Abstract
Background
Coral reefs around the world are experiencing large-scale degradation, largely due to global climate change, overfishing, diseases and eutrophication. Climate change models suggest increasing frequency and severity of warming-induced coral bleaching events, with consequent increases in coral mortality and algal overgrowth. Critically, the recovery of damaged reefs will depend on the reversibility of seaweed blooms, generally considered to depend on grazing of the seaweed, and replenishment of corals by larvae that successfully recruit to damaged reefs. These processes usually take years to decades to bring a reef back to coral dominance.
Methodology/Principal Findings
In 2006, mass bleaching of corals on inshore reefs of the Great Barrier Reef caused high coral mortality. Here we show that this coral mortality was followed by an unprecedented bloom of a single species of unpalatable seaweed (Lobophora variegata), colonizing dead coral skeletons, but that corals on these reefs recovered dramatically, in less than a year. Unexpectedly, this rapid reversal did not involve reestablishment of corals by recruitment of coral larvae, as often assumed, but depended on several ecological mechanisms previously underestimated.
Conclusions/Significance
These mechanisms of ecological recovery included rapid regeneration rates of remnant coral tissue, very high competitive ability of the corals allowing them to out-compete the seaweed, a natural seasonal decline in the particular species of dominant seaweed, and an effective marine protected area system. Our study provides a key example of the doom and boom of a highly resilient reef, and new insights into the variability and mechanisms of reef resilience under rapid climate change.
Introduction
Coral reefs are among the most biologically diverse and economically important ecosystems. However, reefs are rapidly degrading at a global scale, due to a combination of pressures, including climate change, overexploitation, coral diseases, and declining water quality [1]–[4]. Rising ocean temperatures have triggered mass coral bleaching events that have devastated many coral reefs around the world [5] and caused ecological phase or state shifts, from coral-dominance to dominance by seaweeds (fleshy algae) [6]–[8]. Current climate change models suggest increasing frequency and severity of mass coral bleaching events [5], so that phase shifts to algal dominated states are expected to occur more frequently and last longer [9]–[11].
Critically, the recovery of degraded reefs depends on the reversibility of seaweed dominance [12], [13]. However, all previously documented cases have found dominance by seaweeds difficult to reverse, because the algae prevent settlement of new corals, and because the algae persist, usually due to overfishing or mass mortality of key herbivorous species and to relative unpalatability of algae to herbivores [14], [15]. Examples of natural reversals from algal dominance to coral dominated states are extremely rare (but see [16], [17]) and take years to decades to occur (e. g. Kaneohe Bay, Hawaii [18]; Dairy Bull Jamaica [19]). Rapid reversals from algal dominated states to dominance by corals and small algae have only been demonstrated at a very small scale after experimentally induced herbivore exclusion [20]. In that experiment, artificially enhanced algal biomass was rapidly consumed by grazers upon removal of exclusion cages, and reef recovery was dependent on recovery of herbivory, a process extrinsic to the corals and algae.
Inshore, high latitude coral reefs of the largest reef system in the world, the Great Barrier Reef (GBR), Australia, suffered severe mass bleaching of coral in early 2006. Reefs in the area exhibit low coral species diversity and are widely dominated by Acropora corals, with branching Acropora accounting for more than 90% of the coral species [21]. Sea surface temperatures in the inshore reefs of the Keppel Islands (23°10′S, 151°00′E) in the southern GBR rose rapidly in late 2005, with some locations reaching temperatures in December that are not normally found until February. The onset of high sea temperatures early in the season triggered coral bleaching by mid January 2006 [22]. Overall, bleaching damage was severe, affecting 77–95% of coral colonies [22], [23]. The purpose of this paper was to document some novel mechanisms for coral reef resilience based on changes in coral and seaweed abundance following the 2006 mass coral bleaching event that affected reefs of the Keppel Islands.
Results and Discussion
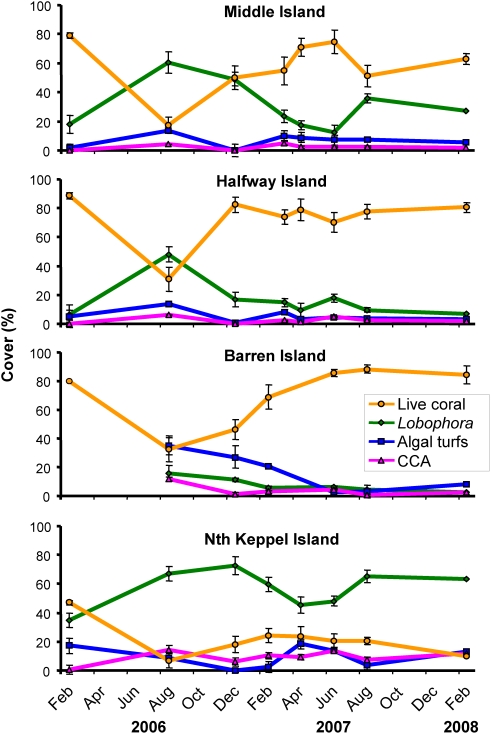
Abundance of corals and seaweeds showed strong dynamics in response to the warming-induced mass coral bleaching event (Figs. 1, 2). Cover of bleached but living coral (mainly branching Acropora spp.) on the reef slopes of Middle Island, Halfway Island, and Barren Island was high (77%–89%) during the bleaching event in January/February 2006. Five months after the onset of bleaching, coral cover was severely reduced, to values around 20–30% by July–August 2006. The coral mortality was followed by an extraordinary bloom of the brown seaweed Lobophora variegata, apparently unprecedented in magnitude on the GBR (GDP and LM personal observations, Fig. 2). This alga commonly grows between the branches of most Acropora colonies in the area, but under normal (i.e. undisturbed) conditions it is not able to grow beyond the base of the branches, probably due to competitive inhibition by the corals. Previous work on L. variegata growing amongst branching Porites cylindrica corals showed that the interaction is competitive, with both coral and alga inhibiting growth of the other [24], [25]. However, seaweeds and algal turfs were apparently released from space competition with the corals due to the bleaching mortality [9] and dramatically increased in cover (200–300% increase on Middle Island and Halfway Island) by August 2006. Importantly, coral bleaching preceded L. variegata overgrowth, and overgrowth only took place on bleached or dead corals at a range of spatial scales (from cm to 10 s of kilometers; careful inspection showed negligible overgrowth of healthy coral). Nonetheless, the seaweed apparently exacerbated coral mortality by overgrowing stressed coral tissue [24]–[26] (Figure S1D). Algal competitiveness may have been enhanced by uptake of nutrients and carbon generated by the coral mortality [27]. There are no previous observations of such an extensive bloom of L. variegata, or indeed any single species of fleshy alga, on the GBR, although large-scale blooms of filamentous algal turfs have occurred following coral mortality [9], [28], [29], and a small-scale bloom of a red seaweed was recorded in response to a ship-grounding [30]. Blooms of L. variegata are common in the Caribbean, particularly after the die-off of the sea urchin Diadema [14], [31] and following coral mortality [32], [33] (also personal observations in Islas del Rosario, Colombia and Flower Garden Banks, Gulf of Mexico, GDP and LM).
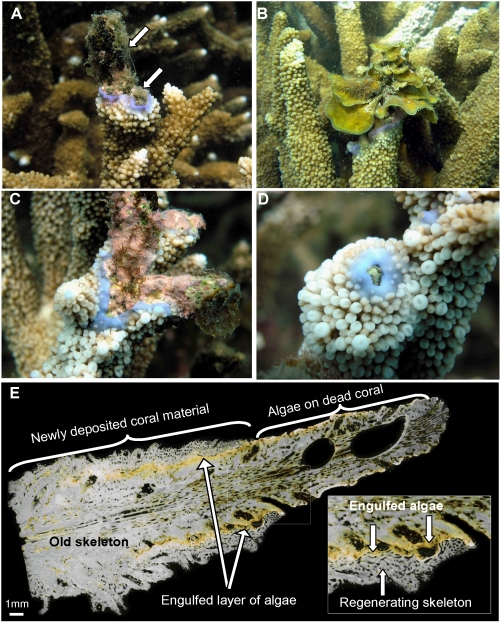
Figure 1. Coral bleaching, algal overgrowth of corals and coral recovery.
A) Bleached corals in the Keppel Islands, Great Barrier Reef, during the mass bleaching event in January 2006. The fleshy brown seaweed Lobophora variegata grows at the base of the branches of Acropora spp. corals. B) L. variegata is released from space competition by coral mortality and overgrows coral skeletons as well as some coral tissue, causing an unprecedented algal bloom. C) Seaweed bloom on North Keppel Island after coral bleaching. The reef has lost its structural complexity and has experienced little coral recovery. D) Recovered reef on Barren Island, showing high coral cover and low cover of seaweeds.
Figure 2. Coral – algal dynamics in response to the 2006 warming-induced coral bleaching event.
Data from the reef slopes of four islands in the Keppel Islands, southern Great Barrier Reef. % cover data are means (n = 10) ±SE, except for Feb 2006 (n = 25–26). CCA: Crustose calcareous algae.
Surprisingly however, the cover of branching Acropora corals at most sites showed an extremely rapid recovery after the seaweed bloom, reaching pre-bleaching levels by December 2006–April 2007 (ca 12–14 months after the onset of bleaching, Fig. 2, Table 1). This represents a 100 to 200% increase in cover of Acropora in approximately 6 months, thereby returning the system to coral dominance (P = 0.004, 0.001 and 0.006 for Tukey's comparisons of August 2006 c.f. February/March 2007 for Middle, Halfway and Barren Islands respectively).
Table 1. Two-way analyses of variance for the effects of sampling date and site on % cover of corals, brown seaweed Lobophora variegata, algal turfs and crustose calcareous algae (CCA).
| Source of variation | df | Mean-Square | F-ratio | p |
| Coral cover | ||||
| Date (D) | 5 | 1.265 | 20.238 | <0.001 |
| Site (S) | 3 | 5.257 | 84.100 | <0.001 |
| D×S | 15 | 0.204 | 3.262 | <0.001 |
| Error | 214 | 0.063 | ||
| Lobophora cover | ||||
| Date (D) | 5 | 0.541 | 14.450 | <0.001 |
| Site (S) | 3 | 5.249 | 140.224 | <0.001 |
| D×S | 15 | 0.121 | 3.244 | <0.001 |
| Error | 214 | 0.037 | ||
| Algal turf cover | ||||
| Date (D) | 5 | 0.161 | 5.207 | <0.001 |
| Site (S) | 3 | 0.096 | 3.094 | 0.028 |
| D×S | 15 | 0.184 | 5.929 | <0.001 |
| Error | 214 | 0.031 | ||
| CCA cover | ||||
| Date (D) | 5 | 0.203 | 15.964 | <0.001 |
| Site (S) | 3 | 0.424 | 33.336 | <0.001 |
| D×S | 15 | 0.018 | 1.378 | 0.160 |
| Error | 214 | 0.013 | ||
Data were Arc-sin transformed. Interactions between date and site were significant; therefore, data were analysed for site effects within dates and date effects within sites, using a one-way ANOVAs and Tukey's post-hoc comparisons (results not shown).
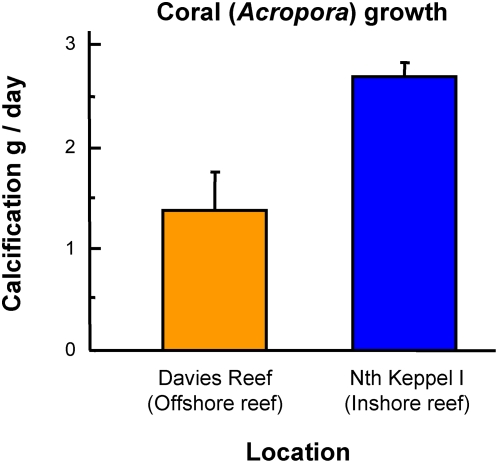
Unexpectedly, the rapid reversal and increase in coral cover did not involve settlement and recruitment of coral larvae. Coral recruitment was generally very low throughout the course of the study at all sites [recruit densities for Middle, Halfway, Barren and North Keppel Islands were 0, <1, <1 and 4 recruits m−2 respectively; Kruskal-Wallis Test indicated no increases in recruit densities through time after the bleaching event, Table 2]. Instead, coral recovery involved a rapid regeneration and regrowth of remnant coral tissue after bleaching mortality, with branches of Acropora emerging from the algal mat to reestablish high cover much faster than could occur from growth of new recruits (Figs. 2, 3). Growth rates of branching Acropora from the Keppel Islands appear unusually high, with rates of calcification nearly 100% faster than those of corals from offshore the GBR (Fig. 4). Linear extension rates of branching Acropora from other Pacific inshore reefs are also extraordinarily high, with mean values of 333 (±42 SD) mm/year [34]. This rapid, vegetative regeneration allowed the corals to out-compete and overgrow the algae settled on dead skeletons.
Table 2. Kruskal-Wallis one-way analyses of variance for the effects of sampling date on density of coral recruits of four islands.
| Source of variation | df | H | p |
| Middle I | |||
| Date | 5 | 0.000 | 1.000 |
| Halfway I | |||
| Date | 5 | 5.000 | 0.416 |
| North Keppel I | |||
| Date | 4 | 4.387 | 0.356 |
| Barren I | |||
| Date | 5 | 11.308 | 0.046* |
Data were log transformed. H: Statistic of the Kruskal-Wallis test; df: degrees of freedom. Dates included in the analyses are: Aug 06, Dec 06, Feb 07, Jun 07, Aug 07, Jan 08. Data missing for Dec 06 in North Keppel Island.
Recruit density was slightly higher in February 2007 but declined afterwards.
Figure 3. Coral recovery following algal overgrowth.
Branches of Acropora corals died after bleaching and were subsequently colonized by a variety of benthic algae. Remnant coral tissue at the base of the coral colonies regrew upward and deposited new skeleton along the old dead coral branch, overgrowing A) algal turfs (arrows), B) fleshy seaweed Lobophora variegata, and C) crustose coralline algae. D) Coral tissue has all but completely overgrown the colonizing algae. E) Thin section of coral showing benthic algae sandwiched between old coral skeleton and a thin layer of new skeleton. Examination using a compound microscope showed that coral tissue overgrew a range of algal types.
Figure 4. Coral growth (calcification).
Calcification rates of Acropora millepora at North Keppel Island and Davies Reef (an offshore reef). Data are means±SE (n = 12 for North Keppel Island and 8 for Davies Reef), and show unusually high growth rates in the Keppel Islands.
We propose that this unusually rapid and successful regrowth stems from several key factors: i. the strong competitive ability of the corals; ii. the corals' ability to regrow from relatively small amounts of live tissue; iii. and a seasonal dieback in the single species of dominant seaweed. Although overgrowth by seaweeds probably inhibited coral growth, a natural seasonal decline in L. variegata, between December 2006 and March/April 2007 (Fig. 2), markedly reduced the apparent effects of this competitive inhibition. Cover of L. variegata decreased significantly from 50% to <20% in Middle Island and from 75% to 45% in North Keppel Island during that period of time (Table 1; P<0.005 for Tukey's comparisons of August 2006 and March/April 2007 for both islands).
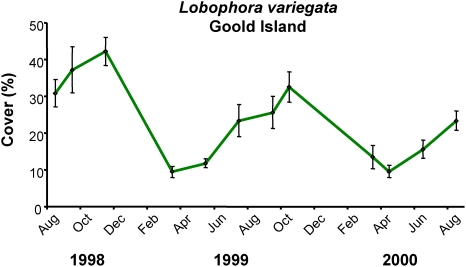
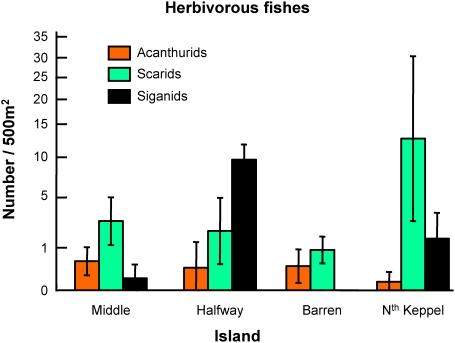
Removal of the seaweed L. variegata in this study appears to have been largely due to inherent seasonal dieback. Large amounts of loose L. variegata were observed at the time of the dieback, and similar seasonal changes in L. variegata have been previously observed in the GBR (Fig. 5) and nearby areas [35], apparently related to elevated seawater temperature during the austral spring and summer (GDP unpublished data). Herbivorous fishes, although largely unfished, are not generally abundant in the Keppel Islands, being generally about an order of magnitude less than on mid and outer shelf reefs [36]. Careful observations did not indicate grazing damage to the L. variegata, despite the extent of the bloom and decline, and patterns of herbivore abundance among the study reefs were not consistent with the growth and decline in L. variegata at these sites (Fig. 6). The site with lowest herbivore densities had lowest L. variegata abundance (Barren Island). The site with most abundant scarids had most abundant L. variegata (North Keppel Island), while siganids were most abundant on Halfway Island, which had intermediate abundance of L. variegata. Large invertebrate herbivores, such as sea urchins, were virtually absent across all sites. Thus, whilst herbivory could have contributed to some degree, and is likely important to algal abundance on these reefs generally, the extent of decline in L. variegata in this study appears largely due to seasonality.
Figure 5. Seasonality in Lobophora variegata on Goold Island, inshore central GBR.
Abundance of L. variegata consistently shows strong declines during the austral summer. Data are means±SE of 5 replicate quadrats.
Figure 6. Herbivore abundance.
Herbivore density data from the study sites; data are square root transformed, means+/−SE of 5 transects per site.
However, the increase in coral cover was apparently also due to strong growth rates and consequent competitive ability of the coral, and not dependent on the seasonal decline in the algal competitor, L. variegata. This is suggested by results for Middle Island (Fig. 2; August–December 2006) where strong coral recovery preceded decline in L. variegata, and from Barren Island, where coral recovery involved overgrowth of non-seasonal algal turfs and crustose calcareous algae. Tissue growth may have been enhanced by heterotrophic feeding [37], as shown elsewhere on GBR reefs [38].
Regeneration of the coral tissue apparently derived from tissue reservoirs, or areas of live coral tissue that persisted at the very base of the coral branches, underneath the seaweed canopy (Figure S1A, B; the “phoenix effect” in which apparently dead coral branches regenerate live tissue [39]–[41]). Removal of the dominant seaweed mat showed that coral tissue mortality was extensive under the seaweed at all sites. However, there did remain small fragments of live coral tissue. The remnant surviving coral tissue rapidly expanded upwards along the dead coral branches (Fig. 3) and actively overgrew L. variegata, as well as a range of other algal types, including filamentous algal turfs, fleshy seaweeds and crustose coralline algae (Fig. 3A–D). Thin sections of Acropora corals show overgrowth of several algae by new coral material, and show that overgrowth involved direct horizontal contact as well as overtopping, resulting in a “seaweed sandwich [42]”, with algae engulfed between new and old layers of skeleton (Fig. 3E). Regeneration over existing coral skeletons offers an energetically efficient and rapid mechanism for recovery, by limiting the calcification required for regrowth. Whilst regeneration of corals has been observed elsewhere [42], [43], our findings are significant because they demonstrate the potential importance of this process for large-scale, rapid recovery even after severe climate-related mass bleaching. The rate and scale of recovery is increasingly critical as climate change causes more frequent mass bleaching events.
Coral recovery and algal dynamics were not uniform in this study. Although most reefs showed rapid recovery, coral cover on North Keppel Island declined from 46% to <10% after bleaching and had recovered relatively little after two years (Fig. 2), despite a marked seasonal decline in L. variegata. Coral cover on North Keppel Island prior to the bleaching event was low compared to the other reefs in the area (46% vs. 75–90% respectively) and cover of L. variegata higher. These differences may reflect differences in disturbance history, conditions less conducive to coral growth, or differences in the extent of coral mortality due to floods from the Fitzroy River (the largest river catchment along the GBR) [44]. Recovery of the reef on North Keppel Island may also have been limited by the loss of three-dimensional structure of the reef framework (most branching Acropora corals have been broken into rubble due to bioerosion, Fig. 1C; habitat complexity has been shown to be critical for the rapid recovery of damaged reefs [19], [45]).
At the other extreme, coral recovery at Barren Island was very strong, and abundance of L. variegata remained much lower than other sites, even after coral mortality (Fig. 2). However, abundance of L. variegata was still highest following coral mortality (18%), and declined as the coral recovered (although not significantly: P = 0.131 for Tukey's comparison of August 2006 and February 2007). Barren Island is further offshore and in deeper water than the other sites, and dead coral tissue was colonized predominantly by algal turfs more typical of offshore reefs. Detailed analyses of the species composition of the algal turfs in this locality (data not shown) revealed a very different species composition of turfing algae, mainly dominated by calcareous turfing species (e.g. Jania and Amphiroa).
Recent events in the Keppel Islands provide an exceptional, but important example of the doom and boom of highly resilient reefs, and thereby provide new insight into the potential variability in mechanisms of reef resilience. Most degraded reefs globally have either failed to recover from events such as coral bleaching and other human induced disturbances [3], or have taken several years to decades to return to pre-disturbance condition [14], [15], [18], [19], [29], [46], [47]. In contrast, the Keppel Islands have shown rapid recovery of coral dominance, despite repeated coral bleaching events (1998, 2002, and 2006 [48]), severe flood plumes (e.g. 1991, 2008 [44]), and dense algal overgrowth. If they allow recovery of coral populations within one year, instead of ten, such exceptional processes may be disproportionately important to larger-scale reef resilience.
Resilience of reef coral populations is typically considered in terms of removal of algal blooms by herbivores, combined with replenishment by coral larvae. Whilst these factors are no doubt vital for reef persistence [7], [13], [49], both abundance of herbivorous fishes and coral recruitment were apparently limited on the reef slopes studied here during these events. There is considerable evidence that algal abundance on coral reefs is generally related to herbivory [6], [7], [50]–[54], and herbivory can be important to interactions between L. variegata and corals on the GBR [25]. However, in this instance, removal of the seaweed L. variegata appears to have been largely due to inherent seasonal dieback, more than consumption by herbivores, although experimental studies would be required to be conclusive. Importantly, this dieback is apparently species specific ([35], GDP unpublished data), so that its ecological significance presumably depends on the nature of the seaweed bloom as a single species. In more typical multi-species seaweed blooms, it is unlikely that all species would have similar seasonality, and competitive effects on coral regrowth would probably be stronger. In this sense, given the apparent limited abundance of herbivores, the reduction in seaweed during our study may be a fortunate coincidence of monospecific bloom and seasonal dieback in that one species. Further, had the decline in L. variegata not coincided with rapid coral growth, it is likely that a range of other algae would have colonized, potentially stabilizing the phase shift. Thus, the seasonal decline was clearly important to the resilience of these reefs in these circumstances, but should not be seen as diminishing the general importance of herbivory to reef resilience.
Our results stand in contrast with many previous studies, especially studies of coral and algal dynamics on Caribbean reefs in the early 80 s, where a combination of coral mortality and hurricane damage followed by mortality of sea urchins, caused massive algal blooms (including L. variegata) that still continue today [14], [15], [55]. Although L. variegata was involved in both circumstances, there are several fundamental differences that probably contribute to the different outcomes. First, the Keppel Islands are dominated by rapidly growing, branching Acropora, apparently better suited to competing with a mat-like algal growth than the massive and plate-like corals that were dominant on Caribbean reefs [26], [55], [56]. Coral-algal interactions will depend considerably on the particular species involved. Second, the monospecific algal bloom in the Keppel Islands was exceptionally vulnerable; most macroalgal blooms are much more diverse, imbuing the algal-dominated state with greater resilience. Studies of Caribbean reefs typically note 5–10 genera of benthic macroalgae (e.g. [31], [57], [58]); after long-term herbivore exclusion on the GBR, at least 10 algal genera were abundant in algal dominated plots [7].
Third, coral recovery may be strongly influenced by the nature of the disturbance regime. Reefs subject to acute disturbances, such as the rapid bleaching in the Keppel Islands, may often recover more effectively than those subject to chronic disturbances such as in the Caribbean [46], [59]. Similarly, the spatial scale of disturbance in our study was much smaller than that in the Caribbean. Numerous other factors can contribute to the resilience or vulnerability of a reef (e.g. [3], [60]).
In summary, unusually rapid coral recovery in the Keppel Islands apparently stemmed from synergistic effects of factors not previously recognized as important to resilience. These factors included robust tissue regeneration, high competitive ability of the corals and a seasonal dieback in the monospecific seaweed bloom, all against a backdrop of an effective marine protected area system and moderate water quality. Understanding the variability in mechanisms underlying resilience is critical for reef management under climate change. Settlement and recruitment of new corals requires years to decades to re-establish abundant corals, whereas recovery in the Keppel Islands took less than one year. Frequent, large-scale damage may mean that reefs able to rapidly recover abundant corals may serve as key refugia, or sources of larvae for reef recovery at broader scales. Diversity in processes may well be critical to the overall resilience and persistence of coral reef ecosystems globally.
Materials and Methods
We monitored the dynamics of corals and benthic algae on the reef slopes (4–7 m depth) of four islands [Middle (North side, Surprise Rock: 23°09.896 S; 150°55.420 E), Halfway (Southwest side: 23°12.193 S; 150°58.187 E), North Keppel (Southeast side: 23°05.123 S; 150°53.983 E) and Barren (South East side, Coral Gardens: 23°09.796 S; 151°05.507 E)]. On each reef slope, the % cover of corals (using functional forms, e.g. branching, massive, mushroom, and genera) and benthic algae (functional forms and genera) was quantified in an area of ca 20 m×4 m using 10, 50×50 cm randomly allocated quadrats (with 10×10 grids) in August and December 2006, March, April, June and August 2007, and February 2008. The number of coral recruits (colonies <5 cm diameter) in each quadrat was also scored. % cover of benthic organisms during the onset of the bleaching event (January–February 2006) was estimated from 25–26 photo-quadrats (1×1.3 m) along 50 m transects. Cover of bleached coral on Barren Island in January 2006 was estimated visually and from an aerial photograph (projected onto a grid of 100 quadrats, with each quadrat scored for bleaching). Although different methods were used to quantify corals and algae during the onset of the bleaching (first sample date) and the rest of the sampling dates (7 dates), the extent of any differences due to methods are likely to be minor compared to the differences between dates due to ecological changes.
Cover and coral recruitment data were analyzed for differences between sampling dates and sites using a two-way analysis of variance, with dates and localities as fixed factors and quadrats as replicates. Data were checked for normality using stem and leaf plots and probability plots; and for homogeneity of variances with Cochran's test. Coral recruitment data were analyzed using a non-parametric Kruskal-Wallis test.
Coral growth data (represented by calcification rate) was determined at North Keppel Island and, for comparison, at an offshore reef in the central GBR (Davies Reef, 18.8°S, 147.6°E). 8 to 12 colonies (15–20 cm size) of branching Acropora millepora were collected at each location, wet weighed on land and returned to the water. The experiment was set up in February 2003 and the corals reweighed in June and September 2003. Although less accurate than the buoyant-weight method [61], the wet-weight method is adequate for estimating relative and gross differences between locations over a relatively long time frame (7 months).
Seasonality in L. variegata was also measured on Goold Island on the inshore, central Great Barrier Reef (18°10.9′S, 146°10.2′E) from 1998 to 2000. Cover was estimated using 5 replicate, fixed 50×50 cm quadrats randomly located on the reef flat.
Density of herbivorous fish was measured using underwater visual census by scuba. Five 50 m×10 m (500 m2) replicate belt transects were censused at each site in March 2007. Transects were laid haphazardly along the reef slope [62].
Coral regrowth was examined using thin sections prepared from Acropora branches (10–15 cm), air-dried and then sectioned in the laboratory, using a bench saw. Longitudinal and latitudinal sections of regenerating axial branches were cut into slices approximately 5 mm thick (n = 10). Select sections were prepared for thin sectioning and fixed in Epoxicure resin (Buehler Ltd, Lake Bluff, IL, USA) onto 50 mm×76 mm slides. Sections were polished to an approximate thickness of 25 µm and analysed under light microscopy.
Supporting Information
Coral mortality and tissue remnants following coral bleaching. A) Dead Acropora sp. colony colonized by algal turfs and with Lobophora variegata seaweed at the base. B) Dead Acropora colony with part of the L. variegata canopy removed, showing remnant pigmented coral tissue (inset). C) L. variegata overgrowing Acropora corals. D) Identical to C) but with the algae removed, showing variable localized bleaching of live coral tissue and some coral mortality occurring underneath the algal canopy. Live coral tissue at the base of the branches may act as tissue reservoirs for future rapid coral recovery.
(9.97 MB TIF)
Acknowledgments
Thanks to P. Williams, A. Crawley, A. Money, D. Venera-Ponton, P. Bongaerts, N. Englebert and Queensland Park and Wildlife Services for help in the field, to J. Johnson and P. Marshall from the GBRMPA for valuable discussions and to K. Anthony, S. Sandin, and to the reviewers for valuable discussion of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This project was funded by the Australian Research Council Centre of Excellence for Coral Reef Studies, the Centre for Marine Studies (The University of Queensland), the PEW Program in Marine Conservation and the Great Barrier Reef Marine Park Authority. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 2.Bruno JF, Selig ER. Regional decline of coral cover in the Indo-Pacific: Timing, extent, and subregional comparisons. PLoS ONE. 2007;28:e711. doi: 10.1371/journal.pone.0000711. doi:10.1371/journal.pone.0000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellwood DR, Hughes TP, Folke C, Nystrom M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 4.Pandolfi JM, Jackson JBC, Baron N, Bradbury RH, Guzmán HM, et al. Are U.S. coral reefs on the slippery slope to slime? Science. 2005;307:1725–1726. doi: 10.1126/science.1104258. [DOI] [PubMed] [Google Scholar]
- 5.Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 6.McCook LJ. Macroalgae, nutrients and phase shifts on coral reefs: scientific issues and management consequences for the Great Barrier Reef. Coral Reefs. 1999;18:357–367. [Google Scholar]
- 7.Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli DM, Hoegh-Guldberg O, et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol. 2007;17:1–6. doi: 10.1016/j.cub.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 8.Scheffer M, Carpenter SR, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Pulido G, McCook LJ. The fate of bleached corals: patterns and dynamics of algal recruitment. Mar Ecol Prog Ser. 2002;232:115–128. [Google Scholar]
- 10.Diaz-Pulido G, McCook LJ, Larkum AWD, Lotze HK, Raven JA, et al. Vulnerability of macroalgae of the Great Barrier Reef to climate change. In: Johnson JE, Marshall PA, editors. Climate change and the Great Barrier Reef. Townsville: Great Barrier Reef Marine Park Authority & Australian Greenhouse Office; 2007. pp. 153–192. [Google Scholar]
- 11.Ledlie MH, Graham NAJ, Bythell JC, Wilson SK, Jennings S, et al. Phase shifts and the role of herbivory in the resilience of coral reefs. Coral Reefs. 2007;26:641–653. [Google Scholar]
- 12.Mumby PJ, Hastings A, Edwards HJ. Thresholds and the resilience of Caribbean coral reefs. Nature. 2007;450:98–101. doi: 10.1038/nature06252. [DOI] [PubMed] [Google Scholar]
- 13.Birrell CL, McCook LJ, Willis BL, Diaz-Pulido G. Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr Mar Biol Annu Rev. 2008;46:25–64. [Google Scholar]
- 14.Hughes TP. Catastrophes, phase-shifts and large-scale degradation of a Caribbean coral reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 15.Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- 16.Done TJ. Phase shifts in coral reef communities and their ecological significance. Hydrobiologia. 1992;247:121–132. [Google Scholar]
- 17.Brown BE, Le Tissier MDDD, Dunne RP, Scoffin TP. Natural and anthropogenic disturbances on intertidal reefs of SE Phuket, Thailand 1979–1992. In: Ginsburg RN, editor. Proceedings of the Colloquium on Global Aspects of Coral Reefs: Health, Hazards, and History. Miami: Rosenstiel School of Marine and Atmospheric Science, University of Miami; 1994. pp. 279–285. [Google Scholar]
- 18.Hunter CL, Evans CW. Reefs in Kaneohe Bay, Hawaii: Two centuries of Western influence and two decades of data. In: Ginsburg RN, editor. Proceedings of the Colloquium on Global Aspects of Coral Reefs: Health, Hazards, and History. Miami: Rosenstiel School of Marine and Atmospheric Science, University of Miami; 1994. pp. 339–345. [Google Scholar]
- 19.Idjadi JA, Lee SC, Bruno JF, Precht WF, Allen-Requa L, et al. Rapid phase-shift reversal on a Jamaican coral reef. Coral Reefs. 2006;25:209–211. [Google Scholar]
- 20.Bellwood DR, Hughes TP, Hoey AS. Sleeping functional group drives coral-reef recovery. Curr Biol. 2006;16:2434–2439. doi: 10.1016/j.cub.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Van Woesik R, Done TJ. Coral communities and reef growth in the southern Great Barrier Reef. Coral Reefs. 1997;16:103–115. [Google Scholar]
- 22.Weeks SJ, Anthony KRN, Bakun A, Feldman GC, Hoegh-Guldberg O. Improved predictions of coral bleaching using seasonal baselines and higher spatial resolution. Limnol Oceanogr. 2008;53:1369–1375. [Google Scholar]
- 23.Jones AM, Berkelmans R, van Oppen MJH, Mieog JC, Sinclair W. A community shift in the symbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc R Soc Lond, Ser B: Biol Sci. 2008;275:1359–1365. doi: 10.1098/rspb.2008.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jompa J, McCook LJ. The effects of nutrients and herbivory on competition between a hard coral (Porites cylindrica) and a brown alga (Lobophora variegata). Limnol Oceanogr. 2002;47:527–534. [Google Scholar]
- 25.Jompa J, McCook LJ. Effects of competition and herbivory on interactions between a hard coral and a brown alga. J Exp Mar Biol Ecol. 2002;271:25–39. [Google Scholar]
- 26.McCook LJ, Jompa J, Diaz-Pulido G. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs. 2001;19:400–417. [Google Scholar]
- 27.Davey M, Holmes G, Johnstone R. High rates of nitrogen fixation (acetylene reduction) on coral skeletons following bleaching mortality. Coral Reefs. 2008;27:227–236. [Google Scholar]
- 28.Endean R, Stablum W. The apparent extent of recovery of reefs of Australia's Great Barrier Reef devastated by the crown-of-thorns starfish. Atoll Res Bull. 1973;168:1–41. [Google Scholar]
- 29.Pearson RG. Recovery and recolonization of coral reefs. Mar Ecol Prog Ser. 1981;4:105–122. [Google Scholar]
- 30.Hatcher BG. A maritime accident provides evidence for alternate stable states in benthic communities on coral reefs. Coral Reefs. 1984;3:199–204. [Google Scholar]
- 31.Steneck RS. Is herbivore loss more damaging to reefs than hurricanes? Case studies from two Caribbean reef systems (1978–1988). In: Ginsburg RN, editor. Proceedings of the Colloquium on Global Aspects of Coral Reefs: Health, Hazards, and History. Miami: Rosenstiel School of Marine and Atmospheric Science, University of Miami; 1994. pp. 220–226. [Google Scholar]
- 32.Diaz-Pulido G, Díaz JM. Algal assemblages in lagoonal reefs of Caribbean oceanic atolls. Proc 8th Int Coral Reef Symp. 1997;1:827–832. [Google Scholar]
- 33.McClanahan TR, Aronson RB, Precht WF, Muthiga NA. Fleshy algae dominate remote coral reefs of Belize. Coral Reefs. 1999;18:61–62. [Google Scholar]
- 34.Crabbe MJC, Smith DJ. Sediment impacts on growth rates of Acropora and Porites corals from fringing reefs of Sulawesi, Indonesia. Coral Reefs. 2005;24:437–441. [Google Scholar]
- 35.Banks SA, Harriott VJ. Coral communities of the Gneering Shoals and Mudjimba Island, southeast Queensland. Mar Freshw Res. 1995;46:1137–1144. [Google Scholar]
- 36.Russ GR. Distribution and abundance of herbivorous fishes in the central Great Barrier Reef I. Levels of variability across the entire continental shelf. Mar Ecol Prog Ser. 1984;20:23–34. [Google Scholar]
- 37.Grottoli AG, Rodrigues LJ, Palardy JE. Heterotrophic plasticity and resilience in bleached corals. Nature. 2006;440:1186–1189. doi: 10.1038/nature04565. [DOI] [PubMed] [Google Scholar]
- 38.Anthony KRN. Enhanced energy status of corals on coastal high-turbidity reefs. Mar Ecol Prog Ser. 2006;319:111–116. [Google Scholar]
- 39.Krupp DA, Jokiel PL, Chartrand TS. Asexual reproduction by the solitary scleractinian coral Fungia scutaria on dead parent coralla in Kaneohe Bay, Oahu, Hawaiian Islands. Proc 7th Int Coral Reef Symp. 1993;1:527–534. [Google Scholar]
- 40.Jokiel PL, Hunter CL, Taguchi S, Watarai L. Ecological impact of a fresh-water “reef kill” in Kaneohe Bay, Oahu, Hawaii. Coral Reefs. 1993;12:177–184. [Google Scholar]
- 41.Riegl B, Piller WE. “Cryptic” tissues inside Acropora frameworks (Indonesia): a mechanism to enhance tissue survival in hard times while also increasing framework density. Coral Reefs. 2001;20:67–68. [Google Scholar]
- 42.Fishelson L. Ecological and biological phenomena influencing coral-species composition on the reef tables at Eilat (Gulf of Aqaba, Red Sea). Mar Biol. 1973;19:183–196. [Google Scholar]
- 43.Loya Y. Skeletal regeneration in a Red Sea scleractinian coral population. Nature. 1976;261:490–491. doi: 10.1038/261490a0. [DOI] [PubMed] [Google Scholar]
- 44.Van Woesik R, De Vantier LM, Glazebrook JS. Effects of cyclone ‘Joy’ on nearshore coral communities of the Great Barrier Reef. Mar Ecol Prog Ser. 1995;128:261–270. [Google Scholar]
- 45.Colgan MW. Coral reef recovery on Guam (Micronesia) after catastrophic predation by Acanthaster placi. Ecology. 1987;68:1592–1605. doi: 10.2307/1939851. [DOI] [PubMed] [Google Scholar]
- 46.Connell JH. Disturbance and recovery of coral assemblages. Coral Reefs. 1997;16 S:S101–S113. [Google Scholar]
- 47.Golbuu Y, Victor S, Penland L, Idip DJ, Emaurois C, et al. Palau's coral reefs show differential habitat recovery following the 1998-bleaching event. Coral Reefs. 2007;26:319–332. [Google Scholar]
- 48.Berkelmans R, De'ath G, Kininmonth S, Skirving WJ. A comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: spatial correlation, patterns, and predictions. Coral Reefs. 2003;23:74–83. [Google Scholar]
- 49.Knowlton N, Jackson JBC. Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol. 2008;6:e54. doi: 10.1371/journal.pbio.0060054. doi:10.1371/journal.pbio.0060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steneck RS. Herbivory on coral reefs: a synthesis. Proc 6th Int Coral Reef Symp. 1988;1:37–49. [Google Scholar]
- 51.McCook LJ. Effects of herbivores and water quality on Sargassum distribution on the central Great Barrier Reef: cross-shelf transplants. Mar Ecol Prog Ser. 1996;139:179–192. [Google Scholar]
- 52.Smith JE, Smith CM, Hunter CL. An experimental analysis of the effects of herbivory and nutrient enrichment on benthic community dynamics on a Hawaiian reef. Coral Reefs. 2001;19:332–342. [Google Scholar]
- 53.Diaz-Pulido G, McCook LJ. Relative roles of herbivory and nutrients in the recruitment of coral reef seaweeds. Ecology. 2003;84:2026–2033. [Google Scholar]
- 54.Newman MJH, Paredes GA, Sala E, Jackson JBC. Structure of Caribbean coral reef communities across a large gradient of fish biomass. Ecol Lett. 2006;9:1216–1227. doi: 10.1111/j.1461-0248.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- 55.Hughes TP. Community structure and diversity of coral reefs: the role of history. Ecology. 1989;70:275–279. [Google Scholar]
- 56.Nugues MM, Bak RPM. Differential competitive abilities between Caribbean coral species and a brown alga: a year of experiments and a long-term perspective. Mar Ecol Prog Ser. 2006;315:75–86. [Google Scholar]
- 57.Hughes TP, Reed DC, Boyle MJ. Herbivory on coral reefs: community structure following mass mortalities of sea urchins. J Exp Mar Biol Ecol. 1987;113:39–59. [Google Scholar]
- 58.Carpenter RC. Mass mortality of Diadema antillarum. I. Long-term effects on sea urchin population-dynamics and coral reef algal communities. Mar Biol. 1990;104:67–77. [Google Scholar]
- 59.Connell JH, Hughes TP, Wallace CC. A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol Monogr. 1997;67:461–488. [Google Scholar]
- 60.McCook LJ, Folke C, Hughes TP, Nystrom M, Obura D, et al. Ecological resilience, climate change and the Great Barrier Reef: An Introduction. In: Johnson J, Marshall PA, editors. Climate change impacts on the Great Barrier Reef. Townsville: Great Barrier Reef Marine Park Authority & Australian Greenhouse Office; 2007. pp. 75–96. [Google Scholar]
- 61.Jokiel PL, Maragos JE, Franzisket L. Coral growth: buoyant weight technique. In: Stoddart DR, Johannes RE, editors. Coral Reefs: research methods. Paris: UNESCO; 1978. pp. 529–541. [Google Scholar]
- 62.Russ GR, Cheal AJ, Dolman AM, Emslie MJ, Evans RD, et al. Rapid increase in fish numbers follows creation of world's largest marine reserve network. Curr Biol. 2008;18:R514–R515. doi: 10.1016/j.cub.2008.04.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coral mortality and tissue remnants following coral bleaching. A) Dead Acropora sp. colony colonized by algal turfs and with Lobophora variegata seaweed at the base. B) Dead Acropora colony with part of the L. variegata canopy removed, showing remnant pigmented coral tissue (inset). C) L. variegata overgrowing Acropora corals. D) Identical to C) but with the algae removed, showing variable localized bleaching of live coral tissue and some coral mortality occurring underneath the algal canopy. Live coral tissue at the base of the branches may act as tissue reservoirs for future rapid coral recovery.
(9.97 MB TIF)