Abstract
l-Methionine (Met) is hepatotoxic at high concentrations. Because Met toxicity in freshly isolated mouse hepatocytes is gender-dependent, the goal of this study was to assess the roles of Met accumulation and metabolism in the increased sensitivity of male hepatocytes to Met toxicity compared with female hepatocytes. Male hepatocytes incubated with Met (30 mM) at 37°C exhibited higher levels of intracellular Met at 0.5, 1.0, and 1.5 h, respectively, compared to female hepatocytes. Conversely, female hepatocytes had higher levels of S-adenosyl-l-methionine compared to male hepatocytes. Female hepatocytes also exhibited higher l-methionine-l-sulfoxide levels relative to control hepatocytes, whereas the increases in l-methionine-d-sulfoxide (Met-d-O) levels were similar in hepatocytes of both genders. Addition of aminooxyacetic acid (AOAA), an inhibitor of Met transamination, significantly increased Met levels at 1.5 h and increased Met-d-O levels at 1.0 and 1.5 h only in Met-exposed male hepatocytes. No gender differences in cytosolic Met transamination activity by glutamine transaminase K were detected. However, female mouse liver cytosol exhibited higher methionine-dl-sulfoxide (MetO) reductase activity than male mouse liver cytosol at low (0.25 and 0.5 mM) MetO concentrations. Collectively, these results suggest that increased cellular Met accumulation, decreased Met transmethylation, and increased Met and MetO transamination in male mouse hepatocytes may be contributing to the higher sensitivity of the male mouse hepatocytes to Met toxicity in comparison with female mouse hepatocytes.
Keywords: Methionine, S-adenosylmethionine, sulfoxidation, transamination, transmethylation, methionine sulfoxide reduction
Introduction
l-Methionine (Met), while an essential amino acid, is hepatotoxic when present at high concentrations. Met toxicity has been linked to total parenteral nutrition-associated cholestasis in infants [1] and may exacerbate hepatocellular necrosis and fibrogenesis in patients with chronic liver disease who often develop hypermethionemia [2]. Laboratory animals dosed with or fed high levels of Met may develop cholestatic liver disease [1,3], hepatocellular ATP and GSH depletion [3-5], and exhibit increased markers of lipid peroxidation [6]. In mice, Met adenosyltransferase (MAT) 1A knockout strains are hypermethionemic, have reduced GSH levels and altered gene expression, and are more prone to oxidative stress in the liver than wild type mice [7,8]. The mechanisms responsible for Met-induced hepatotoxicity, however, are not clear.
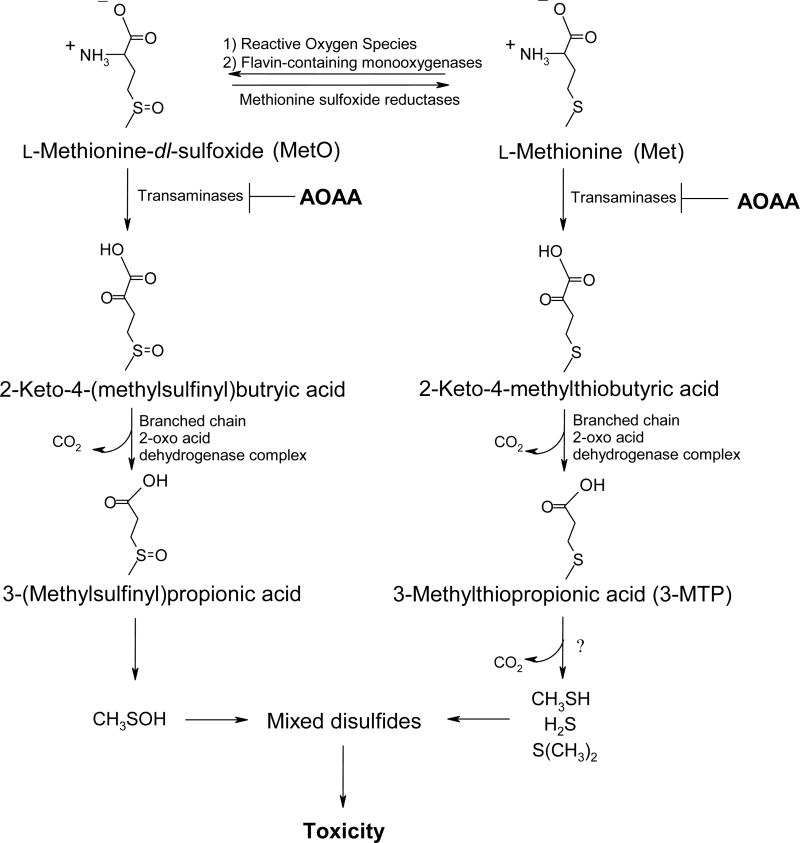
Our laboratory recently used freshly isolated mouse hepatocytes (FIMHs) to investigate Met toxicity. The results obtained regarding Met cytotoxicity in this model are summarized in Table 1. In male mouse hepatocytes, increased lactate dehydrogenase (LDH) leakage and decreased trypan blue (TB) exclusion, preceded by GSH depletion, were detected at Met concentrations ≥ 20 mM [9]. In contrast, female hepatocytes were completely insensitive to Met toxicity at Met concentrations as high as 30 mM and had increased cellular GSH levels compared to female hepatocytes incubated with vehicle alone. Addition of 3-deazaadenosine (3-DA), an inhibitor of S-adenosyl-l-homocysteine (SAH) hydrolase and the Met transmethylation (TM) pathway [10] (Figure 1) potentiated Met toxicity in male FIMHs while addition of aminooxyacetic acid (AOAA), an inhibitor of Met transamination (TA) [11] partially reduced Met toxicity (Figure 2) [9]. The effect of AOAA and 3-DA were not investigated in female FIMHs due to the lack of toxicity of Met in that gender. These results and the finding that 3-methylthiopropionic acid (3-MTP) was nearly 100-fold more cytotoxic than Met in male FIMHs [9] provided evidence for the involvement of Met TA metabolites in the cytotoxicity of Met. However, the biochemical basis for female FIMHs being less sensitive to Met toxicity was unclear. Since GSH depletion was detected in female FIMHs exposed to 0.3 mM 3-MTP, but not 30 mM Met, lack of 3-MTP formation rather than resistance to 3-MTP toxicity may have been a factor. Thus, events upstream of 3-MTP formation may have been contributing to the lower sensitivity of female FIMHs to Met toxicity in comparison with male mouse hepatocytes.
Table 1. Time Course of the effect of Met (30 mM) with or without AOAA (0.2 mM) on the viability of male or female mouse FIMHs as measured by LDH leakage. These data are adapted from a previous publication (9).
| Gender | % Viability | |||
|---|---|---|---|---|
| 2 h | 3 h | 4 h | 5 h | |
| Female | ||||
| Vehicle alone | 79±7a | 68±13 | 67±5 | 61±1 |
| Met | 78±2 | 69±3 | 65±8 | 65±8 |
|
| ||||
| Male | ||||
| Vehicle alone | 83±4 | 75±9 | 70±11 | 60±14 |
| Met | 66±11b | 45±14b | 26±19b | 20±12b |
| Met + AOAA | 74±8 | 67±8c | 50±22 | 40±17 |
Data are expressed as mean ± SD (n = 3-4).
Values are significantly lower than the corresponding values obtained in FIMHs of the same gender exposed to vehicle alone (p<0.05).
Values are significantly higher than the corresponding values obtained in FIMHs of the same gender incubated with Met only (p<0.05).
Figure 1.
Schematic of the Met transmethylation pathway, a proposed detoxification pathway for excess Met in FIMHs.
Figure 2.
Schematic of the Met transamination and sulfoxidation pathways, proposed bioactivation pathways for excess Met in FIMHs.
Met sulfoxidation (SO) was also a significant metabolic pathway in Met-dosed male and female mice with methionine-d-sulfoxide (Met-d-O) being the primary diastereomer detected in both genders [12]. Methionine-dl-sulfoxide (MetO) toxicity in male FIMHs was characterized by increased LDH leakage, decreased TB exclusion, and GSH depletion at MetO concentrations ≥ 20 mM whereas female FIMHs were completely resistant to MetO toxicity. Because MetO toxicity in male FIMHs was also inhibited by AOAA [13], these results suggested a potential role for MetO TA metabolites in the cytotoxicity of MetO and implicated the Met SO pathway in the gender-dependent toxicity of Met in FIMHs (Figure 2).
In summary, the mechanisms leading to gender differences in Met toxicity in FIMHs are not clear, but could be due to gender differences in Met accumulation and metabolism. In the present study, levels of Met, SAM, Met-d-O and methionine-l-sulfoxide (Met-l-O) were determined in male and female FIMHs exposed to vehicle only, 30 mM Met only, or 30 mM Met and 0.2 mM AOAA, for 0-1.5 h at 37°C. The Met concentration used (30 mM) caused toxicity in male, but not female FIMHs (Table 1). The AOAA concentration used (0.2 mM) reduced Met toxicity in male FIMHs (Table 1). The time course (0-1.5 h) for analysis of Met and its metabolites was selected because it preceded Met-induced GSH depletion and cytotoxicity in male FIMHs [9]. Met (30 mM) transamination activity due to glutamine transaminase K (GTK) and reduction of MetO (0-5 mM) to Met were also measured in male and female mouse liver cytosol to clarify the role of these pathways in Met metabolism and toxicity in FIMHs.
Methods and Materials
Reagents
Trypsin inhibitor (type II-O), collagenase (Type IV), Met, l-methionine-dl-sulfoxide (MetO), SAM, AOAA, dithiothreitol (DTT), and 2,4-dinitro-1-fluorobenzene, were obtained from Sigma Chemical Co. (St. Louis, MO). 1-Fluoro-2-4-dinitrophenyl-5-L-alanine amide (Marfey's reagent) was obtained from Pierce Chemical Co. Inc. (Rockford, IL). Hank's balanced salt solution was obtained from Gibco (Grand Island, NY). Dulbecco's modified Eagle's Medium (DMEM) (1×) with 4500 mg/L glucose, and sodium pyruvate but without L-glutamine, Met, and cystine was purchased from HyClone (Logan, UT). All other chemicals and reagents were of the highest quality commercially available.
Isolation and incubation of hepatocytes
Male and female B6C3F1 mice (7-11 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME). Hepatocytes were isolated using the two-step EDTA / collagenase perfusion method and incubated as described previously [9,13]. For all experiments, hepatocyte samples had an initial viability of >85%. All reported data represents the average values obtained from hepatocytes of 3-4 separate animals.
Analysis of intracellular Met-d-O, Met-l-O, and Met
Samples were collected to measure intracellular levels of Met-d-O, Met-l-O and Met via derivatization with 1-fluoro-2-4-dinitrophenyl-5-l-alanine amide as described previously [13,14].
Analysis of intracellular SAM
Cell samples (2.63 mL each) were centrifuged at 50 g for 2 min to gently pellet the cells. The supernatant was removed and the cells washed with 10 mL of ice-cold phosphate buffered saline (137 mM NaCl, 10 mM phosphate, 2.7 mM KCl, pH=7.4) three times. Cell samples were deproteinized by addition of 0.8 mL of ice-cold ethanol and centrifuged at 3000 rpm for 10 min. The supernatant was placed in a separate tube and dried via nitrogen stream. The dried residue was then redissolved in 200 μL deionized water and filtered with an Acrodisc LC-13 membrane filter (Pall Gelman Sciences, Ann Arbor, MI). Samples were analyzed by HPLC at 254 nm described by Wise and colleagues [15]. The mobile phase on pump A consisted of 50 mM NaH2PO4 and 10 mM heptanesulfonic acid with the pH adjusted to 3 with phosphoric acid. Pump B contained 100% of methanol. The flow rate was 1 mL/min. SAM was eluted using an isocratic method of 33% pump B. The typical retention time for SAM was 5.5 min. Quantification of SAM was accomplished using a SAM standard curve of which the limits of quantitation were 0.1 nmol SAM / 106 cells.
Analysis of SAM, Met-d-O, and Met-l-O in medium
To analyze for SAM, Met-d-O and Met-l-O levels in the medium (2.63 mL) of cell samples, medium samples (520 μL) were filtered using an Acrodisc LC-13 membrane filter and fraction collected by HPLC as previously described [12]. SAM, Met, and MetO fractions were taken to dryness, redissolved in 100 μL H2O, and analyzed for SAM, Met, or MetO as described above. The limits of quantitation for SAM, Met-d-O, and Met-l-O in medium were 1 nmol / mL medium for each metabolite.
Analysis of Met transamination activity
Met transamination activity by GTK in mouse liver cytosol was analyzed using a method adapted from Cooper and Pinto [16] as previously described [13]. All reaction mixtures contained PhP (0.6 mM), buffer or Met (30 mM), buffer or AOAA (0.2 mM), and cytosol (0.4 mg protein).
Analysis of MetO reduction activity
MetO reduction to Met in mouse liver cytosol was quantitated using a method adapted from Moskovitz et al. [17]. Briefly, 1 mL of cytosol was dialyzed in buffer (0.1 M KH2PO4, 0.1 M KCl, 5 mM EDTA, pH=7.4). The protein concentration of the dialyzed cytosol was measured as previously described [18]. In an eppendorf tube, 50 μL aliquots of buffer, 80 mM DTT, and 50 μL cytosol (0.8 mg protein) were combined and preincubated for 4 min at 37°C. The assay was started by addition of 50 μL MetO solution (final concentration of 0-5 mM) and incubated for 0 or 60 min. Samples were then placed on ice. Excess DTT was removed from each sample by three extractions with ethyl acetate (500 μL). Following removal of the final organic layer, 200 μL of ice cold ethanol was added. The deproteinized samples were centrifuged at 10000 g for 5 min and the supernatants (400 μL) placed in a separate tube to which 10 μL of 2,4-dinitro-1-fluorobenzene (10%, v/v, in ethanol) and 6 μL of 1 M NaHCO3 were added. The samples were allowed to derivatize overnight and were then analyzed by HPLC at 360 nm as previously described [19] with slight modifications to the gradient. Using a flow a rate of 1 mL / min, mobile phase (A = 1% acetonitrile, 0.1% trifluoroacetic acid, pH 4.5 with sodium hydroxide; B = same as A except with 75% acetonitrile) was run at 30% B from 0 to 3 min and then increased to 75% B over 6 min where it was held for 3 min. The gradient was then reduced back to 30% B over 4 min and held for an additional 4 min. The typical retention time for Met was 9.2 min. The Met peak was quantitated using a standard curve with the limits of quantitation being 1.3 nmol. Total Met formation in each cytosolic sample was calculated by subtracting Met levels at 0 min from Met levels at 60 min.
Statistics
Metabolite areas under the curve (AUC) were calculated by trapezoidal approximation using the AREA transform of the SigmaPlot software package (SPSS Inc., Chicago, IL). Statistical analyses were carried out using the SigmaStat software program (SPSS Inc., Chicago, IL). Comparisons of means were done by paired or unpaired t-test where indicated.
Results
Analysis of intracellular levels of Met and its major metabolites (SAM, Met-d-O, and Met-l-O) at 0, 0.5, 1, and 1.5 h in FIMHs incubated with 30 mM Met, 30 mM Met and 0.2 mM AOAA, or vehicle only (Figures 3-5) allowed for calculation of areas under the curves for the concentrations of Met and its major metabolites (AUC0-1.5 h) (Table 2).
Figure 3.
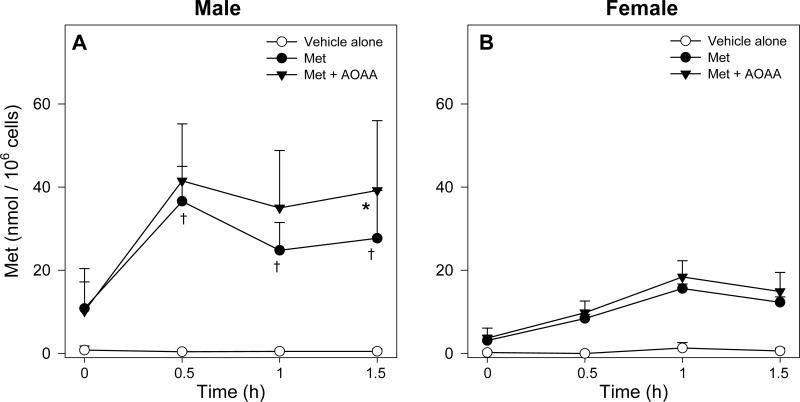
Time course of intracellular Met levels in male (A) and female (B) FIMHs incubated with vehicle only, 30 mM Met only, or 30 mM Met and 0.2 mM AOAA. The symbol * indicates values that are significantly higher than the corresponding values obtained in FIMHs incubated with Met (p<0.05). The symbol † indicates values that are significantly higher than the corresponding values obtained in FIMHs of the opposite gender (p<0.05). Data are expressed as mean ± SD and are from 3-4 separate experiments.
Figure 5.
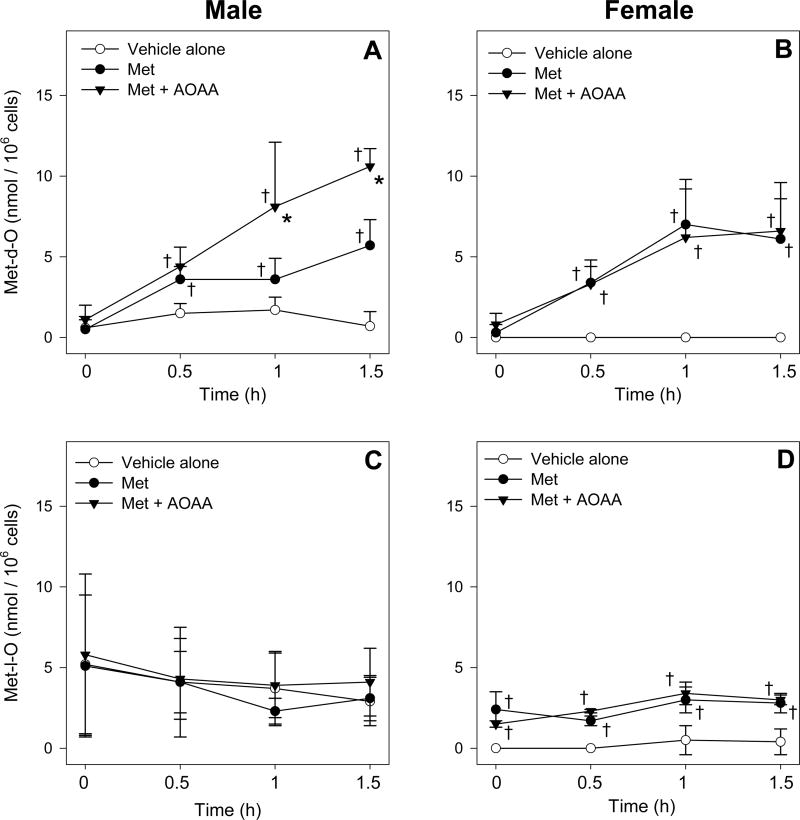
Time course of intracellular Met-d-O (A, B) and Met-l-O (C, D) levels in male and female FIMHs incubated with vehicle only, 30 mM Met only, or 30 mM Met and 0.2 mM AOAA. The symbol * indicates values that are significantly higher than the corresponding values obtained in FIMHs incubated with Met (p<0.05). The symbol ‡ indicates values that are significantly higher than the corresponding values obtained in FIMHs incubated with vehicle alone. Data are expressed as mean ± SD and are from 3-4 separate experiments.
Table 2. Area under the curve (AUC) analysis for intracellular Met and its metabolites (SAM, Met-d-O, and Met-l-O) in male or female FIMHs incubated with 30 mM Met for 1.5 h with or without 0.2 mM AOAA.
| Treatment | AUC0-1.5h (nmol · h / 106 cells)a | |||
|---|---|---|---|---|
| Met | SAM | Met-d-O | Met-l-O | |
| Met | ||||
| Male | 40.3±11.4b | 2.9±1.1 | 5.2±1.2 | 5.2±2.7 |
| Female | 15.8±1.4 | 7.4±3.3c | 6.8±0.8 | 3.7±0.2 |
|
| ||||
| Met + AOAA | ||||
| Male | 50.6±11.3b | 3.1±1.4 | 9.2±2.6c | 6.6±4.0 |
| Female | 18.7±4.4 | 8.0±4.8 | 6.6±2.1 | 4.0±0.4 |
Data are expressed as mean ± SD (n = 3-4).
Values are significantly higher than the corresponding values obtained in FIMHs of the opposite gender (p<0.05).
Values are significantly higher than the corresponding values obtained in FIMHs of the same gender incubated with Met only (p<0.05).
The Met concentrations in Met-treated male and female hepatocytes were much higher than the corresponding values in control hepatocytes (Figure 3). Male FIMHs had significantly higher intracellular Met levels at 0.5, 1, and 1.5 h that were approximately 4-, 1.5-, and 2-fold higher, respectively, than the corresponding values obtained with female FIMHs (Figure 3). Overall Met concentrations in male FIMHs incubated with Met were approximately 2.5-fold higher than Met concentrations in female FIMHs as indicated by the Met AUC0-1.5 h (Table 2). Less than 1% of the Met present in the medium could be accounted for as intracellular Met and its metabolites.
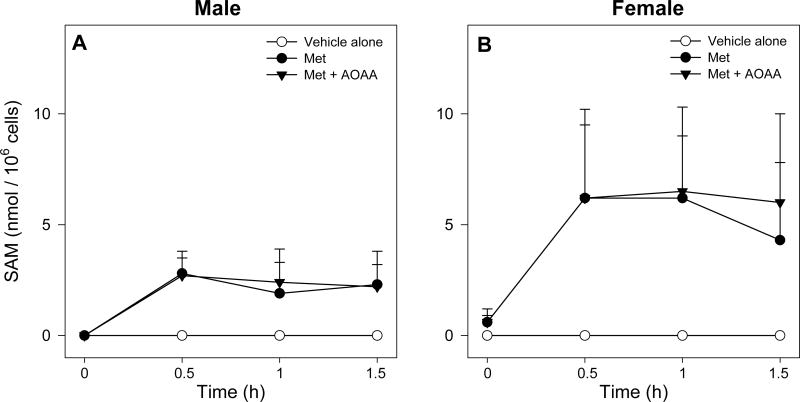
Intracellular SAM levels, while undetectable in control FIMHs, were higher at all time points in Met-dosed FIMHs of both genders (Figure 4) and corresponded to a nearly 2.5-fold higher SAM AUC0-1.5 h in female FIMHs compared to male FIMHs (Table 2). Trace amounts of SAM were detected in the medium of some female, but not male, FIMH samples after Met exposure. However, medium SAM levels were less than the limits of quantitation of the detection method (data not shown).
Figure 4.
Time course of intracellular SAM levels in male (A) and female (B) FIMHs incubated with vehicle only, 30 mM only, or 30 mM Met and 0.2 mM AOAA. Data are expressed as mean ± SD and are from 3-4 separate experiments.
Intracellular Met-d-O levels in Met-exposed male and female FIMHs at 0.5, 1, and 1.5 h were higher than the corresponding control values and rose to similar levels by 1.5 h (Figure 5) resulting in a similar Met-d-O AUC0-1.5 h for both genders (Table 2). Small, but significant, increases in Met-l-O levels were detected at all time points in Met-exposed female FIMHs only (Figure 5), however, Met-l-O values in vehicle-treated female FIMHs were lower than those in vehicle-treated male FIMHs. Trace amounts of Met-d-O and Met-l-O were detected in the medium in some experiments with Met-exposed FIMHs, however, these levels were less than the limits of quantitation of the method.
Addition of AOAA to Met-exposed FIMHs significantly increased the cellular Met levels at 1.5 h (Figure 3), but not the Met AUC0-1.5h (Table 2), in comparison with the corresponding values obtained from male FIMHs incubated with Met only. The AOAA treatment had no effect on detected Met levels in female FIMHs (Figure 3). Whereas addition of AOAA did not alter intracellular SAM levels in Met-exposed FIMHs of either gender (Figure 4), the AOAA treatment nearly doubled Met-d-O levels at 1 h and 1.5 h in Met-exposed male FIMHs (Figure 5) and resulted in a higher Met-d-O AUC0-1.5 h (Table 2) compared to male FIMHs exposed to Met only. AOAA had no effect on Met-d-O levels in Met-dosed female FIMHs, and it did not alter the intracellular Met-l-O levels in FIMHs of either gender (Figure 5).
No increases in Met or its metabolites were detected in control female hepatocytes exposed to AOAA alone (data not shown). This result was unexpected since addition of AOAA to control male hepatocytes previously resulted in significant increases in intracellular Met-d-O levels [13].
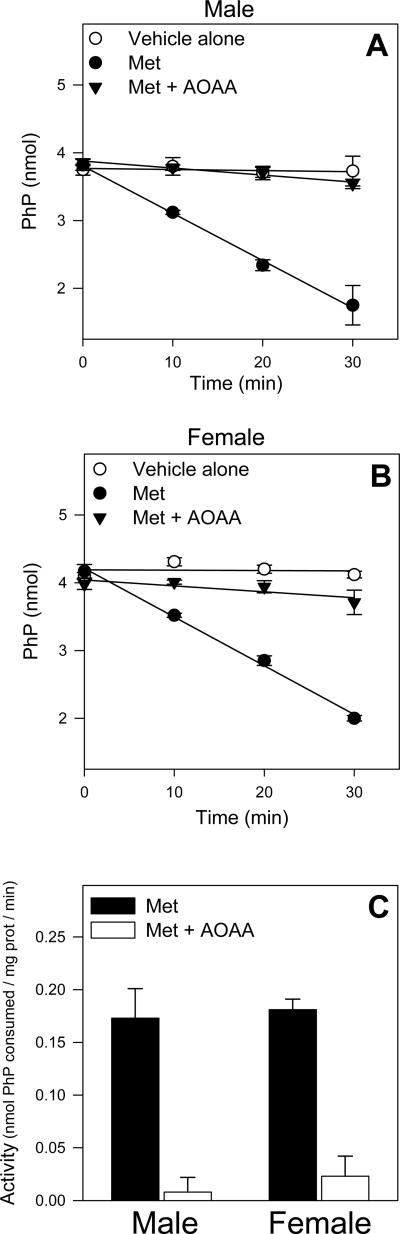
Met TA activity by GTK was measured in male and female mouse liver cytosol as a function of the depletion of PhP, an amino acceptor substrate for GTK. In cytosol of both genders incubated with Met (30 mM), similar linear depletions of PhP from 0-30 min were detected (Figures 6A and 6B) suggesting similar specific activities for PhP depletion due to Met TA (Figure 6C). No significant PhP depletion was detected in male or female cytosol incubated without Met. With cytosol of both genders, addition of AOAA resulted in nearly complete inhibition of Met-induced PhP depletion.
Figure 6.
Time course of PhP (0.6 mM) depletion in male or female cytosol (A, B) after incubations at 37°C with vehicle only, 30 mM Met only, or 30 mM Met and 0.2 mM AOAA. These time course data were then used to calculate specific activity of Met-induced PhP depletion in each gender (C). Data are expressed as mean ± SD and are from 3-4 separate experiments.
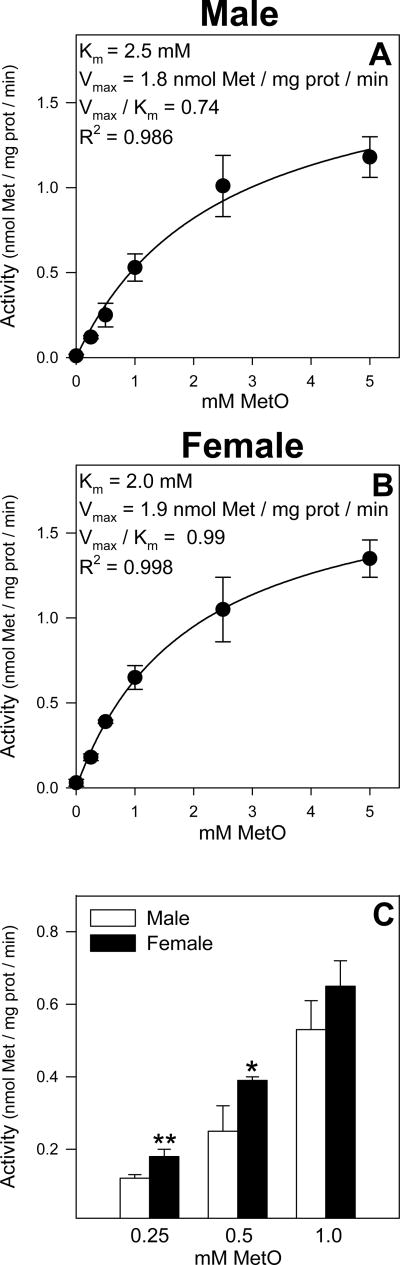
Kinetic constants for MetO reduction to Met were determined in male and female mouse liver cytosol incubated with MetO (0-5 mM) by plotting substrate concentration versus velocity and then using nonlinear regression to solve for the Michaelis-Menten equations (Figures 7A and 7B). Similar Vmax values for MetO reduction to Met (1.8 and 1.9 nmol Met / mg prot / min) were detected with the cytosol of both genders, however, female mouse liver cytosol had a slightly lower Km value (2.0 mM) compared with male mouse liver cytosol (2.5 mM). Consistent with these results, MetO reduction in female mouse liver cytosol incubated with 0.25 and 0.5 mM MetO was approximately 1.5-fold higher than the corresponding activity detected in male mouse liver cytosol (Figure 7C); no gender differences in MetO reduction were detected in cytosol incubated with 1, 2.5, or 5 mM Met (Figures 7A and 7B).
Figure 7.
MetO reduction kinetics in male (A) and female (B) mouse liver cytosol. Met formation was measured after incubating cytosol with MetO (0-5 mM) for 0 or 60 min at 37°C. Data are plotted using nonlinear regression for a one-enzyme system which gave higher R2 values with both genders than plots for a two-enzyme system. These data also allowed for a gender comparison of the MetO reduction activity at individual MetO concentrations in cytosol (C). The symbols * and ** indicate values that are significantly higher than the corresponding values obtained in cytosol of the opposite gender incubated with MetO (*p<0.05, **p<0.01). Data are expressed as mean ± SD and are from 3 separate experiments.
Discussion
Cellular Met levels in male hepatocytes exposed to 30 mM Met reached steady state levels at 0.5 h, whereas steady-state Met levels in Met-exposed female FIMHs were not achieved until 1.0 h and were much lower than the levels in male hepatocytes. The net difference in cellular SAM levels at 0.5 h between Met-exposed male and female FIMHs was approximately 4 nmol / 106 cells whereas the net difference in cellular Met levels was nearly 30 nmol / 106 cells. While levels of secondary Met TM metabolites were not determined, gender differences in Met TM metabolites seem not sufficient to account for the much higher cellular Met levels in Met-exposed male FIMHs. Collectively, these results suggested increased Met uptake in male compared to female hepatocytes.
Met is a substrate for amino acid transport systems A and L [20], but the expression levels of these transporters in male and female mouse liver have not been characterized. Inhibition of amino acid transport system A by 17β-estradiol has been detected in rat mammary adenocarcinoma cells [21], suggesting that hormone-mediated gender differences in the activity or expression level of Met transport systems is possible and could result in lower rates of Met transport into female FIMHs.
Because overall cellular levels of Met in male hepatocytes were higher than in female hepatocytes, it was hypothesized that Met TA may be more prominent in that gender. Addition of AOAA to Met-exposed male hepatocytes resulted in significant increases in cellular Met levels at 1.5 h compared to male hepatocytes exposed to Met alone, however, addition of AOAA did not significantly alter the Met AUC0-1.5h suggesting that the Met TA pathway became significant after 1 h. In contrast, AOAA had no effect on cellular Met levels in Met-exposed female hepatocytes. Since Met TA activity by GTK in male and female mouse liver cytosol was comparable, lower cellular concentrations of Met rather than a decreased capacity for Met TA in female FIMHs may have been responsible for the lack of any measured effect of AOAA on Met levels in that gender. The Km values for Met TA (3.3 mM for GTK) [22] are higher than those for SAM formation (0.003-1.3 mM) [23,24]. In humans with homocystinuria, plasma concentrations of Met TA metabolites only increased significantly when plasma concentrations of Met exceeded 350 μmol/L [25,26]. In a similar fashion, Met TA may only be a significant pathway in FIMHs above a particular threshold concentration of Met that was not achieved in female hepatocytes.
Met-exposed male, but not female FIMHs, incubated with AOAA also had nearly doubled cellular Met-d-O levels at 1.0 and 1.5 h compared to FIMHs incubated with only Met. Significant increases in cellular Met-d-O levels were previously detected in control male FIMHs incubated with AOAA [13], but in the present study, no increases in cellular Met-d-O levels were detected in control female FIMHs incubated with AOAA. We investigated whether the decreased Met-d-O TA in female hepatocytes may be due, in part, to an increased rate of MetO reduction to Met in that gender. Indeed, higher MetO reduction activity was detected in female as compared to male mouse liver cytosol incubated with 0.25 mM and 0.5 mM MetO.
The Km values obtained for MetO reduction in this study (2.5 mM and 2.0 mM in male and female mouse liver cytosol, respectively) are higher than previously reported Km values (0.12 mM and 0.17 mM) for reduction of free MetO by Escherichia Coli methionine sulphoxide reductase A (MsrA) [27,28]. The Km values for the cytosolic male and female mouse liver MetO reductase activities are based on Met formation from a racemic mixture of Met-d-O and Met-l-O, however, several recent studies indicate that Met-d-O is the primary MetO diastereomer reduced in mammals [17,29]. Further support for this hypothesis was provided by our recent finding that Met-l-O was the major MetO diastereomer detected (80-85% total MetO) in male and female FIMHs incubated with a racemic mixture of MetO [13].
The evidence obtained for increased Met and MetO TA in male FIMHs compared to female FIMHs after exposure to Met correlates well to the greatly increased sensitivity of male hepatocytes to Met toxicity relative to female hepatocytes since these pathways have both been implicated as Met bioactivation pathways [9,13]. Furthermore, the lower SAM levels detected in male hepatocytes may indicate a less active Met TM pathway in that gender which would also be consistent with their increased sensitivity to Met toxicity since this pathway has been implicated in Met detoxification [9]. In MAT1A knockout mice, high Met levels and low SAM levels were associated with an abnormal liver gene expression profile similar to that seen in conditions associated with steatohepatitis, and these mice were predisposed to oxidative stress in the liver [8]. In a human with SAH hydrolase deficiency, high plasma Met and SAM levels were detected in association with liver injury [30]. Thus, hypermethionemia associated with a deficient Met TM pathway may lead to hepatotoxicity potentially due to increases in Met TA metabolites similar to those detected in humans with MAT I/III or cystathionine β-synthase deficiency [27,31].
The data obtained using this in vitro model were consistent with the detection of increased liver SAM levels in female mice compared to male mice dosed with Met (400 mg/kg) and the detection of mostly Met-d-O in the livers of Met-dosed mice of both genders [12]. In that study, however, no gender differences in liver Met levels were observed in the Met-dosed mice. A more sustained Met exposure level may be necessary to observe in vivo gender differences in Met accumulation and/or increased Met TA.
In summary, evidence has been obtained for increased Met accumulation and increased Met and MetO TA in male FIMHs relative to female FIMHs after exposure to high levels of Met which may play a role in the increased sensitivity of male hepatocytes to Met toxicity. The in vivo metabolic and hepatotoxicological relevance of these results warrant further investigation.
Acknowledgments
This research is supported by NIH R01 DK044295 and T32-ES-007015.
Abbreviations
- Met
l-methionine
- Met-d-O
l-methionine-d-sulfoxide
- Met-l-O
l-methionine-l-sulfoxide
- MetO
l-methionine-dl-sulfoxide
- MsrA
methionine sulphoxide reductase A
- MAT
methionine adenosyltransferase
- SAM
S-adenosyl-l-methionine
- SAH
S-adenosyl-l-homocysteine
- 3-MTP
3-methylthiopropionic acid
- GSH
glutathione
- GSSG
glutathione disulfide
- DTT
dithiothreitol
- FIMHs
freshly isolated mouse hepatocytes
- ATP
adenosine triphosphate
- 3-DA
3-deaazadenosine
- AOAA
aminooxyacetic acid
- TA
transamination
- TM
transmethylation
- SO
sulfoxidation
- TB
trypan blue
- LDH
lactate dehydrogenase
- PhP
phenylpyruvate
- DMEM
Dulbecco's modified Eagle's Medium
- AUC
area under the curve
- HPLC
high performance liquid chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moss RL, Haynes AL, Pastuszyn A, Glew RH. Methionine infusion reproduces liver injury of parenteral nutrition cholestasis. Pediatr Res. 1999;45:664–668. doi: 10.1203/00006450-199905010-00009. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein JD. Methionine metabolism in liver diseases. Am J Clin Nutr. 2003;77:1094–1095. doi: 10.1093/ajcn/77.5.1094. [DOI] [PubMed] [Google Scholar]
- 3.Shinozuka H, Estes LW, Farber E. Studies on acute methionine toxicity. I. Nucleolar disaggregation in guinea pig hepatic cells with methionine or ethionine and its reversal with adenine. Amer J Path. 1971;64:241–249. [PMC free article] [PubMed] [Google Scholar]
- 4.Cox R, Martin JT, Shinozuka H. Studies on acute methionine toxicity. II. Inhibition of ribonucleic acid synthesis in guinea pig liver by methionine and ethionine. Lab Invest. 1973;29:54–59. [PubMed] [Google Scholar]
- 5.Heyman MB, Tseng HC, Thaler MM. Total parenteral nutrition decreases hepatic glutathione concentration in weanling rats. Hepatology. 1984;4:1049. Abstract. [Google Scholar]
- 6.Mori N, Hirayama K. Long-term consumption of methionine-supplemented diet increases iron and lipid peroxide levels in rat liver. J Nutr. 2000;130:2349–2355. doi: 10.1093/jn/130.9.2349. [DOI] [PubMed] [Google Scholar]
- 7.Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, et al. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez-chantar ML, Corrales FJ, Martinez-Cruz LA, Garcia-Trevijano ER, Huang Z, Chen L, et al. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002;16:1292–1294. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- 9.Dever JT, Elfarra AA. l-methionine toxicity in freshly isolated mouse hepatocytes is gender-dependent and mediated in part by transamination. J Pharmacol Exp Ther. 2008;326:809–17. doi: 10.1124/jpet.108.141044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang PK, Richards HH, Cantoni GL. S-Adenosyl-l-homocysteine hydrolase: Analogues of S-adenosyl-l-homocysteine as potential inhibitors. Mol Pharmacol. 1977;13:939–947. [PubMed] [Google Scholar]
- 11.Mitchell AD, Benevenga NJ. The role of transamination in methionine oxidation in the rat. J Nutr. 1978;108:67–78. doi: 10.1093/jn/108.1.67. [DOI] [PubMed] [Google Scholar]
- 12.Dever JT, Elfarra AA. In vivo metabolism of l-methionine in mice: Evidence for stereoselective formation of methionine-d-sulfoxide and quantitation of other major metabolites. Drug Metab Dispos. 2006;34:2036–2043. doi: 10.1124/dmd.106.012104. [DOI] [PubMed] [Google Scholar]
- 13.Dever JT, Elfarra AA. l-Methionine-dl-sulfoxide metabolism and toxicity in freshly isolated mouse hepatocytes: Gender differences and inhibition with aminooxyacetic acid. Drug Metab Dispos. 2008;36:2252–2260. doi: 10.1124/dmd.108.023390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marfey P. Determination of d-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsburg Res Commun. 1984;49:591–596. [Google Scholar]
- 15.Wise CK, Cooney CA, Ali SF, Poirier LA. Measuring S-adenosylmethionine in whole blood, red blood cells and cultured cells using a fast preparation method and high-performance liquid chromatography. J Chromatogr B. 1997;696:145–152. doi: 10.1016/s0378-4347(97)00213-2. [DOI] [PubMed] [Google Scholar]
- 16.Cooper AJL, Pinto JT. Aminotransferase, l-amino acid oxidase and β-lyase reactions involving l-cysteine S-conjugates found in allium extracts: Relevance to biological activity? Biochem Pharmacol. 2005;69:209–220. doi: 10.1016/j.bcp.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 17.Moskovitz J, Singh VK, Requena J, Wilkinson BJ, Jayaswal RK, Stadtman ER. Purification and characterization of methionine sulfoxide reductases from mouse and staphylococcus aureus and their substrate stereospecificity. Biochem Biophys Res Commun. 2002;290:62–65. doi: 10.1006/bbrc.2001.6171. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Ripp SL, Itagaki K, Philpot RM, Elfarra AA. Methionine S-oxidation in human and rabbit liver microsomes: Evidence for a high-affinity methionine S-oxidase activity that is distinct from flavin-containing monooxygenase 3. Arch Biochem Biophys. 1999;367:322–332. doi: 10.1006/abbi.1999.1247. [DOI] [PubMed] [Google Scholar]
- 20.Matthews RH, Zand R. Methionine transport in S37 cells: Substrate-Dependent function of amino acid transport system A in exchange processes. Biochim Biophys Acta. 1979;554:227–233. doi: 10.1016/0005-2736(79)90020-8. [DOI] [PubMed] [Google Scholar]
- 21.Hissin PJ, Hilf R. Effects of estrogen to alter amino acid transport in R3230AC mammary carcinomas and its relationship to insulin action. Cancer Res. 1979;39:3381–3387. [PubMed] [Google Scholar]
- 22.Cooper AJL, Meister A. Isolation and properties of a new glutamine transaminase from rat kidney. J Biol Chem. 1974;249:2554–2561. [PubMed] [Google Scholar]
- 23.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 24.Mato JM, Corrales FJ, Lu SC, Avila MA. S-Adenosylmethionine: a control switch that regulates liver function. FASEB J. 2002;16:15–26. doi: 10.1096/fj.01-0401rev. [DOI] [PubMed] [Google Scholar]
- 25.Blom HJ, Boers G, Tribels J, van Roessel J, Tangerman A. Cystathionine-synthase-deficient patients do not use the transamination pathway of methionine to reduce hypermethionemia and homocystineria. Metabolism. 1989;38:577–582. doi: 10.1016/0026-0495(89)90220-5. [DOI] [PubMed] [Google Scholar]
- 26.Tangerman A, Wilcken B, Levy HL, Boers GHJ, Mudd SH. Methionine transamination in patients with homocystinuria due to cystathionine β-synthase deficiency. Metabolism. 2000;49:1071–1077. doi: 10.1053/meta.2000.7709. [DOI] [PubMed] [Google Scholar]
- 27.Grimaud R, Ezratz B, Mitchell JK, Lafitte D, Briand C, Derrick PJ, et al. Repair of oxidized proteins. J Biol Chem. 2001;276:48915–48920. doi: 10.1074/jbc.M105509200. [DOI] [PubMed] [Google Scholar]
- 28.Moskovitz J, Poston JM, Berlett BS, Nosworthy NJ, Szczepanowski R, Stadtman ER. Identification and characterization of a putative active site for peptide methionine sulfoxide reductase (MsrA) and its substrate stereospecificity. J Biol Chem. 2000;275:14167–14172. doi: 10.1074/jbc.275.19.14167. [DOI] [PubMed] [Google Scholar]
- 29.Lee BC, Le DT, Gladyshev VN. Mammals reduce methionine-S-sulfoxide with MsrA, are unable to reduce methionine-R-sulfoxide, and this function can be restored with a yeast reductase. J Biol Chem. 2008;283:28361–28369. doi: 10.1074/jbc.M805059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baric I, Fumic K, Cuk M, Schulze A, Finkelstein JD, James J, et al. S-Adenosylhomocysteine hydrolase deficiency in a human: A genetic disorder of methionine metabolism. Proc Natl Acad Sci. 2004;101:4234–4239. doi: 10.1073/pnas.0400658101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gahl WA, Bernardini I, Finkelstein JD, Tangerman A, Martin JJ, Blom HJ, et al. Transsulfuration in an adult with hepatic methionine adenosyltransferase deficiency. J Clin Invest. 1988;81:390–397. doi: 10.1172/JCI113331. [DOI] [PMC free article] [PubMed] [Google Scholar]