Abstract
Recent reports suggest dyslipidemia impairs dendritic cell (DC) function and adaptive immunity. This study aimed to characterize the effect of hypercholesterolemia on antigen-presenting cell function of DCs and DC-dependent CD4+ T-cell responses. DCs incubated in vitro with acetylated low-density lipoprotein cholesterol with or without an acyl-coenzyme A:cholesterol acyl-transferase inhibitor maintained their ability to prime CD4+ T cells. Analysis of T-cell proliferation and interferon-γ and tumor necrosis factor-α production after ex vivo coculture of naïve CD4+ T cells with splenic, inguinal, or iliac DCs from low-density lipoprotein receptor–deficient (LDLR−/−) or apolipoprotein E–deficient (ApoE−/−) mice fed an atherogenic diet highlighted DC efficacy in effector T-cell generation under hypercholesterolemic conditions. Adoptive transfer of carboxyfluorescein diacetate, succinimidyl ester (CFSE)-labeled naïve CD4+ T cells in LDLR−/− recipients and subsequent immunization demonstrated effective priming of naïve T cells in hypercholesterolemic mice. CFSE dilution analyses revealed that hypercholesterolemic DCs were equipotent in naïve CD4+ T-cell priming efficacy with normocholesterolemic DCs. Quantitative real-time PCR and flow cytometric analyses demonstrated that DC expression of multiple molecules involved in antigen processing, presentation, and T-cell stimulation remained unaltered by dyslipidemia. Finally, endogenous antigen-primed CD4+ T cells responded equivalently to a secondary ex vivo antigenic challenge, regardless of whether they were primed in vivo under hypercholesterolemic or control conditions, demonstrating that all essential steps in CD4+ T-cell responses remain intact under atherogenic conditions. This study affirms that the adaptive immune response prevails under the hypercholesterolemic conditions present in atherosclerosis. In particular, DCs remain functional antigen-presenting cells and maintain their ability to prime CD4+ T cells even when cholesterol-loaded.
Keywords: dendritic cells, hypercholesterolemia, atherosclerosis, adaptive immunity
Adaptive immunity can strongly modulate atherogenesis and atherothrombosis,1 because CD4+ T cells promote inflammatory processes that enhance lesion development, undermine the stability of the mature atherosclerotic plaque,2,3 and enhance its prothrombotic state.4 Dendritic cells (DCs) are the only “professional” antigen-presenting cells (APCs) that efficiently stimulate the differentiation of effector CD4+ T cells from naïve T-cell precursors.5
Several reports have questioned the ability of DCs to migrate from peripheral tissues6,7 to draining lymph nodes under hypercholesterolemic conditions associated with atherosclerosis. One report suggested that dyslipidemia inhibits the activation of certain DCs.8 Furthermore, some data suggest that hypercholesterolemia inhibits immune responses and enhances susceptibility to infections.9–11
Given the pivotal function of DCs in initiating T-cell responses,5,12 and the knowledge that T cells contribute to atherosclerotic disease, we hypothesized that contrary to suggestions from certain studies, DCs would maintain their APC function in dyslipidemic states. Therefore, we probed the ability of DCs to induce a T-cell response and prime naïve CD4+ T cells under hypercholesterolemic conditions, mimicking those often encountered during atherogenesis, as well as test the antigen-presenting ability of cholesterol-loaded DCs to stimulate CD4+ T cells, as might occur within the cholesterol-rich microenvironment of the plaque. Using in vitro, ex vivo, and in vivo immunologic approaches, the present study affirms that DCs maintain fully their ability to prime naïve CD4+ T cells efficiently and to restimulate them even after exposure to excessive cholesterol, as might occur within plaques. We demonstrate that the machinery required by DCs to conduct these processes remains intact under dyslipidemic conditions. Finally, we illustrate that antigen-specific priming of a T-cell response in vivo remains unimpaired under hypercholesterolemic conditions that drive atherosclerosis. Our results show that DCs potently activate CD4+ T cells during atherogenesis, a central component in the development and complication of atherosclerotic plaques.
Materials and Methods
Mice
All mice were obtained from The Jackson Laboratory (Bar Harbor, Me) and were on the C57BL/6 background. Experiments were conducted with wild-type, low-density lipoprotein receptor–deficient (LDLR−/−), apolipoprotein E–deficient (ApoE−/−), and OT-II transgenic mice, which express a T-cell receptor (TCR) specific for a peptide fragment of ovalbumin (OVA323–339) presented by the I-Ab class II major histocompatibility complex (MHC-II) molecule. LDLR−/− and ApoE−/− mice were fed either a control chow diet or a high-fat 1.25% cholesterol– containing (hypercholesterolemic) diet. 13 The mice were housed and bred in accordance with institutional guidelines, and experimental protocols were approved by the institutional review board of the Harvard Medical School.
Isolation of DCs and T Cells
Secondary lymphoid organs (spleen, inguinal, and iliac lymph nodes) were crushed and treated with 1 mg/mL collagenase type 3 (Worthington Biochemical Corp) for 1 hour. CD11c+ DCs, CD4+CD11c+ DCs, CD8+CD11c+ DCs, plasmacytoid CD11clo DCs, and CD4+ T cells were isolated using antibodies coated with magnetic beads (Miltenyi Biotec).
Lipid Loading of DCs
Splenic DCs from wild-type mice were incubated in cell culture medium enriched with 100 µg/mL acetylated LDL cholesterol (acLDL) (laboratory of I.T. and Biomedical Technologies Inc) and 10 µg/mL compound 58035 (acyl-coenzyme A:cholesterol acyl-transferase [ACAT] inhibitor) (laboratory of I.T.) for 24 hours and subsequently washed.14
In Vitro and Ex Vivo CD4+ T-Cell Proliferation and Cytokine Secretion Assays
To assess the effect of in vitro loading of acLDL with or without ACAT inhibitor on splenic DCs from wild-type mice, coculture was conducted with splenic CD4+ OT-II T cells. To determine in vivo the effect of hypercholesterolemia on DC function, splenic, inguinal, or iliac DCs from LDLR−/− or ApoE−/− mice after 2 weeks, 1 month, 6 weeks, 2.5 months, or 6 months of high-fat or control diet were cocultured ex vivo with splenic CD4+ OT-II T cells. Finally, to test a complete in vivo T-cell response and DC-dependent T-cell priming under in vivo hypercholesterolemic conditions, LDLR−/− or ApoE−/− mice fed a high-fat or control diet for 2.5 months were then immunized in their left footpad with 100 µL of a 1:1 volume ratio of ovalbumin/complete Freund’s adjuvant (CFA) (Sigma) (concentration of injected ovalbumin, 5 mg/mL). After an additional 8 days on the same diets following immunization (priming), polyclonal effector CD4+ T cells were isolated from the ipsilateral inguinal lymph nodes and restimulated with splenic DCs isolated from wild-type mice. All DC and T-cell cocultures were conducted in a 1:1 ratio (50 000 cells/100 µL each) using cell culture media in 96-well cell culture plates (Falcon), except for experiments with CD4+CD11c+ DCs (14 000 DCs:50 000 T cells), CD8+CD11c+ DCs (20 000 DCs: 50 000 T cells), and plasmacytoid CD11clo DCs (5000 DCs:50 000 T cells). Ovalbumin 100 µg/mL (Sigma), OVA peptide 10 µg/mL (laboratory of A.H.L.), or no antigen (negative controls) was added to the wells and left throughout coculture. After 48 hours of coculture, 50 µL of supernatant per well was removed for fluorescence-activated cell sorting (FACS)-based cytokine analysis of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin (IL)-2, IL-4, and IL-5 (BD Biosciences), and cells were pulsed with 1 µCi per well [3H]-thymidine (PerkinElmer) for 24 hours to assess proliferation.
In Vivo CD4+ T-Cell Proliferation Assay
Splenic CD4+ T cells from wild-type C57/BL6 mice were labeled with carboxyfluorescein diacetate, succinimidyl ester (CFSE) following the instructions of the manufacturer (Molecular Probes/Invitrogen). Cells were then resuspended in PBS 1× at a concentration of 5×106/100 µL. A total of 5×106 CFSE-labeled T cells were adoptively transferred through retroorbital injection into LDLR−/− recipients on diet. Forty-eight hours after adoptive transfer, LDLR−/− mice were immunized in their left footpad with 100 µL of a 1:1 volume ratio of ovalbumin/CFA (concentration of injected ovalbumin, 5 mg/mL). Seventy-two hours after immunization, CD4+ T cells were isolated from the ipsilateral inguinal lymph nodes and stained with CD4-APC (BD Biosciences), and in vivo proliferative response was determined by FACS analysis of CFSE dilution.
Statistics
All statistical analyses were performed using Prism software. Differences were analyzed by Student’s t test and expressed as means±SEM. A probability value of ≤0.05 was considered significant for all analyses.
Confocal microscopy, immunofluorescence, Western blotting, FACS, quantitative PCR, and serum assays are described in the expanded Materials and Methods section, available in the online data supplement at http://circres.ahajournals.org.
Results
DCs Maintain APC Function Under In Vitro Conditions That Promote Cholesterol Uptake and Accumulation Typical of Atherosclerotic Plaques
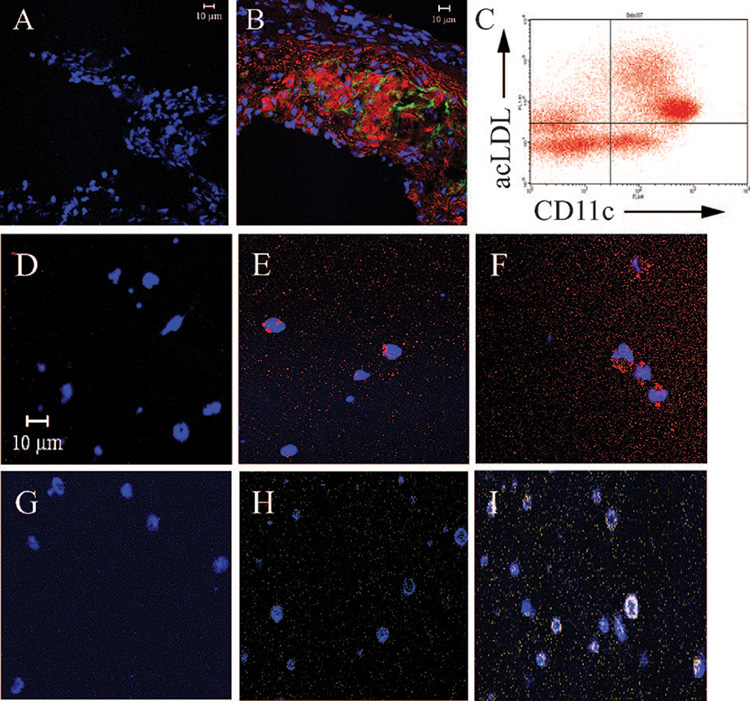
CD11c+ cells with dendritic processes accumulate lipids in vivo within atherosclerotic lesions, suggesting that DCs can form foam cells in vivo (Figure 1A and 1B and Figure I in the online data supplement). To simulate the cholesterol-enriched microenvironment of DCs within atherosclerotic plaques, we exposed DCs in vitro to 100 µg/mL acetylated LDL cholesterol (acLDL) in the presence (10 µg/mL) or absence of an acyl-coenzyme A:cholesterol acyl-transferase (ACAT) inhibitor. These conditions promote the accumulation of cholesterol in cells, ie, cholesteryl esters (acLDL without ACAT inhibitor) mimicking foam cell formation, and unesterified cholesterol (acLDL+ACAT inhibitor), recreating conditions of extreme cholesterol loading in advanced atherosclerotic lesions, similar to the environment encountered by macrophages at various stages of atherosclerotic plaque formation.14
Figure 1.
CD11c+ cells accumulate lipids within atherosclerotic plaques and when exposed to cholesterol in vitro. A and B, Confocal microscopy images (×400) of representative aortic sinuses from LDLR−/− mice after 2.5 months on a high-fat or control diet. Sinuses were stained for CD11c (Alexa-488, green), cholesteryl esters (oil red O [ORO], red), and nuclei (DAPI, blue). A, Aortic sinus from control animal demonstrates absence of CD11c and ORO staining (representative picture; n=4). B, Aortic sinus from a high-fat animal demonstrates positive CD11c and ORO staining. The cells colocalize with cholesteryl esters and CD11c+ dendritic processes (representative picture; n=4). C, CD11c+ DCs were isolated from the spleen of wild-type animals and incubated with 50 µg/mL acLDL for 24 hours. A total of 79.9±0.9% (upper right) of CD11c+ DCs (upper and lower right) stained positive for acLDL, indicating robust uptake (n=3). D through I, In vitro cholesterol-loaded DCs were stained for cholesteryl esters (ORO, red), unesterified cholesterol (filipin, yellow/white), and nuclei (DAPI or To-Pro 3 iodide, blue). D through F, Cholesteryl ester staining; acLDL-treated DCs (E) and acLDL+ACAT inhibitor–treated DCs (F) stained positive, in contrast to control DCs (D). G through I, Unesterified cholesterol staining; acLDL+ACAT inhibitor–treated DCs (I) stained positive, in contrast to acLDL-treated (H) and control (G) DCs.
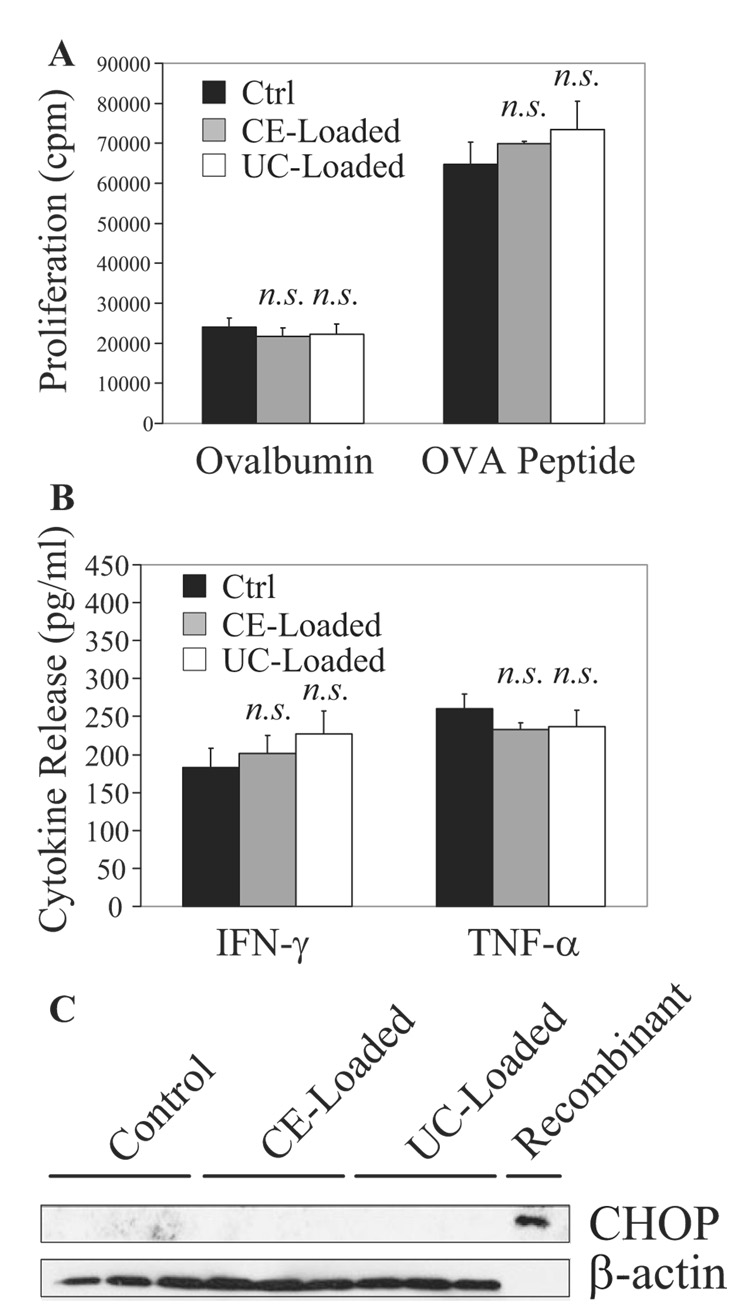
CD11c+ DCs take up large amounts of acLDL (Figure 1C) and become loaded with cholesteryl ester (Figure 1E and 1F) and unesterified cholesterol (Figure 1I). Even after accumulation of cholesterol, CD11c+ DCs have the same capability as control DCs in processing and presenting intact ovalbumin or presenting exogenously added ovalbumin-derived peptide and subsequently stimulating T-cell receptor transgenic ovalbumin peptide–specific CD4+ OT-II T cells (Figure 2A; n=4 experiments). Furthermore, coculture of naïve CD4+ OT-II T cells with lipid-loaded or control DCs releases equivalent levels of 2 key proinflammatory cytokines, IFN-γ and TNF-α (Figure 2B; n=4).
Figure 2.
DCs conserve T-cell stimulation efficacy when exposed to cholesterol in vitro. A, DCs preincubated for 24 hours with 100 µg/mL acLDL (cholesteryl ester [CE]-loaded) with or without 10 µg/mL ACAT inhibitor (unesterified cholesterol [UC]-loaded) were cocultured with CD4+ OT-II T cells for 72 hours in the presence of ovalbumin 100 µg/mL or OVA peptide 10 µg/mL. T-cell [3H]-thymidine incorporation from 48 to 72 hours, indicative of proliferation rate, was similar in coculture with lipid-loaded or control DCs. B, Supernatants isolated at 48 hours from A indicate equivalent levels of IFN-γ and TNF-α released in coculture with lipid-loaded or control DCs. C, CHOP expression was not induced in cholesteryl ester–loaded or unesterified cholesterol–loaded DCs.
Intact intracellular cholesterol trafficking to the endoplasmic reticulum contributes to cholesterol-induced apoptosis of macrophages in advanced atherosclerotic lesions.15 In unesterified cholesterol-loaded macrophages, the endoplasmic reticulum accumulates free cholesterol, leading to CHOP (C/EBP-homologous protein) (also known as GADD 153, growth arrest, and DNA damage-inducible gene 153)-induced apoptosis.14 In contrast to the situation in macrophages, we did not detect CHOP expression in unesterified cholesterol-loaded DCs using Western blotting (Figure 2C; n=3).
DCs Maintain APC Function During Hypercholesterolemia In Vivo
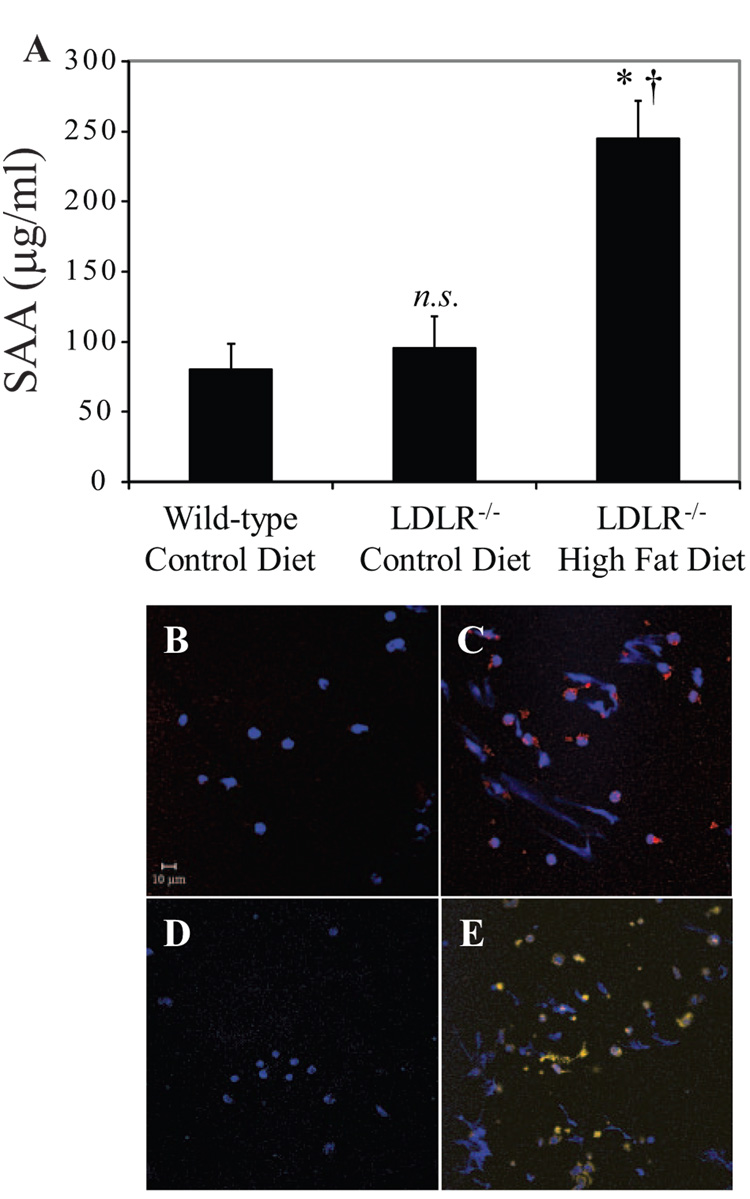
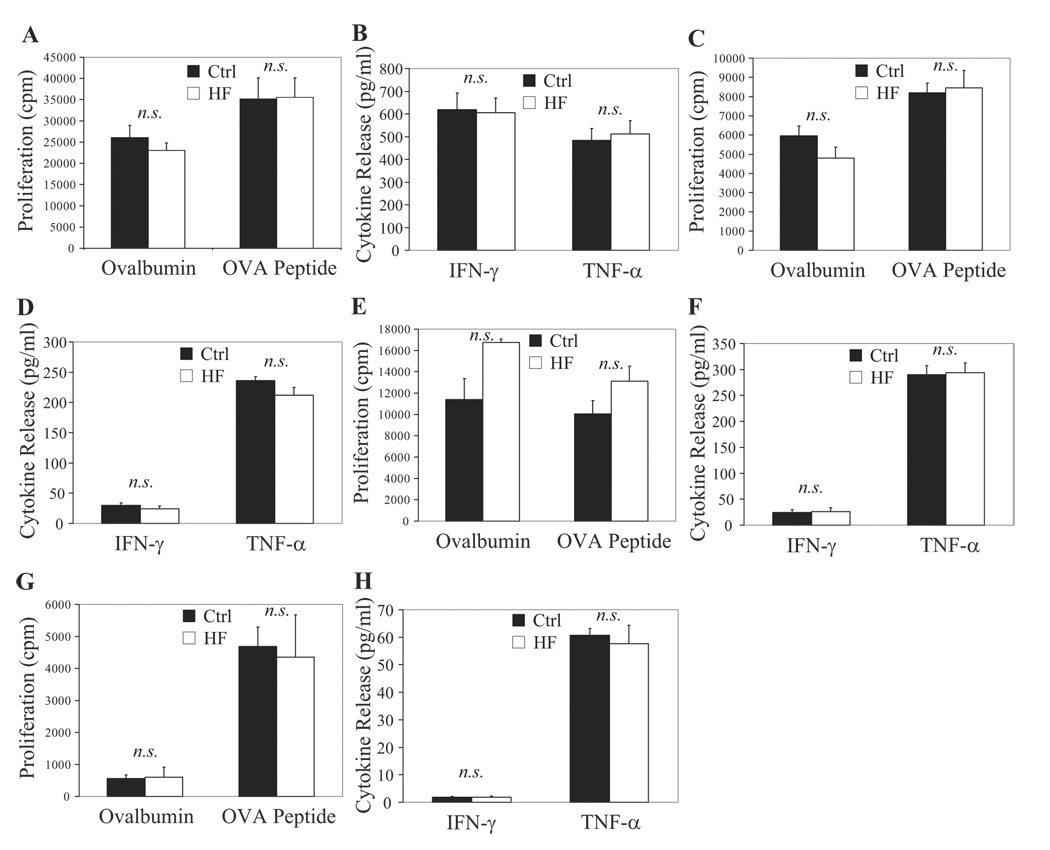
To test the hypothesis that DCs retain their ability to prime T cells under hypercholesterolemic conditions associated with atherosclerosis, we isolated CD11c+ DCs from secondary lymphoid organs of LDLR−/− or ApoE−/− mice fed a high-fat or control diet. Consumption of a high-fat diet for 2.5 or 4 months significantly increased circulating total cholesterol levels in LDLR−/− mice (Table) (n=6 mouse pairs per time point). Serum amyloid A levels rose in parallel (Figure 3A), indicating the induction of acute-phase reactants by inflammation after 2.5 months of diet consumption (n=7 mice per group). Splenic CD11c+ DCs from hypercholesterolemic LDLR−/− mice, despite accumulating cholesteryl esters (Figure 3C) and unesterified cholesterol (Figure 3E), had similar ability as their control counterparts to prime naïve CD4+ OT-II T cells ex vivo. This was true with either intact ovalbumin (which DCs must process) or the ovalbumin-peptide specifically recognized by OT-II (which bypasses the need for processing) (Figure 4A; n=8 experiments). Splenic CD11c+ DCs from LDLR−/− mice isolated after 2 weeks (n=1), 1 month (n=3), or 6 months (n=4) of diet feeding showed similar results (data not shown). Coculture of splenic DCs from cholesterol-fed or control diet–fed LDLR−/− mice with naïve CD4+ OT-II T cells yielded equivalent levels of IFN-γ and TNF-α release (Figure 4B; n=8). Similar results emerged with splenic DCs from ApoE−/− mice after 6 weeks (supplemental Figure IIA and IIB) or 6 months (data not shown) of diet feeding.
Table.
Total Serum Cholesterol in LDLR −/− Mice After a High-Fat Diet
| LDLR−/− | |||
|---|---|---|---|
| Length of Diet | Control (mg/dL) | High Fat (mg/dL) | P |
| 2.5 months | 177±15 | 520±40 | 0.00034 |
| 4 months | 145±14 | 801±39 | 0.000007 |
Figure 3.
Systemic inflammatory response and DC lipid accumulation in vivo during hypercholesterolemia. A, Circulating serum amyloid A (SAA) levels in wild-type and LDLR−/− animals after 2.5 months of high-fat diet. *P=0.0003 vs wild-type control, †P=0.019 vs LDLR−/− control. CD11c+ DCs were isolated from LDLR−/− mice on a 2.5-month atherogenic or control diet. B through E, In vivo cholesterol-loaded DCs were stained for cholesteryl esters (ORO, red), unesterified cholesterol (filipin, yellow/white), and nuclei (DAPI or To-Pro 3 iodide, blue). B and C, Cholesteryl ester staining; DCs from hypercholesterolemic animals (C) stained positive, in contrast to control DCs (B). D and E, Unesterified cholesterol staining; DCs from hypercholesterolemic animals (E) stained positive, in contrast to control DCs (D).
Figure 4.
DCs maintain T-cell priming potency in vivo during hypercholesterolemia. Splenic CD11c+ DCs from high-fat (HF) animals were equipotent to controls ex vivo in eliciting a proliferative response (A) and IFN-γ and TNF-α release in supernatants (B) in coculture. T-cell proliferation and cytokine release were unaltered also when stimulated by CD4+CD11c+ DCs (C and D), CD8+CD11c+ DCs (E and F), and plasmacytoid CD11clo DCs (G and H) exposed in vivo to hypercholesterolemia.
To evaluate the individual functionality of specific DC subtypes, we isolated 3 well-characterized members of this family: CD4+CD11c+ DCs, the most numerous DCs in the spleen; CD8+CD11c+ DCs, which specialize in the cross-presentation of antigens to CD8+ T cells; and plasmacytoid CD11clo DCs, the major source of type I interferons that play a critical role in the clearance of viruses.16 CD4+CD11c+ DCs (Figure 4C and 4D; n=4), CD8+CD11c+ DCs (Figure 4E and 4F; n=4), and plasmacytoid CD11clo DCs (Figure 4G and 4H; n=4) isolated from the spleens of LDLR−/− mice after 2.5 months of hypercholesterolemic diet retained their ability to prime CD4+ OT-II T cells. Plasmacytoid CD11clo DCs, whether from control or high-fat diet–fed mice, displayed a comparatively weak induction of T-cell proliferation and IFN-γ production, a finding consistent with previous reports demonstrating very low expression of MHC-II molecules under unstimulated conditions by this particular DC subtype.16,17
The relative proportions and contributions of individual DC subtypes vary greatly between different secondary lymphoid organs,17 leading us to characterize CD11c+ DCs isolated from inguinal and iliac lymph nodes of LDLR−/− mice that consumed control or atherogenic diets for 2.5 months. DCs from pooled inguinal lymph node pairs of cholesterol-fed animals demonstrated a modest but statistically significantly enhanced ability to process and present ovalbumin and stimulate naïve CD4+ OT-II T cells (supplemental Figure IIC; n=5 experiments). A similar but statistically insignificant trend surfaced for the capacity of inguinal DCs from cholesterol-fed mice to prime naïve CD4+ OT-II T cells ex vivo using ovalbumin peptide as antigen, with IFN-γ and TNF-α levels following a similar pattern (supplemental Figure IID; n=5). No differences between dietary groups emerged after 2 weeks of diet (n=1; data not shown). Iliac lymph nodes may drain CD11c+ monocyte-derived cells from aortic atherosclerotic plaques.7 Therefore, we investigated whether hypercholesterolemic conditions associated with atherogenesis might disturb the ability of iliac DCs to prime CD4+ T cells, pooling bilateral iliac lymph nodes from 8 mice (=16 iliac lymph nodes total per experiment and per diet condition) and isolating CD11c+ DCs. Consumption of a cholesterol-enriched diet did not impede the ability of DCs to prime and activate naïve CD4+ OT-II T cells, using either intact ovalbumin or ovalbumin peptide as a source of antigen, either at 2 weeks (n=1 experiment) or 2.5 months (n=2) of diet feeding (data not shown).
Taken together, these results suggest that CD11c+ DCs from splenic, inguinal, or iliac sources under hypercholesterolemic conditions associated with atherosclerosis have at least equal ability as DCs under normocholesterolemic conditions to process, present antigens, and prime naïve CD4+ T cells ex vivo.
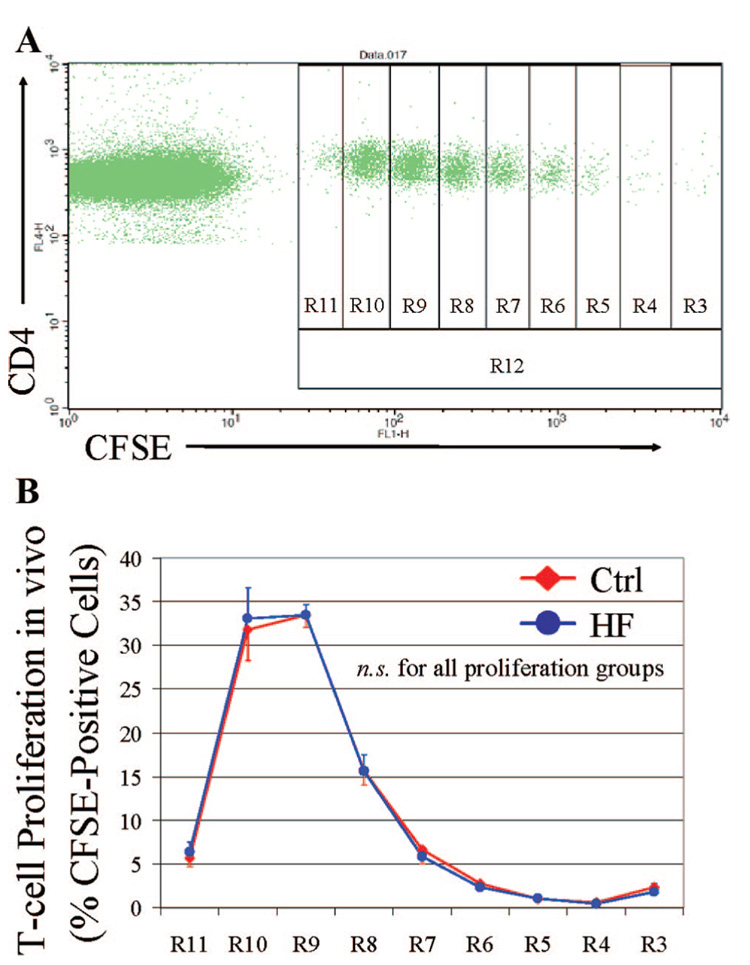
To characterize further the influence of hypercholesterolemia on antigen presentation in vivo, CFSE-labeled naïve CD4+ OT-II T cells were adoptively transferred into LDLR−/− recipients after 2.5 months on control or atherogenic diets. Forty-eight hours after T-cell transfer, footpad immunization was performed with ovalbumin in CFA. Draining ipsilateral inguinal lymph nodes were harvested 72 hours after immunization, and CD4+ T cells were isolated to assess in vivo the proliferative response to DCs by dilution of CFSE fluorescence. An example from a mouse that consumed a control diet is depicted in Figure 5A. R3 represents the fraction of T cells that did not respond to the antigenic stimulus and R11 those that proliferated most, illustrated by decreased CFSE concentrations. DCs under hypercholesterolemic conditions characteristic of atherosclerosis in vivo have equivalent ability to stimulate an antigen-specific proliferative response of naïve CD4+ T cells in lymph nodes compared to DCs under control conditions (Figure 5B; n=10 experiments). Analysis of in vivo T-cell proliferative responses in inguinal lymph nodes after immunization of mice after 6 months on high-fat versus control diet (n=9) also showed equivalent DC antigen processing and presentation efficacy and T-cell stimulation potency between the 2 dietary groups (data not shown).
Figure 5.
DCs conserve complete antigen-processing and T-cell priming capabilities within lymph nodes under in vivo hypercholesterolemia. CFSE-labeled CD4+ OT-II T cells were adoptively transferred into LDLR−/− recipients on a 2.5-month atherogenic or control diet. After immunization with ovalbumin/CFA, CD4+ OT-II T cells were isolated from draining inguinal lymph nodes and stained with CD4-APC, and then in vivo proliferative response corresponding to CFSE dilution was determined by FACS. A, Example drawn from a mouse on control diet. R12 represents the total fraction of CFSE-positive T cells; R3, the fraction that did not respond to the antigenic stimulus; R11, those that proliferated most, illustrated by decreased CFSE concentrations. B, Pooled results demonstrate that DCs under in vivo hypercholesterolemic conditions (HF) have equivalent ability to stimulate an antigen-specific proliferative response of naïve CD4+ T cells as DCs under control conditions.
Hypercholesterolemic Conditions Do Not Alter DC Expression of Molecules Required for Antigen Processing, Presentation, and T-Cell Stimulation
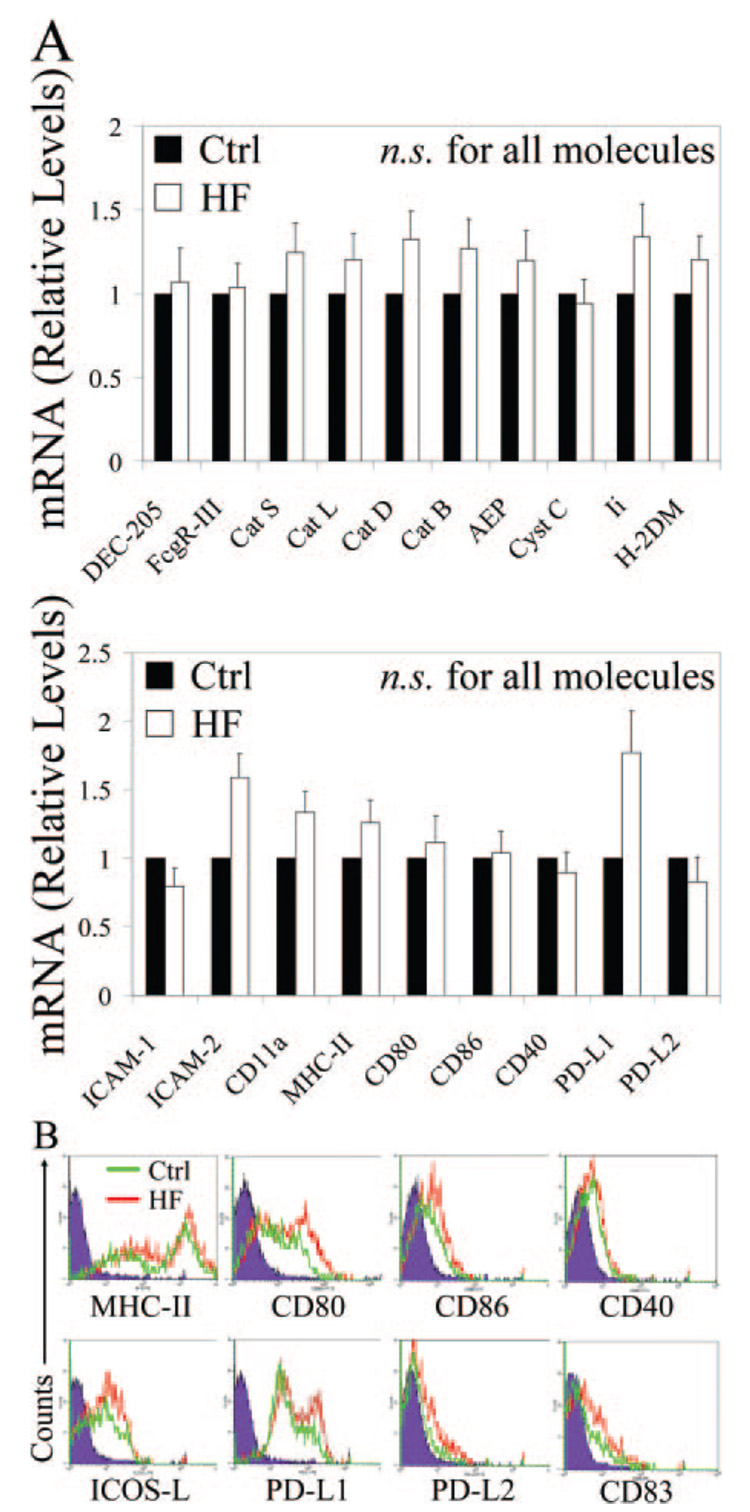
Because our findings indicate that DCs retain their ability to prime CD4+ T cells under atherosclerotic conditions, we predicted that expression of molecules by DCs important for antigen processing and presentation would not significantly change under these conditions. To confirm this, we isolated RNA from inguinal lymph node DCs of control or high-fat diet-fed mice and performed a broad analysis of mRNA levels of several genes important in DC activation of T cells. Expression of genes encoding key molecules involved in antigen uptake (DEC-205, FCγR-III), antigen degradation (cathepsins S, L, D, B, cystatin C), antigen processing (AEP, Ii, H-2DM), immunologic synapse formation (intercellular adhesion molecule [ICAM]-1, ICAM-2, CD11a/lymphocyte function-associated antigen [LFA]-1), and T-cell stimulation (MHC-II, CD80, CD86, CD40, programmed cell death ligand [PD-L]1, PD-L2) indicate that these professional APCs preserve all the machinery crucial to T-cell priming under hypercholesterolemic conditions driving atherogenesis (Figure 6A; n=3 mouse pairs). We further investigated the cell surface expression of key proteins involved in DC/T-cell interaction by FACS analysis. Splenic DCs from LDLR−/− animals on a 3.5-month diet had similar levels of expression of class II MHC, costimulatory molecules such as CD80, CD86, CD40, inducible costimulator ligand [ICOS-L], PDL1, and PD-L2, as well as the DC activation marker CD83 (Figure 6B; n=3 mouse pairs).
Figure 6.
Preserved DC machinery involved in antigen processing and T-cell stimulation during hypercholesterolemia in vivo. A, RT-PCR measurement of relative mRNA levels of key participants in antigen uptake (DEC-205, FCγR-III), antigen degradation (cathepsins S, L, D, B, cystatin c), antigen processing (AEP, Ii, H-2DM), immunologic synapse formation (ICAM-1, ICAM-2, CD11a/LFA-1), and T-cell stimulation (MHC-II, CD80, CD86, CD40, PD-L1, PD-L2) show that inguinal DCs from LDLR−/− animals on a 4-month diet (HF) preserve all the machinery crucial to T-cell priming under hypercholesterolemic conditions driving atherogenesis. B, FACS determination of cell surface proteins involved in DC stimulation of T cells as well as the nonspecific DC activation marker CD83 in splenic DCs from LDLR−/− animals on a 3.5-month diet (HF) demonstrates their conserved expression under hypercholesterolemia.
Hypercholesterolemia Does Not Alter the Adaptive Immune Response In Vivo
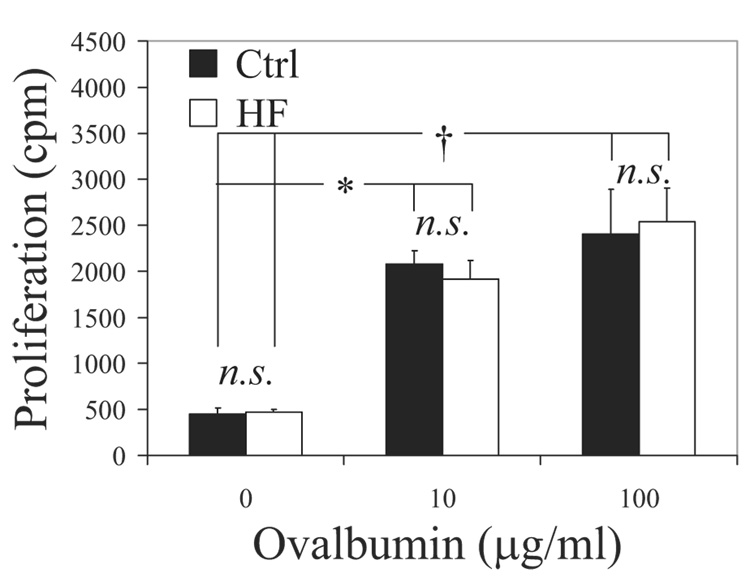
Finally, we tested for potential differences in T-cell priming in response to an exogenous antigen in LDLR−/− and ApoE−/− animals fed control or cholesterol diets for 2.5 months. The mice were immunized in their footpads with ovalbumin and CFA, draining ipsilateral inguinal lymph nodes were harvested, and polyclonal CD4+ T cells were isolated. To assess the in vivo ability of DCs to prime and elicit an adaptive immune response, effector CD4+ T cells were restimulated ex vivo with DCs from wild-type donors. Polyclonal effector CD4+ T cells responded equivalently to a second ex vivo antigen-specific stimulation, regardless of whether they were primed in vivo under hypercholesterolemic or control conditions (Figure 7; n=4 experiments). Similar results emerged in ApoE−/− animals (supplemental Figure III; n=3 experiments).
Figure 7.
Preserved CD4+ T-cell priming in vivo under dyslipidemic conditions. LDLR−/− animals on a 2.5-month diet were immunized with ovalbumin/CFA, and polyclonal CD4+ T cells were isolated from draining lymph nodes and restimulated ex vivo with DCs from wild-type donors. Regardless of the dose of antigen presented (ovalbumin 10 or 100 µg/mL), effector CD4+ T-cell proliferation in response to a second ex vivo antigenic stimulation was unaltered, whether priming occurred in vivo under atherosclerotic (HF) or control conditions. *P<0.01, †P<0.05.
These findings demonstrate that the multiple steps required for a CD4+ T-cell response remain intact under proatherogenic dyslipidemic conditions, including delivery of protein antigen to draining lymph nodes, processing and presentation of the antigen by APCs such as DCs, and activation of antigen-specific naïve T cells.
Discussion
The chronic inflammation that characterizes atherosclerosis involves both the innate and adaptive arms of immunity.18 CD4+ T cells regulate crucial aspects of atherogenesis and atherothrombosis.1,19,20 CD4+ T cells participate in atherosclerosis, as demonstrated by their presence in human21 and rodent22 lesions at all stages of development and modulation of mouse lesion progression by ablation of genes required for T-cell development19,23,24 or effector differentiation25–27 or the adoptive transfer of CD4+ T cells into immunodeficient hypercholesterolemic mice.19 Expression of CD40 ligand (CD40L) by CD4+ T cells induces production of the procoagulant tissue factor by macrophages,3 endothelial cells, and vascular smooth muscle cells.4 In addition, CD4+ T cells orchestrate the weakening of the fibrous cap through IFN-γ–dependent inhibition of interstitial collagen production by smooth muscle cells2 and catabolism of existing collagen fibers through CD40L-dependent interstitial collagenase induction by macrophages.28 These combined effects of CD4+ T cells illustrate their critical role in impairing the integrity of the protective fibrous cap of the plaque and the promotion of a prothrombotic state within lesions: key characteristics of rupture-prone plaques, the usual culprits in such clinical manifestations as unstable angina, acute myocardial infarction, and many ischemic strokes.29
Antigen-specific CD4+ T cells undergo stimulation in peripheral tissues when they encounter disease-specific antigens associated with MHC-II molecules on the surface of APCs.5 DCs, the prototypic antigen-presenting cell type, populate atherosclerotic plaques,30 particularly in the rupture-prone shoulder region of lesions,31 in part under the control of CX3CR132 and granulocyte–macrophage colony-stimulating factor.33 Importantly, stimuli known to accelerate atherogenesis, such as oxidized LDL cholesterol or TNF-α, increase DC adhesion to the endothelium and their subsequent transmigration. 34 In addition, recent results demonstrate the presence of CD11c+ leukocytes with dendritic processes in regions of the normal arterial intima predisposed to atherosclerosis. 35 Some prior studies have suggested that under hypercholesterolemic conditions, DCs display impaired emigration from peripheral tissues such as the skin6 or atherosclerotic plaques.7 Furthermore, a recent report posits that dyslipidemia inhibits Toll-like receptor–induced production of proinflammatory cytokines, as well as the induction of costimulatory molecules by CD11c+CD8α− DC subtypes.8 Numerous reports have also suggested that hypercholesterolemia produces an immunodeficient state and enhances susceptibility to yeast,9 viral,10 and bacterial11 infections. Because cholesterol uptake by DCs and macrophages can theoretically alter the microenvironment of endosomes and endoplasmic reticulum, where antigen processing events take place, predicting that hypercholesterolemia may impact APC function is reasonable. Therefore, the present study directly examined whether cholesterol loading and/or hypercholesterolemic conditions associated with atherosclerosis affect APC functions of DCs and ensuing activation of CD4+ T cells. Our data illustrate that DCs loaded with excess cholesterol retain full T-cell stimulatory capacity, as demonstrated by intact T-cell proliferative responses and production of the Th1 cytokine IFN-γ, as well as TNF-α. Th2 cytokines such as IL-4 and IL-5 were not induced during coculture assays, as expected in C57BL/6 mice, which have a strong bias toward Th1 responses. These findings suggest that, contrary to macrophages,14,15 DCs remain entirely functional under conditions typical of atherosclerotic plaques, and their ability to activate T cells does not change. This difference between DCs and macrophages may result from the superior defenses against oxidative stress of DCs, displayed by elevated levels of superoxide dismutase and peroxiredoxin-1, compared to macrophages.36 Also in contrast to macrophages,14 we found that unesterified cholesterol-loaded DCs do not express the transcription factor CHOP, a marker of unfolded protein response induction. Under normal cellular conditions, this protein is not expressed at detectable levels, but it is highly induced by a variety of endoplasmic reticulum stresses, for example, free cholesterol accumulation. Our observation suggests either a resistance of DCs to cholesterol-induced cytotoxicity or the induction of alternate unfolded protein response pathways in these cells, hypotheses that merit careful future evaluation given the central role DCs play in immunity and tolerance.
Naïve T cells do not enter peripheral tissues.37 Indeed, these cells require priming in secondary lymphoid organs by DCs, and only after antigen-specific differentiation will effector T cells target select tissues in the periphery such as atherosclerotic vessels.5 DCs patrol tissues, sense their environment, and emigrate to tissue-draining lymph nodes, where they activate T-cell immunity or, under some circumstances, T-cell tolerance.12 DCs constitute a heterogeneous family, with varying ratios of subtypes between secondary lymphoid organs.16,17 We demonstrate that “whole” CD11c+ DCs isolated from spleens and inguinal and iliac lymph nodes, which differ in their DC subtype compositions, as well as various individual DC subtypes, all isolated from hypercholesterolemic animals, remain completely functional and unaltered in their ability to prime naïve CD4+ T cells in an antigen-specific manner.
The combination of ovalbumin and CFA we used for immunization creates a depot, which theoretically requires local uptake by DCs and subsequent migration to draining lymph nodes. Given the concentration of antigen used for immunization, a fraction of the antigen may have circulated freely in soluble form through the lymphatics and been endocytosed by DCs directly in the draining lymph nodes. Regardless of their mechanism of entry into secondary lymphoid organs, protein antigens, including putative atherosclerotic antigens, require obligatory processing and presentation by DCs with intact stimulatory capabilities for priming of naïve CD4+ T cells. Our data demonstrate that hypercholesterolemic DCs can successfully generate monoclonal and polyclonal effector CD4+ T cells that may subsequently leave secondary lymphoid organs and target atherosclerotic vessels.
DCs express a variety of proteins that endow them with specialized antigen-processing and presentation functions.38,39 Our mRNA and FACS analyses further illustrate that dyslipidemia does not affect the expression of these proteins. These combined results demonstrate that DCs resist hypercholesterolemia-induced modification and remain fully capable of activating the adaptive arm of the immune response.
Previous reports questioned the occurrence of an adequate and efficient immune response under dyslipidemic conditions. 6–11 The present study affirms that hypercholesterolemia associated with atherosclerotic disease does not impede DC ability to activate CD4+ T cells and initiate the adaptive immune response central to atherothrombosis. Our findings demonstrate that both within the plaque microenvironment and in secondary lymphoid organs under dyslipidemic conditions, DCs maintain their full functional capacity and CD4+ T cell–stimulation potency. These results highlight the ability of DCs to maintain their functions as gatekeepers for adaptive immunity even when lipid-laden and thus remain capable of inducing both pathogenic and protective T-cell responses.
Supplementary Material
Acknowledgments
We thank Thomas Christen for help with cell isolation, Eduardo Folco for help with Western blotting, Elissa Simon-Morrissey for administrative assistance, and Joan Perry for editorial assistance (in the laboratory of P.L.); Paurene Duramad for help with CFSE experiments and Hong Pang and Maggie Tarrio for mouse breeding (in the laboratory of A.H.L.); Connie Woo for help with Western blotting, Cecilia Devlin for help with lipid staining of DCs and for providing the ACAT inhibitor, and Yvan Zhang for providing acLDL (in the laboratory of I.T.).
Sources of Funding
This study was supported by NIH grant HL034636 and Fondation Leducq, Paris, France (to P.L.); NIH grant HL087282 (to A.H.L.); NIH grant HL057560 (to I.T.); and Foundation Alfonso Martin Escudero, Madrid, Spain (to E.M.-G.).
Footnotes
Presented in part at the Arteriosclerosis, Thrombosis, and Vascular Biology Annual Conference, Atlanta, Ga, April 16–18, 2008, and published in abstract form (Arterioscler Thromb Vasc Biol. 2008;28:e-56).
Disclosures
None.
References
- 1.Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol. 2006;26:2421–2432. doi: 10.1161/01.ATV.0000245830.29764.84. [DOI] [PubMed] [Google Scholar]
- 2.Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991;11:1223–1230. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- 3.Mach F, Schonbeck U, Bonnefoy JY, Pober JS, Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40: induction of collagenase, stromelysin, and tissue factor. Circulation. 1997;96:396–399. doi: 10.1161/01.cir.96.2.396. [DOI] [PubMed] [Google Scholar]
- 4.Steffel J, Luscher TF, Tanner FC. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation. 2006;113:722–731. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 6.Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shamshiev AT, Ampenberger F, Ernst B, Rohrer L, Marsland BJ, Kopf M. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8alpha-negative dendritic cells and protective Th1 type immunity. J Exp Med. 2007;204:441–452. doi: 10.1084/jem.20061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netea MG, Demacker PN, de Bont N, Boerman OC, Stalenhoef AF, van der Meer JW, Kullberg BJ. Hyperlipoproteinemia enhances susceptibility to acute disseminated Candida albicans infection in low-density-lipoprotein-receptor-deficient mice. Infect Immun. 1997;65:2663–2667. doi: 10.1128/iai.65.7.2663-2667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludewig B, Jaggi M, Dumrese T, Brduscha-Riem K, Odermatt B, Hengartner H, Zinkernagel RM. Hypercholesterolemia exacerbates virus-induced immunopathologic liver disease via suppression of antiviral cytotoxic T cell responses. J Immunol. 2001;166:3369–3376. doi: 10.4049/jimmunol.166.5.3369. [DOI] [PubMed] [Google Scholar]
- 11.de Bont N, Netea MG, Demacker PN, Verschueren I, Kullberg BJ, van Dijk KW, van der Meer JW, Stalenhoef AF. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. J Lipid Res. 1999;40:680–685. [PubMed] [Google Scholar]
- 12.Moser M. Dendritic cells in immunity and tolerance-do they display opposite functions? Immunity. 2003;19:5–8. doi: 10.1016/s1074-7613(03)00182-1. [DOI] [PubMed] [Google Scholar]
- 13.Lichtman AH, Clinton SK, Iiyama K, Connelly PW, Libby P, Cybulsky MI. Hyperlipidemia and atherosclerotic lesion development in LDL receptor-deficient mice fed defined semipurified diets with and without cholate. Arterioscler Thromb Vasc Biol. 1999;19:1938–1944. doi: 10.1161/01.atv.19.8.1938. [DOI] [PubMed] [Google Scholar]
- 14.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 15.Feng B, Zhang D, Kuriakose G, Devlin CM, Kockx M, Tabas I. Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:10423–10428. doi: 10.1073/pnas.1732494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 17.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 18.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 19.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 20.Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosono M, de Boer OJ, van der Wal AC, van der Loos CM, Teeling P, Piek JJ, Ueda M, Becker AE. Increased expression of T cell activation markers (CD25, CD26, CD40L and CD69) in atherectomy specimens of patients with unstable angina and acute myocardial infarction. Atherosclerosis. 2003;168:73–80. doi: 10.1016/s0021-9150(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 22.Roselaar SE, Kakkanathu PX, Daugherty A. Lymphocyte populations in atherosclerotic lesions of apoE−/− and LDL receptor−/− mice. Decreasing density with disease progression. Arterioscler Thromb Vasc Biol. 1996;16:1013–1018. doi: 10.1161/01.atv.16.8.1013. [DOI] [PubMed] [Google Scholar]
- 23.Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest. 2001;108:251–259. doi: 10.1172/JCI11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X, Robertson AK, Rudling M, Parini P, Hansson GK. Lesion development and response to immunization reveal a complex role for CD4 in atherosclerosis. Circ Res. 2005;96:427–434. doi: 10.1161/01.RES.0000156889.22364.f1. [DOI] [PubMed] [Google Scholar]
- 25.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 26.Buono C, Pang H, Uchida Y, Libby P, Sharpe AH, Lichtman AH. B7-1/B7-2 costimulation regulates plaque antigen-specific T-cell responses and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109:2009–2015. doi: 10.1161/01.CIR.0000127121.16815.F1. [DOI] [PubMed] [Google Scholar]
- 27.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sukhova GK, Schonbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, Libby P. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99:2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- 29.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 30.Bobryshev YV. Dendritic cells in atherosclerosis: current status of the problem and clinical relevance. Eur Heart J. 2005;26:1700–1704. doi: 10.1093/eurheartj/ehi282. [DOI] [PubMed] [Google Scholar]
- 31.Yilmaz A, Lochno M, Traeg F, Cicha I, Reiss C, Stumpf C, Raaz D, Anger T, Amann K, Probst T, Ludwig J, Daniel WG, Garlichs CD. Emergence of dendritic cells in rupture-prone regions of vulnerable carotid plaques. Atherosclerosis. 2004;176:101–110. doi: 10.1016/j.atherosclerosis.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 32.Liu P, Yu YR, Spencer JA, Johnson AE, Vallanat CT, Fong AM, Patterson C, Patel DD. CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler Thromb Vasc Biol. 2008;28:243–250. doi: 10.1161/ATVBAHA.107.158675. [DOI] [PubMed] [Google Scholar]
- 33.Shaposhnik Z, Wang X, Weinstein M, Bennett BJ, Lusis AJ. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2007;27:621–627. doi: 10.1161/01.ATV.0000254673.55431.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weis M, Schlichting CL, Engleman EG, Cooke JP. Endothelial determinants of dendritic cell adhesion and migration: new implications for vascular diseases. Arterioscler Thromb Vasc Biol. 2002;22:1817–1823. doi: 10.1161/01.atv.0000036418.04998.d5. [DOI] [PubMed] [Google Scholar]
- 35.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203:2073–2083. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivollier A, Perrin-Cocon L, Luche S, Diemer H, Strub JM, Hanau D, van Dorsselaer A, Lotteau V, Rabourdin-Combe C, Rabilloud T, Servet-Delprat C. High expression of antioxidant proteins in dendritic cells: possible implications in atherosclerosis. Mol Cell Proteomics. 2006;5:726–736. doi: 10.1074/mcp.M500262-MCP200. [DOI] [PubMed] [Google Scholar]
- 37.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 38.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 39.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.