Abstract
In our laboratory the domestic ram is used as an experimental model to study the early programming of neural mechanisms underlying same-sex partner preference. This interest developed from the observation that ∼8% of domestic rams are sexually attracted to other rams (male-oriented) in contrast to the majority of rams that are attracted to oestrous ewes (female-oriented). One prominent feature of sexual differentiation in many species is the presence of a sexually dimorphic nucleus (SDN) in the preoptic/anterior hypothalamus that is larger in males than in females. Lesion studies in rats and ferrets implicate the SDN in the expression of sexual preferences. We discovered an ovine SDN (oSDN) in the preoptic/anterior hypothalamus that is smaller in male- than in female-oriented rams and similar in size to the oSDN of ewes. Neurons of the oSDN show abundant aromatase expression that is also reduced in male-oriented compared to female-oriented rams. This observation suggests that sexual partner preferences are neurologically hard-wired and could be influenced by hormones. Aromatase-containing neurons constitute a nascent oSDN as early as d 60 of gestation, which becomes sexually dimorphic by d 135 of gestation when it is 2 times larger in males than in females. Exposure of fetal female lambs to exogenous testosterone from d 30 to 90 of gestation resulted in a masculinised oSDN. These data demonstrate that the oSDN develops prenatally and may influence adult sexual preferences. Surprisingly, inhibition of aromatase activity in the brain of ram fetuses during the critical period did not interfere with defeminisation of adult sexual partner preference or oSDN volume. These results fail to support an essential role for neural aromatase in the sexual differentiation of sheep brain and behaviour. Thus, we propose that oSDN morphology and male-typical partner preferences may instead be programmed through an androgen receptor mechanism not involving aromatisation.
Keywords: sexual maturation, sexual partner preference, copulatory behaviour, puberty, aromatase inhibitor, ATD, sheep, LH surge, fertility
Introduction
Sexual partner preference refers to an animal’s sexual attraction to partners of the same or opposite sex when given a choice. Sexual partner preferences are enduring and highly sexually dimorphic. In all species, most males court, mount, and mate with females and most females solicit and mate with males. For the past 50 years, the organisational/activational hypothesis of sexual differentiation has provided the key framework for understanding the development of sexual behaviour (1). According to this hypothesis, a critical period of susceptibility exists in early perinatal life during which circulating testosterone produced from the fetal testis masculinises and defeminises the neuroendocrine and behavioural potential of the brain. In the absence of testosterone the brain is feminised and can support cyclic gonadotrophin-releasing hormone secretion required for ovulation and the expression of feminine sexual behaviours. The sex-typical programmes established early in life can be disrupted or reprogrammed by experimental changes in the hormonal environment during perinatal development (2). Several animal models (e.g. mice, rats, ferrets, zebra finches) have been used to study the impact of perinatal hormone manipulation on sexual partner preferences (3-5). While this approach provides important information about the processes involved in brain differentiation they are invariably complicated by unwanted effects on genital morphology. Within this framework, animals that exhibit same-sex mate preferences are assumed to have experienced perinatal hormonal environments that are more typical of the opposite sex (3). Thus, males that prefer same-sex sexual partners are thought to have experienced a subthreshold exposure or response to testosterone that is more typical of females leading, in turn, to incomplete masculinisation and/or defeminisation during development. The ability to identify and study a small proportion of rams that exhibit exclusive same sex preference has provided us with an opportunity to test the organisation/activational hypothesis in unmanipulated animals by examining correlations between endocrine responses, brain morphology, and sexual preferences. The current review will provide a synthesis of recent research on this novel and important animal model.
The Ram Model
Sheep are seasonally breeding animals that are most active during the autumn in the Northern Hemisphere. The decrease in day length at this time elicits increases in gonadotrophin, which stimulates gamete production and gonadal steroid secretion in both rams and ewes. Cyclic elevations in progesterone and the increase in oestradiol that accompanies ovulation trigger the expression of oestrous behaviour in ewes, while tonic secretion of testosterone by the testis activates copulatory behaviour in rams. Ewes in oestrous transmit sexually stimulating olfactory cues that together with other sensory and behavioural signals attract sexually interested rams. Most adult domestic rams court, mount, and mate with oestrous ewes. However, there are significant numbers of adult rams that are healthy but show very little or no sexual interest in oestrous ewes. These rams have been called ‘homosexual’ (6), ‘non-workers’ (7), ‘low response rams’ (8) to distinguish them from rams with a typically vigorous sexual attraction to ewes. Zenchak et al. (9) first reported that some of these seeming low libido rams actually show considerable sexual behaviour directed towards rams and concluded their failure to mate oestrous ewes was a consequence of their preference for rams as sexual partners. Mounting and genital contact occurs between rams in wild populations and appears to be exhibited within the context of dominance encounters in all-male groups during the non-breeding and early breeding seasons (10). In free-ranging sheep flocks rams remain in mixed sex groups until puberty. However, under farm conditions, rams are usually segregated from ewes between weaning and their first mating experience at 18 to 24 months. Although it has been suggested that the expression of same sex behaviour in rams accompanies these management practices (11), research clearly shows that prepubertal exposure of rams to ewes enhances sexual performance, but does not prevent the same-sex attractions from developing in rams (12,13).
There are now a substantial number of reports documenting the occurrence of same-sex sexual preferences in domestic rams (14-21). We have adopted the terminology “male-oriented ram” to distinguish rams with exclusive preferences for same-sex sexual partners from “female-oriented rams” that exhibit male-typical preferences for oestrous ewes. Male-orientation differs from occasional displays of male-male mounting behaviour observed in most rams. Male-oriented rams will ignore an oestrous ewe and instead mount and sometimes ejaculate with rams when they are presented with both stimuli in a sexual partner preference test.
Ram sexual preferences have been characterised in our programme by extensive testing over a two to three year period. Initially a performance test is administered during which 18-mo-old rams are exposed individually to three oestrous ewes on 9 -18 separate occasions during a two month period. The tests last 30 minutes and the number of courtship behaviours, mounts, and ejaculations are recorded. Rams that fail to perform are given overnight exposure to ewes to assure that they are not inhibited by the testing situation. After the performance test rams are classified as potentially male-oriented, female-oriented, or asexual. Each ram is then exposed to two restrained rams and two restrained oestrous ewes in a standardised 30 minutes sexual preference test during which all behavioural interactions are recorded. This test is given when the rams are ∼16 - 18 month-old and again one year later.
At the end of this testing sequence, rams are assigned to one of four classifications based on their mounting preferences. Male-oriented rams exclusively mount other rams. Female-oriented exclusively mount oestrous ewes. Bisexual rams mount both rams and ewes, although not necessarily to the same extent, and asexual rams mount neither stimulus. By far the largest group of rams is female-oriented (∼60%), while ∼8 -10% are male-oriented, ∼12-18% are asexual, and ∼18-22% are bisexual (18,22,23).
1. Endocrine Correlates of Partner Preference Behaviour
a. Endocrine responses of male oriented rams to sexual stimuli
Olfactory signals have an important role in facilitating reproduction in both rams and ewes. The odor of ram’s fleece stimulates LH secretion in ewes at the start of the breeding season (ram effect), whereas the presence of an oestrous ewe induces LH and testosterone secretion in sexually active female-oriented rams (ewe effect) (24-26). Sexually inactive and male-oriented rams do not show enhanced hormone secretion when exposed to an oestrous ewe or a sexually mature ram (15,16). Interestingly, sexually active rams also display greater levels of investigatory olfactory behaviour toward stimulus animals, which may account for their ability to discriminate between sexes and exhibit a neuroendocrine response only when exposed to oestrous ewes. These results suggest that asexual rams and male-oriented rams do not detect and/or process olfactory cues exactly like female-oriented rams. However, despite these behavioural and neuroendocrine differences, Alexander et al. (19) found no differences between male-oriented and female-oriented rams in numbers of Fos- and Fra-positive immunoreactive neurons in the hypothalamus 3 h after exposure to oestrous ewes.
b. Relationship of serum steroid concentrations to male preferences
Testosterone is critical for the expression of masculine sexual behaviours in all vertebrates (27). Minimal threshold concentrations of serum testosterone are required for expression of mating behaviour in sheep (28). Several reports have shown that the concentration of serum testosterone is not different between male- and female-oriented rams indicating that variations in androgen concentrations cannot account for differences in sexual partner preference and sexual drive in adult rams (16,21,29). In contrast, we observed that in anaesthetised rams concentrations of testosterone in both jugular and testicular vein serum are greater in female-oriented than in male-oriented and asexual rams (14,29). The difference in androgen concentrations between conscious and anaesthetised rams may relate to either a direct effect of anaesthesia on the central nervous system, or to acute elevations in cortisol induced by the endocrine response to anaesthesia and immobilisation. Cortisol can, in turn, suppress androgen synthesis by acting on the hypothalamic and pituitary (30,31) or directly on the testis (32,33). Cortisol levels were higher in male-oriented and asexual rams than in female-oriented rams when blood samples were taken under anaesthesia. These results indicate that male-oriented and asexual rams experienced more anaesthesia-related stress than female-oriented rams. In contrast, cortisol levels measured under control conscious conditions did not differ among groups. However, this distinction in stress response between ram classes was not observed in a subsequent study that used restraint as the acute stressor (34). The discrepancy indicates that anaesthesia unmasks dissimilarities between ram classes that cannot be explained simply by a variation in stress responsiveness but could entail differences in the direct effect that anaesthesia exerts on the central nervous system, further supporting the idea that functional differences exist between the brains of rams that differ in sexual behaviour expression and partner preference.
c. Oestrogen responsiveness of male-oriented rams
The positive feedback effect of oestrogen on gonadotrophin secretion is sexually dimorphic in sheep (35). Treatment of intact, ovariectomised or anoestrous ewes with oestradiol initially decreases serum LH (negative feedback), but is followed by an LH surge within 16 - 22 h (positive feedback). Oestrogens exert negative feedback, but fail to evoke positive feedback, in rams and ewes exposed prenatally to androgen. Male- and female-oriented rams, whether intact or castrated, exhibit similar feedback responses to oestradiol (17,36). Both classes of rams exhibit an initial negative feedback response to oestradiol injections, followed by a prolonged recovery and overshoot of LH concentrations that does not meet the criterion of an LH surge (i.e. LH values exceeding twice the average pre-oestradiol baseline concentration for a minimum of six consecutive hours (37)).
Reproductive behaviours in sheep are strongly influenced by gonadal hormones and are also sexually dimorphic. Sexual activity in ewes is under the control of progesterone and oestradiol. Progesterone derived from the corpus luteum primes the central nervous system for the subsequent induction of oestrous behaviour by oestradiol. When paired with a ram, a ewe in oestrous will permit a male to investigate her genital region and eventually stand still. Once the ewe stands for the ram she will usually tail-fan and turn her head back to the ram while remaining immobile. This behaviour is quickly followed by the ram mounting. Oestrogens fail to promote oestrous behaviours in rams or prenatally androgenised ewes. Similar to their inability to express an LH surge, male- and female-oriented rams do not exhibit female-typical behaviours in response to progesterone and oestrogen (36). Taken together these data suggest that the neural mechanisms controlling LH surge and female-typical responses to oestradiol are defeminised in male-oriented rams.
2. Neurobiological correlates of ram sexual partner preference
Perkins et al. (17) were first to suggest that male-oriented rams exhibited neurobiological attributes more typical of ewes than of female-oriented rams when they reported that the oestrogen receptor content of the amygdala was significantly higher in female-oriented rams than in male-oriented rams and ewes. This observation is interesting because the amygdala, especially the medial amygdala, has been suggested as one candidate site for integrating chemosensory and hormonal cues important for the expression of opposite-sex odor preferences and copulatory behaviours (38,39). Further evidence that the amygdala is important for the expression of sexual partner preference is the recent observation that amygdala connectivity is sex-atypical in homosexual men and women (40).
Resko et al. (14) reported that aromatase activity is significantly higher in the medial preoptic area of female-oriented rams than of male-oriented rams (ewes were not used for comparison in this study). Previous studies in rats demonstrated that the capacity for aromatisation in the medial preoptic is greater in males than in females and this difference is sexually differentiated in rats (41). Thus, it can be speculated that the reason for lower levels of aromatase activity in adult male-oriented rams was the result of processes that occurred prenatally.
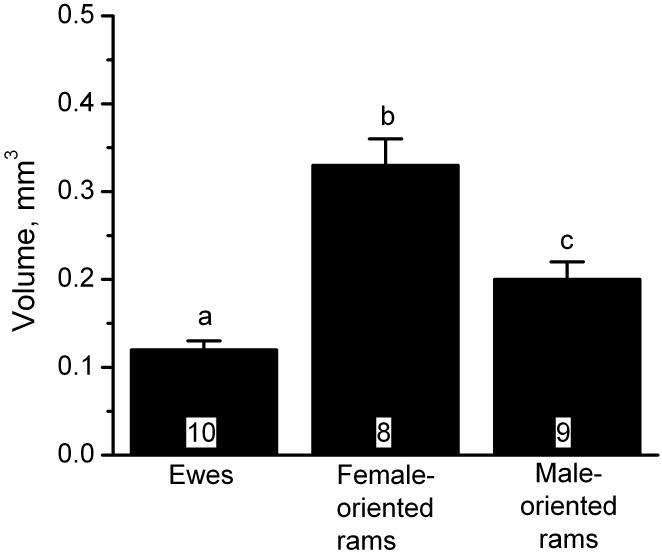
In 2004, we reported that male-oriented rams, like ewes (in midluteal phase), have a smaller cluster of cells in the preoptic area/anterior hypothalamus than female-oriented rams (42). This area was named the ovine sexually dimorphic nucleus (oSDN) because of its neuroanatomical similarity to the sexually dimorphic nucleus of the preoptic area in rats. The oSDN is also reminiscent of the INAH3 in humans, which is smaller in homosexual men and heterosexual women than in heterosexual men (43). The oSDN is an oblong group of cells situated bilaterally adjacent to the third ventricle comprising the central component of the medial preoptic nucleus. It can be identified by both its dense Nissl stain and high expression of aromatase mRNA (Figure 1). The correlation between oSDN size and sexual preferences are believed to be functionally important because the medial preoptic area/anterior hypothalamus is essential for the expression of male copulatory behaviours and has been shown to play an important role in male typical sexual partner preferences (44). Differences in the size of the oSDN among female-oriented rams, male-oriented rams and ewes are not due to differences in adult exposure to testosterone because these differences persist even after adult sheep are gonadectomised and treated with physiological concentrations of T. Rather, recent observations from our laboratory suggest that the oSDN develops prior to birth.
Figure 1.
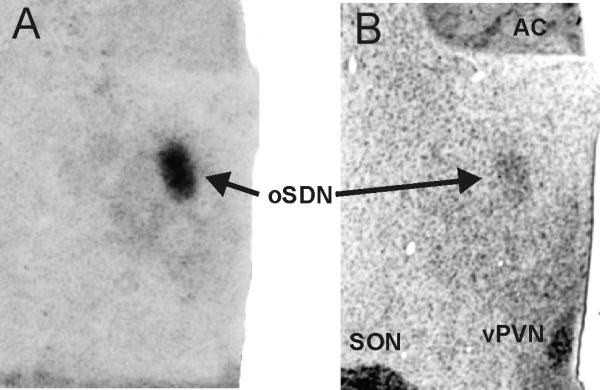
Coronal view through the medial preoptic area-anterior hypothalamus showing the location of the oSDN. (A) Digitised autoradiogram illustrating aromatase mRNA expression. (B) Thionin stained adjacent brain section. Abbreviations: AC, anterior commissure; SON, supraoptic nucleus; vPVN, ventral paraventricular nucleus. Reprinted with permission from (23)
3. Developmental origins of adult sexual partner preferences
To better understand the process of brain differentiation in sheep and its role in sexual preference, we studied the ontogeny of the oSDN in fetal lambs and found that aromatase-containing neurons constitute a nascent oSDN as early as day 60 of gestation, which clearly becomes sexually dimorphic by d 135 when it is two times larger in males than in females. Exposure of fetal female lambs to exogenous testosterone from day 30 to 90 of gestation resulted in an enlarged or masculinised oSDN typical of genetic males (45). These data indicate that sexual differentiation of the oSDN occurs prenatally. These results raise the question of whether differences in oSDN volume between adult male- and female-oriented rams also arise from individual variations in prenatal hormone exposure. This question cannot be answered directly since there is no way of determining which male fetuses will express same-sex attractions as adults. Nonetheless, the larger volume of the oSDN in males and testosterone exposed females suggests that the numbers of neurons and/or volume of neurophil comprising the oSDN are established by androgen exposure during early development and may later predispose adult rams to express a sexual attraction to either ewes or other rams.
Given the correlation between sexually differentiated structure of the oSDN and adult sexual partner preferences, we attempted to influence the organisation of adult sexual partner preferences in rams by pharmacologically disrupting testosterone’s action in the developing brain, using the aromatase inhibitor ATD (1,4,6-androstatriene-3,17-dione) during the period of gestation when the sheep brain is most sensitive to the behaviour modifying effects of exogenous testosterone, i.e. gestational days 50 - 80 (46). We reported initially that aromatase activity is high within the fetal lamb hypothalamus during the critical period and that ATD injections given to pregnant ewes inhibited aromatase activity in the fetal lamb brain by >85% (47). However, ATD exposure under this experimental paradigm did not interfere with the expression of male-typical sexual partner preferences, or defeminisation of receptive behaviour and LH surge mechanism. We next evaluated higher doses of ATD administered for longer periods of gestation that encompassed the entire critical period of gestational days 30 - 90. Again, ATD exposed rams did not differ from control rams in terms of sexual partner preferences, age of sexual maturation, copulatory behaviours, or behavioural and LH surge responses to oestrogen. Moreover, the volume of the oSDN was not reduced after exposure to ATD in either of these experiments (our unpublished observations). These results indicate that aromatisation is unlikely to account for brain masculinisation and defeminisation in the ram. Instead, brain sexual differentiation is possibly controlled through an androgen receptor mechanism. Further studies are needed to test this hypothesis by determining whether pharmacological treatments which block androgen receptors during fetal development can disrupt male-typical sexual differentiation in rams.
Conclusions
The domestic ram is a unique animal model that exhibits exclusive same-sex sexual partner preferences. Male-oriented rams actively court other rams using male-typical sexual solicitations (e.g. genital sniffs, licks, nudges, and leg kicks), while completely ignoring oestrous ewes. Yet, with respect to their responses to gonadal hormones and capacity to show sex-typical consummatory behaviours, male-oriented rams show male-typical mounting, not oestrogen-induced receptivity and LH surge secretion. These observations can be interpreted to suggest that male-oriented rams, like female-oriented rams, are masculinised and defeminised with respect to mounting, receptivity, and gonadotrophin secretion, but are not defeminised for sexual partner preferences. This is one of few examples, other than humans and nonhuman primates (48), where sexual behaviours and sexual partner preferences are dissociated suggesting that these behaviours may be programmed differently. Together with their female-typical mate preference, male-oriented rams have a small, i.e., female-typical, oSDN. This observation reinforces the notion that there are aspects of brain structure and function which are also not completely defeminised in male-oriented rams. Although the exact function of the oSDN is not yet known, its volume, length, and cell number correlate with sexual partner preference and, as such, a dimorphism in its volume and of cells could bias the processing of sexually relevant sensory cues involved in mate choice. Finally, although our research suggests that the oSDN develops prenatally, more research is needed to understand the requirements and timing of its development and ultimately whether and how prenatal programming effects the expression of sexual partner preferences in adults.
Figure 2.
Differences in the volume of the oSDN among luteal phase ewes and gonadally-intact female- and male-oriented rams. Data are presented as means ± SEM. Bars marked with different letters are significantly different; P < 0.05. Reprinted with permission from (42).
Acknowledgements
Support for this research was provided by NIH grant R01 RR 14270. The authors gratefully acknowledge their collaborators, students, and technicians for their many contributions to the work discussed in this review.
References
- 1.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 2.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:253–286. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 3.Adkins-Regan E, Mansukhani V, Thompson R, Yang S. Organizational actions of sex hormones on sexual partner preference. Brain Res Bull. 1997;44:497–502. doi: 10.1016/s0361-9230(97)00231-1. [DOI] [PubMed] [Google Scholar]
- 4.Baum MJ. Mammalian animal models of psychosexual differentiation: When is ‘translation’ to the human situation possible? Horm Behav. 2006;50:579–588. doi: 10.1016/j.yhbeh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Bakker J. Sexual differentiation of the neuroendocrine mechanisms regulating mate recognition in mammals. J Neuroendocrinol. 2003;15:615–621. doi: 10.1046/j.1365-2826.2003.01036.x. [DOI] [PubMed] [Google Scholar]
- 6.Price EO. Male sexual behavior. Vet Clin North Am Food Anim Pract. 1987;3:405–422. doi: 10.1016/s0749-0720(15)31161-0. [DOI] [PubMed] [Google Scholar]
- 7.Fisher A, Matthews L. The social behavior of sheep. In: Keeling LJ, Gonyou HW, editors. Social Behaviour in Farm Animals. CAB Publishing; New York: 2001. pp. 211–245. [Google Scholar]
- 8.Zenchak JJ, Anderson GC. Sexual performance levels of rams (ovis aries) as affected by social experiences during rearing. J Anim Sci. 1980;50:167–174. [Google Scholar]
- 9.Zenchak JJ, Anderson GC, Schein MW. Sexual partner preferences of adult rams (ovis aries) as affected by social experiences during rearing. Appl Anim Ethol. 1981;7:157–167. [Google Scholar]
- 10.Geist V. Mountain Sheep A Study in Behavior and Evolution. The University of Chicago Press; Chicago: 1974. [Google Scholar]
- 11.Lynch JJ, Hinch GN, Adams DB. The Behaviour of Sheep: Biological Principles and Implications for Production. CSIRO Publications; East Melbourne: 1992. The reproductive behaviour of sheep; pp. 96–125. [Google Scholar]
- 12.Katz LS, Price EO, Wallach SJR, Zenchak JJ. Sexual performance of rams reared with and without females after weaning. J Anim Sci. 1988;33:1166–1171. doi: 10.2527/jas1988.6651166x. [DOI] [PubMed] [Google Scholar]
- 13.Price EO, Borgwardt R, BLACKSHAW JK, BLACKSHAW A, Dally MR, Erhard H. Effect of early experience on the sexual performance of yearling rams. Appl Anim Behav Sci. 1994;42:41–48. [Google Scholar]
- 14.Resko JA, Perkins A, Roselli CE, Fitzgerald JA, Choate JVA, Stormshak F. Endocrine correlates of partner preference behavior in rams. Biol Reprod. 1996;55:120–126. doi: 10.1095/biolreprod55.1.120. [DOI] [PubMed] [Google Scholar]
- 15.Perkins A, Fitzgerald JA. Luteinizing hormone, testosterone, and behavioral response of male-oriented rams to estrous ewes and rams. J Anim Sci. 1992;70:1787–1794. doi: 10.2527/1992.7061787x. [DOI] [PubMed] [Google Scholar]
- 16.Alexander BM, Stellflug JN, Rose JD, Fitzgerald JA, Moss GE. Behavior and endocrine changes in high-performing, low-performing, and male-oriented domestic rams following exposure to rams and ewes in estrus when copulation is precluded. J Anim Sci. 1999;77:1869–1874. doi: 10.2527/1999.7771869x. [DOI] [PubMed] [Google Scholar]
- 17.Perkins A, Fitzgerald JA, Moss GE. A comparison of LH secretion and brain estradiol receptors in heterosexual and homosexual rams and female sheep. Horm Behav. 1995;29:31–41. doi: 10.1006/hbeh.1995.1003. [DOI] [PubMed] [Google Scholar]
- 18.Price EO, Katz LS, Wallach SJR, Zenchak JJ. The relationship of male-male mounting to the sexual preferences of young rams. Appl Anim Behav Sci. 1988;21:347–355. [Google Scholar]
- 19.Alexander BM, Rose JD, Stellflug JN, Fitzgerald JA, Moss GE. Fos-like immunoreactivity in brain regions of domestic rams following exposure to rams or ewes. Physiol Behav. 2001;73:75–80. doi: 10.1016/s0031-9384(01)00441-3. [DOI] [PubMed] [Google Scholar]
- 20.Alexander BM, Rose JD, Stellflug JN, Fitzgerald JA, Moss GE. Low-sexually performing rams but not male-oriented rams can be discriminated by cell size in the amygdala and preoptic area: a morphometric study. Behav Brain Res. 2001;119:15–21. doi: 10.1016/s0166-4328(00)00335-1. [DOI] [PubMed] [Google Scholar]
- 21.Pinckard K, Stellflug J, Williams M, Stormshak F. Influence of castration and estrogen replacement on sexual behavior of female-oriented, male-oriented, and asexual rams. J Anim Sci. 1998;78:1947–1953. doi: 10.2527/2000.7871947x. [DOI] [PubMed] [Google Scholar]
- 22.Perkins A, Fitzgerald JA, Price EO. Luteinizing hormone and testosterone response of sexually active and inactive rams. J Anim Sci. 1992;70:2086–2093. doi: 10.2527/1992.7072086x. [DOI] [PubMed] [Google Scholar]
- 23.Roselli CE, Larkin K, Schrunk JM, Stormshak F. Sexual partner preference, hypothalamic morphology and aromatase in rams. Physiol Behav. 2004;83:233–245. doi: 10.1016/j.physbeh.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Booth WD, Signoret JP. Olfaction and reproduction in ungulates. Oxf Rev Reprod Biol. 1992;14:263–301. [PubMed] [Google Scholar]
- 25.Perkins A, Fitzgerald JA, Price EO. Luteinizing Hormone and Testosterone Response of Sexually Active and Inactive Rams. J Ani Sci. 1992;70:2086–2093. doi: 10.2527/1992.7072086x. [DOI] [PubMed] [Google Scholar]
- 26.Rosa HJD, Juniper DT, Bryant MJ. The effect of exposure to oestrous ewes on rams’ sexual behaviour, plasma testosterone concentration and ability to stimulate ovulation in seasonally anoestrous ewes. Appl Anim Behav Sci. 2000;67:293–305. doi: 10.1016/s0168-1591(00)00086-1. [DOI] [PubMed] [Google Scholar]
- 27.Hull EM, Meisel RL, Sachs BD. Male sexual behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; San Diego: 2002. pp. 3–137. [Google Scholar]
- 28.D’Occhio MJ, Brooks DE. Threshold of plasma testosterone required for normal mating activity in male sheep. Horm Behav. 1982;16:383–394. doi: 10.1016/0018-506x(82)90047-2. [DOI] [PubMed] [Google Scholar]
- 29.Roselli CE, Stormshak F, Stellflug JN, Resko JA. Relationship of serum testosterone concentrations to mate preferences in rams. Biol Reprod. 2002;67:263–268. doi: 10.1095/biolreprod67.1.263. [DOI] [PubMed] [Google Scholar]
- 30.Matteri RL, Watson JG, Moberg GP. Stress or acute adrenocorticotrophin treatment suppresses LHRH-induced LH release in the ram. J Reprod Fertil. 1984;72:385–393. doi: 10.1530/jrf.0.0720385. [DOI] [PubMed] [Google Scholar]
- 31.Juniewicz pe, Johnson BH, Bolt DJ. Effect of adrenal steroids on testosterone and luteinizing hormone secretion in the ram. J Androl. 1987;8:190–196. doi: 10.1002/j.1939-4640.1987.tb02430.x. [DOI] [PubMed] [Google Scholar]
- 32.Orr TE, Mann DR. Role of glucocorticoids in the stress-induced suppression of testicular steroidogenesis in adult male rats. Horm Behav. 1992;26:350–363. doi: 10.1016/0018-506x(92)90005-g. [DOI] [PubMed] [Google Scholar]
- 33.Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- 34.Stellflug JN. Comparison of cortisol, luteinizing hormone, and testosterone responses to a defined stressor in sexually inactive rams and sexually active female-oriented and male-oriented rams. J Anim Sci. 2006;84:463–468. doi: 10.2527/2006.8461520x. [DOI] [PubMed] [Google Scholar]
- 35.Karsch FJ, Foster DL. Sexual differentiation of the mechanism controlling the preovulatory discharge of luteinizing hormone in sheep. Endocrinoolgy. 1974;97:373–379. doi: 10.1210/endo-97-2-373. [DOI] [PubMed] [Google Scholar]
- 36.Stormshak F, Estill CT, Resko JA, Roselli CE. Changes in LH secretion in response to an estradiol challenge in male- and female-oriented rams and in ewes. Reproduction. 2008;135:733–738. doi: 10.1530/REP-07-0505. [DOI] [PubMed] [Google Scholar]
- 37.Masek KS, Wood RI, Foster DL. Prenatal dihydrotestosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in sheep. Endocrinology. 1999;140:3459–3466. doi: 10.1210/endo.140.8.6913. [DOI] [PubMed] [Google Scholar]
- 38.Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur J Neurosci. 2006;24:3541–3552. doi: 10.1111/j.1460-9568.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- 39.Wood RI. Integration of chemosensory and hormonal input in the male Syrian hamster brain. Ann N Y Acad Sci. 1998;855:362–372. doi: 10.1111/j.1749-6632.1998.tb10594.x. [DOI] [PubMed] [Google Scholar]
- 40.Savic I, Lindstrom P. PET and MRI show differences in cerebral asymmetry and functional connectivity between homo- and heterosexual subjects. Proc Natl Acad Sci USA. 2008;105:9403–9408. doi: 10.1073/pnas.0801566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roselli CE, Klosterman SA. Sexual differentiation of aromatase activity in the rat brain: Effects of perinatal steroid exposure. Endocrinology. 1998;139:3193–3201. doi: 10.1210/endo.139.7.6101. [DOI] [PubMed] [Google Scholar]
- 42.Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145:478–483. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- 43.LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science. 1991;253:1034–1037. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- 44.Paredes RG. Medial preoptic area/anterior hypothalamus and sexual motivation. Scand J Psychol. 2003;44:203–212. doi: 10.1111/1467-9450.00337. [DOI] [PubMed] [Google Scholar]
- 45.Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology. 2007;148:4450–4457. doi: 10.1210/en.2007-0454. [DOI] [PubMed] [Google Scholar]
- 46.Roselli CE, Schrunk JM, Stadelman HL, Resko JA, Stormshak F. The effect of aromatase inhibition on the sexual differentiation of the sheep brain. Endocrine. 2006;29:501–512. doi: 10.1385/ENDO:29:3:501. [DOI] [PubMed] [Google Scholar]
- 47.Roselli CE, Resko JA, Stormshak F. Estrogen synthesis in fetal sheep brain: effect of maternal treatment with an aromatase inhibitor. Biol Reprod. 2003;68:370–374. doi: 10.1095/biolreprod.102.007633. [DOI] [PubMed] [Google Scholar]
- 48.Vasey PL. Same-sex sexual partner preference in hormonally and neurologically unmanipulated animals. Annu Rev Sex Res. 2002;13:141–179. [PubMed] [Google Scholar]