding to ER-X, a putative membrane-bound ER that may mediate estradiol-enhanced ERK activation (Toran-Allerand et al., 2002). Therefore, if progesterone blocks estradiol-induced ERK activation by binding to ER-X, then increasing doses of progesterone should decrease the extent to which estradiol is capable of activating ERK. Indeed, Figure 2 suggests that increasing doses of progesterone gradually decrease estradiol-induced activation of both p44 and p42 phospho-ERK levels in the dorsal hippocampus. Finally, although we have interpreted the behavioral data to suggest that more time with the novel object indicates enhanced memory for the familiar object, an alternate hypothesis is that the stress from injections immediately after training produced a negative affect towards the familiar object which caused the mice to avoid that object during testing. This response would likely be mediated by the amygdala rather than the hippocampus, which could explain the lack of effect of progesterone on hippocampal ERK activation. However, we find this explanation unlikely, given that avoidance learning requires hippocampal ERK activation (Alonso et al., 2002). Thus, even if progesterone had enhanced object recognition through avoidance learning in the present study, this effect should still have increased hippocampal ERK activation.
The fact that all doses of progesterone reduced the beneficial effects of E2 on hippocampal ERK activation may suggest that the combination of estradiol plus progesterone enhances memory through a pathway other than ERK. However, progesterone alone has been shown to significantly increase hippocampal phospho-42 and -p44 ERK protein levels in young ovariectomized rats 24 hrs after a single i.p. injection (Guerra-Araiza et al., 2008). It is possible that the lack of an increase in ERK in the present study reflects a biphasic activation of ERK by progesterone, where the decrease observed 1 hour after injection takes place either before or after an increase at other time points. This possibility is suggested by the biphasic effect of combined estradiol plus progesterone treatment on CA1 dendritic spine density, such that a combined hormone treatment initially increases spine density, but then significantly decreases spine density (Woolley and McEwen, 1993). This intriguing possibility must be addressed with regard to ERK activation with additional time points both before and after the 1 hr point used in the present study. Another possible explanation for the results in the present study is that the hormones enhance memory by activating other signaling pathways, such as the phosphoinositide-3 kinase (PI3-K) pathway. Progesterone increases phosphorylation of Akt, a key effector of the PI3-K pathway, in cerebral cortical explants (Singh, 2001) and in the hippocampus of young ovariectomized rats (Guerra-Araiza et al., 2008). In addition, progesterone-induced neuroprotection against glutamate toxicity has been shown to be dependent on the PI3-K pathway (Kaur et al., 2007). As such, future investigations into the molecular mechanisms underlying the mnemonic effects of estradiol plus progesterone should include this pathway.
In conclusion, the present study is the first to report that the beneficial effects of combined estradiol and progesterone treatment are dependent on an effective dose of progesterone. Results from our previous report indicate that 10 and 20 mg/kg P, but not 5 mg/kg P, enhance memory consolidation in young ovariectomized mice (Harburger et al., 2008). This study extends these findings and demonstrates that E2 plus 10 or 20 mg/kg P, but not 5 mg/kg P, enhances memory consolidation in young ovariectomized mice. Results from the present study also suggest that activation of the ERK pathway may not be necessary for combined estradiol and progesterone to enhance memory consolidation in young ovariectomized mice. Future work in our laboratory will further investigate the role of this, and other, pathways to determine the underlying mechanism responsible for the beneficial effects of estradiol and progesterone on memory.
Acknowledgments
This work was sponsored by NIH grant RO1 AG022525 to KMF, the Samuel K. Bushnell Dissertation Fellowship to LLH from Yale Graduate School of Arts and Sciences, and Yale University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso M, Vianna MR, Depino AM, Mello e Souza T, Pereira P, Szapiro G, Viola H, Pitossi F, Izquierdo I, Medina JH. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12:551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn Mem. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz EE, Kennell JS, Czambel RK, Rubin RT, Rhodes ME. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav. 2003;76:481–486. doi: 10.1016/j.pbb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Nelson ME, Granholm AC. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15:2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3 alpha-hydroxy-5 alpha[beta]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Ardenghi P, Paratcha G, Levi de Stein M, Izquierdo I, Medina JH. Learning-associated activation of nuclear MAPK, CREB and Elk-1, along with Fos production, in the rat hippocampus after a one-trial avoidance learning: abolition by NMDA receptor blockade. Brain Res Mol Brain Res. 2000;76:36–46. doi: 10.1016/s0169-328x(99)00329-0. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Farr SA, Flood JF, Scherrer JF, Kaiser FE, Taylor GT, Morley JE. Effect of ovarian steroids on footshock avoidance learning and retention in female mice. Physiol Behav. 1995;58:715–723. doi: 10.1016/0031-9384(95)00124-2. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behav Neurosci. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000a;101:931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000b;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128:459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Kerr KM, Frick KM. Life-long environmental enrichment differentially affects the mnemonic response to estrogen in young, middle-aged, and aged female mice. Neurobiol Learn Mem. 2007a;88:393–408. doi: 10.1016/j.nlm.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Kerr KM, Frick KM. Short-term environmental enrichment decreases the mnemonic response to estrogen in young, but not aged, female mice. Brain Res. 2007b;1160:91–101. doi: 10.1016/j.brainres.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Amorim MA, Pinto-Almazan R, Gonzalez-Arenas A, Campos MG, Garcia-Segura LM. Regulation of the phosphoinositide-3 kinase and mitogen-activated protein kinase signaling pathways by progesterone and its reduced metabolites in the rat brain. J Neurosci Res. 2008 doi: 10.1002/jnr.21848. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28:602–610. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Pechenino AS, Saadi A, Frick KM. Post-training progesterone dose-dependently enhances object, but not spatial, memory consolidation. Behav Brain Res. 2008;194:174–180. doi: 10.1016/j.bbr.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85:2441–2449. doi: 10.1002/jnr.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;23:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur J Pharmacol. 2000;400:205–209. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008a;122:716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MC, Orr PT, Frick KM. Differential effects of acute progesterone administration on spatial and object memory in middle-aged and aged female C57BL/6 mice. Horm Behav. 2008b;54:455–462. doi: 10.1016/j.yhbeh.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100:10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr PT, Lewis MC, Frick KM. Dorsal hippocampal progesterone infusions enhance object recognition in young female mice. doi: 10.1016/j.pbb.2009.05.012. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitha J. Amorphous water-soluble derivatives of cyclodextrins: nontoxic dissolution enhancing excipients. J Pharm Sci. 1985;74:987–990. doi: 10.1002/jps.2600740916. [DOI] [PubMed] [Google Scholar]
- Pitha J, Harman SM, Michel ME. Hydrophilic cyclodextrin derivatives enable effective oral administration of steroidal hormones. J Pharm Sci. 1986;75:165–167. doi: 10.1002/jps.2600750213. [DOI] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Ramsden M, Pike CJ. Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res. 2006;1099:206–210. doi: 10.1016/j.brainres.2006.03.127. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14:407–415. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz R, Roesler R, Barros DM, de Souza MM, Rodrigues C, Sant’Anna MK, Quevedo J, Choi HK, Neto WP, DeDavid e Silva TL, Medina JH, Izquierdo I. Effects of post-training infusions of a mitogen-activated protein kinase kinase inhibitor into the hippocampus or entorhinal cortex on short- and long-term retention of inhibitory avoidance. Behav Pharmacol. 1999;10:723–730. doi: 10.1097/00008877-199912000-00003. [DOI] [PubMed] [Google Scholar]
- Wilson MA. GABA physiology: modulation by benzodiazepines and hormones. Crit Rev Neurobiol. 1996;10:1–37. doi: 10.1615/critrevneurobiol.v10.i1.10. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shima N, Yuri K. Age-related changes in the expression of ER-beta mRNA in the female rat brain. Brain Res. 2007;1155:34–41. doi: 10.1016/j.brainres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Zheng G, Zhang X, Chen Y, Zhang Y, Luo W, Chen J. Evidence for a role of GABAA receptor in the acute restraint stress-induced enhancement of spatial memory. Brain Res. 2007;1181:61–73. doi: 10.1016/j.brainres.2007.08.077. [DOI] [PubMed] [Google Scholar]