Abstract
Mice lacking the dopamine transporter (DAT−/−) are characterized by high extracellular dopamine levels and spontaneous hyperlocomotion. We performed a detailed analysis of the behavioral phenotype of DAT−/− mice in order to identify other behavioral impairments associated with the hyperdopaminergic tone of these mutant mice. In particular, we investigated locomotor activity, exploration, social and maternal behaviors, which are known to be regulated by dopamine. DAT−/− mice were easily aroused by novelty and always responded with an hyperlocomotion, which interfered with habituation to the testing environment, exploratory behavior in an open field and coping response to forced swimming stress. Social behaviors such as interaction with an unknown congener or aggressiveness were not modified in DAT−/− mice compared to DAT+/− and DAT+/+ mice, although the maternal behavior of the mutant females was severely disturbed. Haloperidol and clozapine reversed hyperactivity in DAT−/− mice with a rightward shift of the dose-response curve compared to control animals, suggesting a dopamine-mediated effect. These results emphasize the role of dopamine regulation in locomotion, exploration, and maternal behaviors and suggest that mice with a genetic deletion of the DAT may represent an useful model to elucidate the altered behavioral processes accompanying pathological conditions associated with hyperdopaminergic function.
Keywords: Aggression, Animals, Behavior, Animal, Carrier Proteins, genetics, Circadian Rhythm, Dopamine, analysis, metabolism, Dopamine Plasma Membrane Transport Proteins, Female, Locomotion, Male, Maternal Behavior, Membrane Glycoproteins, Membrane Transport Proteins, Mice, Mice, Knockout, Nerve Tissue Proteins, Physical Conditioning, Animal, Social Behavior
Keywords: hyperlocomotion, circadian activity, exploratory behavior, forced swimming test, antipsychotic, social interaction, aggressive behavior, maternal behavior
INTRODUCTION
Considerable evidence has established the role of dopamine (DA) neurotransmission in the regulation of motor activity, emotion, motivation and cognition (Le Moal, 1995). Imbalance of DA systems is thought to underlie conditions such as schizophrenia, drug addiction, Parkinson’s disease, tardive dyskinesia, and attention-deficit hyperactivity disorder (Hornykiewicz, 1986; Carlsson, 1988; Hechtman, 1994; Koob and Nestler, 1997). Traditionally, animal experiments have used pharmacological agents that modulate neurotransmitter activity, or neurotoxin-induced lesions of specific structures or DA pathways. Recently, a strain of mice that lacks the DA transporter (DAT−/−) has been developed (Giros et al., 1996), providing a valuable tool for the study of the long-term behavioral consequences of the dysregulation of DA neurotransmission. The DAT is a key regulator of DA synaptic transmission and the constitutive deletion of the DAT gene results in increased extracellular DA, decreased neuronal DA stores and decreased DA receptors (Giros et al., 1996; Jones et al., 1998).
Spontaneous hyperlocomotion is the most obvious behavioral characteristic of DAT−/− mice. The hyperactivity shows minimal intensity decrease over time, compared to the locomotor habituation displayed by control animals and is present during both phases of the light-dark cycle (Giros et al., 1996). In the present study, we further investigated the impact of the deletion of the DAT on locomotion, as well as on other behaviors which are known to be regulated by DA, including exploration, social and maternal behaviors, to identify the behavioral dysfunctions associated to the hyperdopaminergic phenotype of DAT−/− mice. We first examined the ability of DAT−/− mice to habituate to a novel environment after repeated exposure and measured circadian activity during 48 hours to gain insight into the rythmicity of locomotor behavior in DAT−/− mice. Moreover, we studied the reversion of hyperlocomotion by the typical and atypical antipsychotic drugs, haloperidol and clozapine, to test the involvement of DA transmission in the observed effects. Indeed, a recent study reported that novelty-induced hyperlocomotion in DAT−/− mice is not associated to increased DA levels in the striatum and can be inhibited by drugs increasing serotonin levels, suggesting a role for serotonergic transmission (Gainetdinov et al., 1999). Locomotor activity and exploratory behavior were evaluated in an open field under high illumination to measure the influence of emotion on these behaviors (Archer, 1973; Walsh and Cummins, 1976). We also evaluated motor reactivity and coping reaction in response to an inescapable stress, using the forced swimming test.
Numerous animal studies have reported that chronic treatment with psychomimetic drugs such as amphetamine, phencyclidine or hallucinogens disrupt normal social interactions (Ellenbroek and Cools, 1990; Geyer and Markou, 1995). Given that DAT−/− mice have been proposed as an extreme example of long-term use of psychostimulants (Jones et al., 1998), we investigated whether the deletion of the DAT gene affects social relations in these mice. We thus examined the interaction with an unknown congener and aggressiveness in DAT−/− mice and control littermates. In addition, DAT removal results in anterior pituitary hypoplasia, dwarfism and inability to lactate (Bossé et al., 1997). Despite normal circulating prolactin and growth hormone levels in DAT−/− mice, the hypoplastic pituitary is unable to produce and secrete sufficient quantities of prolactin and growth hormone to support lactation and normal growth. Accordingly, Giros et al. (1996) reported a deficit in weight and size in DAT−/− mice and an impairment in maternal behavior so that offsprings need to be transferred to foster mothers in order to survive. We further studied this altered behavior in DAT−/− mice by using the maternal pup-care test.
METHODS
Animals
Homozygous DAT−/− mice were obtained by genetic manipulation as described (Giros et al., 1996). These mice were then backcrossed for more than two years (12 generations) on a C57BL/6 background. DAT−/−, heterozygous DAT+/− and wild-type DAT+/+ littermates were obtained from the mating of DAT+/− mice. Before the behavioral experiments, the genotype of the mice was determined by Southern (DNA) blot analysis as previously described (Giros et al., 1996). Mice were housed 4–5 per cage after weaning under standard housing conditions with food and water available ad libitum. All mice used were 8–10 weeks old, drug-naive and were only used in one test. When not stated otherwise, experiments were conducted during the light phase of the light/dark cycle (lights on 07:30–19:30). All behavioral and pharmacological studies were performed in a sound-proof room by an observer who did not know either the genotype of the mice or the pharmacological treatment. All experiments were conducted in accordance with standard ethical guidelines (European Communities Council Directive for the care and use of laboratory animals) and approved by the local ethical committee.
Drugs
Haloperidol was obtained from Sigma and clozapine was donated by Sandoz Pharmaceuticals. Both neuroleptics were solubilized in a drop of Tween, dissolved in 0.9% NaCl solution and injected subcutaneously (s.c.) in a volume of 0.01 ml/g.
Behavioral analysis
Spontaneous locomotion
Locomotion was evaluated in activity boxes (20 × 15 × 25 cm) under moderate illumination (<5 lux). The animal’s displacements were individually measured by photocell beams located across the long axis, 15 mm (horizontal activity) and 30 mm (vertical activity) above the floor. Each box was connected by an interface to a computer (Immetronic, Bordeaux, France). In the locomotor habituation test, mice were placed in the activity boxes for 60 min (at 14:00 h) for four consecutive days. The locomotor circadian rhythm was measured during 48 h. DAT−/− and DAT+/+ mice were placed in the activity boxes at 08:00 h. The floor of each box was covered with sawdust and there was food and water on disposal.
Effects of haloperidol and clozapine on locomotor activity
Mice were injected (at 14:00 h) with saline, haloperidol (0.05, 0.10, 0.15, 0.20 or 0.30 mg/kg, s.c.) or clozapine (1, 2 or 3 mg/kg, s.c.) 30 min before the introduction in the activity boxes, and locomotor activity was measured for 60 min.
Motor activity and exploratory behavior in the open field
Locomotor, rearing and exploratory activities were examined in an open field (55 × 55 × 20 cm) under high illumination (100 lux lamp placed 45 cm above the floor). The floor and the walls of the open field were white, and care was taken to minimize shadows in the box. Eight wooden cylinders (10×3 × 3 cm) were placed either at the center or the periphery of the box, depending on the test situation. The behavior of each animal was videotaped for 6 min and scored from a video monitor placed in a nearby room. The floor of the box was divided in 16 squares of equal dimension. Locomotion was rated as the number of squares crossed (when the animal penetrated a square with its two forepaws) and rearings were expressed as the number of times the animal raised on its two hind legs sniffing in the air, whether it leant on the wall or not. The latency to the first visit to an unknown object and the number of visits were also measured. A visit to an object was considered as any approach, sniffing or investigatory behavior. Other behaviors, including grooming, sniffing, chewing and head bobbing, were investigated via direct visual observation.
Forced swimming stress
The forced swimming test was adapted from Porsolt et al. (1979). Animals were forced to swim individually in a vertical glass cylinder (height: 18 cm; diameter: 15 cm) containing 13.5 cm of water maintained at 25±1°C. After 6 min in the water they were removed and allowed to dry in a heated enclosure. The latency and total duration of immobility were measured. An animal was judged to be immobile whenever it remained floating in the water in a slightly hunched but upright posture, the head just above the water. An investigator present in the room, seated 1 m away from the glass cylinder, performed the behavioral measurements, in order to correctly evaluate the paw movements of the animal.
Social behavior
Social interactions were evaluated in a box (55 × 55 × 20 cm) under red illumination (25 W), using the procedure described by Sams-Dodd et al. (1997). The floor was covered with sawdust that had been exposed to other mice before testing, to provide a constant odor level in the box. The test was performed at 20:00 h, i.e., 30 min after the light to dark transition, a period characterized by a high level of general activity. Two mice of the same genotype and sex, but unfamiliar to each other, were introduced simultaneously in the box at diametrically opposing places. Mice were videotaped for 6 min and scored from a video monitor placed in a nearby room. Social interaction was expressed as the time spent in exploration of the congener.
The resident/intruder test was performed in the home cage (45 × 25 × 15 cm) of a resident male mouse. Each resident male was housed with a female and left undisturbed for four weeks in a quiet room. Thirty min before the test, the female and the pups were removed from the cage. A group-housed male (intruder) of the same genotype was introduced in the cage of the resident for 10 min. This intruder mouse was distinguished by a hole in each ear. An investigator present in the room scored the latency to attack and the number of attacks exhibited by the resident mouse.
Maternal behavior
The pup-care-behavior test was adapted from Carlier et al. (1982). Each female was mated with a male in a cage (45 × 25 × 15 cm) and the mice were left undisturbed until testing. Mice had on disposal 1 g cotton to build a nest. When the female appeared to be visibly close to parturition, the male was removed from the mating cage. Observations were made 24±12 h after parturition. The female was removed briefly from the cage and the pups were placed in the side of the cage opposite to the nest emplacement. The female was placed back on the nest and maternal behavior was scored for 10 min by an investigator present in the room. The parameters examined were: 1) number of living pups the day of the test; 2) number of living pups 24 h after the test; 3) first contact: time between the moment when the female was placed on the nest and the moment when the snout contacted one of the pups; 4) first retrieval: time between the first contact and taking into the mouth the first pup; 5) first carrying: time between the first retrieval and placing of the first offspring in the nest; 6) time with first pup: time spent in the nest with the first pup; 7) move away: number of times the female moved away from one of the pups, situated outside the nest, without transporting them; 8) time employed to carry back all the pups into the nest; 9) time spent with all the pups in the nest; and 10) mean interval time between retrieval of each pup.
Statistical analysis
An analysis of variance (ANOVA) was used to compare the distribution of the values between groups; post-hoc individual comparisons were made using Tukey’s test. The results of the pup-care test were analyzed with the Mann-Whitney test. Data are presented as mean ± SEM.
RESULTS
Spontaneous hyperlocomotion
As previously reported (Giros et al., 1996), DAT−/− mice displayed a high level of locomotion when introduced in a novel environment, compared to DAT+/− and DAT+/+ mice [day 1: F(2,31)=55.97, p<0.0001] (Figure 1). The genotype effect persisted during the four daily sessions of the locomotor habituation test [F(2,31)=159.50, p<0.0001]. DAT+/+ mice exhibited a decrease in motor activity after daily exposure to the activities boxes [day effect: F(3,33)=9.51, p<0.0001], whereas DAT−/− and DAT+/− mice failed to show habituation of locomotor activity (Figure 1).
Figure 1.
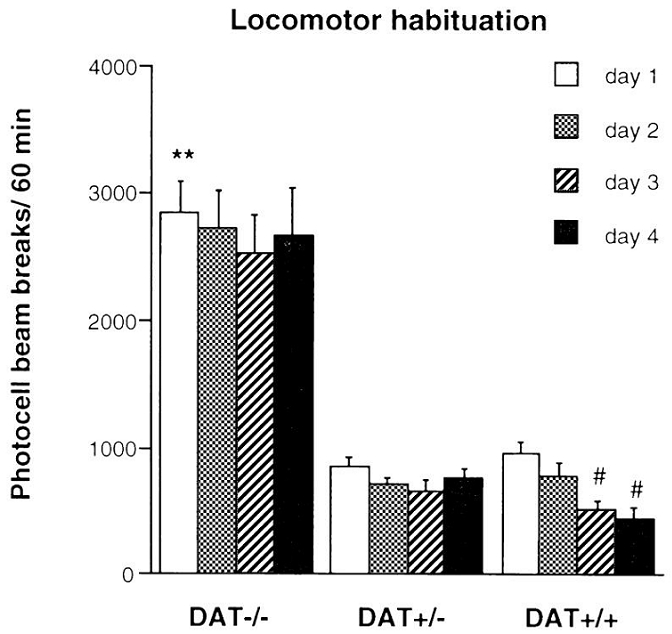
Locomotor response of DAT−/−, DAT+/− and DAT+/+ mice tested during 60 min in the same environment for four consecutive days, n = 9–12 per group. ** p<0.01 vs. DAT+/+ mice, the same day; # p<0.01 vs. first day, the same genotype.
DAT−/− mice also exhibited increased horizontal and vertical activity when evaluated during a 48 h continuous session, under a 12-h light/dark cycle [genotype effect: F(1,15)=4.80, p<0.05, and F(1,15)=18.36, p<0.0008, for horizontal and vertical activity, respectively] (Figure 2). Motor activity of both DAT−/− and DAT+/+ mice varied according to the light/dark cycle, increasing significantly during the night [time effect: F(47,15)=11.81, p<0.0001; and F(47,15)=9.69, p<0.0001, for horizontal and vertical activity, respectively]. There was a significant interaction between genotype and circadian motor activity [horizontal activity: F(47,658)=3.33, p<0.0001; vertical activity: F(47,658)=4.01, p<0.0001], because the hyperlocomotion and increased rearing behavior displayed by DAT−/− mice when first placed in the activity boxes or after the transition to the dark cycle lasted longer than in DAT+/+ mice. However, as shown in Figure 2, DAT−/− mice showed habituation of horizontal activity after the first hour of exposure to the novel environment, with the same time course as that of the wild-type controls. Furthermore, the horizontal and vertical activities of DAT−/− mice no longer differed from those observed in DAT+/+ controls 3 h after introduction into the novel environment until the beginning of the dark cycle (i.e., 11:00–19:30), as well as during the second part of each dark cycle (i.e., 23:00–07:30) and during the second light cycle (i.e., 07:30–19:30).
Figure 2.
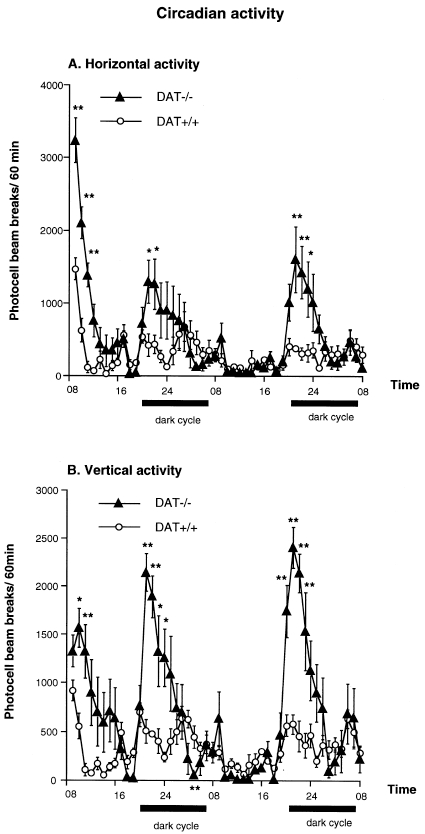
Horizontal (A) and vertical (B) circadian activity of DAT−/− and DAT+/+ mice measured in the same environment during 48 h. n = 6–10 per group. * p<0.05, and ** p<0.01 vs. DAT+/+ mice at the same time point.
Reversion of locomotion by haloperidol or clozapine
DAT−/− mice pretreated with saline displayed a higher level of locomotion when introduced in a novel environment than DAT+/− and DAT+/+ littermates (genotype effect: [F(2,40)=38.82, p<0.0001], Figure 3A; [F(2,40)=56.58, p<0.0001], Figure 3B). Haloperidol administration (0, 0.05, 0.10, 0.15, 0.20 or 0.30 mg/kg, s.c.) reduced the locomotor activity of DAT−/−, DAT+/− and DAT+/+ mice. The ANOVA indicated a genotype effect [F(2,177)=117.13, p<0.0001], a dose effect [F(5,177)=65.93, p<0.0001] and a genotype × dose interaction [F(10,177)=16.15, p<0.0001]. Indeed, there was a right shift of the dose-response curve in DAT−/− and DAT+/− mice. Specifically, the first efficient dose of haloperidol to reduce novelty-induced locomotor activity was 0.05 mg/kg in DAT+/+ mice, 0.10 mg/kg in DAT+/− mice and 0.15 mg/kg in DAT−/− mice (Figure 3A). The latter dose of haloperidol totally abolished the hyperlocomotion of DAT−/− mice, reducing it to a level similar to that observed in DAT+/− and DAT+/+ mice.
Figure 3.
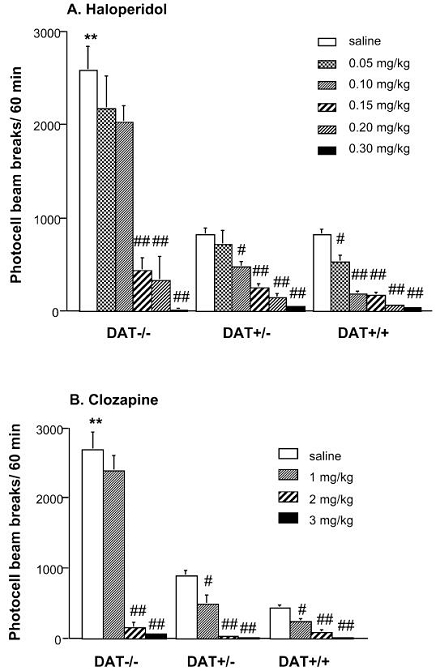
Effect of acute administration of different doses of haloperidol (A) or clozapine (B) on the locomotor activity of DAT−/−, DAT+/− and DAT+/+ mice tested in a novel environment. Drugs were administered s.c., 30 min before the introduction in the activity boxes, n = 10–15 per group. ** p<0.01 vs. saline-treated DAT+/+ mice; # p<0.05 and ## p<0.01 vs. saline-treated mice of the same genotype.
Pretreatment with clozapine (0, 1, 2 or 3 mg/kg, s.c.) also induced a reversion of motor activity in DAT−/−, DAT+/− and DAT+/+ mice. The ANOVA revealed a genotype effect [F(2,121)= 174.26, p<0.0001], a dose effect [F(3,121)=54.84, p<0.0001] and a genotype × dose interaction [F(6,121)=18.05, p<0.0001] (Figure 3B). The clozapine dose-response curve of DAT−/− mice also showed a rightward shift, the first efficient dose being 1 mg/kg in DAT+/+ and DAT+/− mice, and 2 mg/kg in DAT−/− mice. The administration of 2 or 3 mg/kg of clozapine completely inhibited locomotion in the three groups of mice.
Decreased exploratory behavior in the open field
Figure 4 shows horizontal and vertical activity of DAT mutant mice and control littermates in a highly illuminated open field. DAT−/− mice displayed a higher level of horizontal activity than DAT+/− and DAT+/+mice, independently of the location of the novel objects [F(2,130)=59.79, p<0.0001] (Figure 4A). However, horizontal activity decreased in the three groups when the objects were placed in the periphery of the open field, compared to the center [F(1,130)=23.09, p<0.0001]. The amplitude of the decrease was more marked in DAT−/− mice than in control littermates [F(2,130)=12.07, p<0.0001]. Surprisingly, DAT−/− mice did not display increased vertical activity in the open field and the number of rearings was not affected by the location of the objects in neither genotype (Figure 4B).
Figure 4.
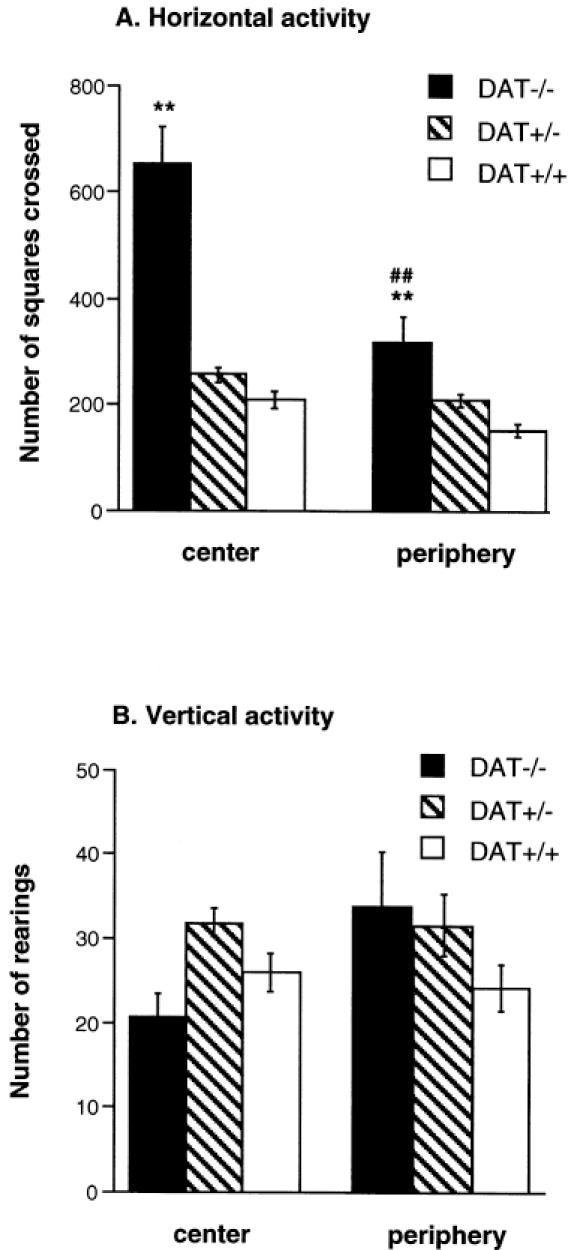
Horizontal (A) and vertical activity (B) in DAT−/−, DAT+/− and DAT+/+ mice measured in an open field test during 6 min. The open field arena was highly illuminated and eight novel objects were placed in the central (center) or the peripheral (periphery) zone of the box. n = 18–38 per group. ** p<0.01 vs. DAT+/+ mice tested with the same location of the objects; ## p<0.01 vs. mice of the same genotype tested with the other location of the objects.
The exploratory behavior toward the unknown objects placed in the center or the periphery of the open field is presented in Figure 5. DAT+/− mice showed a decreased latency to explore the objects compared to DAT−/− mice [genotype effect: F(2,130)=4.56, p<0.02]. The three groups of mice exhibited a reduced latency when the objects were placed in the periphery of the open field [location effect: F(1,130)=30.31, p<0.0001], but there was no genotype × location interaction (Figure 5, top panel). Measurement of the number of visits to the unknown objects revealed that, independently of the location of the objects, DAT−/− mice showed a weak exploratory behavior compared to DAT+/− and DAT+/+ mice [genotype effect: F(2,130)=53.99, p<0.0001] (Figure 5, bottom panel). In all groups, the number of visits was higher when the objects were placed in the periphery of the open field [location effect: F(1,130)=13.54, p<0.0003], and there was no genotype × location interaction. Relative to wild-type mice, neither DAT+/− nor DAT−/− mice exhibited enhanced levels of other spontaneous behaviors such as grooming, sniffing, chewing or head bobbing (data not shown).
Figure 5.
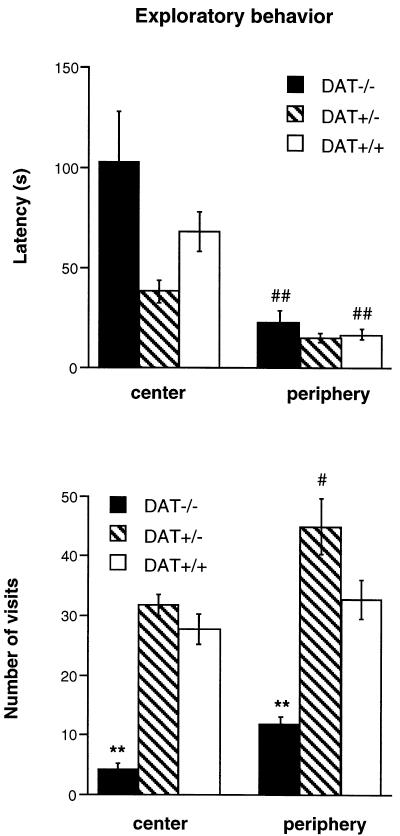
Exploratory behavior (latency and number of visits) in the presence of eight novel objects placed in the central (center) or peripheral (periphery) zone of a highly illuminated open field in DAT−/−, DAT+/− and DAT+/+ mice. The test lasted 6 min. n = 12–20 per group. ** p<0.01 vs. DAT+/+ mice tested with the same location of the objects; # p<0.05, and ## p<0.01 vs. mice of the same genotype tested with the other location of objects.
Continuous swimming during the forced swimming test
The behavioral response of DAT−/− mice to a situation of inescapable stress was investigated using the forced swimming test (Figure 6). In contrast to DAT+/+ mice, DAT−/− mice continued to swim during the entire duration of the test [genotype effect: F(2,57)=31.68, p<0.0001]. DAT+/− mice showed an intermediate behavior, with a longer latency to interrupt their swimming behavior (data not shown) and a lower immobilization time compared to DAT+/+ mice (Tukey’s test: p<0.01).
Figure 6.
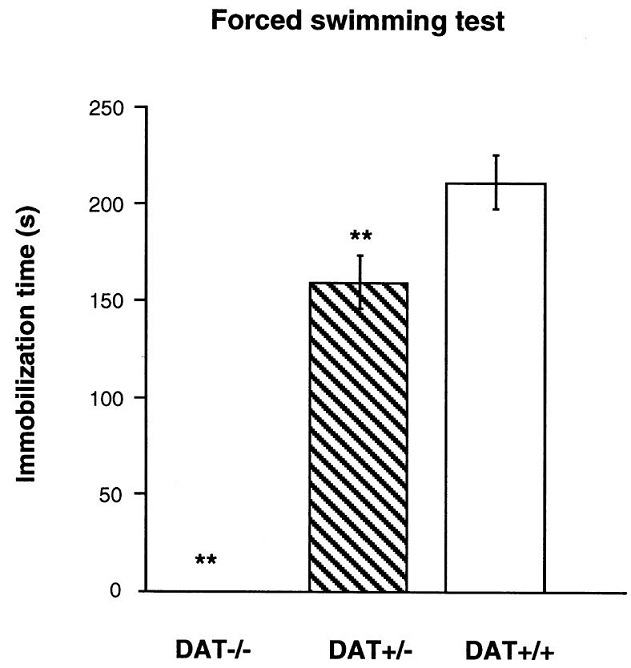
Behavioral responses of DAT−/−, DAT+/− and DAT+/+ mice to the forced swimming test. Time spent immobile was measured during 6 min. n = 8–30 per group. ** p<0.01 vs. DAT+/+ mice.
Normal social behavior
Investigation of social interactions between mice of the same genotype showed a similar level of time spent in congener exploration in DAT−/−, DAT+/− and DAT+/+ mice (Figure 7A). The resident/intruder test, performed with mice of the same genotype, revealed no significant differences in the latency to attack between the three groups of mice (data not shown). The number of attacks to the intruder was also similar in the three genotypes (Figure 7B).
Figure 7.

(A) Social interaction between unfamiliar pairs of mice of the same genotype and sex. (B) Aggressive behavior (number of attacks) of male resident mice confronted to unfamiliar intruder mice of the same genotype. The tests lasted 6 min. n = 10–23 per group.
Disturbed maternal behavior
Table 1 shows the results of the maternal pup-care test in DAT mutant mice and their control littermates. Although DAT−/− mice were fertile, the percentage of pregnant females was lower and they had smaller litters compared to DAT+/+ females at birth and 24 h after the test (Mann-Whitney tests: p=0.05 and p<0.01, respectively). We observed no genotype differences in the first contact, first retrieval and first carrying behaviors. However, DAT−/− females spent less time in the nest with the first pup (p=0.06 vs. DAT+/+ mice) as well as with all pups (p<0.05 vs. DAT+/− and DAT+/+ females). They also employed longer time to regroup all the pups in the nest, spent more time between each retrieval, and three out of five females failed to retrieve all pups during the 10 min that lasted the test, although these differences were not statistically significant. Moreover, DAT−/− females displayed increased levels of parallel activities during the test, such as burying, rearing, and grooming (data not shown), which interfered with mother-pup interactions.
Table 1.
Maternal pup-care test in DAT−/−, DAT+/− and DAT+/+ females.
| DAT+/+ | DAT+/− | DAT−/− | |
|---|---|---|---|
| percentage of pregnant females | 91 (10/11) | 90 (9/10) | 36 (5/14) |
| number of pups at day 0 | 6.80±0.47 | 6.56±0.65 | 4.80±0.80* |
| number of pups at day 1 | 3.80±0.80 | 2.11±0.81 | 0.40±0.40** |
| first contact | 13.00±3.82 | 16.44±4.35 | 17.00±9.70 |
| first retrieval | 19.50±7.54 | 33.22±21.15 | 24.00±11.77 |
| first carrying | 74.50±34.10 | 32.00±15.01 | 62.00±55.76 |
| time in the nest with the first pup | 33.00±22.16 | 6.67±3.44 | 0 |
| number of move away | 4.10±2.01 | 3.11±1.22 | 5.20±2.60 |
| time to carry all the pups into the nest | 342.50±59.19 | 301.67±67.25 | 423.00±108.42 |
| percentage of females retrieving all the pups | 80 (8/10) | 78 (7/9) | 40 (2/5) |
| time in the nest with all pups | 88.70±41.86 | 123.89±48.46 | 0§ |
| mean interval time between each retrieval | 90.80±56.81 | 22.33±3.57 | 356.40±136.43 |
Mann-Whitney test:
p<0.05 and
p<0.01 vs. DAT+/+;
p<0.05 vs. DAT+/+ and DAT+/−.
DISCUSSION
The purpose of the present study was to investigate the behavioral phenotype of DAT−/− mice to highlight the behavioral impairments associated with the increased extracellular DA levels present in these mutant mice. We showed that DAT−/− mice were easily aroused by environmental stimuli and responded with a predominant behavior, hyperlocomotion. This motor hyperactivity disturbed the expression of normal locomotor habituation, exploratory behavior and response to an inescapable stress. It was compatible with social behavior such as congener interactions and aggressiveness, although maternal pup care behavior was dramatically disturbed. The hyperlocomotion of DAT−/− mice was reversed by typical and atypical antipsychotics.
Motor activation in response to a novel environment is stimulated by the attractive and arousal salience of the novel stimulus (Berlyne et al., 1966; Hughes, 1997), and the behavior decreases when rodents are habituated to the stimulus (Hooks and Kalivas, 1995). Our results showed that the alterations induced by the DAT gene deletion impaired locomotor reactivity to a novel as well as a familiar environment. Thus, in addition to the previously reported increase in novelty-induced motor hyperactivity (Giros et al., 1996; Gainetdinov et al., 1999), DAT−/− mice showed no locomotor habituation when repetitively exposed to the same environment. This lack of locomotor habituation is probably not due to the relatively short duration of exposure to the environment (i.e. 1 h), because these mutant mice did not show habituation when exposed during 10 h to the same environment for four consecutive days (data not shown). It is possible that the strong hyperactivity of DAT−/− mice interferes with the establishment of a normal exploratory behavior of the novel environment, thereby preventing locomotor habituation during the following exposure to the environment. Alternatively, the daily handling stress before introduction of the animals into the testing environment and the release of DA associated with the transfer of the mice from their home cage to the novel environment, together with the slower clearance of DA observed in DAT−/− mice (Jones et al., 1998), may all contribute to the inability of these mice to develop locomotor habituation after repeated testing. Interestingly, DAT+/− mice also failed to show habituation over consecutive days of testing, although they did not differ significantly in their level of locomotor activity from wild-type animals (Giros et al., 1996; Jones et al., 1999). This observation suggests that environmental locomotor habituation after daily testing is a process highly sensitive to the changes in synaptic DA regulation, which are otherwise unable to induce significant modifications in the intensity of spontaneous locomotion in DAT+/− mice.
In a previous study, Giros et al. (1996) showed that the locomotor activity of DAT−/− mice, measured for 3 h during the light and the dark cycles, was significantly higher during the night cycle compared to the day cycle, an effect which may be related to the activation of DA neurotransmission during the night (Paulson and Robinson, 1994). The study of the locomotor circadian activity during 48 h confirmed that DAT−/− mice were highly reactive to arousal stimuli such as novelty and light/dark transition and showed a delayed locomotor habituation, indicating that their capacity to adapt to novel stimuli is disturbed. Although DAT−/− mice were markedly hyperactive during the dark cycle, during the light phase DAT−/− mice displayed a level of locomotion similar to DAT+/+ controls when observed in their home cage or after several hours of exposure to the testing environment, indicating that when not stimulated by environmental stimuli, DAT−/− mice showed normal locomotor activity. Locomotor habituation occurred despite the constitutive hyperdopaminergic tone of these mice, suggesting the development of compensatory mechanisms at the level of pre- and postsynaptic DA receptors (Giros et al., 1996; Jones et al., 1999).
Novelty-induced hyperlocomotion in DAT−/− mice was reversed by pretreatment with haloperidol or clozapine. The right shift of the dose-response curve observed in DAT−/− mice after treatment with the DA receptor antagonist haloperidol suggests that higher doses of the drug may be required in order to displace the increased levels of endogenous DA found in these mice (Giros et al., 1996). However, both 0.05 and 0.10 mg/kg of haloperidol are able to decrease the motor hyperactivity induced by DA agonists such as apomorphine (Puech et al., 1981). Thus, the enhanced levels of synaptic DA shown in DAT−/− mice cannot totally explain why these doses of haloperidol failed to reverse the hyperactivity of these mice. It is possible that the decrease in D2 receptor mRNA and protein observed in DAT−/− mice (Giros et al., 1996; Jones et al., 1999) contributes to the diminished potency of haloperidol. Clozapine is an atypical antipsychotic with a low DA/serotonin affinity ratio (Meltzer et al., 1989). The mechanism implicated in clozapine’s inhibition of DA-agonist induced locomotor activity is controversial and could involve either DA or serotonin antagonist effects (Kinon and Lieberman, 1996). However, the fact that higher doses of clozapine were required to reverse hyperlocomotion in DAT−/− mice than in DAT+/+ mice supports a major role of DA blockade in the observed effects. The hypothesis of DA-mediated novelty-induced hyperlocomotion in DAT−/− mice is in accordance with in vivo voltammetry and electrophysiological studies demonstrating modifications in the firing rate of DA mesocorticolimbic neurons in response to a novel environmental stimulus (Schultz et al., 1992; Rebec et al., 1997). Hence, it is possible that the recently reported lack of increase in DA concentrations in the striatum of DAT−/− mice after exposure to a novel environment (Gainetdinov et al., 1999) may be related to the structure chosen to sample DA levels. Indeed, the striatum is a structure of the DA circuitry not directly implicated in the reactivity to novelty, in contrast to the nucleus accumbens (Hooks and Kalivas, 1995).
Because behavioral alterations are particularly visible in stressful situations and the DA mesocorticolimbic system is involved in the control of emotional states (Bertolucci-d’Angio et al., 1990), we investigated the behavior of DAT−/− mice under aversive conditions. Our results showed that the aversive high illumination of the open-field test did not affect horizontal hyperactivity in DAT−/− mice. Interestingly, although DAT−/− mice have been reported to display increased rearing in parallel to the horizontal hyperlocomotion when tested in photobeam cages (Figure 2; Gainetdinov et al., 1999), they did not differ from DAT+/− and DAT+/+ controls in their level of vertical activity in the open-field test. This observation could be related to the aversiveness of the high illumination, which has been reported to diminish rearing behavior (Walsh and Cummins, 1976). Therefore, the level of vertical activity measured in the open field may be an indication of the emotional state of DAT−/− mice induced by high illumination. In addition, DAT−/− mice displayed a weak exploratory behavior toward the novel objects compared to DAT+/− and DAT+/+ mice. Emotional experiences depend on the intervention of three factors to engage a behavior: one determining the motivation toward the stimulus, the second controlling the arousal state and the last one mediating the motor activation with approach or avoidance (Heilman, 1997). Thus, the low number of visits to the objects performed by DAT−/− mice may be explained by the arousal state produced by the open-field test, leading to an avoidance behavior toward the objects, as well as a lack of motivation to approach them. The establishment of a motivational state implicates an activation of DA mesocorticolimbic neurons (Cador et al., 1991), which could be impaired in DAT−/− mice. Another explanation for the decrease in exploratory behavior in DAT−/− mice could be the hyperlocomotion of these animals, which may be incompatible with the exploration of the objects. Indeed, DA agonists have been reported to reduce exploratory behavior in rodents while increasing locomotion (Robbins and Iversen, 1973), indicating that locomotor activation is not necessarily correlated to exploration.
The behavioral dysfunction of DAT−/− mice was also observed after exposure to an inescapable stress. When DAT−/− mice were evaluated in the forced swimming test, they remained engaged in an inappropriate behavior (i.e., swimming) and did not develop what has been interpreted as an adaptive strategy (i.e., floating) (Borsini et al., 1986). The hyperactivity of DAT−/− mice during a stressful situation may be the result of increased DA release, leading to the expression of inappropriate behaviors in regard to the situation (Finlay and Zigmond, 1997; Inglis and Moghaddam, 1999).
By contrast, no social deficits were revealed in DAT−/− mice in the present study. Despite their marked hyperactivity, DAT−/− mice spent the same amount of time in encounter relations and social exploration as DAT+/+ control animals. Although previous studies have shown disturbed social behaviors after chronic treatment with psychostimulant drugs (Ellenbroek and Cools, 1990; Geyer and Markou, 1995), Sams-Dodd (1995) reported that amphetamine-treated rats are able to maintain a normal level of social behavior while they are hyperactive and performing stereotyped behavior. Taken together, these results indicate that locomotor hyperactivity is not always correlated to a deficit in social behavior and that alterations in DA transmission induced by DAT gene deletion or amphetamine administration are not necessarily associated with abnormal social interaction. Moreover, haloperidol and clozapine are not able to restore normal social behavior in rats treated with amphetamine (Steinpreis et al., 1994), further questioning the implication of DA in social behavior. Recent findings suggest that alterations of the glutamate neurotransmitter system result in abnormal social interactions, supporting the glutamate dysfunction hypothesis for the etiology of schizophrenia. For instance, social deficits are more easily induced in rats by chronic phencyclidine, an NMDA receptor antagonist, than by chronic amphetamine (Sams-Dodd, 1995). Moreover, mice with reduced NMDA receptor expression display severe deficits in social interactions (Mohn et al., 1999).
We observed no significant differences in the aggressive behavior displayed by DAT−/−, DAT+/− and DAT+/+ mice toward an unfamiliar intruder of the same genotype and sex. However, we noticed a tendency in DAT−/− and DAT+/− groups to express increased aggressiveness, which may be masked by the experimental design chosen. Indeed, in mice treated with amphetamine, temporal analysis of the attacks revealed a fragmentation and repetition of motor routines with a simultaneous reduction in the influence of environmental cues on the control of behavior (Miczek and Haney, 1994; Moro et al., 1997). Thus, our negative results on a quantitative measure do not exclude possible temporal differences in the aggressive behavior displayed by DAT−/− and DAT+/− mice. Furthermore, we used an experimental design confronting two unfamiliar mice of the same genotype. Because the genotype of the intruder may differently interfere with the aggressive behavior of the resident mice, additional studies using DAT+/+ mice as intruders are necessary to answer this question.
The low survival rate of newborns from DAT−/− females is probably associated to the inability of females to lactate postpartum (Giros et al., 1996). No genotype differences were observed during the maternal pup-care test concerning the first contact, first retrieval and first carrying behaviors. These parameters are closely dependent upon parturition-induced prolactin secretion, which acts on brain mechanisms to either decrease fear and aversion of infant stimuli or increase attraction and approach toward infant stimuli, or both (Numan and Sheehman, 1997). Despite their failure to release prolactin due to the inhibitory action of DA on prolactin synthesis and secretion (Bossé et al., 1997), DAT−/− females reacted correctly to the infant stimulus and carried normally the first pup back into the nest. However, DAT−/− females spent more time between each carrying than DAT+/− and DAT+/+ mice, thus employing longer time to regroup all the pups in the nest. Therefore, the deficit in prolactin secretion may have affected motivation to maintain retrieval behavior in DAT−/− females, as already reported after DA mesencephalic lesions in rats (Numan and Sheehman, 1997). Moreover, DAT−/− females spent considerable time in parallel activities (burying, rearing, grooming) that disturbed mother-pup interactions. These behaviors may result from the heightened arousal state of the mice during the test, leading to distractibility, as already described when DAT−/− mice are tested in a complex situation (Gainetdinov et al., 1999). Pups are also active participants to mother-pup interactions and numerous differences have been reported depending on the sex, the capacity to emit ultrasonic vocalization and the integrity of their DA system (Carlier et al., 1982; Wilkins et al., 1997). For instance, litters composed of DA-depleted pups disturb maternal behavior and induce a high level of burying (Wilkins et al., 1997). Hence, it is possible that DAT−/− females were in addition affected by the behavior of homozygous pups.
In conclusion, the automated locomotor behavior displayed by DAT−/− mice in reaction to novel stimuli may result from an altered environmental perception and sensitized arousal state. Social investigation and aggressive behavior were not affected, suggesting that the modifications of DA transmission in DAT−/− mice did not impair the perception of affectively significant external stimuli. However, the behavioral dysfunctions of DAT−/− mice were progressively exacerbated by the stressfulness of the test, so that another social behavior studied under stressful conditions, the maternal pup-care behavior, was disrupted. The hyperactivity, hypoexploration, difficulty in adapting to novel stimuli and inflexible behaviors displayed by DAT−/− mice do not resemble the behavioral responses of mice injected with high doses of psychostimulant drugs, but rather are similar to the results obtained after lesions of DA mesocorticolimbic projections, as well as after administration of low doses of DA receptor agonists affecting presynaptic release of DA (Eilman et al., 1989; Le Moal, 1995) Therefore, the changes in presynaptic homeostasis described in DAT−/− mice concerning the control of DA synthesis and release by D2 autoreceptors (Jones et al., 1998; 1999) may be strongly implicated in the behavioral profile of DAT−/− mice observed in the present study. Finally, the lack of social deficits in DAT−/− mice questions the role of DA in the social-withdrawal symptoms observed in human psychostimulant-induced paranoia and in the negative symptoms of schizophrenia.
Acknowledgments
This work was supported by grants from INSERM to M.H. and B.G. and Mission Interministérielle de Lutte centre les Drogues et la Toxicomanie (convention 96D04) to B.G. C.S. was supported by a fellowship from the Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche, and C.R. by Sanofi Research and the Fondation pour la Recherche Médicale.
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
References
- Archer J. Tests for emotionality in rats and mice: a review. Anim Behav. 1973;21:205–235. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- Berlyne DE, Koenig DV, Hirota T. Novelty, arousal and the reinforcement of diversive exploration in the rat. J Comp Physiol Psychol. 1966;62:222–226. doi: 10.1037/h0023681. [DOI] [PubMed] [Google Scholar]
- Bertolucci d’Angio M, Serrano A, Driscoll P, Scatton B. Involvement of mesocorticolimbic dopaminergic systems in emotional states. Prog Brain Res. 1990;85:405–416. doi: 10.1016/s0079-6123(08)62692-8. [DOI] [PubMed] [Google Scholar]
- Borsini F, Volterra G, Meli A. Does the behavioral “despair” test measure “despair”? Physiol Behav. 1986;38:385–386. doi: 10.1016/0031-9384(86)90110-1. [DOI] [PubMed] [Google Scholar]
- Bossé R, Fumagalli F, Jaber M, Giros B, Gainetdinov RR, Wetsel WC, Missale C, Caron MG. Anterior pituitary hypoplasia and dwarfism in mice lacking the dopamine transporter. Neuron. 1997;19:127–138. doi: 10.1016/s0896-6273(00)80353-0. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ, Simon H, Le Moal M, Stinus L. Limbic-striatal interactions in reward-related processes: modulation by the dopaminergic system. In: Willner P, Scheel-Krüger J, editors. The mesolimbic dopamine system: from motivation to action. Cambridge: J Wiley & Sons; 1991. pp. 225–250. [Google Scholar]
- Carlier M, Roubertoux P, Cohen-Salmon C. Differences in patterns of pup care in Mus Musculus Domesticus. Comparisons between eleven inbred strains. Behav Neural Biol. 1982;35:205–210. doi: 10.1016/s0163-1047(82)91213-4. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1:179–186. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- Eilman D, Golani I, Szechtman H. D2-agonist quinpirole induces perseveration of routes and hyperactivity but no perseveration of movements. Brain Res. 1989;26:255–267. doi: 10.1016/0006-8993(89)90243-6. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR. Animals models with construct validity for schizophrenia. Behav Pharmacol. 1990;1:469–490. [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ. The effects of stress on central dopaminergic neurons: possible clinical implications. Neurochem Res. 1997;22:1387–1394. doi: 10.1023/a:1022075324164. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. New York: Raven Press; 1995. pp. 787–798. [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Hechtman L. Genetic and neurobiological aspects of attention deficit hyperactive disorder: a review. J Psychiatry Neurosci. 1994;19:193–201. [PMC free article] [PubMed] [Google Scholar]
- Heilman KM. The neurobiology of emotional experience. J Neuropsychiatry Clin Neurosci. 1997;9:439–448. doi: 10.1176/jnp.9.3.439. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Kalivas PW. The role of mesoaccumbens-pallidal circuitry in novelty-induced behavioral activation. Neuroscience. 1995;64:587–597. doi: 10.1016/0306-4522(94)00409-x. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. A quarter century of brain dopamine research. In: Woodruff GN, Poat JA, Roberts PJ, editors. Dopaminergic systems and their regulation. London: Macmillan; 1986. pp. 3–19. [Google Scholar]
- Hughes RN. Intrinsic exploration in animals: motives and measurement. Behav Process. 1997;41:213–226. doi: 10.1016/s0376-6357(97)00055-7. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu X-T, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Lieberman JA. Mechanisms of action of atypical antipsychotic drugs: a critical analysis. Psychopharmacology. 1996;124:2–34. doi: 10.1007/BF02245602. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. Neuropsychiatry Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Le Moal M. Mesocorticolimbic dopaminergic neurons. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. New York: Raven Press; 1995. pp. 283–294. [Google Scholar]
- Meltzer HY, Matsubara S, Lee J. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D1, D2 and 5-HT2 pKi values. J Pharmacol Exp Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- Miczek A, Haney M. Psychomotor stimulant effects of d-amphetamine, MDMA and PCP: aggressive and schedule-controlled behavior in mice. Psychopharmacology. 1994;115:358–365. doi: 10.1007/BF02245077. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Roller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Moro M, Salvador A, Simon VM. Changes in the structure of the agonistic behavior of mice produced by D-amphetamine. Pharm Biochem Behav. 1997;56:47–54. doi: 10.1016/S0091-3057(96)00153-0. [DOI] [PubMed] [Google Scholar]
- Numan M, Sheehan TP. Neuroanatomical circuitry for mamalian maternal behavior. Ann N Y Acad Sci. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Relationship between circadian changes in spontaneous motor activity and dorsal versus ventral striatal dopamine neurotransmission assessed with on-line microdialysis. Behav Neurosci. 1994;108:624–635. doi: 10.1037//0735-7044.108.3.624. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Blavet N, Deniel M, Jalfre Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. Eur J Pharmacol. 1979;57:201–210. doi: 10.1016/0014-2999(79)90366-2. [DOI] [PubMed] [Google Scholar]
- Puech AJ, Rioux P, Poncelet M, Brochet D, Chermat R, Simon P. Pharmacological properties of new antipsychotic agents: use of animal models. Neuropharmacology. 1981;20:1279–1284. [PubMed] [Google Scholar]
- Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997;76:707–714. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- Robbins T, Iversen SD. A dissociation of the effects of d-amphetamine on locomotor activity and exploration in rats. Psychopharmacologia. 1973;28:155–164. doi: 10.1007/BF00421400. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Distinct effects of d-amphetamine and phencyclidine on the social behaviour of rats. Behav Pharmacol. 1995;6:55–65. [PubMed] [Google Scholar]
- Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology. 1997;132:303–310. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–4606. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinpreis RE, Sokolowski JD, Papanikolaou A, Salamone JD. The effects of haloperidol and clozapine on the PCP- and amphetamine-induced suppression of social behavior in the rat. Pharm Biochem Behav. 1994;47:579–585. doi: 10.1016/0091-3057(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The open field test: a critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- Wilkins A, Logan M, Kehoe P. Postnatal pup brain dopamine depletion inhibits maternal behavior. Pharm Biochem Behav. 1997;58:867–873. doi: 10.1016/s0091-3057(97)00051-8. [DOI] [PubMed] [Google Scholar]


