Abstract
Aims
Elevated homocysteinaemia is associated not only with an increased risk for cardiovascular disease but also for increased morbidity and mortality in patients with established coronary artery or cerebrovascular disease. Whether elevated homocysteine further increases the morbidity and mortality in patients undergoing cardiac surgery on cardiopulmonary bypass (CPB) (a prothrombotic state itself) remains less known.
Methods and results
Accordingly, we conducted a prospective observational study with pre-operative measurement of plasma homocysteine levels in 531 consecutive patients undergoing cardiac operations on CPB. The association of pre-operative plasma homocysteine levels with post-operative morbidity and hospital mortality was evaluated. Elevated homocysteine levels (>15 µmol/L) were observed in 209 patients (39.4%), and homocysteinaemia was associated with a higher mortality and perioperative morbidity (major morbidity, low cardiac output, acute renal failure, mesenteric infarction, and thrombo-embolic events). Even after accounting for the differences in baseline clinical features, EuroSCORE, and CPB time, pre-operative homocysteine levels remained independently associated with hospital mortality [odds ratio (OR) 1.06, 95% confidence interval (CI) 1.03–1.11], major morbidity (OR 1.04, 95% CI 1.01–1.07), low cardiac output (OR 1.04, 95% CI 1.02–1.08), mesenteric infarction (OR 1.06, 95% CI 1.01–1.11), and thrombo-embolic events (OR 1.09, 95% CI 1.04–1.13). This association of homocysteine with increased risk of morbidity and mortality was observed particularly in CABG patients.
Conclusion
Elevated pre-operative homocysteine level is independently associated with increased morbidity and mortality, particularly in patients undergoing CABG. Specific post-operative antithrombotic strategies may be advisable in hyperhomocysteinaemic patients.
Keywords: Homocysteine, Risk factors, Cardiac surgery, Outcomes
Introduction
Prior investigations have shown that hyperhomocysteinaemia (HHC) is associated with an increased risk for cardiovascular disease, including coronary artery disease, peripheral arterial disease, stroke, and venous thrombosis.1–5 Similarly, elevated homocysteine levels have been demonstrated to be associated with increased risk of morbidity and mortality in patients with established coronary artery or cerebrovascular disease.6–8 Evidence exists supporting significant impact of homocysteine on endothelial resistance to thrombosis as well as on vasodilatory and antithrombotic effects of nitric oxide, potentially accounting for some of the increased risks of these recurrent events in patients with cardiovascular disease.9–11
Patients undergoing cardiac surgery are at significant risk for serious adverse arterial and venous thrombotic events, including myocardial infarction, unstable angina, mesenteric ischaemia, stroke, pulmonary embolism, and deep vein thrombosis, resulting in high operative morbidity and mortality.12–15 However, the prevalence and/or the relationship of pre-operative homocysteine with perioperative cardiovascular events have not been previously explored adequately in this high-risk cohort. Accordingly, the major goal of the current study was to ascertain the prevalence of pre-operative HHC as well as evaluate its relationship with perioperative adverse events in patients undergoing cardiac surgery at our institution. We hypothesized that many patients undergoing cardiac surgery are likely to have elevated homocysteine levels because of significant epidemiological evidence linking homocysteine with cardiovascular disease.1–5 Additionally, we theorized that the high levels of plasma homocysteine may be potentially associated with an increased thrombo-embolic risk in these patients undergoing cardiac operations, and therefore may represent an independent risk factor for perioperative morbidity and mortality.
Methods
Patient population and study design
Patients scheduled for cardiac operations at our institution (IRCCS Policlinico S. Donato, Milan, Italy) have a plasma homocysteine level measurement as a part of pre-operative assessment since the beginning of the year 2007. For the purpose of this study, we included all adult patients ≥18 years of age undergoing cardiac surgery requiring cardiopulmonary bypass (CPB) who had pre-operative homocysteine measured. Patients were excluded if they had an off-pump surgery or did not have a homocysteine measurement. All the patients gave written informed consent to the scientific treatment of their clinical data. Surgical and medical treatments were at the discretion of surgical team in accordance with evidence-based guidelines, with no specific treatment mandated or new intervention applied as a part of this study. This observational study was approved by the local ethical committee in May 2007. As a result, only patients who underwent cardiac surgery after 1 June 2007 were considered for this study.
Data collection
Data on patient demographic, past medical history, comorbid conditions, details of surgical procedural information, perioperative laboratory values, management, and treatments, and post-operative outcomes including length of stay and 30-day mortality are routinely collected for all patients undergoing cardiac surgery at our institution. For the purpose of this analysis, we linked this routinely collected information from the institutional database to the homocysteine laboratory data. The data elements are routinely filled out at three different sites: ward (for pre- and late post-operative data); operating room (for intra-operative data); and in the Intensive Care Unit (ICU). The operative risk was determined according to the logistic EuroSCORE; all the risk conditions were defined according to the EuroSCORE definitions.16
Anaesthesia, surgery, and cardiopulmonary bypass
Pre-medication included atropine sulphate (0.5 mg), prometazine (50 mg), and fentanyl (50–100 µg according to the patient’s weight) intra-muscularly administered 1 h before the induction of anaesthesia. Anaesthesia was induced with an intra-venous infusion of remifentanil (starting dose 0.5 µg kg−1 min−1) and a midazolam bolus of 0.2 mg/kg. Cisatracurium besylate (0.2 mg/kg) was subsequently administered to allow tracheal intubation. Subsequently, anaesthesia was maintained with a continuous infusion of remifentanil (dose ranging from 0.05 to 1 µg kg−1 min−1, titrated on the basis of the haemodynamic response) and midazolam (0.1 mg kg−1 h−1) or propofol (3 mg kg−1 h−1). Cardiopulmonary bypass was conducted using either closed or open circuits, standard or phosphorylcholine-coated hollow-fibre oxygenators, and roller or centrifugal pumps according to availability. Regardless of the circuit used, the priming volume was always minimized to 800–1000 mL. Lowest core body temperature during CPB varied from 27 to 37°C as requested by the surgeon. Antegrade intermittent cold crystalloid or cold blood cardioplegia was used according to the surgeon’s preference. The pump flow was targeted between 2.0 and 2.8 L min−1 m−2, and the target mean arterial pressure was settled at 60 mmHg. Anticoagulation was established with an initial dose of 300 IU per kilogram of body weight of porcine intestinal heparin injected into a central venous line 10 min before the initiation of CPB and a target-activated clotting time of 480 s; patients receiving closed and biocompatible circuits received a reduced dose of heparin with a target-activated clotting time settled at 300 s. At the end of CPB, heparin was reversed by protamine chloride at a 1:1 ratio of the loading dose, regardless of the total heparin dosage.
Homocysteine measurement
To determine the pre-operative serum homocysteine value, venous blood samples were taken between 7.30 and 9.00 a.m. (after overnight fast), usually the day before the operation. Blood samples were collected in tubes containing EDTA, immediately placed on ice, and plasma was separated within 1 h. Plasma was stored at –20°C until analysis. Total plasma homocysteine levels were determined using an immunofluorescence method (AxSYM, Abbott) already validated for this type of analysis,17 and applied in a previous large randomized controlled trial.18 The method included a standard 6-points calibration; for patients with levels of homocysteine exceeding 50 µmol/L, an automated dilution protocol was applied. The inter-day coefficient of variation of the analytic method was 4.36, 5.64, and 6.55%, respectively, for control samples containing a high, medium, and low homocysteine concentration. Hyperhomocysteinaemia was defined as a plasma level of homocysteine exceeding 15 µmol/L.19
Sample size estimation
The primary endpoint was to investigate the possible association between plasma homocysteine levels and complications and mortality following cardiac operations. The sample size was determined by a power analysis targeted on thrombo-embolic complications and based on the available data and some assumptions outlined as follows:
The post-operative thrombo-embolic complication rate (myocardial infarction, stroke, mesenteric infarction, pulmonary and peripheral embolism) was ascertained using the Institutional Database for patients operated between 2000 and 2006 (n = 8,126 patients). The total thrombo-embolic complication rate was 4.8% (myocardial infarction 3.0%, stroke 1.4%, mesenteric infarction 0.3%, pulmonary and peripheral embolism 0.1%).
The experimental hypothesis was that patients with HHC would have a higher rate of post-operative thrombo-embolic events than patients without HHC. The rate of thrombo-embolic events in patients with HHC was assumed to be twice that in those without HHC.
The prevalence of HHC in the cardiac surgical population is unknown. However, it is reasonable to assume it to be higher in patients with cardiovascular disease than the 11% prevalence of HHC described in the European population.18 Considering this basic information, and looking retrospectively at our data from the first three months of 2007, we assumed a prevalence of HHC of 30% in our patient population.
Based on the overall event rate of 4.8% in the 8126 patients undergoing cardiac surgery between 2000 and 2006 and on these assumptions for the prevalence of HHC (30%) as well as twice the expected event rate in HHC patients compared with those without it, we estimated an event rate of 3.7% for non-HHC patients and 7.4% for HHC patients.
Therefore, the sample size was settled at 520 patients, with a predicted distribution of 364 non-HHC and 156 HHC subjects, with predicted thrombo-embolic event numbers of, respectively, 13 (3.7%) and 12 (7.4%), relative risk 2.2, 95% confidence interval 1.003–5.05, and P < 0.05.
Statistical analysis
Data are expressed as median with interquartile range for continuous variables and as number and percentage for binary categorical variables. Homocysteine values were treated as a continuous variable in all the statistical tests. For display purpose in the tables, homocysteine was grouped into quintiles to show the distribution and values of perioperative and outcome variables.
The following perioperative variables were tested for univariate association with homocysteine levels: demographics and past medical history (hypertension, diabetes on medication, chronic obstructive pulmonary disease, peripheral vascular disease, heart failure, recent myocardial infarction, unstable angina, congestive heart failure, left ventricular ejection fraction, pre-operative use of intra-aortic balloon pump, prior vascular surgery, prior cardiac surgery, prior cerebrovascular accident, chronic dialysis, serum creatinine value, creatinine clearance, haematocrit). Mortality risk was assessed with the logistic EuroSCORE. Operative variables considered were: type of operation, CPB duration, aortic cross-clamping duration, lowest haematocrit on CPB, and lowest temperature on CPB.
The univariate association of homocysteine levels with the following outcome variables was also assessed: time on ventilator, ICU stay, hospital stay; in-hospital mortality; acute renal failure, peak serum creatinine value, atrial fibrillation (new onset), ventricular arrhythmias, pneumonia, sepsis, mediastinitis, stroke, re-operation for bleeding, severe bleeding, need for allogeneic blood transfusions, low cardiac output syndrome, myocardial infarction, lung dysfunction, mesenteric infarction, any thrombo-embolic event (myocardial infarction, stroke, mesenteric infarction, pulmonary or systemic embolism), and major morbidity (any of the following: need for re-operation, sternal wound infection, permanent stroke, renal failure, mechanical ventilation for >48 h).
Differences in homocysteine values were tested using a Student’s t-test for independent samples (binary variables) or an analysis of variance for continuous variables. The association between pre-operative homocysteine levels and outcome variables was tested with a multivariable logistic regression or linear regression analysis (as appropriate), generating the odds ratio (for 1 U change in homocysteine level) with 95% confidence interval.
To avoid overfitting of the models, a limited number of independent variables were admitted after accounting for different comorbidities, the absence of multicollinearity, and their clinical importance as defined by previous studies. These variables included EuroSCORE and CPB duration for all outcome variables and pre-operative serum creatinine for acute renal failure. The risk for Type I error due to multiple testing was addressed using a Bonferroni correction for the P-values required to reject the null hypothesis. Multicollinearity within multivariable models was addressed with a multicollinearity diagnostics and tolerance statistics.
Sensitivity analysis was also performed to evaluate the association of pre-operative homocysteine with outcomes in patients undergoing CABG and/or valves and in patients with isolated valve surgery.
The predictive value of pre-operative homocysteine in determining the thrombo-embolic events and the hospital mortality rate was tested with a receiver operating characteristics (ROC) analysis, with assessment of the area under the curve (AUC) and standard error of the AUC. Cut-off values were searched and tested for sensitivity and specificity (with 95% confidence interval). The cut-off point assessment was based on the pre-requisite of a sensitivity of at least 70%, based on the coordinates of the ROC curve. The cut-off values were settled at the point where the sum of sensitivity and specificity was the highest, according to Youden’s index: (sensitivity + specificity)−1.
The ROC estimates were validated with bootstrapping techniques, which involved drawing a random sample of 531 cases (with replacement) from the original sample of 531 cases. A P-value <0.05 was considered significant for all the statistical tests. All the statistical tests were two-sided. Statistical calculations were performed using a computerized statistical program (SPSS 13.0, Chicago, IL, USA).
Results
Study population and patients' demographic and surgical data
Between June 2007 and January 2008, 820 patients underwent cardiac surgical procedures at our institution. Of these, 205 patients who had paediatric cardiac surgery, 35 patients who received an off-pump operation, and 49 cases with no pre-operative value of homocysteine available due to technical laboratory problems or to the emergent condition of the operation were excluded from this analysis. The remaining 531 patients formed the basis of this study, fulfilling the estimated sample size.
The 49 cases excluded due to unavailability of the pre-operative homocysteine value had significantly higher rate of emergent operations (20 vs. 0.2%, P < 0.001), EuroSCORE (7.5 ± 2 vs. 6.4 ± 7.6, P = 0.017), heart failure (20 vs. 0.2%, P < 0.001), and intra-aortic balloon pump use (6 vs. 0.2%, P = 0.002) and lower left ventricular ejection fraction (48 ± 13 vs. 52 ± 11, P = 0.022). The other pre- and intra-operative factors were not significantly different.
Demographics, pre-operative and operative data of our patient population are reported in Tables 1 and 2 for the overall population and for quintiles of distribution of the homocysteine level. The mean pre-operative homocysteine level in our patient population was 15.4 ± 8.3 µmol/L and the median value was 13.7 µmol/L (IQR 10.5–17.4 µmol/L, range 1.7–92 µmol/L). In the subgroup of patients with coronary artery disease, the baseline values were 15.7 ± 8.6 µmol/L and the median value was 13.9 µmol/L (IQR 10.6–17.5 µmol/L, range 1.7–92 µmol/L).
Table 1.
Demographics and clinical features in the overall population and homocysteine quintiles
| Factor | All patients | Homocysteine subgroups (quintiles)–range (μmol/L) |
P-value | ||||
|---|---|---|---|---|---|---|---|
| Median (IQR) or n(%) | I (1.7–9.8) | II (9.9–12.5) | III (12.6–14.8) | IV (14.9–19.1) | V (19.2–92.3) | ||
| Demographics | |||||||
| Age (years) | 68 (59–74) | 65 (56–70) | 66 (55–71) | 69 (59–70) | 70 (59–76) | 71 (61–77) | 0.001 |
| Gender, male | 375 (70.6) | 59 (56) | 74 (69) | 79 (74) | 79 (74) | 84 (78) | 0.021 |
| Height (cms) | 168 (160–174) | 165 (160–171) | 168 (162–174) | 168 (160–173) | 168 (162–174) | 170 (161–175) | 0.179 |
| Weight (kg) | 73 (65–80) | 74 (53–83) | 74 (63–80) | 72 (66–79) | 75 (65–83) | 72 (65–80) | 0.317 |
| Past medical history | |||||||
| Hypertension | 220 (41.4) | 41 (39) | 41 (38) | 46 (43) | 46 (44) | 46 (43) | 0.182 |
| Diabetes mellitus | 57 (10.7) | 15 (14) | 6 (6) | 14 (13) | 7 (7) | 15 (14) | 0.939 |
| COPD | 21 (4.0) | 2 (2) | 5 (5) | 4 (4) | 3 (3) | 7 (6) | 0.146 |
| PVD | 48 (9.0) | 8 (8) | 10 (9) | 9 (9) | 11 (10) | 10 (9) | 0.128 |
| Heart failure | 1 (0.2) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0.895 |
| Prior cardiac surgery | 22 (4.1) | 2 (2) | 5 (4) | 4 (4) | 7 (7) | 4 (4) | 0.983 |
| Prior vascular surgery | 21 (4.0) | 1 (1) | 1 (1) | 7 (7) | 3 (3) | 9 (8) | 0.092 |
| Prior CVA | 15 (2.8) | 1 (1) | 4 (4) | 4 (4) | 2 (2) | 4 (4) | 0.408 |
| Chronic dialysis | 9 (1.7) | 1 (1) | 0 (0) | 1 (1) | 4 (4) | 3 (3) | 0.216 |
| Recent MI (<30 days) | 45 (8.5) | 13 (12) | 3 (3) | 8 (8) | 9 (9) | 12 (11) | 0.387 |
| Unstable angina | 30 (5.6) | 6 (6) | 10 (9) | 5 (5) | 3 (3) | 6 (6) | 0.176 |
| Pre-operative CHF | 16 (3.0) | 4 (4) | 1 (1) | 1 (1) | 4 (4) | 6 (6) | 0.301 |
| Pre-operative IABP | 1 (0.2) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0.895 |
| Left ventricular EF (%) | 54 (45–60) | 55 (50–60) | 55 (48–60) | 53 (45–60) | 53 (41–60) | 50 (38–57) | 0.001 |
| Serum creatinine (mg/dL) | 1 (0.8–1.2) | 0.8 (0.7–1.0) | 0.8 (0.8–1.0) | 1.0 (0.8–1.1) | 1.0 (0.8–1.2) | 1.2 (1.0–1.6) | 0.001 |
| Creatinine clearance (mL/min) | 75.5 (54.7–95.5) | 85 (71–105) | 88 (67–114) | 72 (58–91) | 72 (50–93) | 57 (34–86) | 0.001 |
| Haematocrit (%) | 39 (36–42) | 39 (36–42) | 40 (37–42) | 39 (35–42) | 40 (37–42) | 38 (34–42) | 0.110 |
| Homocysteine level (μmol/L) | 13.7 (10.5–17.4) | 8.3 (7–9) | 11.2 (11–12) | 13.8 (13–14) | 16.2 (16–17) | 24.2 (21–28) | 0.001 |
| Hyperhomocysteinaemia | 209 (39.4) | 0 (0) | 0 (0) | 0 (0) | 100 (96) | 109 (100) | 0.001 |
| EuroSCORE | 4 (2.1–7) | 3.1 (2–5) | 3.5 (2–7) | 4 (2–7) | 5 (3–7) | 6 (3–10) | 0.001 |
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; EF, ejection fraction; IABP, intra-aortic balloon pump; IQR, interquartile range; MI, myocardial infarction; PVD, peripheral vascular disease.
Table 2.
Cardiac surgical data in the overall population and homocysteine quintiles
| Factor | All patients | Homocysteine subgroups (quintiles)—range (μmol/L) |
P-value | ||||
|---|---|---|---|---|---|---|---|
| Median (IQR) or n (%) | I (1.7–9.8) | II (9.9–12.5) | III (12.6–14.8) | IV (14.9–19.1) | V (19.2–92.3) | ||
| Isolated coronary surgery | 262 (49.3) | 49 (46) | 51 (48) | 61 (57) | 44 (41) | 57 (52) | 0.637 |
| Isolated valve surgery | 156 (29.4) | 43 (41) | 29 (27) | 24 (22) | 32 (30) | 28 (26) | 0.317 |
| Combined procedure | 113 (21.3) | 16 (15) | 26 (24) | 21 (19) | 30 (28) | 20 (18) | 0.102 |
| CPB duration (min) | 68 (54–95) | 62 (52–84) | 68 (52–92) | 65 (51–101) | 75 (57–95) | 70 (55–96) | 0.048 |
| Closed, biocompatible circuits | 145 (27) | 28 (27) | 33 (31) | 28 (27) | 28 (27) | 28 (27) | 0.965 |
| Aortic cross-clamp time | 45 (35–66) | 42 (35–62) | 47 (36–64) | 42 (34–62) | 49 (37–69) | 46 (33–45) | 0.424 |
| Nadir haematocrit on CPB (%) | 27 (24–29) | 27 (24–29) | 28 (25–30) | 27 (24–29) | 28 (24–31) | 26 (24–29) | 0.966 |
| Nadir temperature on CPB (°C) | 32 (31.7–33) | 32 (32–33) | 32 (32–33) | 32 (32–33) | 32 (31–33) | 32 (32–33) | 0.747 |
CPB, cardiopulmonary bypass; IQR, interquartile range.
A total of 209 patients (39.4%) had a pre-operative plasma homocysteine level >15 µmol/L, fulfilling the definition for HHC. There was a significant positive association between homocysteine level and age, gender male, serum creatinine value, EuroSCORE, and CPB duration; and an inverse association with left ventricular ejection fraction and creatinine clearance.
Association of homocysteinaemia with perioperative events
Pre-operative homocysteine level (Table 3) was significantly associated with the time on ventilator, the ICU stay, and the peak post-operative serum creatinine value. It was significantly higher in patients who developed acute renal failure, low cardiac output, and major morbidity. A trend towards higher homocysteine values was observed in patients suffering from thrombo-embolic events and hospital mortality. Results of multivariable linear or logistic regression models having outcome variables as dependent variables and pre-operative homocysteine level as independent variable are shown in Table 4. Only events occurring in at least five patients (1%) have been considered. The tolerance values for each independent variable were always >0.8, thus excluding multicollinearity risk. After the Bonferroni correction, the P-value to reject the null hypothesis in the setting of 18 different outcomes comparison was determined to be 0.0028.
Table 3.
Clinical events in the overall population and homocysteine quintiles
| Factor | All patients | Homocysteine subgroups (quintiles)—range (μmol/L) |
P-value | ||||
|---|---|---|---|---|---|---|---|
| Median (IQR) or n(%) | I (1.7–9.8) | II (9.9–12.5) | III (12.6–14.8) | IV (14.9–19.1) | V (19.2–92.3) | ||
| Length of stay | |||||||
| Time on ventilator (h) | 12 (9–20) | 11 (7–15) | 11.5 (8–16) | 12 (10–24) | 13 (10–20) | 16 (11–38) | 0.004 |
| ICU stay (days) | 2 (1–4) | 2 (1–3) | 2 (1–3) | 2 (1–4) | 2 (1–3) | 3 (1–6) | 0.004 |
| Hospital stay/days) | 7 (1–11) | 7 (1–10) | 8 (1–12) | 7 (1–10) | 7 (1–10) | 8 (1–12) | 0.924 |
| In-hospital events | |||||||
| Death | 18 (3.4) | 1 (1) | 2 (2) | 3 (3) | 5 (5) | 7 (6) | 0.073 |
| Mediastinitis | 6 (1.1) | 1 (1) | 0 (0) | 2 (2) | 2 (2) | 1 (1) | 0.972 |
| Peak serum creatinine (mg/dL) | 1 (0.8–1.3) | 0.8 (0.7–1.0) | 0.9 (0.7–1.1) | 1.0 (0.9–1.3) | 1.1 (0.8–1.6) | 1.2 (0.9–1.8) | 0.001 |
| Acute renal failure | 23 (3.8) | 1 (1) | 3 (3) | 4 (4) | 3 (3) | 12 (11) | 0.016 |
| Atrial fibrillation | 27 (5.1) | 6 (6) | 6 (6) | 4 (4) | 3 (3) | 8 (7) | 0.870 |
| Ventricular arrhythmias | 3 (0.6) | 1 (1) | 0 (0) | 2 (2) | 2 (2) | 1 (1) | 0.836 |
| Pneumonia | 4 (0.8) | 1 (1) | 0 (0) | 1 (1) | 1 (1) | 1 (1) | 0.287 |
| Sepsis | 9 (1.7) | 0 (0) | 0 (0) | 3 (3) | 3 (3) | 3 (3) | 0.522 |
| Stroke | 2 (0.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 0.381 |
| Re-operation for bleeding | 9 (1.7) | 2 (2) | 2 (2) | 1 (1) | 1 (1) | 3 (3) | 0.652 |
| Bleeding >1000 mL | 29 (5.5) | 6 (6) | 4 (4) | 5 (5) | 4 (4) | 10 (9) | 0.128 |
| Allogeneic transfusions | 105 (19.8) | 15 (14) | 13 (12) | 20 (19) | 19 (18) | 38 (35) | 0.001 |
| Low output syndrome | 70 (13.2) | 7 (7) | 9 (8) | 17 (16) | 10 (9) | 27 (25) | 0.001 |
| Myocardial infarction | 7 (1.3) | 1 (1) | 1 (1) | 2 (2) | 2 (2) | 1 (1) | 0.893 |
| Lung dysfunction | 8 (1.6) | 1 (1) | 0 (0) | 2 (2) | 2 (2) | 2 (2) | 0.675 |
| Mesenteric infarction | 5 (0.9) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 3 (3) | 0.087 |
| Any thrombo-embolic event | 16 (3.0) | 1 (1) | 2 (2) | 2 (2) | 3 (3) | 8 (8) | 0.052 |
| Major morbiditya | |||||||
| Overall patients | 34 (6.4) | 3 (3) | 4 (4) | 7 (7) | 4 (4) | 16 (15) | 0.007 |
| CABG patients (n=332) | 24 (7.1) | 2 (3.4) | 2 (2.9) | 5 (6.7) | 3 (5.2) | 12 (16) | 0.012 |
| Non-CABG patients (n=199) | 10 (5.2) | 1 (2.1) | 2 (5.4) | 2 (6.5) | 1 (2.2) | 4 (12.1) | 0.438 |
CABG, coronary artery bypass graft; ICU, intensive care unit; IQR, interquartile range.
aDefined as the presence of any one of the following: need for re-operation, sternal wound infection, permanent stroke, renal failure, and/or prolonged ventilation (defined as >48 h).
Table 4.
Multivariable analyses (linear or logistic regression) for outcome variables association with pre-operative homocysteinaemia
| Outcome variable | Multivariable analysisa (overall patients) |
Multivariable analysisa (CABG patients) |
||||
|---|---|---|---|---|---|---|
| b (95% CI) | OR (95% CI) | P-value | b (95% CI) | OR (95% CI) | P-value | |
| Mechanical ventilation | 0.796 (0.178–1.415) | – | 0.012b | 0.956 (0.154–1.759) | – | 0.020b |
| ICU stay | 0.053 (0.003–0.109) | – | 0.063 | 0.057 (−0.022–0.137) | – | 0.159 |
| Hospital mortality | 0.063 (0.03–0.104) | 1.06 (1.03–1.11) | 0.001 | 0.091 (0.02–0.157) | 1.09 (1.02–1.17) | 0.010b |
| Allogeneic transfusions | 0.045 (0.02–0.068) | 1.05 (1.02–1.07) | 0.001 | 0.047 (0.01–0.086) | 1.05 (1.01–1.09) | 0.022b |
| Low cardiac output | 0.045 (0.02–0.077) | 1.04 (1.02–1.08) | 0.002 | 0.058 (0.02–0.104) | 1.06 (1.02–1.11) | 0.010b |
| Acute renal failurec | 0.038 (0.01–0.068) | 1.04 (1.01–1.07) | 0.031b | 0.084 (0.01–0.157) | 1.09 (1.01–1.17) | 0.031b |
| Mesenteric infarction | 0.056 (0.01–0.104) | 1.06 (1.01–1.11) | 0.014b | 0.104 (0.03–0.174) | 1.11 (1.03–1.19) | 0.006b |
| Thrombo-embolic events | 0.082 (0.039–0.122) | 1.09 (1.04–1.13) | 0.001 | 0.085 (0.02–0.148) | 1.09 (1.02–1.16) | 0.007b |
| Major morbidity | 0.041 (0.01–0.068) | 1.04 (1.01–1.07) | 0.008b | 0.067 (0.02–0.113) | 1.07 (1.02–1.12) | 0.008b |
aAdjusted for EuroSCORE and cardiopulmonary bypass duration.
bNot significant after Bonferroni correction.
cAdjusted for EuroSCORE, cardiopulmonary bypass duration, and pre-operative serum creatinine value.
Even after accounting for differences in baseline EuroSCORE and time of CPB (and in case of acute renal failure, also pre-operative serum creatinine), homocysteine level was an independent risk factor for prolonged mechanical ventilation time, in-hospital need for transfusion(s), low cardiac output syndrome, mesenteric infarction, any thrombo-embolic events, and mortality. Furthermore, despite the conservative P-value after the Bonferroni correction, the association between homocysteine and various outcomes persisted (significantly or showed a non-significant trend). Particularly, any thrombo-embolic event and mortality still remained highly statistically significant despite this conservative P-value accounting for multiple comparisons.
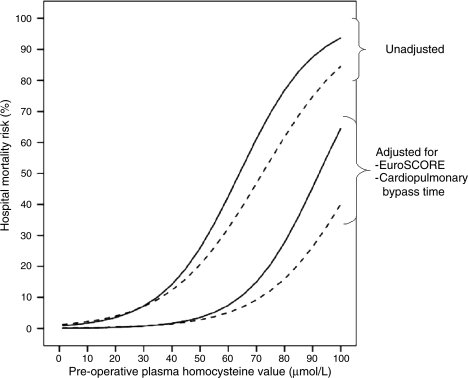
Although, our estimate of sample size was not based exclusively on CABG patients, sensitivity analysis demonstrated that the risk of any thrombo-embolic event (adjusted OR 1.09, 95% CI 1.02–1.16, P = 0.007) and in-hospital mortality (adjusted OR 1.09, 95% CI 1.02–1.17, P = 0.01) were significantly associated with homocysteine level in the subgroup of patients undergoing CABG with or without valve surgery (Table 4). Trends were also observed for the association between homocysteine and outcomes in the subgroup of patients with CABG after the Bonferroni correction. Whereas similar higher trends for any thrombo-embolism (adjusted OR 1.05, 95% CI 0.96–1.12, P = 0.31) and in-hospital mortality (adjusted OR 1.02, 95% CI 0.93–1.12, P = 0.67) were noted among patients undergoing valvular surgery, these differences were not statistically significant. Figure 1 depicts the unadjusted and adjusted mortality rates (derived using multivariable logistic regression analysis) as a function of the pre-operative homocysteine value in patients undergoing any cardiac surgery and in those undergoing CABG.
Figure 1.
The relationship between pre-operative plasma homocysteine values and in-hospital mortality (undajusted and adjusted-derived using multivariable logistic regression analysis) for patients undergoing any cardiac surgery (dashed lines) and for those undergoing coronary artery bypass graft surgery (continuous lines).
Finally, we explored the value of pre-operative homocysteine levels in predicting the mortality and thrombo-embolic complications and in-hospital mortality risk applying an ROC analysis. The results for the crude patient population and for the internal validation obtained with a bootstrap technique are reported in Table 5. Pre-operative homocysteine levels have an acceptable predictive ability for thrombo-embolic events (AUC 0.71), but lower for in-hospital mortality (AUC 0.68). Similar values were detected in the bootstrap series. Values of homocysteine around 15 µmol/L were associated with a sensitivity and specificity of 70 and 64% (thrombo-embolic events) and 69 and 61% (in-hospital mortality), respectively. However, the low level of specificity and the large 95% CI preclude robust confirmation of these values as being clinically relevant.
Table 5.
Receiver operating characteristics analysis for pre-operative homocysteine level as predictor for thrombo-embolic complications and mortality: derivation series and bootstrap series
| Development series |
Bootstrap series |
|||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | AUC (95% CI) | Cut-off value (µmol/L) | Sensitivity, % | Specificity, % | AUC (95% CI) | Cut-off value (95% CI) | Sensitivity (95% CI), % | Specificity (95% CI), % |
| In-hospital mortality | 0.68 (0.56–0.80) | 14.8 | 72 | 61 | 0.67 (0.55–0.78) | 14.8 (14.5–15.1) | 69 (61–80) | 61 (55–67) |
| Thrombo-embolic events | 0.71 (0.56–0.84) | 14.3 | 75 | 56 | 0.70 (0.57–0.84) | 15.4 (14.9–16.0) | 70 (61–83) | 64 (55–82) |
AUC, area under the curve; CI, confidence interval.
Discussion
Our study findings
Our data suggest that the prevalence of pre-operative HHC is high, with two of every five patients undergoing cardiac surgery having elevated homocysteine values >15 µmol/L. Importantly, HHC was independently associated with increased risk of perioperative morbidity and mortality. This increased risk of adverse events was only partially explained by older age and greater comorbid conditions such as lower left ventricular ejection fraction and lower creatinine clearance in this cohort (accounting for their higher EuroSCORE) as even after adjustment for these baseline confounders, HHC remained an independent correlate of higher need for transfusion, acute renal failure, low cardiac output syndrome, any thrombo-embolic event, but more importantly, in-hospital mortality. The association of elevated homocysteine level with mortality was observed predominantly among patients undergoing CABG, but not in patients undergoing isolated valve surgery.
Comparison with previous studies
The association between homocystinuria, a genetic disorder with extremely high plasma homocysteine levels (in excess of 100 µmol/L), and premature atherosclerosis, increased risk of thrombo-embolism and premature death has been known for more than last five decades.20,21 This knowledge subsequently lead to series of epidemiological investigations that have consistently shown that even moderate elevation of homocysteine is associated with increased risk of coronary artery disease, peripheral arterial disease, stroke, and venous thrombo-embolic disease.1–5 Additionally, in patients with established cardiovascular disease, elevated homocysteine levels are related to higher morbidity and mortality risk.6–8
Storti et al.22 studied the changes in homocysteine levels in a population of patient who underwent coronary revascularization. They detected a mean pre-operative value (17.3 µmol/L) higher than the one found in the present study for coronary patients. The homocysteine levels significantly decreased after the operation. No outcome analyses were performed in this study, which included a limited (48) number of patients.
Our data in the CABG population suggest heightened risk of perioperative morbidity and mortality in patients with elevated homocysteine undergoing surgical coronary revascularization. However, the previously quoted studies suggested that in patients with cardiovascular disease, elevated homocysteine levels confer heightened risk of adverse events over intermediate or long-term follow-up.1–8 Our data complement these results, indicating that this increased risk is observed even acutely (in perioperative period) in CABG patients. This is not surprising if one were to consider the prevailing hypothesis on the mechanisms underlying the association of elevated homocysteine with adverse outcomes in patients with cardiovascular disease. A high level of homocysteine impairs endothelial function9 and decreases the bioavailability of nitric oxide from endothelial cells.10 It also reduces the affinity of antithrombin (an anticoagulant) to bind to endothelial cells.23 Finally, it appears that homocysteine may limit the coagulation inhibitory effect normally exerted by the thrombomodulin-protein C complex through a reduction in protein C activation rate.24 These mechanisms decrease endothelial resistance to thrombosis, reduce vasodilatory effects of nitric oxide, and promote thrombosis. Similarly, cardiac operations with CPB itself lead to the hypercoaguable state by the generation of massive doses of thrombin that consumes antithrombin,25 by decreasing nitric oxide synthesis,25,26 and by reducing protein C and tissue factor pathway inhibitor.27 Therefore, it is easy to comprehend why elevated homocysteine may further aggravate the hypercoaguable state observed among patients undergoing CABG, thereby compounding the thrombo-embolic risk in these patients.
That perhaps these mechanisms are operative is supported to some extent by the fact that the great majority of perioperative complications observed in patients with elevated homocysteine undergoing cardiac surgery seem to be thrombo-embolic events resulting from either macrovascular clots in mesenteric and cerebral circulation (mesenteric infarction and a non-significant increase in stroke) or microvascular thrombosis in coronary and renal microcirculation (low cardiac output and acute renal failure). As a matter of fact, the highest odd of an adverse event associated with elevated homocysteine level was with total thrombo-embolic events (9% probability increase for each 1 µmol/L increment).
Clinical implications
Is there a role for routine measurement of homocysteine in the management of patients undergoing cardiac surgery? Our data suggest that perhaps homocysteine imparts additional prognostic information for morbidity and mortality risk in cardiac surgery. Whether expense of routine measurement of homocysteine can be justified will depend on whether modification (or reduction) of this risk marker will improve outcomes in this cohort and the extent to which it is improved. Supplementation of diet with folic acid, vitamin B6, and vitamin B12 has been shown to decrease homocysteine levels.28 Despite this, treating patients who have elevated homocysteine with these vitamins has failed to decrease cardiovascular morbidity and mortality in two recently completed large randomized controlled trials.18,29 In view of these findings, a similar dietary approach in patients with elevated homocysteine levels scheduled for cardiac operations is not expected to decrease their operative risks. However, this remains to be proven in future. Alternatively, it is possible that early identification of patients at high-risk of perioperative thrombo-embolic events may allow modification of the surgical approach, i.e. the use of off-pump surgery that may improve outcomes of these patients. Furthermore, a number of studies have demonstrated that after cardiac operations the endothelial anticoagulant properties are exhausted (low levels of antithrombin and protein C-S complex),25,26,30 and some investigations have suggested that pharmacological supplementation with antithrombin may in fact be beneficial in improving outcomes of these individuals.25,30 Since the risk of thrombotic complications after cardiac surgery is increased by the presence of elevated pre-operative homocysteine levels, the present studies generate a hypothesis that patients with HHC may perhaps benefit from supplementation of such natural anticoagulants. Additionally, strategies such as aggressive treatment with antiplatelet or anticoagulant drugs in this high-risk subset for perioperative thrombotic events may also have the potential for reducing the risk of these events in this cohort. However, these hypotheses remain merely speculative at this time and need to be tested and proved in future investigations.
Limitations
This observational study should be regarded as hypothesis generating and caution should be exerted while inferring causation. This single tertiary centre study needs validation in future prospective investigation involving large number of patients undergoing cardiac surgery at large number of hospitals. The influence of elevated homocysteine levels on intermediate and long-term outcomes was not assessed. Finally, in patients undergoing emergent procedures, collecting information on homocysteine was not a priority. As such, there was a higher prevalence of emergent procedures in the 49 patients with missing homocysteine data. Thus, the prognostic significance of homocysteine in patients undergoing emergent CABG needs to be confirmed in future studies.
Conclusions
Elevated homocysteine levels are commonly observed in patients undergoing cardiac surgery. This increase is independently associated with higher perioperative thrombo-embolic events and mortality in these patients, particularly those undergoing CABG. Future studies evaluating strategies and alternative surgical approaches that would allow reduction in the high perioperative risk in patients with elevated homocysteine are in order.
Funding
R.H.M. is funded by the Duke Clinical Research Institute, Durham, NC, USA. Funding to pay the Open Access publication charges for this article was provided by IRCCS Policlinico S. Donato, Milan, Italy.
Conflict of interest: none declared.
Acknowledgement
We would like to thank Miss Valeria Pistuddi (from the Cardiothoracic Surgery Database Secretariat, IRCCS Policlinico S.Donato) for data collection and the management of the database; and Miss Anna Balduini (Scientific Documentation and Library, IRCCS Policlinico S.Donato) for help with literature search and retrieval of the relevant scientific information.
References
- 1.The Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 2.Eikelboom JW, Lonn E, Genest J, Jr, Hankey G, Yusuf S. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann Intern Med. 1999;131:363–375. doi: 10.7326/0003-4819-131-5-199909070-00008. [DOI] [PubMed] [Google Scholar]
- 3.Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337:230–236. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Smith SJ, Stroup DF, Steinberg KK, Mueller PW, Thacker SB. Homocyst(e)ine and cardiovascular disease: a systematic review of the evidence with special emphasis on case-control studies and nested case-control studies. Int J Epidemiol. 2002;31:59–70. doi: 10.1093/ije/31.1.59. [DOI] [PubMed] [Google Scholar]
- 5.Moghadasian MH, McManus BM, Frohlich JJ. Homocyst(e)ine and coronary artery disease. Clinical evidence and genetic and metabolic background. Arch Intern Med. 1997;157:2299–2308. [PubMed] [Google Scholar]
- 6.Perry IJ, Refsum H, Morris RW, Ebrahim SB, Ueland PM, Shaper AG. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet. 1995;346:1395–1398. doi: 10.1016/s0140-6736(95)92407-8. [DOI] [PubMed] [Google Scholar]
- 7.Stubbs PJ, Al-Obaidi MK, Conroy RM, Collinson PO, Graham IM, Noble MIM. Effect of plasma homocysteine concentration on early and late events in patients with acute coronary syndromes. Circulation. 2000;102:605–610. doi: 10.1161/01.cir.102.6.605. [DOI] [PubMed] [Google Scholar]
- 8.Bostom AG, Silbershatz H, Rosenberg IH, Jacques PF, Selhub J, D’Agostino RB, Wilson PW, Wolf PA. Nonfasting plasma total homocysteine levels and all-cause and cardiovascular disease mortality in elderly Framingham men and women. Arch Intern Med. 1999;159:1077–1080. doi: 10.1001/archinte.159.10.1077. [DOI] [PubMed] [Google Scholar]
- 9.Lentz SR. Mechanisms of homocysteine-induced atherothrombosis. J Thromb Haemost. 2005;3:1646–1654. doi: 10.1111/j.1538-7836.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- 10.Stamler JS, Osborne JA, Jaraki O, Rabbani LE, Mullins M, Singel D, Loscalzo J. Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest. 1993;91:308–318. doi: 10.1172/JCI116187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harpel PC, Zhang X, Borth W. Homocysteine and hemostasis: pathogenetic mechanisms predisposing to thrombosis. J Nutr. 1996;126:1285S–1289S. doi: 10.1093/jn/126.suppl_4.1285S. [DOI] [PubMed] [Google Scholar]
- 12.The PREVENT IV Investigators. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery-PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 13.Edwards FH, Clark RE, Schwartz M. Coronary artery bypass grafting: the Society of Thoracic Surgeons National Database experience. Ann Thorac Surg. 1994;57:12–19. doi: 10.1016/0003-4975(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 14.Desai ND, Cohen EA, Naylor CD, Fremes SE for the Radial Artery Patency investigators. N Engl J Med. 2004;351:2302–2309. doi: 10.1056/NEJMoa040982. [DOI] [PubMed] [Google Scholar]
- 15.Anyanwu AC, Filsoufi F, Salzberg SP, Bronster DJ, Adams DH. Epidemiology of stroke after cardiac surgery in the current era. J Thorac Cardiovasc Surg. 2007;134:1121–1127. doi: 10.1016/j.jtcvs.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, Cortina J, David M, Faichney A, Gabrielle F, Gams E, Harjula A, Jones MT, Pintor PP, Salamon R, Thulin L. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–822. doi: 10.1016/s1010-7940(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 17.Brunelli T, Pepe G, Marcucci R, Giusti B, Prisco D, Abbate R, Fedi S. Comparison of three methods for total homocysteine plasma determination. Clin Lab. 2001;47:393–397. [PubMed] [Google Scholar]
- 18.The Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 19.de Bree A, van der Put NM, Mennen LI, Verschuren WM, Blom HJ, Galan P, Bates CJ, Herrmann W, Ullrich M, Dierkes J, Westphal S, Bouter LM, Heine RJ, Stehouwer CD, Dekker JM, Nijpels GN, Araújo F, Cunha-Ribeiro LM, Refsum H, Vollset S, Nygard O, Ueland PM. Prevalences of hyperhomocysteinemia, unfavorable cholesterol profile and hypertension in European populations. Eur J Clin Nutr. 2005;59:480–488. doi: 10.1038/sj.ejcn.1602097. [DOI] [PubMed] [Google Scholar]
- 20.Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, Andria G, Boers GH, Bromberg IL, Cerone R, Fowler B, Grobe H, Schmidt H, Schweitzer H. The natural history of homocystinuria due to cystathionine b-synthase deficiency. Am J Hum Genet. 1985;37:1–31. [PMC free article] [PubMed] [Google Scholar]
- 21.Schimke RN, McKusick VA, Huang T, Pollack AD. Homocystinuria: studies of 20 families with 38 affected members. JAMA. 1965;193:711–719. doi: 10.1001/jama.1965.03090090017003. [DOI] [PubMed] [Google Scholar]
- 22.Storti S, Cerrillo AG, Rizza A, Giannelli I, Fontani G, Glauber M, Clerico A. Coronary artery bypass grafting surgery is associated with a marked reduction in serum homocysteine and folate levels in the early postoperative period. Eur J Cardiothorac Surg. 2004;26:682–686. doi: 10.1016/j.ejcts.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Nishinaga M, Ozawa T, Shimada K. Homocysteine, a thrombogenic agent, suppresses heparan sulfate expression in cultured porcine aortic endothelial cells. J Clin Invest. 1993;92:1381–1386. doi: 10.1172/JCI116712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodgers GM, Conn MT. Homocysteine, an atherogenic stimulus, reduces protein C activation by arterial and venous endothelial cells. Blood. 1990;75:895–901. [PubMed] [Google Scholar]
- 25.Ranucci M, Frigiola A, Menicanti L, Ditta A, Boncilli A, Brozzi S. Postoperative antithrombin levels and outcome in cardiac operations. Crit Care Med. 2005;33:355–360. doi: 10.1097/01.ccm.0000153409.55645.58. [DOI] [PubMed] [Google Scholar]
- 26.Ranucci M, Ditta A, Boncilli A, Cotza M, Carboni G, Brozzi S, Bonifazi C, Tiezzi A. Determinants of antithrombin consumption in cardiac operations requiring cardiopulmonary bypass. Perfusion. 2004;19:47–52. doi: 10.1191/0267659104pf711oa. [DOI] [PubMed] [Google Scholar]
- 27.Boyle EM, Jr, Morgan EN, Verrier ED. The endothelium disturbed: the procoagulant response. In: Spiess BD, editor. The Relationship Between Coagulation, Inflammation and Endothelium—a Pyramid Towards Outcome. Baltimore, MD: Lippincot Williams and Wilkins; 2000. pp. 79–89. [Google Scholar]
- 28.Homocysteine Lowering Trialists’ Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82:806–812. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- 29.Bønaa KH, Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K NORVIT Trial Investigators. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 30.Koster A, Chew D, Kuebler W, Habazettl H, Hetzer R, Kuppe H. High antithrombin III levels attenuate hemostatic activation and leukocyte activation during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2003;126:906–907. doi: 10.1016/s0022-5223(03)00392-1. [DOI] [PubMed] [Google Scholar]